Abstract
COVID-19, occurring due to SARS-COV-2 infection, is the most recent pandemic disease that has led to three million deaths at the time of writing. A great deal of effort has been directed towards altering the virus trajectory and/or managing the interactions of the virus with its subsequent targets in the human body; these interactions can lead to a chain reaction-like state manifested by a cytokine storm and progress to multiple organ failure. During cytokine storms the ratio of pro-inflammatory to anti-inflammatory mediators is generally increased, which contributes to the instigation of hyper-inflammation and confers advantages to the virus. Because cytokine expression patterns fluctuate from one person to another and even within the same person from one time to another, we suggest a road map of COVID-19 management using an individual approach instead of focusing on the blockbuster process (one treatment for most people, if not all). Here, we highlight the biology of the virus, study the interaction between the virus and humans, and present potential pharmacological and non-pharmacological modulators that might contribute to the global war against SARS-COV-2. We suggest an algorithmic roadmap to manage COVID-19.
1. Introduction
Deaths due to SARS-COV-2 infection have officially surpassed 3 million, with the probable number of victims being much higher. The latest surges of contagion are perhaps stronger than the original wave and seem to be unstoppable. While vaccination is considered the best solution to eradicating the virus and halting the pandemic, the number of people vaccinated worldwide is very small, especially in the nations currently hardest hit, and probably will proceed very slowly. This leaves the health services that must save the lives of people already infected with few options at their disposal. The need is particularly critical given that the available evidence suggests the current treatment modalities have only a minimal impact on survival. Indeed, the significant variability in the clinical picture and outcomes in COVID-19 patients means that any single, comprehensive approach to treatment is a dead-end road. Further, our perceptions of COVID-19 and what we know of the virus, diagnosis, disease symptoms, course, treatment, and aftermath of its infection have changed with time.
If patients are to be treated with new possible therapeutic options, the basic biology of the infection process, especially the proteins and physiological conditions associated with the phases of the development of the disease, must be delineated to understand the challenges and tailor treatment to each individual. In this review, we highlight the biology of the virus, discuss the interaction between the virus and humans, and present an algorithmic roadmap of potential pharmacological and non-pharmacological modulators that might reduce the clinical effects of SARS-COV-2 infection. Adopting individualized medicine and pharmacodiagnostics might represent a more effective rationale, at least in severely ill patients.
2. Historical Preface
In 1930, a newly isolated virus strain caused acute respiratory infection in chickens, termed infectious bronchitis virus (IBV) [1,2]. Then, two additional animal viruses were isolated: In 1946, transmissible gastroenteritis virus (TGEV) affected pigs, and in 1949, mouse hepatitis virus (MHV) affected mice [3,4]. In the 1960s, the era of human coronaviruses started when David Tyrrell and ML Bynoe isolated a viral strain, called virus B814, from respiratory samples from a schoolboy with a common cold [5,6]. In the same decade, two additional species were isolated including human coronavirus 229E and human coronavirus organ culture 43 (HCoV-OC43) [7,8]. Almeida et al. called this group of viruses “coronavirus” as they resemble the “solar corona” or they are “in a crown” [9]. Feline infectious peritonitis virus in was isolated in 1963 [10], canine virus was isolated in 1971 [11,12], and porcine epidemic diarrhea virus (PEDV) was isolated in 1978 [13,14]. Finally, at the end of December 2019, three patients from the seafood and wet animal wholesale market in Wuhan, China were admitted to the hospital with pneumonia of unknown causes [15]. Later, Zhu et al. reported a novel beta-coronavirus, “CoV (2019-nCoV),” as the cause of this pneumonia. This novel virus was named “novel coronavirus-infected pneumonia” (NCIP), later known as SARS-COV-2, [15], and has evolved, consequently, into ten subtypes thus far [16] (See Figure 1).
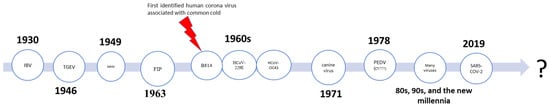
Figure 1.
Summary of the timeline of discovery of many of coronavirus family members.
3. Virus Biology
The entry process depends on several factors, including virus-related factors (e.g., binding proteins) and host-related factors (e.g., tissue tropism or the tissue’s ability to receive and accommodate the virus) [17]. The process of virus fusion and entry is more complex, but, in general, it depends on acidification and/or proteolytic activation [18,19]; therefore, it is not surprising if systemic alkalization would be a rational approach to disrupt virus entry [20]. The fusion process occurs between the interaction of virus proteins and host receptor proteins. There are many viral proteins, one example being the S protein. The S protein is one of four structural proteins encoded by the CoV single-stranded, positive-sense RNA genome. In the viral membrane, the S protein functions to (i) interact with the cellular receptor and (ii) induce viral fusion with the cell via the plasma membrane protein angiotensin-converting enzyme 2 (ACE2) [21,22,23,24].
The S protein needs to be primed by an appropriate protease at the S1 and S2 interface (S1/S2) to catalyze the membrane fusion reaction and trigger the FPs immediately upstream (S2′). What is interesting about this triggering event is that several proteases can trigger it and it is the protease requirements that drive viral tropism.
Both SARS-CoV and MERS-CoV S can be triggered to fuse at either the plasma membrane or the endosomal membrane, and their route of access is determined by protease availability while being governed by the attachment of the surface unit, S1, of the S protein to a cellular receptor, facilitating viral attachment to a target cells’ surface. Additionally, access involves S protein priming by cellular proteases, which involves S protein cleavage at the S1/S2 and the S2′ site and allows fusion of viral and cellular membranes, a process driven by the S2 subunit [25]. The SARS-COV-2 engages ACE2 as the entry receptor and employs the cellular serine protease TMPRSS2 for S protein priming [25].
Angiotensin-converting enzyme 2 (ACE2) receptors were shown to be the SARS-CoV-2 gateway to the cell [20,21,22]. ACE2 is an enzyme affixed to the plasma membrane (outer membrane) and is widely expressed among various human tissues. Expression levels differ from higher expression (small intestine, testis, kidneys, heart, thyroid, and adipose tissue), to medium expression (lungs, colon, liver, bladder, and adrenal gland), and to the lowest expression (blood, spleen, bone marrow, brain, blood vessels, and muscle) [26]. While lungs show medium expression compared to other tissues, SARS-COV-2 initially infects the lung [27]. However, we do not know whether the virus is disseminated to other parts of the body or not [21]. If yes, it will be of significant progress to know whether the multiple organ failure is associated with the side effect of drugs, ARDS, or with virus entry to that tissue [28,29,30,31,32].
After viral fusion with the plasma membrane, virus internalization can occur through various routes (Figure 2) [33,34,35]:
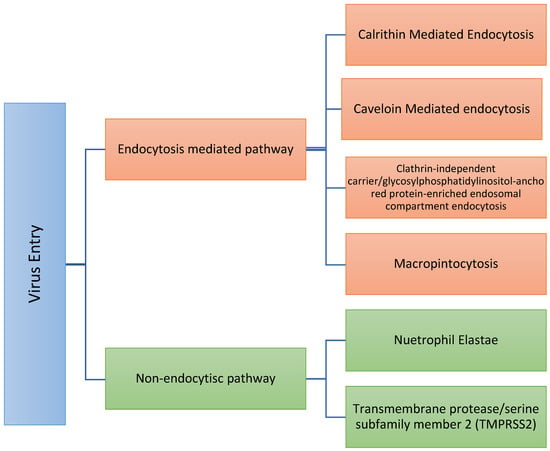
Figure 2.
Summary of the different routes of virus entry. Therefore, proper determination of the exact route of entry might enhance the selection rationale for which targeted therapy should be used.
Non-endocytic pathway: This pathway could also be termed a “proteases-directed internalization pathway.” After S protein binding to the receptor (not before), host proteases mediate virus–cell fusion [21,35,36]. Some of these proteases are trans-plasma membrane proteins. They play a crucial role in COVID-19. Some of these proteases include the following:
- -
- Neutrophil elastase (NE): Elastase is a serine protease that also hydrolyzes amides. Neutrophil elastase is one of eight elastases found in the body. That NE could mediate entry is clinically vital since elastase is produced by neutrophils in the lungs during SARS-CoV-2 infection and could promote the progression of SARS-CoV-2 infection [16]. Therefore, as the hallmark of COVID-19, further neutrophils recruitment supports the release of a massive amount of NE and, subsequently, further virus entry [37,38,39] in a positive feedback cycle. Sivelestat is a NE inhibitor [40,41] and, so, might limit SARS-COV-2 entry. In this respect, we are not sure if the medications that decrease neutrophil counts will represent a potential treatment or not. Some of these medications include Carbimazole, Clozapine, Dapsone, Dipyrone, Methimazole, Penicillin G, Procainamide, Propylthiouracil, Rituximab, Sulfasalazine, and Ticlopidine;
- -
- Type II transmembrane serine proteases, including transmembrane protease/serine subfamily member 2 (TMPRSS2) [42], also known as human epitheliasin [43]. Human TMPRSS2 mRNA is expressed in several tissues, including the prostate, ovary, breast, lung, kidney, pancreas, bile duct, salivary gland, stomach, small intestine, and colon [43,44,45]. Transient expression of TMPRSS2 enhances SARS-CoV-2 S-mediated cell–cell fusion [21]. S protein is primed by TMPRSS2 and, therefore, TMPRSS2 supports virus entry, viral fusion, and spread and further increases the virus [46]. Nafamostat and Camostat are transmembrane protease inhibitors; serine 2 acts as a TMPRSS2 inhibitor [25,47,48,49]. Further, Nafamostast acts as an anticoagulant drug, which might shed light on how the virus induces thrombosis. Camostast is used for the treatment of pancreatitis. TMPRSS2 expression is subjected to population variability, e.g., TMPRSS2 expression is relatively lower in darker skin than in the white populations, suggesting that the probability of TMPRSS2-dependent virus internalization might be higher in a white population [50]. In addition, the expression of TMPRSS2 increases with aging [49], and TMPRSS2 expression is positively correlated with androgen levels, i.e., the male population [50].
Although TMPRSS2 inhibition could be presented as a promising approach in managing SARS-COV-2 [25], we are not sure if blocking of TMPRSS2 will stop the viral infectivity or if the virus will adapt to such inhibition and undergo cellular internalization through another endocytic pathway, because if the plasma membrane-route proteases are available, the virus can fuse via an “early pathway” at the plasma membrane and, if not, the virus can fuse via a “late pathway” at the endosomal membrane [25].
The Endocytic pathway also could be termed as a receptor-mediated endocytosis pathway. The endocytosis pathway can be carried out through different routes, e.g., clathrin-mediated endocytosis, caveolin-mediated endocytosis, flotillin-dependent endocytosis, macropinocytosis, and clathrin-independent carrier/glycosylphosphatidylinositol-anchored protein-enriched endosomal compartment endocytosis [33,51,52,53,54,55]. An acidic environment activates endosomal proteases such as cathepsin B and cathepsin L, which are known activators of other CoV family members, that become active in the early and late endosomes. To rationally target each approach, it will be wise to determine which route the virus follows to enter the cell endocytotically and then target it as follows.
