1. Introduction
Blood transfusion is a standard medical intervention with well-documented benefits in a range of clinical practices. In Europe, Directive 2002/98/EC [
1] lays down quality and safety standards for the collection and testing of human blood and blood components and for their processing, storage, and distribution in order to safeguard public health and to ensure a high level of human protection. In this context, hemovigilance, as a set of organized surveillance procedures relating to serious adverse or unexpected events or reactions in donors or recipients, must be implemented. The International Society of Blood Transfusion Working Party on Hemovigilance, in partnership with other professional associations, has developed definitions of types of adverse reactions [
2].
One rare adverse reaction associated with transfusion is acute painful transfusion reaction (APTR), characterized by the sudden onset of severe pain shortly after the transfusion has commenced. This review summarizes current knowledge about APTR, focusing on clinical manifestations, underlying mechanisms, diagnostic criteria and management strategies.
2. Background
APTR has been described as a rare and distinct transfusion-related complication. Its characteristics were analyzed in detail by Jennane et al. [
3], who highlighted clinical presentations and emphasized the need for increased awareness and reporting. Davenport et al. [
4] contributed to understanding its potential association with HLA class II antibodies, providing important insights into its pathophysiology. It is a very rare syndrome characterized only by acute pain during the transfusion of blood or blood components, and it is not related to any other adverse reactions, such as Febrile Non-hemolytic Transfusion Reaction (FNHTR) and Acute Hemolytic Transfusion Reaction (AHTR) due to immunohematological incompatibility (most frequently due to antigen–antibody (ABO) incompatibility), but severe reactions can also occur with other blood group antibodies, such as anti-Jka, anti-Fya, or anti-U. Transfusion-Related Acute Lung Injury (TRALI) or Septic Reaction are further examples. APTR is reported as an “Unclassified Reaction”. It manifests with acute pain in the joints, particularly in the lumbar region and the torso, but may also be accompanied by hypertension, tachycardia, and shortness of breath. The most significant characteristic of this syndrome is the cessation of pain immediately upon discontinuation of the transfusion.
The exact etiology of this reaction is unknown; however, it has been associated with the transfusion of leuko-reduced erythrocytes. Leuko-reduced red blood cell products contain minimal concentrations of leukocytes; however, studies such as those reported in
