Abstract
Thalassemia, a genetic condition characterized by defective hemoglobin synthesis, is often managed with transfusion therapy, which can lead to iron overload—a significant contributor to morbidity and mortality due to organ damage and pathogenic infections. Iron chelation therapy, the cornerstone of managing iron toxicity, may inadvertently influence the gut microbiome, a critical modulator of immunity and metabolism. This review provides new insights into the interplay between iron chelation therapy and gut microbiome dynamics in thalassemia patients. It synthesizes findings on how chelators such as deferoxamine, deferasirox, and deferiprone influence microbial composition, iron availability, and systemic inflammation. Emerging evidence highlights alterations in gut microbial diversity, with reduced beneficial taxa and increased pathogenic populations, driven by changes in luminal iron levels. This imbalance contributes to immune dysregulation, systemic inflammation, and susceptibility to infections. The review advocates for tailored treatment strategies that integrate microbiome-targeted interventions alongside traditional chelation therapy to improve patient outcomes. By combining genetic profiling, dietary adjustments, and microbiome modulation, this approach offers a promising avenue for personalized medicine in thalassemia care.
1. Introduction
Thalassemia is a group of inherited hematologic disorders characterized by defective synthesis of hemoglobin subunits, leading to chronic anemia and systemic complications. The genetic mutations responsible for the condition affect the genes that encode for α- or β-globin chains, leading to imbalances in the production of hemoglobin and ineffective erythropoiesis. This imbalance precipitates a cascade of pathological events, including oxidative stress, hemolysis, and iron overload, which together contribute to significant morbidity and mortality. The clinical manifestations range from asymptomatic carrier states to severe anemia, reflecting the genetic diversity and complexity of the disease [1].
Thalassemia might be divided into two major forms: α-thalassemia, due to mutations or deletions in the HBA1 and HBA2 genes, and β-thalassemia, due to mutations in the HBB gene. These molecular defects disrupt hemoglobin synthesis and lead to varying degrees of anemia. However, iron overload mechanisms differ; while β-thalassemia (especially TDT and NTDT) results in increased intestinal iron absorption due to hepcidin suppression, α-thalassemia patients typically develop iron overload only if they undergo frequent transfusions [2]. In β-thalassemia, excess iron results from transfusions and increased intestinal absorption due to hepcidin suppression. In contrast, α-thalassemia patients do not typically develop iron overload unless they require frequent transfusions. When present, transfusion-related iron overload can lead to complications such as cardiomyopathy, cirrhosis, and endocrine dysfunction [3].
Recent attention has centered on the participation of oxidative stress and dysregulated hepcidin in the pathophysiology of thalassemia. Oxidative damage due to unpaired globin chains has highlighted the promise of antioxidant therapies, while hepcidin suppression favors iron overload and dysbiosis in the gut microbiome. These findings have stimulated the development of novel treatment options, including gene therapy, pharmacologic erythropoiesis-targeting agents, and microbiome-modulating strategies, which are expected to allow for more personalized and effective management [4].
Iron chelation therapy is necessary in cases of thalassemia presenting with iron overload. However, while chelators such as deferoxamine, deferasirox, and deferiprone reduce iron toxicity and improve patient outcomes, they also have effects on the gut microbiome, which has a very important role in systemic health [5]. Dysbiosis, promoted by altered luminal iron levels, enhances inflammation, impairs gut barrier function, and predisposes patients to infections. The interaction of chelation therapy with the gut microbiome may provide new opportunities for understanding how better to undertake therapy [6].
Recent studies highlight oxidative stress and hepcidin suppression as central mechanisms in thalassemia pathophysiology. Ineffective erythropoiesis in β-thalassemia leads to increased reactive oxygen species (ROS) generation, contributing to cellular damage and systemic inflammation [7,8,9]. Simultaneously, hepcidin suppression results in unregulated intestinal iron absorption, worsening iron overload even in non-transfused patients [3,5,10]. These dysregulations not only impact organ function but also influence gut microbiota composition, favoring the growth of pathogenic species and increasing susceptibility to infections [11,12].