- -
- Clathrin-mediated endocytosis, the endosomal/lysosomal pathway, is the uptake of material (e.g., ferritin, LDL particles) into the cell from its surface using clathrin-coated vesicles [56], and the clathrin-coated vesicle changes its geometry to accommodate endocytosis [57,58]. Clathrin-mediated endocytosis can be inhibited by Pentamidine [20], dynasore [59], monodansylcadaverine (MDC) [20], depletion of intracellular potassium [20], phenylarside oxide (PAO) [60], cytosolic acidification (ammonium chloride (NH4Cl)) [61], hypertonic shock (sucrose) [62], and chlorpromazine [63]. Importantly, in this respect, a group of French scientists observed a lower prevalence of symptomatic and severe forms of COVID-19 infections in psychiatric patients treated with the anti-psychotic drug chlorpromazine, and formulated the hypothesis that chlorpromazine might be a preventive against COVID-19 [20]. Following this observation, a clinical trial has been set-up [64].
- -
- Caveolin-mediated endocytosis: Caveolae were described first in 1953 by Palade [65,66]. Caveolae and caveolin-containing membrane domains on the plasma membrane have various curvatures and shapes [66]. When the clathrin vesicles fuse with endosomes/lysosomes, the caveosome (multi-caveolar complexes) never fuses with lysosomes and, hence [67,68], it will not be surprising if SARS-COV-2 acts more through caveolin-mediated endocytosis [33] as that represents a successful anti-predator strategy. Vanadate, a tyrosine phosphatase inhibitor, stimulates caveolin-mediated endocytosis, while Nystatin (anti-fungal drug) suppresses the caveolin-mediated endocytosis, and chlorpromazine is non-specific [69]. Brefeldin A (antiviral drug) [70] and Nocodazole (anti-neoplastic drug) [71] also inhibit the caveolin pathway [72].
- -
- Cathepsin B (catB): Cathepsin B is a lysosomal cysteine protease that belongs to the papain family [20,73]. Cathepsin B plays a vital role in intracellular proteolysis. In normal physiological conditions, active cathepsin B is localized to the endosomal/lysosomal compartment and is primarily involved in the normal turnover of intracellular and extracellular proteins, thus maintaining homeostatic metabolic activity within cells [71]. Beyond its effect in the mitochondrial complex I, metformin acts as a catB-inhibitor [73,74]. However, inhibition of catB will result in thyroid dysfunction, as catB is necessary for thyroxin production. Therefore, the targeting of catB should be cautiously monitored.
- -
- Cathepsin L (catL): Cathepsin L can degrade nearly all proteins, including enzymes, receptors, and transcription factors [75]. The physiological function of catL depends on its subcellular localization as follows:
- o
- In endosomes/lysosomes: As it degrades the proteins in lysosomes, catL plays a crucial role in maintaining the lysosome–endosome compartment of the cardiac myocyte. Therefore, any alteration of catL might induce a progressive dilated cardiomyopathy [76,77,78,79]. In addition, disruption of catL might decrease CD+ T cells.
- o
- In the nucleus: Cathepsin L is a double-edged sword, it can either accelerate or inhibit the proliferation based on a couple of factors [75].
- o
- In the cytoplasm: Cathepsin L initiates the lysosomal pathways of apoptosis [80,81,82].
- o
- In the extracellular space: In the inflammatory environment, pro-inflammatory cytokines induce catL expression in endothelial cells, macrophages, and smooth muscle, then the released catL degrades elastin and collagen [75] and so might disrupt the matrix.
Cathepsin L has been identified as a highly effectual enzyme to degrade the viruses’ outermost capsid and underlying proteins as a criterion to initiate an infection. The viruses access the endosomal–lysosomal compartment and thereafter disassemble by endocytosis. The subvirion particles penetrate to the cytoplasm to replicate [75,83]. Therefore, catL plays a critical role in the entry of the SARS-COV-2 virus [84]. Cathepsin L inhibitors such as CTLA-2α [85], the selective N-(benzyloxycarbonyl)-L-phenylalanyl-L-tyrosinal [86], MDL28170 [87], and even slight alkalization show a gradual reduction in fusion due to catL activity [87].
4. Cytokine Storm
It seems that the first introduction of the term cytokine storm was used in early 1993 to describe the role of interleukin 1 on graft-versus-host disease [88]. Cytokine storm might be a synonym to immune attack or massive immune response against foreign bodies, e.g., bacteria [89] and viruses [90,91,92], where SARS-COV-2 is one of those viruses. However, the cytokine storm in COVID-19 surely does not develop directly as an immune response to the virus [93,94] nor as a result of the multiple organ failure [95] due to COVID-19 [93]. A cytokine profile resembling systemic lupus erythematosus (SLE) and/or secondary hemophagocytic lymphohistiocytosis (sHLH), is associated with COVID-19 disease severity and is characterized by an increase in the following:
Interleukin 1 beta (IL-1β), also termed lymphocyte activating factor, leukocytic pyrogen, leukocytic endogenous mediator, or mononuclear cell factor is a pro-protein converted to mature IL-1β by cytosolic caspase 1 [96] and produced by activated macrophages [97]. IL-1β plays a crucial role in mediating autoimmunity [98]. The monoclonal anti-IL-1β antibody, canakinumab, is a suggested inhibitor [99,100]. In this regard, Novartis started a clinical trial to show the effect of Canakinumab against COVID-19 [101]. Curcumin also shows an inhibitory effect on IL-1 [102].
Granulocyte-colony stimulating factor (G-CSF) is a secreted glycoprotein produced by macrophages, endothelial cells, stromal cells, natural killer cells, and T cells [103]. There are four types of CSF: granulocyte-colony stimulating factor, macrophage-colony stimulating factor, granulocyte-macrophage-colony stimulating factor, and multi-colony stimulating factor (interleukin-3) [104]. G-CSF supports production and differentiation of white blood cells. G-CSF supports neutrophil proliferation, and GM-CSF supports macrophages and eosinophils proliferation [105]. Administration of G-CSF is associated with increased lactate dehydrogenase, uric acid, isoenzymes, alkaline phosphatase, and the increase in soluble IL-2 receptors [104], which are found at higher levels in serum of COVID-19 patients [106,107,108,109]. G-CSF plasma levels were also correlated with the severity of COVID-19 (higher in patients in the intensive care unit) [110,111,112]. Therefore, targeting the G-CSF/G-CSF receptor axis to manage COVID-19 should be considered.
Interferon-gamma-inducible protein-10 (IP-10)/CXCL10, also called IP-10, is a small protein chemokine (10kDa) induced by IFN- γ and produced by many cell types, e.g., neutrophils, monocytes, endothelial cells, fibroblasts, and keratinocytes. Physiologically, CXCL10 recruits natural killer cells (NK cells), eosinophils, and leukocytes and increases the Th1/Th2 ratio [113,114,115]. CXCL10 also increases the level of several inflammatory mediators, including TNF-α that promotes inflammation. Therefore, CXCL10 is considered an inflammatory chemokine that contributes to many autoimmune diseases [113,116,117]. The CXCL levels increase 10-fold in COVID-19 and they play a paramount role in its immunopathology [118,119]. CXCL10 inhibition might represent a rational approach to the target, and its inhibitors include Vitamin D [120], Thiazolidinediones [121], Ganodermycin [120], and artemether combined with atorvastatin [120,122,123], although the earlier data about the last example suggested the reverse effect.
Macrophage inflammatory protein 1-α (MIP 1-α) belongs to the family of chemokines, also known as MIP-1alpha chemokine (C-C motif) ligand 3 (CCL3) [124]. Upon activation, they can be expressed by hematopoietic cells and a variety of tissue cells such as fibroblasts, epithelial cells, vascular smooth muscle cells, or platelets [125,126]. MCP 1-α are best known for their chemotactic and pro-inflammatory effects and are crucial for immune responses towards infection and inflammation and are produced by countless cells, particularly macrophages, dendritic cells, and lymphocytes [127]. MIP 1-α is associated with COVID-19 [128,129] and, therefore, targeting MIP 1-α directly or altering it at the receptor level (CCL3 blocker) represents a wise approach to managing COVID-19 [130]. IL-10 inhibits MIP 1-α expression [131].
Monocyte chemoattractant protein 1(MCP-1/CCL2) is also known as the small inducible cytokine A2. Although monocyte/macrophages are the primary sources of CCL2, many cells also produce MCP1, such as endothelial, epithelial, smooth muscle, fibroblasts, mesangial, astrocytic, monocytic, and microglial cells [132,133,134,135]. Those cells are responsible for antiviral immunity. Many factors stimulate MCP-1 production, such as oxidative stress, growth factors, and cytokines. MCP-1 is the critical regulator of migration and infiltration of monocytes and macrophages [132]. MCP1 recruits monocytes, dendritic cells, and T-cells (memory) to the inflammation site because of inflammation and infection [136]. MCP-1 is subject to population variability as it is higher in whiter skin than in darker skin populations [137,138]. MCP-1 is associated with COVID-19 [139,140,141]; therefore, targeting MCP-1 could also be considered in managing SARS-2 infection [130]. Melatonin inhibits MCP-1 expression [142]. Bindarit decreases MCP-1 synthesis [143]. Spiegelmer (L-enantiomeric RNA oligonucleotide mNOX-E36), also called L-ribonucleic acid aptamer, inhibits MCP-1 activity [144]. Furthermore, MCP-1 could be blocked at the receptor level (CCR2 blockage) [143] using compounds such as 747 (a natural combination related in structure to kaempferol) [145] and 15a (an orthostatic CCR2, a small molecule antagonist) [146].
Tumor necrosis factor-α (TNF-α) is also called cachexin or cachectin. TNF-α is a cell-signaling protein produced mainly by macrophages in response to stimuli and mediates the inflammatory response [147,148,149]. A great deal of data showed that TNF-α has a receptor association with COVID-19 and higher TNF-α expression is correlated with disease severity and higher mortality [150,151,152,153]. It is not surprising that anti-TNF-α drugs (adalimumab (A), certolizumab pegol (C), etanercept (E), golimumab (G), and infliximab (I)) are considered anti-COVID-19 treatments [152,153,154,155,156,157] (Table 1).

Table 1.
Cytokines/chemokines that mediate the cytokine storm, with their antagonists.
5. Notes on Some Currently Administered Pharmacological Modulating Agents
Administration off-label of certain drugs (drug repurposing) that target the virus and/or the cytokine storm has become a promising approach in managing COVID-19 [158]; some of these agents include:
Macrolide antibiotics: These antibiotics inhibit bacterial protein synthesis. Therefore, they are clinically used to fight infections by atypical bacterial (bacteria that lack cell walls). Evidence shows their promising activity in the management of COVID-19 [159].