Oncotarget indicate that cytokines such as IL-8 can accumulate during storage and be released during transfusion, which can potentially contribute to transfusion-related reactions. This highlights the importance of monitoring cytokine levels and their effects in the transfusion medicine field (Chang et al., 2018) [
5]. Contrary to misconceptions, leuko-reduced RBC products contain minimal residual leukocytes. However, cytokines such as IL-8 may accumulate during storage due to interactions with Duffy antigens and are released during transfusion [
6]. This phenomenon may explain the observed association of IL-8 with the incidence of APTR.
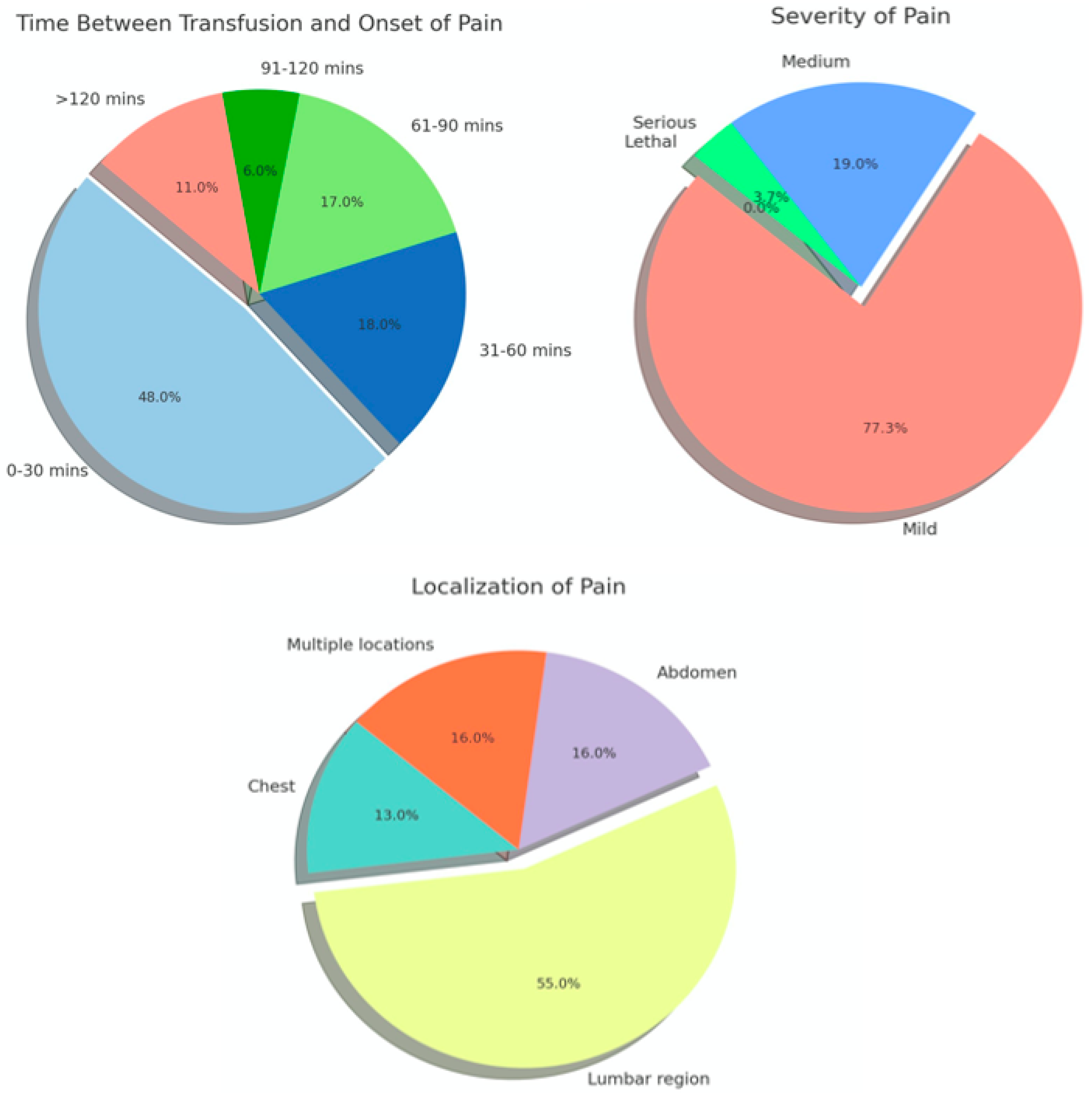
The analysis of notifications of adverse reactions reported in France between 2000 and 2016 revealed 430 diagnosed cases of APTR with overall incidence per 100,000 units of 0.8 for all blood components, 3.7 for platelets, 0.5 for erythrocytes, and 0.2 for plasma. The onset of pain was within 30 min of transfusion in 48% of cases, and the location was the lumbar region in 55%. A 2006 study by Alvarado- Ramy et al. reported the following distribution of APTR cases across underlying health conditions: 50% in leukemia and other hematologic malignancies, 11% in Sickle Cell Disease, 11% in Solid Malignancy, 6% in Thalassemia, and 22% in other conditions (
Figure 1) [
7].
3. Pathophysiology
During the typical storage conditions of red blood cells (RBCs), abundant physiochemical alternations take place, affecting the overall product quality. In addition to this, accumulating data indicate that stored RBCs have increased cytokine content [
7]. Recent studies have highlighted the impact of red blood cell (RBC) storage on the accumulation of bioactive lipids, cytokines, and microparticles. The storage lesion, which is characterized by progressive changes such as hemolysis, increased free iron, and the accumulation of inflammatory mediators, has been implicated in post-transfusion adverse reactions (García-Roa et al., 2017) [
8]. Moreover, variations in red blood cell (RBC) preparation methods have been demonstrated to influence cytokine levels. This is evidenced by a comparative study conducted by Schiroli et al., which revealed that distinct preparation techniques resulted in variations in potassium, iron, and inflammatory marker concentrations [
6]. These findings underscore the necessity for tailored transfusion strategies tailored to specific patient populations.
Increased levels of cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), interleukin-6 (IL-6), and interleukin-8 (IL-8) have been observed in random donors’ RBC concentrates. These white blood cell-derived soluble pro-inflammatory mediators are released in a time-dependent manner and may be associated with transfusion reactions [
9].