While iron overload is controlled through chelation, there will be associated changes in the gut microbial flora balance, immune-related activities, and inflammation. The dysbiosis in thalassemia aggravates a variety of systemic complications, including vulnerability to infection and disturbance in iron metabolism. Because chelators alter the luminal iron levels, they could, secondarily, impact the gut microbiota and, thus, draw interest in a more microbiome-centric approach. Perhaps the integration of probiotics, prebiotics, dietary modifications, and alternative approaches to chelation in treatment could bring optimum treatment more holistic approach toward improvement in systemic health along with gut health in thalassemia. This review underlines how iron chelation affects the gut microbiome in thalassemia patients and discusses related aspects of therapy.
Literature Selection Strategy
We conducted a systematic literature search using established databases and predefined selection criteria to ensure a rigorous and transparent review process. The objective was to identify peer-reviewed studies on the relationship between thalassemia, iron overload, microbiome alterations, and chelation therapy. A summary of the literature selection process is presented in Table 1. Literature selection criteria provide a concise overview of the databases searched, keywords used, and the inclusion/exclusion criteria applied to ensure the relevance and quality of selected studies.

Table 1.
Literature selection criteria.
2. Iron Metabolism and Its Role in Thalassemia
Iron is an absolute requirement for microbial and human metabolism, and the body has no natural means to eliminate excess iron. Iron overload, due to this very reason, constitutes a major complication in thalassemia. Chronic transfusions bring about a progressive accumulation of iron in the liver, heart, and endocrine glands, leading to fibrosis, cardiomyopathy, and endocrine dysfunction [13]. Hepatic iron deposition leads to cirrhosis and hepatocellular carcinoma, while excess cardiac iron increases the risk of arrhythmias and heart failure, significantly affecting patient survival [14].
Iron is another important factor that shapes the compositional gut microbiota. Many gut bacteria use iron during basic physiological processes, including DNA synthesis and respiration. Such pathogenic bacteria usually have a high-affinity iron acquisition system, allowing them to outcompete the beneficial ones in an iron-rich environment and, hence, cause dysbiosis. Iron balance is required for gut homeostasis, as iron-driven dysbiosis may contribute to systemic inflammation and immune dysfunction [15].
Iron Chelation Therapy
The chief strategy for treatment is iron chelation to reduce both circulating and stored iron. This prevents the facilitation of reactive oxygen species generation by excess iron, oxidative stress, and subsequent cellular damage. Currently, three major iron chelators are used clinically: deferoxamine, deferasirox, and deferiprone. All have disparate pharmacokinetics and differ concerning efficacy [16,17].
Deferoxamine is a highly effective parenteral chelator that reduces serum and organ iron, but it requires prolonged infusions and, thus, suffers from poor patient compliance [2]. Defarasirox is an oral chelator that excretes iron via the feces, which improves compliance but requires monitoring due to gastrointestinal and hepatic side effects [10]. Deferiprone is very efficient in cardiac iron removal but does have the drawbacks of agranulocytosis and gastrointestinal disturbances, thus requiring frequent follow-up [18].
Beyond the reduction of iron overload, chelation therapy also minimizes oxidative stress and inflammation, which are equally major contributors to thalassemia complications. Chelators decrease ROS levels and pro-inflammatory cytokines, leading to a decrease in the oxidative lesions in the heart, liver, and endocrine glands [7]. However, iron chelation influences gut microbiota through alteration of luminal iron availability. For example, the use of deferasirox, which increases fecal iron excretion, may affect microbial composition by limiting the availability of iron to pathogenic and beneficial bacteria. This interaction between chelation therapy and gut microbiota health, thus, should be further pursued since optimization of iron management with preservation of microbiome balance will most likely improve patient outcomes [19].
Iron chelation is the cornerstone of thalassemia treatment, but efforts are still ongoing for a personalized approach. The elucidation of systemic effects, which also encompass effects on the gut microbiome, may present the opportunity for more improved therapeutic approaches combining iron management with microbiome-targeting therapy [8,9]. The comparison of different iron chelation therapies is summarized in Table 2.