-Azithromycin exerts its immunomodulatory role via the following:
- (i)
- Suppression of LPS-induced MDC and IP-10 expression through the MAPK–JNK and the NFκB–p65 pathways [160];
- (ii)
- Inhibition of the cytoplasmic phospholipase A2, so it might be equivalent to steroids that suppress the release of eicosanoids (prostaglandins, thromboxane, leukotrienes, and HEPTE) [161].
-Clarithromycin is another macrolide antibiotic that is useful in the management of COVID-19 due to its following properties:
- -
- Influence on two steps in the influenza virus entry process. The drug was found to reduce the expression of sialic acid residues on the surface of airway epithelial cells, reduce virus binding, and reduce the number of acidic endosomes in the cell, inhibiting endosomal escape [34];
- -
- Alteration of endosomal pH. Clarithromycin also inhibits the release of NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) [162] and might support the mortality rate due to SARS-COV-2 [163]. The massive release of cytokines in such conditions raises another possible question: Does the cytokine storm support the viral entry and dissemination across the body or is the cytokine storm just a consequence of massive uncontrolled cell death?
- -
- Stabilization of the mast cells [164]. Mast cells secret both histamine and heparin. Therefore, clarithromycin might prevent the massive release of histamine and prevent anaphylaxis and perhaps the blood coagulation that might result from activation of factor XII through the bradykinin activation. However, the prevailing medical dogma is that heparin is an anticoagulant.
Although macrolides have proven useful in their administration as anti-COVID-19 drugs by attenuating the cytokine storm, they exert additional benefits in the management of patients with COVID-19 by reducing the incidence of bacterial co-infection [165].
Amiloride: Amiloride is a well-known potassium-sparing diuretic. It has been shown to inhibit micropinocytosis [69], which might alter this viral entry pathway. It also inhibits the E protein, which is crucial for viral replication [166].
Sodium bicarbonate: Spike proteins of SARS-COV-2 become more disordered at alkaline pH values while becoming more well-formed at more acidic pH values. Therefore, increasing extracellular pH most probably alters the ACE2–SARS-COV-2 interaction dynamics [167]. Hence, sodium bicarbonate used as a buffer has been suggested as it attenuates the hyper-inflammatory environment [168]. In this respect, potassium citrate as a buffer might also be beneficial in COVID-19 treatment [169].
6. Food Supplements That Might Prevent COVID-19 Complications
There are some food supplements that might prevent or decrease the severity of COVID-19 infection; some of these food supplements include:
N-Acetyl Cysteine (NAC): NAC is a precursor of glutathione used to treat paracetamol overdoses and is a mucolytic (loosens thick mucus in the lungs). NAC is an immune booster and an anti-inflammatory and antiviral agent. Therefore, it is a suitable agent for COVD-19 [170], significantly reducing the cytokine storm [171]. NAC has also been used in the management of critically ill septic patients [170].
Vitamin E has a lysosomal membrane stabilization function [172,173] and has an inhibitory effect on the production of several pro-inflammatory cytokines, including IL-1, IL-6, TNF, and the chemokine IL-8, by monocytes and macrophages. Vitamin E can also stimulate the intracellular type I IFN system, which exercises antiviral activities [174]. Therefore, it might be beneficial in prevention and/or management of COVID-19 complications.
Desferrioxamine is an iron-chelating agent. A higher level of transferrin indicates a higher level of iron, which is toxic in humans. Blocking receptor-mediated endocytosis reduced the internalization of some infectious viruses similarly to the reduction of endocytosis by transferrin [175], suggesting that transferrin might be an indicator of how viruses enter the cells and how some possible inhibitors could function by inducing paralysis of receptor-mediated endocytosis, e.g., amiloride and NH4Cl [176]. In this respect, desferrioxamine might show potential activity as an anti-COVID-19 treatment [177].
Zinc is an essential trace element found in humans, plants, and microorganisms. Nearly 10% of human proteins (enzymes, transcriptional factors, etc.) bind with zinc. In plasma, 60% of zinc is bound with albumin and around 10% with transferrin (iron transporter, see above). Thus, if iron is increased, free zinc is reduced (hypozincemia) [178,179]. In COVID-19, the level of iron is increased significantly, and, therefore, the level of free zinc is reduced, and this is associated with poor outcomes [180,181,182]. Thus, a zinc supplement is a wise choice in the management of COVID-19 [183].
Vitamin C is a known antioxidant and its role in treating sepsis has been reported [184,185], which supports the notion that vitamin C could be considered in COVID-19 treatment [186]. A clinical trial involving 140 patients will be conducted in Wuhan, China. The scientists will be assessing the need for the ventilator, vasopressors, organ failure, the length of ICU stay, and the mortality in response to high doses of vitamin C [187].
Vitamin K With the increasing evidence that microthrombi formation is linked to COVID 19 infection [188,189], low molecular weight heparin, Fondaparinux, oral anticoagulants, or vitamin K antagonists have been recommended to all patients with an active infection unless they have a relevant contraindication [190,191,192,193], therefore, the level of vitamin K should be closely adjusted.
Vitamin D (calcitriol), although it is one of the fat-soluble vitamins, it is considered to be a hormone [194,195]. As a hormone, vitamin D activates innate immunity and suppresses adaptive immunity [196,197]. Vitamin D deficiency is also associated with viral infection and is accompanied by acute respiratory distress syndrome [197,198]. Therefore, many trials have been carried to test it against COVID-19.
Melatonin is a hormone mainly secreted from the pineal gland [199]. Melatonin is an enormously powerful antioxidant hormone. Melatonin inhibits the activation of the primary inflammasome NLRP3, which leads to cytokine storms [200]. Besides its activity in the antioxidant cascade [201,202,203,204], melatonin supports the expression of many antioxidant cellular enzymes, including glutathione reductase, glutathione peroxidase, superoxide dismutase, and catalase [205,206]. As some of these enzymes are mitochondrial enzymes, melatonin is also a mitochondrial antioxidant [207,208] and, thus, it can be used to manage COVID-19 [209,210].
7. Roadmap to Manage COVID-19 and Concluding Remarks
COVID-19 is an emerging pandemic disease threatening human life. COVID-19 manifests as hyper-inflammation and heterogenous increases or decreases in cytokines, chemokines, electrolytes imbalance, etc. The significant variability in the clinical picture and outcomes in COVID-19 patients means that any blockbuster approach to treatment is a dead-end road. Adopting individualized medicine and pharmacodiagnostics (predictive medicine) might represent a more effective rationale, at least in severely ill patients [211,212].
Prevention by implementing facemask usage is important; some public health practitioners and theoreticians are raising the possibility of herd immunity, but that comes at a high cost of more deaths [213]. This roadmap for optimizing decision making to tailor and fine-tune therapy to the characteristics of the disease and specific needs of the patient includes (i) managing the infection via its signs and symptoms (e.g., cytokine, hyper-inflammation) and prevention of co-infection by targeting the virus and/or the cytokine storm to avoid co-infection, whether bacterial, viral, or by other microorganisms; and (ii) delaying the post-infection toxicity, e.g., thromboembolism. Moreover, the administration of pharmacological and/or nutraceutical compounds that support the repairing of the injured lung epithelium, especially among older people, will represent a promising strategy in the war against COVID-19.
Author Contributions
K.O.A. contributed to the conceptualization, data curation, formal analysis, investigation, resources, software, and writing (original draft). L.S. and S.J.R. contributed to the supervision, conceptualization, data curation, formal analysis, investigation, resources, software, and writing (review and editing). S.T.S.A., S.B.M.A. and M.S. contributed to the conceptualization, data curation, resources, and writing (original draft). A.A., S.S.A., A.S.A., A.M.A., A.M.R., M.E.A. and H.S.A. contributed to methodology, resources, and software. A.B., J.D., R.A.C. and M.E.I. contributed to the investigation, methodology, and visualization. S.J.R. and L.S. contributed to investigation, methodology, and resources. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Estola, T. Coronaviruses, a new group of animal RNA viruses. Avian Dis. 1970, 14, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Schalk, A.; Hawn, M. An apparently new respiratory disease of baby chicks. J. Am. Vet. Med. Assoc. 1931, 78, 413–422. [Google Scholar]