APTR is primarily associated with several key cytokines, each of which performs a distinct function in inflammatory response and pain mediation. The pro-inflammatory cytokine IL-1β plays a crucial role in the initiation of inflammatory responses and the onset of pain by enhancing the sensitivity of nociceptive neurons. Similarly, TNF-α has been shown to promote pain perception and inflammation. IL-6 is involved in the acute phase response, making a significant contribution to the development of systemic inflammation. Furthermore, IL-8 is involved in chemotaxis and implicated in pain mechanisms, particularly in inflammatory pain conditions [
7,
9,
10].
These cytokines activate specific pain pathways. For example, IL-1β and TNF-α can render peripheral nociceptors more sensitive, thereby inducing a state of hyperalgesia [
8]. Cytokine-mediated sensitization is a pivotal factor in the severe pain reported by patients experiencing APTR.
The inflammatory response, which is initiated by the release of cytokines, is a key element in the pathophysiology of APTR. This response includes the recruitment of immune cells to the transfusion site, which can intensify the pain experienced. The accumulation of pro-inflammatory mediators, including cytokines, leads to vasodilation, increased vascular permeability and the activation of pain pathways. In clinical practice, patients may present with severe chest, back, or limb pain characteristic of APTR, typically within 30 min of administration of the transfusion. The pain typically subsides shortly after the transfusion has stopped, which demonstrates the dynamic nature of the inflammatory response. The systemic release of cytokines can induce additional symptoms, including tachycardia, hypertension, and respiratory distress, which are indicative of the body’s reaction to the transfused blood components [
9,
10].
Leukapheresis is a procedure aimed at minimizing transfusion reactions and optimizing the effects of transfusion by removing leukocytes and their mediators of inflammation. It can take place by filtration prior to component storage (prestorage leukoreduction), before the transfusion (laboratory filtration) or during the transfusion (bedside filtration). Prestorage leukapheresis has been shown to be the best practice due to the significant reduction in inflammatory mediators in the resulting red cell product [
5,
11].
However, especially with regard to IL-8, the situation seems to be different, as IL-8 accumulates in the erythrocyte unit. This is because Duffy red cell antigens (Fya, Fyb) act as IL-8 receptors which bind IL-8 during storage and release it again during transfusion [
6,
8,
10].
In addition, there are studies suggesting that cytokines (especially IL-8) increase because of the warming of the blood unit that takes place in the time between issuing and transfusion in the clinic [
8,
10].
These specificities of IL-8 are probably related to the occurrence of APTR and explain its increased frequency compared to other transfusion-related adverse reactions when transfusing leuko-reduced red cell products [
6,
7,
8].
4. Clinical Features
APTR typically occurs within 30 min of the onset of transfusion and may be resolved within the same time frame following the discontinuation of transfusion [
12,
13].
The typical presenting symptom is severe pain, which may be localized in the abdomen, back, ribs, or other areas [
13,
14]. Systemic symptoms may include tachypnoea, hypertension, and tachycardia. Additional symptoms such as chills and fever have been observed, as well as tachycardia and hypertension, which are indicative of a systemic inflammatory response. The pain experienced during an acute transfusion reaction is frequently described as intense and painful to the point of debilitation and as sharp, stabbing, or cramping in nature. It may be localized in the lower back and may radiate to the upper back. It may be experienced on one or both sides of the body and may be mistaken for acute hemolysis. Patients may also experience pain in the hips and shoulders (
Figure 2).
Further insights into APTR characteristics have been provided by Murphy et al., who have reported gender and age distributions, as well as ABO blood group involvement in transfusion-associated chest pain syndromes (Jennane, 2014). Similarly, Jennane et al. presented comprehensive case studies of patients who experienced severe pain during transfusion, emphasizing the rapid onset and resolution of symptoms following transfusion cessation. These findings align with the current understanding of APTR as a distinct and under-reported phenomenon that requires further investigation [
13,
15].
A differential diagnosis is an essential component of evaluating a patient with APTR, as it permits the identification of potential alternative causes (
Table 1).
The differential diagnoses are shown in
Table 1.
A comprehensive series of laboratory tests should be performed to eliminate other potential causes of acute pain and to assess for the presence of hemolysis or other transfusion-related complications [
15,
16]. Such tests may include but are not limited to the following: complete blood count (CBC), biochemistry including total and indirect bilirubin, LDH, blood cultures, coagulation profile, direct and indirect Coombs tests, and cytokine modulation (investigational).