Table 2.
Iron chelation therapy comparison. Data from [7,8,9,11,13,14,16,17,18,19,20,21,22].
3. The Gut Microbiome
The gut microbiome plays an important role in digestion, immunity, and metabolism, in which iron is a key factor that influences microbial composition. Most pathogenic bacteria possess high-affinity iron uptake systems that give them a competitive advantage over good microbes in high-iron environments, thus disrupting the microbial balance [14].
Iron levels within the gut are controlled by proteins such as lactoferrin and transferrin, limiting the amount of free iron accessible to microbes. This balance may be disrupted by dietary changes, iron supplementation, or disease, resulting in a dysbiosis state characterized by reduced diversity and increased relative abundance of pathogenic species [20]. Excessive available iron inhibits the growth of beneficial bacteria, including Lactobacillus and Bifidobacterium, and favors the growth of pathogenic bacteria, including Escherichia coli [21]. Dysbiosis impairs gut barrier function, facilitating bacterial entry into the bloodstream and systemic inflammation, thereby increasing the risk of metabolic and immune disorders [22].
Gut microbiota plays a vital role in regulating iron homeostasis for the prevention of inflammation and infection. Understanding this would pave the way to apply microbiome-based therapies such as probiotics and nutritional intervention toward improved health outcomes. The factors of predisposition for gut dysbiosis are summarized in Table 3.

Table 3.
Factors of predisposition for gut dysbiosis. Data from [4,5,14,23,24,25].
3.1. Evidence of Gut Microbiome Changes in Thalassemia Patients
Thalassemia is managed through blood transfusions and iron chelation therapy to prevent anemia and iron overload. Recent studies highlight significant gut microbiome alterations in thalassemia patients, which may impact disease progression and overall health. Compared to healthy individuals, thalassemia patients—especially those not receiving treatment—exhibit reduced microbial diversity and shifts in community structure. Non-transfusion-dependent β-thalassemia/HbE patients show lower levels of beneficial short-chain fatty acid (SCFA)-producing bacteria (Alistipes, Coprococcus, Oscillospira) and an increased abundance of potentially pathogenic taxa (Clostridium, Fusobacteriaceae, Enterobacteriaceae). Frequent infections and iron overload further contribute to gut inflammation and microbiota imbalances [11,12].
SCFAs are essential for gut barrier integrity and immune regulation. In β-thalassemia, excess iron intake from transfusions disrupts these protective mechanisms, exacerbating gut inflammation and epithelial damage. Beneficial probiotic bacteria, such as Lactobacillales, thrive in low-iron environments but can be displaced by pathogenic species when iron levels rise [24,26,27].
A study by Suparan et al. compared transfusion-dependent (TDT) and non-transfusion-dependent (NTDT) thalassemia patients to healthy controls. TDT patients exhibited significantly higher iron overload and distinct microbial shifts, with increased Verrucomicrobiota and Fusobacteriota, further linking iron dysregulation to gut microbiome alterations [11,28]. The comparison of NTDT and TDT is summarized in Table 4.

Table 4.
Comparison of NTDT and TDT. Data from [3,4,5,10,11,12,22,23,24,26,27,28,29,30,31,32,33].
Maintaining microbial balance in thalassemia patients is crucial for mitigating systemic inflammation and improving health outcomes. Future strategies should consider microbiome-targeted therapies alongside traditional iron management.
3.2. Functional Impacts of Microbiome Dysbiosis
The gut microbiome has a high value in the process of iron absorption and storage. Beneficial bacteria support dietary iron conversion into bioavailable forms, whereas dysbiosis disturbs this process. Overgrowth of pathogenic bacteria increases iron uptake and worsens iron overload, while the decline in beneficial microbes may reduce its absorption, thus causing deficiency. In cases of thalassemia, dysbiosis further complicates iron regulation, where there is already a problem of imbalance, and thus points out the necessity of microbiome stability in maintaining iron homeostasis [34].