- McIntosh, K. Coronaviruses: A Comparative Review. In Current Topics in Microbiology and Immunology/Ergebnisse der Mikrobiologie und Immunitätsforschung; Springer: Berlin/Heidelberg, Germany, 1974; pp. 85–129. [Google Scholar]
- Fenner, F.; Bachmann, P.; Gibbs, E.; Murphy, F.; Studdert, M.; White, D. Coronaviridae. In Veterinary Virology; Elsevier: Amsterdam, The Netherlands, 1987; pp. 505–518. [Google Scholar]
- Tyrrell, D.A.J.; Bynoe, M.L. Cultivation of a Novel Type of Common-cold Virus in Organ Cultures. Br. Med. J. 1965, 1, 1467–1470. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, D.A.J. Hunting Common Cold Viruses by Some New Methods. J. Infect. Dis. 1970, 121, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Kahn, J.S.; McIntosh, K. History and Recent Advances in Coronavirus Discovery. Pediatr. Infect. Dis. J. 2005, 24, S223–S227. [Google Scholar] [CrossRef]
- Geller, C.; Varbanov, M.; Duval, R.E. Human coronaviruses: Insights into environmental resistance and its influence on the development of new antiseptic strategies. Viruses 2012, 4, 3044–3068. [Google Scholar] [CrossRef]
- Virology: Coronaviruses. Nature 1968, 220, 650. [CrossRef]
- Pedersen, N.C. A review of feline infectious peritonitis virus infection: 1963–2008. J. Feline Med. Surg. 2009, 11, 225–258. [Google Scholar] [CrossRef] [PubMed]
- Binn, L.N.; Lazar, E.C.; Keenan, K.P.; Huxsoll, D.L.; Marchwicki, R.H.; Strano, A.J. Recovery and characterization of a coronavirus from military dogs with diarrhea. Proc. Annu. Meet. U. S. Anim. Health Assoc. 1974, 359–366. [Google Scholar]
- Pratelli, A. The evolutionary processes of canine coronaviruses. Adv. Virol. 2011, 2011, 562831. [Google Scholar] [CrossRef]
- Pensaert, M.B.; de Bouck, P. A new coronavirus-like particle associated with diarrhea in swine. Arch. Virol. 1978, 58, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.K.; Kim, H.H.; Lee, S.H.; Yoon, S.S.; Park, J.W.; Cho, I.S. Isolation and characterization of a new porcine epidemic diarrhea virus variant that occurred in Korea in 2014. J. Vet. Sci. 2018, 19, 71–78. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, C.; Das, C.; Ghosh, A.; Singh, A.K.; Mukherjee, S.; Majumder, P.P.; Basu, A.; Biswas, N.K. Global Spread of SARS-CoV-2 Subtype with Spike Protein Mutation D614G is Shaped by Human Genomic Variations that Regulate Expression of TMPRSS2 and MX1 Genes. bioRxiv 2020. [Google Scholar] [CrossRef]
- Baron, S.; Fons, M.; Albrecht, T. Viral Pathogenesis. In Medical Microbiology; Baron, S., Ed.; Elsevier Ltd.: Galveston, TX, USA, 1996; ISBN 9780123744104. [Google Scholar]
- Belouzard, S.; Millet, J.K.; Licitra, B.N.; Whittaker, G.R. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses 2012, 4, 1011–1033. [Google Scholar] [CrossRef] [PubMed]
- Chu, V.C.; McElroy, L.J.; Chu, V.; Bauman, B.E.; Whittaker, G.R. The Avian Coronavirus Infectious Bronchitis Virus Undergoes Direct Low-pH-Dependent Fusion Activation during Entry into Host Cells. J. Virol. 2006, 80, 3180–3188. [Google Scholar] [CrossRef]
- Moore, L.L.; Bostick, D.A.; Garry, R.F. Sindbis virus infection decreases intracellular pH: Alkaline medium inhibits processing of sindbis virus polyproteins. Virology 1988, 166, 1–9. [Google Scholar] [CrossRef]
- Tang, T.; Bidon, M.; Jaimes, J.A.; Whittaker, G.R.; Daniel, S. Coronavirus membrane fusion mechanism offers a potential target for antiviral development. Antivir. Res. 2020, 178, 104792. [Google Scholar] [CrossRef]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef]
- Li, W.; Moore, M.J.; Vasllieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greeneugh, T.C.; et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Mü, M.A.; Drosten, C.; Pö, S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-Y.; Li, L.; Zhang, Y.; Wang, X.-S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect. Dis. Poverty 2020, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, S.M.; Yu, X.H.; Tang, S.L.; Tang, C.K. Coronavirus disease 2019 (COVID-19): Current status and future perspectives. Int. J. Antimicrob. Agents 2020, 55, 105951. [Google Scholar] [CrossRef]
- Diao, B.; Feng, Z.; Wang, C.; Wang, H.; Liu, L.; Wang, C.; Wang, R.; Liu, Y.; Liu, Y.; Wang, G.; et al. Human Kidney is a Target for Novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection. medRxiv 2020, 2. [Google Scholar] [CrossRef]
- Maisch, B. SARS-CoV-2 as potential cause of cardiac inflammation and heart failure. Is it the virus, hyperinflammation, or MODS? Herz 2020, 45, 321–322. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, Y.; Gao, R.; Lu, R.; Han, K.; Wu, G.; Tan, W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA 2020, 323, 1843–1844. [Google Scholar] [CrossRef]
- Brancatella, A.; Ricci, D.; Viola, N.; Sgrò, D.; Santini, F.; Latrofa, F. Subacute Thyroiditis After Sars-COV-2 Infection. J. Clin. Endocrinol. Metab. 2020, 105, 2367–2370. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Sun, S.; Xu, C.-H.; Zhang, J.; Xu, Y.; Zhu, H.; Peh, S.-C.; Korteweg, C.; McNutt, M.A.; Gu, J. Pathology of the thyroid in severe acute respiratory syndrome. Hum. Pathol. 2007, 38, 95–102. [Google Scholar] [CrossRef]
- Wang, H.; Yang, P.; Liu, K.; Guo, F.; Zhang, Y.; Zhang, G.; Jiang, C. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 2008, 18, 290–301. [Google Scholar] [CrossRef]
- Thorley, J.A.; McKeating, J.A.; Rappoport, J.Z. Mechanisms of viral entry: Sneaking in the front door. Protoplasma 2010, 244, 15–24. [Google Scholar] [CrossRef]
- Zumla, A.; Chan, J.F.W.; Azhar, E.I.; Hui, D.S.C.; Yuen, K.-Y. Coronaviruses—Drug discovery and therapeutic options. Nat. Rev. Drug Discov. 2016, 15, 327–347. [Google Scholar] [CrossRef] [PubMed]
- Belouzard, S.; Madu, I.; Whittaker, G.R. Elastase-mediated activation of the severe acute respiratory syndrome coronavirus spike protein at discrete sites within the S2 domain. J. Biol. Chem. 2010, 285, 22758–22763. [Google Scholar] [CrossRef]
- Zuo, Y.; Yalavarthi, S.; Shi, H.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.N.; Weber, A.; Barnes, B.J.; Egeblad, M.; et al. Neutrophil extracellular traps in COVID-19. JCI Insight 2020. [Google Scholar] [CrossRef]
- Liu, Y.; Du, X.; Chen, J.; Jin, Y.; Peng, L.; Wang, H.H.X.; Luo, M.; Chen, L.; Zhao, Y. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J. Infect. 2020, 81, e6–e12. [Google Scholar] [CrossRef] [PubMed]
- Lagunas-Rangel, F.A. Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): A meta-analysis. J. Med. Virol. 2020, 92, 1733–1734. [Google Scholar] [CrossRef]
- Benabid, R.; Wartelle, J.; Malleret, L.; Guyot, N.; Gangloff, S.; Lebargy, F.; Belaaouaj, A. Neutrophil elastase modulates cytokine expression: Contribution to host defense against pseudomonas aeruginosa-induced pneumonia. J. Biol. Chem. 2012, 287, 34883–34894. [Google Scholar] [CrossRef]
- Ono, S.; Kuroki, T.; Adachi, T.; Kosaka, T.; Okamoto, T.; Tanaka, T.; Tajima, Y.; Kanematsu, T.; Eguchi, S. The effect of selective neutrophil elastase inhibitor on pancreatic islet yields and functions in rat with hypercytokinemia. Ann. Transplant. 2011, 16, 99–106. [Google Scholar] [CrossRef]
- Antalis, T.M.; Bugge, T.H.; Wu, Q. Membrane-anchored serine proteases in health and disease. In Progress in Molecular Biology and Translational Science; Elsevier B.V.: Amsterdam, The Netherlands, 2011; Volume 99, pp. 1–50. [Google Scholar]
- Paoloni-Giacobino, A.; Chen, H.; Peitsch, M.C.; Rossier, C.; Antonarakis, S.E. Cloning of the TMPRSS2 gene, which encodes a novel serine protease with transmembrane, LDLRA, and SRCR domains and maps to 21q22.3. Genomics 1997, 44, 309–320. [Google Scholar] [CrossRef]
- Jacquinet, E.; Rao, N.V.; Rao, G.V.; Hoidal, J.R. Cloning, genomic organization, chromosomal assignment and expression of a novel mosaic serine proteinase: Epitheliasin. FEBS Lett. 2000, 468, 93–100. [Google Scholar] [CrossRef]
- Lin, B.; Ferguson, C.; White, J.T.; Wang, S.; Vessella, R.; True, L.D.; Hood, L.; Nelson, P.S. Prostate-localized and androgen-regulated expression of the membrane- bound serine protease TMPRSS2. Cancer Res. 1999, 59, 4180–4184. [Google Scholar] [PubMed]
- Matsuyama, S.; Nao, N.; Shirato, K.; Kawase, M.; Saito, S.; Takayama, I.; Nagata, N.; Sekizuka, T.; Katoh, H.; Kato, F.; et al. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc. Natl. Acad. Sci. USA 2020, 117, 7001–7003. [Google Scholar] [CrossRef] [PubMed]
- Kawase, M.; Shirato, K.; van der Hoek, L.; Taguchi, F.; Matsuyama, S. Simultaneous Treatment of Human Bronchial Epithelial Cells with Serine and Cysteine Protease Inhibitors Prevents Severe Acute Respiratory Syndrome Coronavirus Entry. J. Virol. 2012, 86, 6537–6545. [Google Scholar] [CrossRef]