5. Treatment
The objective of APTR treatment is to relieve symptoms and diagnose and treat the underlying cause.
It is recommended that transfusion must be discontinued immediately upon the appearance of symptoms. It is crucial to immediately discontinue the transfusion of the specific blood product involved. However, this does not imply that the patient cannot receive future transfusions with appropriately matched products [
17,
18,
19].
Symptomatic relief: Pain management is provided in the form of the administration of analgesics, such as acetaminophen or non-steroidal anti-inflammatory drugs (NSAIDs), with the objective of providing pain relief. In cases of severe pain, the use of opioids, such as morphine or hydromorphone, is a potential treatment option [
19].
Hydration: It is important to ensure adequate hydration in order to facilitate the flushing out of potential inflammatory mediators and to support kidney function [
18].
Monitoring and supportive care: Continuous monitoring of the patient’s vital signs, including heart rate, blood pressure, and respiratory status, enables the detection of any deterioration [
3].
6. Conclusions
Acute transfusion reactions are transfusion-related events that can significantly affect patient safety and therapeutic management. These reactions, ranging from moderate allergies to severe anaphylactic shock, require timely identification and appropriate management.
The processes underlying APTRs remain insufficiently understood and warrant further investigation. The insights from this review may facilitate more accurate diagnoses and a deeper understanding of the underlying causes and risk factors associated with these reactions. Such advancements may support the development of targeted pharmaceuticals and prophylactic strategies, potentially reducing the incidence and severity of APTRs.
An effective transfusion safety framework is supported by the establishment of robust surveillance and reporting systems. A 1 to 5 scale can standardize transfusion-related pain assessments. This should be based on evidence and expert consensus. The development of such a scale would enhance reliability and validity. It is recommended that healthcare organizations collaborate to create comprehensive databases that track trends and patterns by ensuring the thorough documentation of each APTR and reporting to the relevant transfusion safety officer or blood bank. Aggregated data will provide valuable insights for clinical practice and inform policy actions aimed at improving transfusion safety.
The close monitoring and ongoing management of APTR cases are essential, especially in light of potential long-term implications and the importance of continued follow-up care beyond the acute phase. Additionally, providing patients with detailed information and education about transfusion reactions, including early warning symptoms, is a critical component of care. This patient-centered approach ensures that individuals remain informed and vigilant about their health, enabling timely intervention if a reaction occurs.
APTRs pose a significant challenge in transfusion medicine, requiring a multifaceted approach to therapy and prevention. Healthcare professionals can enhance patient outcomes and transfusion safety by fostering awareness, promoting research, improving surveillance and reporting mechanisms, and prioritizing patient education and follow-up care. Such efforts will deepen the understanding of APTRs and support the development of more effective strategies for their management and prevention.
Author Contributions
Conceptualization, S.D. and C.P.; methodology, S.D. and A.A.; software, S.D.; validation, S.D., A.A. and C.P.; formal analysis, S.M. and A.X.; investigation, A.A. and A.G.; resources, A.G., A.A. and C.P.; data curation, A.X.; writing—original draft preparation, S.D. and C.P.; writing—review and editing, S.D., A.A., S.M. and C.P.; visualization, S.D. and A.X.; supervision, S.M. and C.P.; project administration, S.M., C.P. and A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Directive 2002/98/EC of the European Parliament and of the Council of 27 January 2003 setting standards of quality and safety for the collection, testing, processing, storage, and distribution of human blood and blood components. Off. J. Eur. Union 2003, L33, 30–40.