Gut-associated lymphoid tissue (GALT) is a part of the immune defense that is supported by a balanced microbiome; dysbiosis weakens mucosal immunity, increases inflammation, and elevates pro-inflammatory cytokines, thereby worsening oxidative stress and organ damage in thalassemia [29].
A healthy microbiome maintains the integrity of gut barrier functions and prevents toxic elements’ passage through the bloodstream. In dysbiosis, the barrier becomes disrupted, with increased intestinal permeability “leaky gut”—which permits toxins and pathogens to cause systemic inflammation. In thalassemia, this will contribute to exacerbating gastrointestinal symptoms, malabsorption of nutrients, and immune dysfunction. The microbial balance reduces the level of inflammation, thus helping improve overall health [30].
4. Bacterial Infections in Thalassemia Patients
Patients with thalassemia are highly susceptible to bacterial infections, which include septicemia, pneumonia, and biliary tract infections [25]. This vulnerability arises from iron overload, frequent transfusions, and microbiome imbalances, particularly in β-thalassemia. Many patients undergo splenectomy, which further reduces their immunity due to reduced antigen clearance and antibody production [35,36,37]. Reduced chemotaxis with an increase in oxidative stress due to various etiological factors further deteriorates the infectious state of the patient [38,39,40,41]. The overview of thalassemia infections is given in Table 5.
Excess iron favors the growth of pathogenic bacteria, resulting in infections due to Klebsiella pneumoniae, Streptococcus pneumoniae, Yersinia enterocolitica, E. coli, and Listeria monocytogenes [42,43]. Klebsiella species are particularly dangerous, causing sinusitis, septicemia, and abscesses. Y. enterocolitica infections, commonly occurring in patients treated with desferrioxamine, can be resistant to therapy and require prolonged antibiotic therapy, while L. monocytogenes may cause neurological complications [44,45,46]. The most common cause of infection in post-splenectomy cases is S. pneumoniae, and splenectomized thalassemia patients are more susceptible to infection compared to other patient groups.

Table 5.
Overview of thalassemia infections. Data from [25,44,46,47,48,49,50,51,52].
Table 5.
Overview of thalassemia infections. Data from [25,44,46,47,48,49,50,51,52].
| Pathogen | Associated Infections | Pathogen-Specific Risk Factors | Case Studies |
|---|---|---|---|
| Klebsiella spp. | Sinusitis, septicemia, abscesses (kidneys, liver, lungs, parotid gland). | Iron overload in severe thalassemia with frequent transfusions. | Common in thalassemia patients, with a high mortality rate due to iron overload. |
| Y. enterocolitica | Septicemia, tonsillitis, pharyngitis, focal abscesses. | Desferrioxamine therapy (iron chelator acting as a siderophore). | Infections were significantly associated with desferrioxamine use. |
| L. monocytogenes | Neurological infections and systemic infections in immunocompromised. | Iron overload increases bacterial virulence. | Show increased severity, with high morbidity in immunocompromised patients. |
| S.pneumoniae | Post-splenectomy infections. | Splenectomy reduces clearance of encapsulated bacteria. | The risk of sepsis among asplenic individuals is significantly elevated, with a high case-fatality rate of 50–70%. |
| E. coli | Bacteremia, urinary tract infections (UTIs). | Iron overload enhances virulence; | Iron overload significantly increases susceptibility to bacterial infections, including E. coli UTIs. |
Optimization of chelation strategies with gut microbial balance is critical to minimizing infection risks and improving patient outcomes [53]. A study by Obed et al. showed that thalassemia patients, especially those with higher iron levels, suffered from E. coli infections and urinary tract infections. The iron-rich gut environment indeed favors pathogenic growth. A balanced approach toward iron chelation therapy and antibiotic use is required. While chelators reduce iron overload, some of them, such as deferoxamine, may inadvertently support bacterial growth and increase the risk of bacteremia [52]. The impact of iron chelation therapy in thalassemia is represented in Figure 1.