- Zhou, Y.; Vedantham, P.; Lu, K.; Agudelo, J.; Carrion, R.; Nunneley, J.W.; Barnard, D.; Pöhlmann, S.; McKerrow, J.H.; Renslo, A.R.; et al. Protease inhibitors targeting coronavirus and filovirus entry. Antivir. Res. 2015, 116, 76–84. [Google Scholar] [CrossRef]
- Rensi, S.; Altman, R.B.; Liu, T.; Lo, Y.-C.; McInnes, G.; Derry, A.; Keys, A. Homology Modeling of TMPRSS2 Yields Candidate Drugs That May Inhibit Entry of SARS-CoV-2 into Human Cells. ChemRxiv 2020. [Google Scholar] [CrossRef]
- Schuler, B.A.; Christian Habermann, A.; Plosa, E.J.; Taylor, C.J.; Jetter, C.; Kapp, M.E.; Benjamin, J.T.; Gulleman, P.; Nichols, D.S.; Braunstein, L.Z.; et al. Age-related expression of SARS-CoV-2 priming protease TMPRSS2 in the developing lung. bioRxiv 2020. [Google Scholar] [CrossRef]
- Inoue, Y.; Tanaka, N.; Tanaka, Y.; Inoue, S.; Morita, K.; Zhuang, M.; Hattori, T.; Sugamura, K. Clathrin-Dependent Entry of Severe Acute Respiratory Syndrome Coronavirus into Target Cells Expressing ACE2 with the Cytoplasmic Tail Deleted. J. Virol. 2007, 81, 8722–8729. [Google Scholar] [CrossRef]
- Doherty, G.J.; McMahon, H.T. Mechanisms of Endocytosis. Annu. Rev. Biochem. 2009, 78, 857–902. [Google Scholar] [CrossRef]
- Burkard, C.; Verheije, M.H.; Wicht, O.; van Kasteren, S.I.; van Kuppeveld, F.J.; Haagmans, B.L.; Pelkmans, L.; Rottier, P.J.M.; Bosch, B.J.; de Haan, C.A.M. Coronavirus Cell Entry Occurs through the Endo-/Lysosomal Pathway in a Proteolysis-Dependent Manner. PLoS Pathog. 2014, 10, e1004502. [Google Scholar] [CrossRef]
- Fekri, F.; Abousawan, J.; Bautista, S.; Orofiamma, L.; Dayam, R.M.; Antonescu, C.N.; Karshafian, R. Targeted enhancement of flotillin-dependent endocytosis augments cellular uptake and impact of cytotoxic drugs. Sci. Rep. 2019, 9, 17768. [Google Scholar] [CrossRef]
- Glebov, O.O. Understanding SARS-CoV-2 endocytosis for COVID-19 drug repurposing. FEBS J. 2020, 287, 3664–3671. [Google Scholar] [CrossRef]
- McMahon, H.T.; Boucrot, E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2011, 12, 517–533. [Google Scholar] [CrossRef]
- Rust, M.J.; Lakadamyali, M.; Zhang, F.; Zhuang, X. Assembly of endocytic machinery around individual influenza viruses during viral entry. Nat. Struct. Mol. Biol. 2004, 11, 567–573. [Google Scholar] [CrossRef]
- Ehrlich, M.; Boll, W.; Van Oijen, A.; Hariharan, R.; Chandran, K.; Nibert, M.L.; Kirchhausen, T. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell 2004, 118, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Macia, E.; Ehrlich, M.; Massol, R.; Boucrot, E.; Brunner, C.; Kirchhausen, T. Dynasore, a Cell-Permeable Inhibitor of Dynamin. Dev. Cell 2006, 10, 839–850. [Google Scholar] [CrossRef]
- Gibson, A.E.; Noel, R.J.; Herlihy, J.T.; Ward, W.F. Phenylarsine oxide inhibition of endocytosis: Effects on asialofetuin internalization. Am. J. Physiol. Cell Physiol. 1989, 257, C182–C184. [Google Scholar] [CrossRef] [PubMed]
- Cosson, P.; De Curtis, I.; Pouyssegur, J.; Griffiths, G.; Davoust, J. Low cytoplasmic pH inhibits endocytosis and transport from the trans-Golgi network to the cell surface. J. Cell Biol. 1989, 108, 377–387. [Google Scholar] [CrossRef]
- Heuser, J.E.; Anderson, R.G.W. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J. Cell Biol. 1989, 108, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.H.; Rothberg, K.G.; Anderson, R.G.W. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 1993, 123, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Plaze, M.; Attali, D.; Petit, A.-C.; Blatzer, M.; Simon-Loriere, E.; Vinckier, F.; Cachia, A.; Chrétien, F.; Gaillard, R. Repurposing chlorpromazine to treat COVID-19: The reCoVery study. Encephale 2020, 46, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Palade, G.E. PROGRAM. Proceedings of the Electron Microscope Society of America. J. Appl. Phys. 1953, 24, 1414–1425. [Google Scholar] [CrossRef]
- Kiss, A.L.; Botos, E. Endocytosis via caveolae: Alternative pathway with distinct cellular compartments to avoid lysosomal degradation? J. Cell. Mol. Med. 2009, 13, 1228–1237. [Google Scholar] [CrossRef] [PubMed]
- Pelkmans, L.; Kartenbeck, J.; Helenius, A. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol. 2001, 3, 473–483. [Google Scholar] [CrossRef]
- Nichols, B.J. A distinct class of endosome mediates clathrin-independent endocytosis to the Golgi complex. Nat. Cell Biol. 2002, 4, 374–378. [Google Scholar] [CrossRef]
- Nunes-Correia, I.; Eulálio, A.; Nir, S.; Pedroso de Lima, M.C. Caveolae as an additional route for influenza virus endocytosis in MDCK cells. Cell. Mol. Biol. Lett. 2004, 9, 47–60. [Google Scholar] [PubMed]
- Tamura, G.; Ando, K.; Suzuki, S.; Takatsuki, A.; Arima, K. Antiviral activity of brefeldin A and verrucarin A. J. Antibiot. 1968, 21, 160–161. [Google Scholar] [CrossRef]
- Beswick, R.W.; Ambrose, H.E.; Wagner, S.D. Nocodazole, a microtubule depolymerising agent, induces apoptosis of chronic lymphocytic leukaemia cells associated with changes in Bcl-2 phosphorylation and expression. Leuk. Res. 2006, 30, 427–436. [Google Scholar] [CrossRef]
- Le, P.U.; Nabi, I.R. Distinct caveolae-mediated endocytic pathways target the Golgi apparatus and the endoplasmic reticulum. J. Cell Sci. 2003, 116, 1059–1071. [Google Scholar] [CrossRef] [PubMed]
- Gobec, S.; Frlan, R. Inhibitors of Cathepsin B. Curr. Med. Chem. 2006, 13, 2309–2327. [Google Scholar] [CrossRef]
- Sweeney, D.; Raymer, M.L.; Lockwood, T.D. Antidiabetic and antimalarial biguanide drugs are metal-interactive antiproteolytic agents. Biochem. Pharmacol. 2003, 66, 663–677. [Google Scholar] [CrossRef]
- Kirschke, H.; Cathepsin, L. Handbook of Proteolytic Enzymes; Elsevier Ltd.: Amsterdam, The Netherlands, 2013; Volume 2, pp. 1808–1817. ISBN 9780123822192. [Google Scholar]
- Spira, D.; Stypmann, J.; Tobin, D.J.; Petermann, I.; Mayer, C.; Hagemann, S.; Vasiljeva, O.; Günther, T.; Schüle, R.; Peters, C.; et al. Cell type-specific functions of the lysosomal protease cathepsin L in the heart. J. Biol. Chem. 2007, 282, 37045–37052. [Google Scholar] [CrossRef]
- Petermann, I.; Mayer, C.; Stypmann, J.; Biniossek, M.L.; Tobin, D.J.; Engelen, M.A.; Dandekar, T.; Grune, T.; Schild, L.; Peters, C.; et al. Lysosomal, cytoskeletal, and metabolic alterations in cardiomyopathy of cathepsin L knockout mice. FASEB J. 2006, 20, 1266–1268. [Google Scholar] [CrossRef]
- Stypmann, J.; Gläser, K.; Roth, W.; Tobin, D.J.; Petermann, I.; Matthias, R.; Mönnig, G.; Haverkamp, W.; Breithardt, G.; Schmahl, W.; et al. Dilated cardiomyopathy in mice deficient for the lysosomal cysteine peptidase cathepsin L. Proc. Natl. Acad. Sci. USA 2002, 99, 6234–6239. [Google Scholar] [CrossRef]
- Tang, Q.; Cai, J.; Shen, D.; Bian, Z.; Yan, L.; Wang, Y.X.; Lan, J.; Zhuang, G.Q.; Ma, W.Z.; Wang, W. Lysosomal cysteine peptidase cathepsin L protects against cardiac hypertrophy through blocking AKT/GSK3β signaling. J. Mol. Med. 2009, 87, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Chwieralski, C.E.; Welte, T.; Bühling, F. Cathepsin-regulated apoptosis. Apoptosis 2006, 11, 143–149. [Google Scholar] [CrossRef]
- Fogarty, M.P.; McCormack, R.M.; Noonan, J.; Murphy, D.; Gowran, A.; Campbell, V.A. A role for p53 in the Beta-amyloid-mediated regulation of the lysosomal system. Neurobiol. Aging 2010, 31, 1774–1786. [Google Scholar] [CrossRef] [PubMed]
- Kaasik, A.; Rikk, T.; Piirsoo, A.; Zharkovsky, T.; Zharkovsky, A. Up-regulation of lysosomal cathepsin L and autophagy during neuronal death induced by reduced serum and potassium. Eur. J. Neurosci. 2005, 22, 1023–1031. [Google Scholar] [CrossRef]
- Huang, I.C.; Bosch, B.J.; Li, F.; Li, W.; Kyoung, H.L.; Ghiran, S.; Vasilieva, N.; Dermody, T.S.; Harrison, S.C.; Dormitzer, P.R.; et al. SARS coronavirus, but not human coronavirus NL63, utilizes cathepsin L to infect ACE2-expressing cells. J. Biol. Chem. 2006, 281, 3198–3203. [Google Scholar] [CrossRef]
- Ou, X.; Liu, Y.; Lei, X.; Li, P.; Mi, D.; Ren, L.; Guo, L.; Guo, R.; Chen, T.; Hu, J.; et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020, 11, 1620. [Google Scholar] [CrossRef] [PubMed]
- Sugita, S.; Horie, S.; Nakamura, O.; Maruyama, K.; Takase, H.; Usui, Y.; Takeuchi, M.; Ishidoh, K.; Koike, M.; Uchiyama, Y.; et al. Acquisition of T Regulatory Function in Cathepsin L-Inhibited T Cells by Eye-Derived CTLA-2α during Inflammatory Conditions. J. Immunol. 2009, 183, 5013–5022. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.T.; Yamaguchi, K.; Hayama, T.; Kobori, T.; Sigeizumi, S.; Sugimoto, K.; Kondo, K.; Tsuji, T.; Ohba, Y.; Tagami, K.; et al. Suppressive effect of N-(benzyloxycarbonyl)-L-phenylalanyl-L-tyrosinal on bone resorption in vitro and in vivo. Eur. J. Pharmacol. 1996, 300, 131–135. [Google Scholar] [CrossRef]
- Simmons, G.; Gosalia, D.N.; Rennekamp, A.J.; Reeves, J.D.; Diamond, S.L.; Bates, P. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc. Natl. Acad. Sci. USA 2005, 102, 11876–11881. [Google Scholar] [CrossRef]
- Ferrara, J.L.M.; Abhyankar, S.; Gilliland, D.G. Cytokine storm of graft-versus-host disease: A critical effector role for interleukin-1. Transplant. Proc. 1993, 25, 1216–1217. [Google Scholar]