- Vasudev, R.; Sawhney, V.; Dogra, M.; Raina, T.R. Transfusion-related adverse reactions: From institutional hemovigilance to National Hemovigilance program. Asian J. Transfus. Sci. 2016, 10, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Jennane, S.; Raissi, A.; Mahtat, E.M.; Zahid, H.; Messaoudi, N.; Doghmi, K.; Mikdame, M. Acute pain transfusion reaction. Transfus. Clin. Biol. J. Soc. Fr. Transfus. Sang. 2014, 21, 330–331. [Google Scholar] [CrossRef]
- Davenport, R.D.; Cooling, L.; Newman, B. Acute pain transfusion reaction associated with transfusion of HLA class II antibodies. Transfusion 2003, 43, 111A. [Google Scholar]
- Chang, C.C.; Lee, T.C.; Su, M.J.; Lin, H.C.; Cheng, F.Y.; Chen, Y.T.; Yen, T.H.; Chu, F.Y. Transfusion-associated adverse reactions (TAARs) and cytokine accumulations in the stored blood components: The impact of prestorage versus poststorage leukoreduction. Oncotarget 2018, 9, 4385. [Google Scholar] [CrossRef]
- Schiroli, D.; Merolle, L.; Quartieri, E.; Chicchi, R.; Fasano, T.; De Luca, T.; Molinari, G.; Pulcini, S.; Pertinhez, T.A.; Di Bartolomeo, E.; et al. Comparison of Two Alternative Procedures to Obtain Packed Red Blood Cells for β-Thalassemia Major Transfusion Therapy. Biomolecules 2021, 11, 1638. [Google Scholar] [CrossRef]
- Alvarado-Ramy, F.; Kuehnert, M.J.; Alonso-Echanove, J.; Sledge, L.; Haley, N.R.; Epstein, J.; Vostal, J.; Pearson, M. A multistate cluster of red blood cell transfusion reactions associated with use of a leucocyte reduction filter. Transfus. Med. 2006, 16, 41–48. [Google Scholar] [CrossRef]
- García-Roa, M.; del Carmen Vicente-Ayuso, M.; Bobes, A.M.; Pedraza, A.C.; González-Fernández, A.; Martín, M.P.; Sáez, I.; Seghatchian, J.; Gutiérrez, L. Red blood cell storage time and transfusion: Current practice, concerns and future perspectives. Blood Transfus. 2017, 15, 222. [Google Scholar] [PubMed]
- Sut, C.; Tariket, S.; Chou, M.L.; Garraud, O.; Laradi, S.; Hamzeh-Cognasse, H.; Seghatchian, J.; Burnouf, T.; Cognasse, F. Duration of red blood cell storage and inflammatory marker generation. Blood Transfus. 2017, 15, 145–152. [Google Scholar]
- Shaigan, M.; Pourfath, E.A.; Namiri, M.; Babaee, G. Generation of IL-8 and TNF-alpha in platelet concentrates during storage. Arch. Iran. Med. 2006, 9, 27–31. [Google Scholar]
- Shukla, R.; Patel, T.; Gupte, S. Release of cytokines in stored whole blood and red cell concentrate: Effect of leukoreduction. Asian J. Transfus. Sci. 2015, 9, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Pande, A.; Setya, D.; Kumar, P.; Shanker, A. Comparative Study for Measurement of Residual Leucocytes in Leucodepleted Red Blood Cells by Two Different Methods. Indian J. Hematol. Blood Transfus. 2020, 36, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Savage, W.J. Transfusion reactions. Hematol. Oncol. Clin. N. Am. 2016, 30, 619–634. [Google Scholar] [CrossRef] [PubMed]
- Robertson-Patera, J.; Lu, W. A Case of Recurrent Acute Pain Transfusion Reactions. Am. J. Clin. Pathol. 2020, 154 (Suppl. S1), S167. [Google Scholar] [CrossRef]
- Murphy, C.; Parakh, R.; Metcalf, R.; Pagano, M.B. Transfusion-associated chest pain. Transfusion 2018, 58, 1–7. [Google Scholar] [CrossRef]
- Hillis, C.M.; Shih, A.W.; Heddle, N.M. Best practices in the differential diagnosis and reporting of acute transfusion reactions. Int. J. Clin. Transfus. Med. 2016, 1, 1–14. [Google Scholar]
- Tinegate, H.; Birchall, J.; Gray, A.; Haggas, R.; Massey, E.; Norfolk, D.; Pinchon, D.; Sewell, C.; Wells, A.; Allard, S. Guideline on the investigation and management of acute transfusion reactions. Br. J. Haematol. 2012, 159, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Paudel, P.; Sinha, P. Acute Pain Transfusion Reaction: A Case Report. Cureus 2024, 16, e64206. [Google Scholar] [CrossRef] [PubMed]
- Delaney, M.; Wendel, S.; Bercovitz, R.S.; Cid, J.; Cohn, C.; Dunbar, N.M.; Apelseth, T.O.; Popovsky, M.; Stanworth, S.J.; Tinmouth, A.; et al. Transfusion reactions: Prevention, diagnosis, and treatment. Lancet 2016, 388, 2825–2836. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).