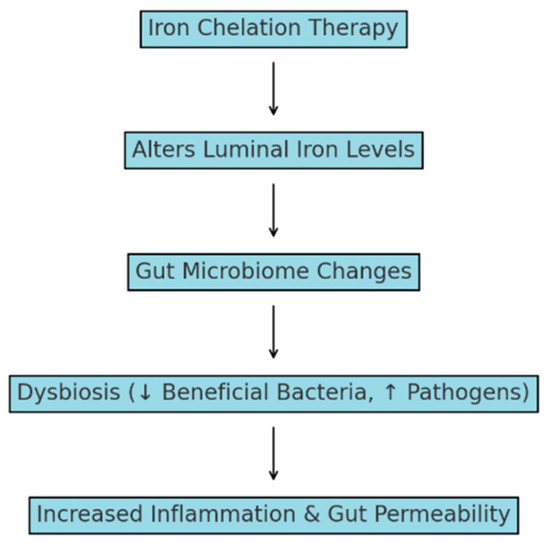
Figure 1.
Impact of iron chelation therapy on the gut microbiome and systemic health in thalassemia. ↑—increased; ↓—decreased.
5. Therapeutic Implications and Future Directions
The link between gut microbiome health and iron metabolism in thalassemia has led to new therapeutic strategies aimed at addressing dysbiosis while managing iron overload. Probiotics (Lactobacillus rhamnosus and Bifidobacterium breve) can help restore microbial balance by reducing inflammation and strengthening gut barrier function [32]. Prebiotics (inulin and fructooligosaccharides) selectively stimulate beneficial bacteria, enhancing microbiome diversity and nutrient absorption. Combining probiotics and prebiotics as synbiotics has shown promise in improving gut health and modulating iron metabolism [19].
Dietary modifications also play a key role in optimizing the microbiome’s response to iron chelation. High-fiber, fermented, and polyphenol-rich foods promote beneficial bacteria and reduce inflammation, whereas high-fat, high-sugar diets contribute to dysbiosis and systemic inflammation. Additionally, iron-binding compounds like phytates and tannins may help regulate iron bioavailability, limiting excess iron’s impact on pathogenic bacteria [33].
To minimize microbiome disruption, alternative chelation strategies are being explored. Deferiprone, which targets intracellular iron, may have less impact on gut luminal iron levels compared to deferasirox, which promotes fecal iron excretion. Novel nanotechnology-based chelation agents and targeted drug delivery systems could further reduce systemic toxicity while preserving gut microbial integrity [18,19]. Balancing iron chelation with microbiome-focused interventions offers a promising approach to improving health outcomes in thalassemia patients [16].
6. Conclusions
Thalassemia remains a problematic global health challenge due to complications caused by transfusion-dependent iron overload that require iron chelation therapies to control its levels. This often leads to an imbalanced gut microbiome, elevating its iron levels and favoring the spread of pathogenic infections, one of the most common causes of mortality in thalassemia patients. The interplay of illness progression, chelation therapy, and microbiota imbalance emphasizes the disease’s complexity. Thus, it highlights the need for novel therapeutic approaches and a customized strategy to address this problem, including all facets of Thalassaemia genetics, iron overload, and chelation and their impact on the microbiota. New therapeutic approaches that address the haematologic and microbiological components of thalassemia can enhance patient results and long-term health.