- Bisno, A.L.; Brito, M.O.; Collins, C.M. Molecular basis of group A streptococcal virulence. Lancet Infect. Dis. 2003, 3, 191–200. [Google Scholar] [CrossRef]
- Yokota, S. Influenza-associated Encephalopathy--Pathophysiology and Disease Mechanisms. Nihon Rinsho Jpn. J. Clin. Med. 2003, 61, 1953–1958. [Google Scholar]
- Jahrling, P.B.; Hensley, L.E.; Martinez, M.J.; LeDuc, J.W.; Rubins, K.H.; Relman, D.A.; Huggins, J.W. Exploring the potential of variola virus infection of cynomolgus macaques as a model for human smallpox. Proc. Natl. Acad. Sci. USA 2004, 101, 15196–15200. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.J.; Su, I.J.; Theron, M.; Wu, Y.C.; Lai, S.K.; Liu, C.C.; Lei, H.Y. An interferon-γ-related cytokine storm in SARS patients. J. Med. Virol. 2005, 75, 185–194. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Wang, H.; Ma, S. The cytokine storm and factors determining the sequence and severity of organ dysfunction in multiple organ dysfunction syndrome. Am. J. Emerg. Med. 2008, 26, 711–715. [Google Scholar] [CrossRef]
- Denes, A.; Lopez-Castejon, G.; Brough, D. Caspase-1: Is IL-1 just the tip of the ICEberg? Cell Death Dis. 2012, 3, e338. [Google Scholar] [CrossRef] [PubMed]
- Beuscher, H.U.; Günther, C.; Röllinghoff, M. IL-1 beta is secreted by activated murine macrophages as biologically inactive precursor. J. Immunol. 1990, 144, 2179–2183. [Google Scholar] [PubMed]
- Sutton, C.E.; Lalor, S.J.; Sweeney, C.M.; Brereton, C.F.; Lavelle, E.C.; Mills, K.H.G. Interleukin-1 and IL-23 Induce Innate IL-17 Production from γδ T Cells, Amplifying Th17 Responses and Autoimmunity. Immunity 2009, 31, 331–341. [Google Scholar] [CrossRef]
- Dhimolea, E. Canakinumab. MAbs 2010, 2, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A.; Simon, A.; van der Meer, J.W.M. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug Discov. 2012, 11, 633–652. [Google Scholar] [CrossRef]
- Sheng, C.C.; Sahoo, D.; Dugar, S.; Prada, R.A.; Wang, T.K.M.; Abou Hassan, O.K.; Brennan, D.; Culver, D.A.; Rajendram, P.; Duggal, A.; et al. Canakinumab to reduce deterioration of cardiac and respiratory function in SARS-CoV-2 associated myocardial injury with heightened inflammation (canakinumab in Covid-19 cardiac injury: The three C study). Clin. Cardiol. 2020, 43, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhao, Y.; Hu, J. Curcumin Inhibits Imiquimod-Induced Psoriasis-Like Inflammation by Inhibiting IL-1beta and IL-6 Production in Mice. PLoS ONE 2013, 8, e67078. [Google Scholar] [CrossRef]
- Alsina, M.; Guise, T.A.; Roodman, G.D. Cytokine Regulation of Bone Cell Differentiation. In Vitamins and Hormones; Academic Press Inc.: Cambridge, MA, USA, 1996; Volume 52, pp. 63–98. [Google Scholar]
- Wood, A.J.; Lieschke, G.J.; Burgess, A.W. Granulocyte Colony-Stimulating Factor and Granulocyte-Macrophage Colony-Stimulating Factor. N. Engl. J. Med. 1992, 327, 28–35. [Google Scholar] [CrossRef]
- Root, R.K.; Dale, D.C. Granulocyte Colony-Stimulating Factor and Granulocyte-Macrophage Colony-Stimulating Factor: Comparisons and Potential for Use in the Treatment of Infections in Nonneutropenic Patients. J. Infect. Dis. 1999, 179, S342–S352. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, H.-T.; Goncalves, J.; Xiao, Y.; Wang, M.; Guo, Y.; Sun, C.; Tang, X.; Jing, L.; Zhang, M.; et al. An interpretable mortality prediction model for COVID-19 patients. Nat. Mach. Intell. 2020, 2, 283–288. [Google Scholar] [CrossRef]
- Cai, Q.; Huang, D.; Yu, H.; Zhu, Z.; Xia, Z.; Su, Y.; Li, Z.; Zhou, G.; Gou, J.; Qu, J.; et al. COVID-19: Abnormal liver function tests. J. Hepatol. 2020, 73, 566–574. [Google Scholar] [CrossRef]
- Han, H.; Ma, Q.; Li, C.; Liu, R.; Zhao, L.; Wang, W.; Zhang, P.; Liu, X.; Gao, G.; Liu, F.; et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg. Microbes Infect. 2020, 9, 1123–1130. [Google Scholar] [CrossRef]
- Filardi, T.; Morano, S. COVID-19: Is there a link between the course of infection and pharmacological agents in diabetes? J. Endocrinol. Investig. 2020, 43, 1053–1060. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Velavan, T.P.; Meyer, C.G. Mild versus severe COVID-19: Laboratory markers. Int. J. Infect. Dis. 2020, 95, 304–307. [Google Scholar] [CrossRef]
- Malek, A.E. Time to revisit the use of G-CSF after allogeneic haematopoietic cell transplantation in COVID-19 era? Br. J. Cancer 2021, 124, 1183. [Google Scholar] [CrossRef]
- Lee, E.Y.; Lee, Z.-H.; Song, Y.W. CXCL10 and autoimmune diseases. Autoimmun. Rev. 2009, 8, 379–383. [Google Scholar] [CrossRef]
- Zhao, Q.; Kim, T.; Pang, J.; Sun, W.; Yang, X.; Wang, J.; Song, Y.; Zhang, H.; Sun, H.; Rangan, V.; et al. A novel function of CXCL10 in mediating monocyte production of proinflammatory cytokines. J. Leukoc. Biol. 2017, 102, 1271–1280. [Google Scholar] [CrossRef]
- Ohta, K.; Naruse, T.; Kato, H.; Ishida, Y.; Nakagawa, T.; Ono, S.; Shigeishi, H.; Takechi, M. Differential regulation by IFN-γ on TNF-α-induced chemokine expression in synovial fibroblasts from temporomandibular joint. Mol. Med. Rep. 2017, 16, 6850–6857. [Google Scholar] [CrossRef]
- Vazirinejad, R.; Ahmadi, Z.; Kazemi Arababadi, M.; Hassanshahi, G.; Kennedy, D. The Biological Functions, Structure and Sources of CXCL10 and Its Outstanding Part in the Pathophysiology of Multiple Sclerosis. Neuroimmunomodulation 2014, 21, 322–330. [Google Scholar] [CrossRef]
- Qi, X.F.; Kim, D.H.; Yoon, Y.S.; Jin, D.; Huang, X.Z.; Li, J.H.; Deung, Y.K.; Lee, K.J. Essential involvement of cross-talk between IFN-γ and TNF-α in CXCL10 production in human THP-1 monocytes. J. Cell. Physiol. 2009, 220, 690–697. [Google Scholar] [CrossRef]
- Vaninov, N. In the eye of the COVID-19 cytokine storm. Nat. Rev. Immunol. 2020, 20, 277. [Google Scholar] [CrossRef]
- Coperchini, F.; Chiovato, L.; Croce, L.; Magri, F.; Rotondi, M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020, 53, 25. [Google Scholar] [CrossRef]
- Jung, M.; Liermann, J.C.; Opatz, T.; Erkel, G. Ganodermycin, a novel inhibitor of CXCL10 expression from Ganoderma applanatum. J. Antibiot. 2011, 64, 683–686. [Google Scholar] [CrossRef] [PubMed]
- Seidel, P.; Alkhouri, H.; Lalor, D.J.; Burgess, J.K.; Armour, C.L.; Hughes, J.M. Thiazolidinediones inhibit airway smooth muscle release of the chemokine CXCL10: In vitro comparison with current asthma therapies. Respir. Res. 2012, 13, 90. [Google Scholar] [CrossRef] [PubMed]
- Castiglione, V.; Chiriacò, M.; Emdin, M.; Taddei, S.; Vergaro, G. Statin therapy in COVID-19 infection. Eur. Heart J. Cardiovasc. Pharmacother. 2020, 6, 258–259. [Google Scholar] [CrossRef]
- Lee, K.C.H.; Sewa, D.W.; Phua, G.C. Potential role of statins in COVID-19. Int. J. Infect. Dis. 2020, 96, 615–617. [Google Scholar] [CrossRef]
- Zlotnik, A.; Yoshie, O. Chemokines: A new classification system and their role in immunity. Immunity 2000, 1, 121–127. [Google Scholar] [CrossRef]
- Menten, P.; Wuyts, A.; Van Damme, J. Macrophage inflammatory protein-1. Cytokine Growth Factor Rev. 2002, 13, 455–481. [Google Scholar] [CrossRef]
- Ren, M.; Guo, Q.; Guo, L.; Lenz, M.; Qian, F.; Koenen, R.R.; Xu, H.; Schilling, A.B.; Weber, C.; Ye, R.D.; et al. Polymerization of MIP-1 chemokine (CCL3 and CCL4) and clearance of MIP-1 by insulin-degrading enzyme. EMBO J. 2010, 29, 3952–3966. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.; von Stebut, E. Macrophage inflammatory protein-1. Int. J. Biochem. Cell Biol. 2004, 36, 1882–1886. [Google Scholar] [CrossRef]
- Xu, Z.-S.; Shu, T.; Kang, L.; Wu, D.; Zhou, X.; Liao, B.-W.; Sun, X.-L.; Zhou, X.; Wang, Y.-Y. Temporal profiling of plasma cytokines, chemokines and growth factors from mild, severe and fatal COVID-19 patients. Signal Transduct. Target. Ther. 2020, 5, 100. [Google Scholar] [CrossRef] [PubMed]
- Tufan, A.; Avanoğlu Güler, A.; Matucci-Cerinic, M. COVID-19, immune system response, hyperinflammation and repurposing antirheumatic drugs. Turk. J. Med. Sci. 2020, 50, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhang, H.; Zhan, M.; Jiang, J.; Yin, H.; Dauphars, D.J.; Li, S.-Y.; Li, Y.; He, Y.-W. Preventing Mortality in COVID-19 Patients: Which Cytokine to Target in a Raging Storm? Front. Cell Dev. Biol. 2020, 8, 677. [Google Scholar] [CrossRef]
- Berkman, N.; John, M.; Roesems, G.; Jose, P.J.; Barnes, P.J.; Chung, K.F. Inhibition of macrophage inflammatory protein-1 alpha expression by IL-10. Differential sensitivities in human blood monocytes and alveolar macrophages. J. Immunol. 1995, 155, 4412–4418. [Google Scholar]
- Barna, B.P.; Pettay, J.; Barnett, G.H.; Zhou, P.; Iwasaki, K.; Estes, M.L. Regulation of monocyte chemoattractant protein-1 expression in adult human non-neoplastic astrocytes is sensitive to tumor necrosis factor (TNF) or antibody to the 55-kDa TNF receptor. J. Neuroimmunol. 1994, 50, 101–107. [Google Scholar] [CrossRef]
- Brown, Z.; Strieter, R.M.; Neild, G.H.; Thompson, R.C.; Kunkel, S.L.; Westwick, J. IL-1 receptor antagonist inhibits monocyte chemotactic peptide 1 generation by human mesangial cells. Kidney Int. 1992, 42, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Cushing, S.D.; Berliner, J.A.; Valente, A.J.; Territo, M.C.; Navab, M.; Parhami, F.; Gerrity, R.; Schwartz, C.J.; Fogelman, A.M. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc. Natl. Acad. Sci. USA 1990, 87, 5134–5138. [Google Scholar] [CrossRef]
- Standiford, T.J.; Kunkel, S.L.; Phan, S.H.; Rollins, B.J.; Strieter, R.M. Alveolar macrophage-derived cytokines induce monocyte chemoattractant protein-1 expression from human pulmonary type II-like epithelial cells. J. Biol. Chem. 1991, 266, 9912–9918. [Google Scholar] [CrossRef]
- Lu, B.; Rutledge, B.J.; Gu, L.; Fiorillo, J.; Lukacs, N.W.; Kunkel, S.L.; North, R.; Gerard, C.; Rollins, B.J. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J. Exp. Med. 1998, 187, 601–608. [Google Scholar] [CrossRef]
- Bielinski, S.J.; Pankow, J.S.; Miller, M.B.; Hopkins, P.N.; Eckfeldt, J.H.; Hixson, J.; Liu, Y.; Register, T.; Myers, R.H.; Arnett, D.K. Circulating MCP-1 levels shows linkage to chemokine receptor gene cluster on chromosome 3: The NHLBI Family Heart Study follow-up examination. Genes Immun. 2007, 8, 684–690. [Google Scholar] [CrossRef]
- Dupuis, J.; Larson, M.G.; Vasan, R.S.; Massaro, J.M.; Wilson, P.W.F.; Lipinska, I.; Corey, D.; Vita, J.A.; Keaney, J.F.; Benjamin, E.J. Genome scan of systemic biomarkers of vascular inflammation in the Framingham Heart Study: Evidence for susceptibility loci on 1q. Atherosclerosis 2005, 182, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Costela-Ruiz, V.J.; Illescas-Montes, R.; Puerta-Puerta, J.M.; Ruiz, C.; Melguizo-Rodríguez, L. SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020, 54, 62–75. [Google Scholar] [CrossRef]
- Hussman, J.P. Cellular and Molecular Pathways of COVID-19 and Potential Points of Therapeutic Intervention. Front. Pharmacol. 2020, 11, 1169. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shen, C.; Li, J.; Yuan, J.; Wei, J.; Huang, F.; Wang, F.; Li, G.; Li, Y.; Xing, L.; et al. Plasma IP-10 and MCP-3 levels are highly associated with disease severity and predict the progression of COVID-19. J. Allergy Clin. Immunol. 2020, 146, 119–127. [Google Scholar] [CrossRef]
- Yu, G.M.; Tan, W. Melatonin Inhibits Lipopolysaccharide-Induced Inflammation and Oxidative Stress in Cultured Mouse Mammary Tissue. Mediat. Inflamm. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Yan, L. Anti-CCL-2/MCP-1: Directed biologicals for inflammatory and malignant diseases. In Target Validation in Drug Discovery; Elsevier Inc.: Amsterdam, The Netherlands, 2007; pp. 103–119. ISBN 9780123693938. [Google Scholar]
- Baeck, C.; Wehr, A.; Karlmark, K.R.; Heymann, F.; Vucur, M.; Gassler, N.; Huss, S.; Klussmann, S.; Eulberg, D.; Luedde, T.; et al. Pharmacological inhibition of the chemokine CCL2 (MCP-1) diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. Gut 2012, 61, 416–426. [Google Scholar] [CrossRef]
- Yao, W.; Ba, Q.; Li, X.; Li, H.; Zhang, S.; Yuan, Y.; Wang, F.; Duan, X.; Li, J.; Zhang, W.; et al. A Natural CCR2 Antagonist Relieves Tumor-associated Macrophage-mediated Immunosuppression to Produce a Therapeutic Effect for Liver Cancer. EBioMedicine 2017, 22, 58–67. [Google Scholar] [CrossRef]
- Bot, I.; Zacarías, N.V.O.; De Witte, W.E.A.; De Vries, H.; Van Santbrink, P.J.; Van Der Velden, D.; Kröner, M.J.; Van Der Berg, D.J.; Stamos, D.; De Lange, E.C.M.; et al. A novel CCR2 antagonist inhibits atherogenesis in apoE deficient mice by achieving high receptor occupancy. Sci. Rep. 2017, 7, 52. [Google Scholar] [CrossRef]
- Parameswaran, N.; Patial, S. Tumor necrosis factor-α signaling in macrophages. Crit. Rev. Eukaryot. Gene Expr. 2010, 20, 87–103. [Google Scholar] [CrossRef]
- Gahring, L.C.; Carlson, N.G.; Kulmer, R.A.; Rogers, S.W. Neuronal expression of tumor necrosis factor alpha in the OVOUJI fme brain. Neuroimmunomodulation 1996, 3, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Kale, V.P.; Gilhooley, P.J.; Phadtare, S.; Nabavizadeh, A.; Pandey, M.K. Role of Gambogic Acid in Chemosensitization of Cancer. In Role of Nutraceuticals in Chemoresistance to Cancer; Elsevier: Amsterdam, The Netherlands, 2018; pp. 151–167. [Google Scholar]
- Mortaz, E.; Tabarsi, P.; Jamaati, H.; Roofchayee, N.D.; Dezfuli, N.K.; Hashemian, S.M.; Moniri, A.; Marjani, M.; Malkmohammad, M.; Mansouri, D.; et al. Increased serum levels of soluble TNF-α receptor is associated with mortality of ICU COVID-19 patients. Front. Immunol. 2020. [Google Scholar] [CrossRef]
- Brito, C.A.; Paiva, J.G.; Pimentel, F.N.; Guimarães, R.S.; Moreira, M.R. COVID-19 in patients with rheumatological diseases treated with anti-TNF. Ann. Rheum. Dis. 2020. [Google Scholar] [CrossRef]
- Perlin, D.S.; Zafir-Lavie, I.; Roadcap, L.; Raines, S.; Ware, C.F.; Neil, G.A. Levels of the TNF-Related Cytokine LIGHT Increase in Hospitalized COVID-19 Patients with Cytokine Release Syndrome and ARDS. mSphere 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, M.; Maini, R.N.; Woody, J.N.; Holgate, S.T.; Winter, G.; Rowland, M.; Richards, D.; Hussell, T. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet 2020, 395, 1407–1409. [Google Scholar] [CrossRef]
- Gerriets, V.; Bansal, P.; Goyal, A.; Khaddour, K. Tumor Necrosis Factor Inhibitors; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Robinson, P.C.; Richards, D.; Tanner, H.L.; Feldmann, M. Accumulating evidence suggests anti-TNF therapy needs to be given trial priority in COVID-19 treatment. Lancet Rheumatol. 2020, 2, e653–e655. [Google Scholar] [CrossRef]
- Duret, P.-M.; Sebbag, E.; Mallick, A.; Gravier, S.; Spielmann, L.; Messer, L. Recovery from COVID-19 in a patient with spondyloarthritis treated with TNF-alpha inhibitor etanercept. Ann. Rheum. Dis. 2020, 79, 1251–1252. [Google Scholar] [CrossRef]
- Stallmach, A.; Kortgen, A.; Gonnert, F.; Coldewey, S.M.; Reuken, P.; Bauer, M. Infliximab against severe COVID-19-induced cytokine storm syndrome with organ failure-a cautionary case series. Crit. Care 2020, 24, 444. [Google Scholar] [CrossRef]
- Buonaguro, F.M.; Ascierto, P.A.; Morse, G.D.; Buonaguro, L.; Puzanov, I.; Tornesello, M.L.; Bréchot, C.; Gallo, R.C. Covid-19: Time for a paradigm change. Rev. Med. Virol. 2020, 30, e2134. [Google Scholar] [CrossRef]
- Pani, A.; Lauriola, M.; Romandini, A.; Scaglione, F. Macrolides and viral infections: Focus on azithromycin in COVID-19 pathology. Int. J. Antimicrob. Agents 2020, 56, 106053. [Google Scholar] [CrossRef]
- Kuo, C.H.; Lee, M.S.; Kuo, H.F.; Lin, Y.C.; Hung, C.H. Azithromycin suppresses Th1- and Th2-related chemokines IP-10/MDC in human monocytic cell line. J. Microbiol. Immunol. Infect. 2019, 52, 872–879. [Google Scholar] [CrossRef]
- Banjanac, M.; Munić Kos, V.; Nujić, K.; Vrančić, M.; Belamarić, D.; Crnković, S.; Hlevnjak, M.; Eraković Haber, V. Anti-inflammatory mechanism of action of azithromycin in LPS-stimulated J774A.1 cells. Pharmacol. Res. 2012, 66, 357–362. [Google Scholar] [CrossRef]
- Yamaya, M.; Shinya, K.; Hatachi, Y.; Kubo, H.; Asada, M.; Yasuda, H.; Nishimura, H.; Nagatomi, R. Clarithromycin inhibits type A seasonal influenza virus infection in human airway epithelial cells. J. Pharmacol. Exp. Ther. 2010, 333, 81–90. [Google Scholar] [CrossRef]
- Huang, J.; Hume, A.J.; Abo, K.M.; Werder, R.B.; Villacorta-Martin, C.; Alysandratos, K.D.; Beermann, M.L.; Simone-Roach, C.; Lindstrom-Vautrin, J.; Olejnik, J.; et al. SARS-CoV-2 Infection of Pluripotent Stem Cell-Derived Human Lung Alveolar Type 2 Cells Elicits a Rapid Epithelial-Intrinsic Inflammatory Response. Cell Stem Cell 2020. [Google Scholar] [CrossRef]
- Kazama, I.; Saito, K.; Baba, A.; Mori, T.; Abe, N.; Endo, Y.; Toyama, H.; Ejima, Y.; Matsubara, M.; Yamauchi, M. Clarithromycin Dose-Dependently Stabilizes Rat Peritoneal Mast Cells. Chemotherapy 2016, 61, 295–303. [Google Scholar] [CrossRef]
- Sterenczak, K.A.; Barrantes, I.; Stahnke, T.; Stachs, O.; Fuellen, G.; Undre, N. Co-infections: Testing macrolides for added benefit in patients with COVID-19. Lancet Microbe 2020, 1, e313. [Google Scholar] [CrossRef]
- Wilson, L.; Gage, P.; Ewart, G. Hexamethylene amiloride blocks E protein ion channels and inhibits coronavirus replication. Virology 2006, 353, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Tsybovsky, Y.; Olia, A.S.; Gorman, J.; Rapp, M.A.; Cerutti, G.; Katsamba, P.S.; Nazzari, A.; Schon, A.; Wang, P.D.; et al. A pH-dependent switch mediates conformational masking of SARS-CoV-2 spike. bioRxiv 2020. [Google Scholar] [CrossRef]
- Ray, S.C.; Baban, B.; Tucker, M.A.; Seaton, A.J.; Chang, K.C.; Mannon, E.C.; Sun, J.; Patel, B.; Wilson, K.; Musall, J.B.; et al. Oral NaHCO3 Activates a Splenic Anti-Inflammatory Pathway: Evidence That Cholinergic Signals Are Transmitted via Mesothelial Cells. J. Immunol. 2018, 200, 3568–3586. [Google Scholar] [CrossRef]
- Vlachakis, D.; Papakonstantinou, E.; Mitsis, T.; Pierouli, K.; Diakou, I.; Chrousos, G.; Bacopoulou, F. Molecular mechanisms of the novel coronavirus SARS-CoV-2 and potential anti-COVID19 pharmacological targets since the outbreak of the pandemic. Food Chem. Toxicol. 2020, 146, 111805. [Google Scholar] [CrossRef]
- Shi, Z.; Puyo, C.A. N-acetylcysteine to combat COVID-19: An evidence review. Ther. Clin. Risk Manag. 2020, 16, 1047–1055. [Google Scholar] [CrossRef]
- Assimakopoulos, S.F.; Marangos, M. N-acetyl-cysteine may prevent COVID-19-associated cytokine storm and acute respiratory distress syndrome. Med. Hypotheses 2020, 140, 109778. [Google Scholar] [CrossRef]
- Bulger, E.M.; Maier, R.V. An Argument for Vitamin E Supplementation in the Management of Systemic Inflammatory Response Syndrome. Shock 2003, 19, 99–103. [Google Scholar] [CrossRef]
- Mukherjee, A.K.; Ghosal, S.K.; Maity, C.R. Lysosomal membrane stabilization by α-tocopherol against the damaging action of Vipera russelli venom phospholipase A2. Cell. Mol. Life Sci. 1997, 53, 152–155. [Google Scholar] [CrossRef]
- Fiorino, S.; Gallo, C.; Zippi, M.; Sabbatani, S.; Manfredi, R.; Moretti, R.; Fogacci, E.; Maggioli, C.; Travasoni Loffredo, F.; Giampieri, E.; et al. Cytokine storm in aged people with CoV-2: Possible role of vitamins as therapy or preventive strategy. Aging Clin. Exp. Res. 2020, 32, 2115–2131. [Google Scholar] [CrossRef]
- Wessling-Resnick, M. Crossing the Iron Gate: Why and How Transferrin Receptors Mediate Viral Entry. Annu. Rev. Nutr. 2018, 38, 431–458. [Google Scholar] [CrossRef]
- Varga, M.J.; Weibull, C.; Everitt, E. Infectious entry pathway of adenovirus type 2. J. Virol. 1991, 65, 6061. [Google Scholar] [CrossRef] [PubMed]
- Dalamaga, M.; Karampela, I.; Mantzoros, C.S. Commentary: Could iron chelators prove to be useful as an adjunct to COVID-19 Treatment Regimens? Metabolism 2020, 108, 154260. [Google Scholar] [CrossRef] [PubMed]
- Banach, W.; Nitschke, K.; Krajewska, N.; Mongiałło, W.; Matuszak, O.; Muszyński, J.; Skrypnik, D. The Association between Excess Body Mass and Disturbances in Somatic Mineral Levels. Int. J. Mol. Sci. 2020, 21, 7306. [Google Scholar] [CrossRef]
- Delima, R.; Trinder, D.; Olynyk, J.K. Potential protective effects of zinc in iron overload. Liver Int. 2007, 27, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Jothimani, D.; Kailasam, E.; Danielraj, S.; Nallathambi, B.; Ramachandran, H.; Sekar, P.; Manoharan, S.; Ramani, V.; Narasimhan, G.; Kaliamoorthy, I.; et al. COVID-19: Poor outcomes in patients with zinc deficiency. Int. J. Infect. Dis. 2020, 100, 343–349. [Google Scholar] [CrossRef]
- Djoko, K.Y.; Cheryl-lynn, Y.O.; Walker, M.J.; McEwan, A.G. The role of copper and zinc toxicity in innate immune defense against bacterial pathogens. J. Biol. Chem. 2015, 290, 1854–1861. [Google Scholar] [CrossRef]
- Rink, L.; Gabriel, P. Zinc and the immune system. Proc. Nutr. Soc. 2000, 59, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Squitti, R.; Picozza, M.; Pawar, A.; Rongioletti, M.; Dutta, A.K.; Sahoo, S.; Goswami, K.; Sharma, P.; Prasad, R. Zinc and COVID-19: Basis of Current Clinical Trials. Biol. Trace Elem. Res. 2020, 1–11. [Google Scholar] [CrossRef]
- Marik, P.E.; Khangoora, V.; Rivera, R.; Hooper, M.H.; Catravas, J. Hydrocortisone, Vitamin C, and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Before-After Study. Chest 2017, 151, 1229–1238. [Google Scholar] [CrossRef]
- Litwak, J.; Cho, N.; Nguyen, H.; Moussavi, K.; Bushell, T. Vitamin C, Hydrocortisone, and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Analysis of Real-World Application. J. Clin. Med. 2019, 8, 478. [Google Scholar] [CrossRef]
- Feyaerts, A.F.; Luyten, W. Vitamin C as prophylaxis and adjunctive medical treatment for COVID-19? Nutrition 2020, 79–80, 110948. [Google Scholar] [CrossRef]
- Carr, A.C. A new clinical trial to test high-dose vitamin C in patients with COVID-19. Crit. Care 2020, 24, 133. [Google Scholar] [CrossRef]
- Ahmed, S.; Zimba, O.; Gasparyan, A.Y. Thrombosis in Coronavirus disease 2019 (COVID-19) through the prism of Virchow’s triad. Clin. Rheumatol. 2020, 39, 2529–2543. [Google Scholar] [CrossRef]
- Loo, J.; Spittle, D.A.; Newnham, M. COVID-19, immunothrombosis and venous thromboembolism: Biological mechanisms. Thorax 2021, 76, 412–420. [Google Scholar] [CrossRef]
- Marongiu, F.; Barcellona, D. Fondaparinux: Should It Be Studied in Patients with COVID-19 Disease? TH Open 2020, 4, e300–e302. [Google Scholar] [CrossRef]
- Cuker, A.; Tseng, E.K.; Nieuwlaat, R.; Angchaisuksiri, P.; Blair, C.; Dane, K.; Davila, J.; DeSancho, M.T.; Diuguid, D.; Griffin, D.O.; et al. American Society of Hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19. Blood Adv. 2021, 5, 872–888. [Google Scholar] [CrossRef]
- Bikdeli, B.; Madhavan, M.V.; Gupta, A.; Jimenez, D.; Burton, J.R.; Der Nigoghossian, C.; Chuich, T.; Nouri, S.N.; Dreyfus, I.; Driggin, E.; et al. Pharmacological Agents Targeting Thromboinflammation in COVID-19: Review and Implications for Future Research. Thromb. Haemost. 2020, 120, 1004–1024. [Google Scholar]
- Russo, V.; Cardillo, G.; Viggiano, G.V.; Mangiacapra, S.; Cavalli, A.; Fontanella, A.; Agrusta, F.; Bellizzi, A.; Amitrano, M.; Iannuzzo, M.; et al. Fondaparinux Use in Patients With COVID-19: A Preliminary Multicenter Real-World Experience. J. Cardiovasc. Pharmacol. 2020, 76, 369–371. [Google Scholar] [CrossRef]
- Demer, L.L.; Hsu, J.J.; Tintut, Y. Steroid Hormone Vitamin D: Implications for Cardiovascular Disease. Circ. Res. 2018, 122, 1576–1585. [Google Scholar] [CrossRef] [PubMed]
- Norman, A.W. From vitamin D to hormone D: Fundamentals of the vitamin D endocrine system essential for good health. Am. J. Clin. Nutr. 2008, 88, 491S–499S. [Google Scholar] [CrossRef]
- Hewison, M. Vitamin D and innate and adaptive immunity. Vitam. Horm. 2011, 86, 23–62. [Google Scholar] [CrossRef]
- Bishop, E.L.; Ismailova, A.; Dimeloe, S.; Hewison, M.; White, J.H. Vitamin D and Immune Regulation: Antibacterial, Antiviral, Anti-Inflammatory. JBMR Plus 2021, 5. [Google Scholar] [CrossRef]
- Quesada-Gomez, J.M.; Entrenas-Castillo, M.; Bouillon, R. Vitamin D receptor stimulation to reduce acute respiratory distress syndrome (ARDS) in patients with coronavirus SARS-CoV-2 infections: Revised Ms SBMB 2020_166. J. Steroid Biochem. Mol. Biol. 2020, 202. [Google Scholar] [CrossRef] [PubMed]
- Aulinas, A. Physiology of the Pineal Gland and Melatonin; MDText.com, Inc., 2000. [Google Scholar]
- Zhang, R.; Wang, X.; Ni, L.; Di, X.; Ma, B.; Niu, S.; Liu, C.; Reiter, R.J. COVID-19: Melatonin as a potential adjuvant treatment. Life Sci. 2020, 250, 117583. [Google Scholar] [CrossRef]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative stress, prooxidants, and antioxidants: The interplay. BioMed Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef]
- Zhang, H.-M.; Zhang, Y. Melatonin: A well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 2014, 57, 131–146. [Google Scholar] [CrossRef]
- Hacışevki, A.; Baba, B. An Overview of Melatonin as an Antioxidant Molecule: A Biochemical Approach. In Melatonin—Molecular Biology, Clinical and Pharmaceutical Approaches; IntechOpen: London, UK, 2018. [Google Scholar]
- Tan, D.-X.; Manchester, L.C.; Esteban-Zubero, E.; Zhou, Z.; Reiter, R.J. Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism. Molecules 2015, 20, 18886–18906. [Google Scholar] [CrossRef] [PubMed]
- Jockers, R.; Delagrange, P.; Dubocovich, M.L.; Markus, R.P.; Renault, N.; Tosini, G.; Cecon, E.; Zlotos, D.P. Update on melatonin receptors: IUPHAR Review 20. Br. J. Pharmacol. 2016, 173, 2702–2725. [Google Scholar] [CrossRef]
- Sharafati-Chaleshtori, R.; Shirzad, H.; Rafieian-Kopaei, M.; Soltani, A. Melatonin and human mitochondrial diseases. J. Res. Med. Sci. 2017, 22, 2. [Google Scholar] [CrossRef]
- Reiter, R.J.; Rosales-Corral, S.; Tan, D.X.; Jou, M.J.; Galano, A.; Xu, B. Melatonin as a mitochondria-targeted antioxidant: One of evolution’s best ideas. Cell. Mol. Life Sci. 2017, 74, 3863–3881. [Google Scholar] [CrossRef]
- Mayo, J.C.; Sainz, R.M.; González-Menéndez, P.; Hevia, D.; Cernuda-Cernuda, R. Melatonin transport into mitochondria. Cell. Mol. Life Sci. 2017, 74, 3927–3940. [Google Scholar] [CrossRef]
- Shneider, A.; Kudriavtsev, A.; Vakhrusheva, A. Can melatonin reduce the severity of COVID-19 pandemic? Int. Rev. Immunol. 2020, 39, 153–162. [Google Scholar] [CrossRef]
- Bahrampour Juybari, K.; Pourhanifeh, M.H.; Hosseinzadeh, A.; Hemati, K.; Mehrzadi, S. Melatonin potentials against viral infections including COVID-19: Current evidence and new findings. Virus Res. 2020, 287, 198108. [Google Scholar] [CrossRef]
- Jørgensen, J.T.; Hersom, M. Companion diagnostics-a tool to improve pharmacotherapy. Ann. Transl. Med. 2016, 4, 482. [Google Scholar] [CrossRef]
- Jørgensen, J.T. Companion diagnostics: The key to personalized medicine. Foreword. Expert Rev. Mol. Diagn. 2015, 15, 153–156. [Google Scholar] [CrossRef]
- Burlea-Schiopoiu, A.; Ferhati, K. The Managerial Implications of the Key Performance Indicators in Healthcare Sector: A Cluster Analysis. Healthcare 2020, 9, 19. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).