Author Contributions
Conceptualization, M.A. and M.H.; methodology, S.D. and N.C.; validation, M.D., M.A. and M.H.; formal analysis, M.A.; investigation, S.D. and N.C.; writing—original draft preparation, S.D., N.C. and M.A.; writing—review and editing, M.H. and M.D.; visualization, M.D.; supervision, M.A.; project administration, M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sadiq, I.Z.; Abubakar, F.S.; Usman, H.S.; Abdullahi, A.D.; Ibrahim, B.; Kastayal, B.S.; Ibrahim, M.; Hassan, H.A. Thalassemia: Pathophysiology, diagnosis, and advances in treatment. Thalass. Rep. 2024, 14, 81–102. [Google Scholar] [CrossRef]
- Cappellini, M.D.; Porter, J.B.; Viprakasit, V.; Taher, A.T. A paradigm shift on beta-thalassaemia treatment: How will we manage this old disease with new therapies? Blood Rev. 2018, 32, 300–311. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, D.G.; Zidova, Z.; Mikhael, M.; Hamdi, A.; Horvathova, M.; Ponka, P. Pathophysiology and treatment of beta-thalassemia: Investigations of heme oxygenase 1 and its inhibitors. Blood 2015, 126, 3373. [Google Scholar] [CrossRef]
- Allali, S.; de Montalembert, M.; Brousse, V.; Chalumeau, M.; Karim, Z. Management of iron overload in hemoglobinopathies. Transfus. Clin. Biol. 2017, 24, 223–226. [Google Scholar] [CrossRef]
- Auger, D.; Pennell, D.J. Cardiac complications in thalassemia major. Ann. N. Y. Acad. Sci. 2016, 1368, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Borgna-Pignatti, C.; Marsella, M. Iron chelation in thalassemia major. Clin. Ther. 2015, 37, 2866–2877. [Google Scholar] [CrossRef]
- Di Paola, A.; Tortora, C.; Argenziano, M.; Marrapodi, M.; Rossi, F. Emerging roles of the iron chelators in inflammation. Int. J. Mol. Sci. 2022, 23, 47977. [Google Scholar] [CrossRef] [PubMed]
- Walter, P.; Macklin, E.; Porter, J.; Evans, P.; Kwiatkowski, J.; Neufeld, E.; Harmatz, P. Inflammation and oxidant stress in β-thalassemia patients treated with iron chelators deferasirox (ICL670) or deferoxamine: An ancillary study of the Novartis CICL670A0107 trial. Haematologica 2008, 93, 817–825. [Google Scholar] [CrossRef]
- Fibach, E.; Rachmilewitz, E. The role of antioxidants and iron chelators in the treatment of oxidative stress in thalassemia. Ann. N. Y. Acad. Sci. 2010, 1202, 10–16. [Google Scholar] [CrossRef]
- Taher, A.; Weatherall, D.; Cappellini, M. Thalassaemia. Lancet 2018, 391, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Suparan, K.; Trirattanapa, K.; Sriwichaiin, S.; Kerdphoo, S.; Tantiworawit, A.; Chattipakorn, N.; Chattipakorn, S.C. Transfusion-dependent thalassemia patients develop cognitive impairment with gut dysbiosis. Alzheimers Dement. 2023, 19, 73541. [Google Scholar] [CrossRef]
- Thiengtavor, C.; Siriworadetkun, S.; Paiboonsukwong, K.; Fucharoen, S.; Pattanapanyasat, K.; Vadolas, J.; Svasti, S.; Chaichompoo, P. Increased ferritin levels in non-transfusion-dependent β°-thalassaemia/HbE are associated with reduced CXCR2 expression and neutrophil migration. Br. J. Haematol. 2019, 189, 162–195. [Google Scholar] [CrossRef] [PubMed]
- Pinto, V.; Forni, G. Management of iron overload in beta-thalassemia patients: Clinical practice update based on case series. Int. J. Mol. Sci. 2020, 21, 22871. [Google Scholar] [CrossRef] [PubMed]
- Parmanand, B.; Kellingray, L.; Gall, L.; Basit, A.; Fairweather-Tait, S.; Narbad, A. A decrease in iron availability to human gut microbiome reduces the growth of potentially pathogenic gut bacteria: An in vitro colonic fermentation study. J. Nutr. Biochem. 2019, 67, 20–27. [Google Scholar] [CrossRef]
- Seyoum, Y.; Baye, K.; Humblot, C. Iron homeostasis in host and gut bacteria—A complex interrelationship. Gut Microbes 2021, 13, e1874855. [Google Scholar] [CrossRef]
- Porter, J.B.; Shah, F.T. Iron overload in thalassemia and related conditions: Therapeutic goals and assessment of response to chelation therapies. Hematol. Oncol. Clin. N. Am. 2010, 24, 1109–1130. [Google Scholar] [CrossRef]
- Abetz, L.; Baladi, J.; Jones, P.; Rofail, D. The impact of iron overload and its treatment on quality of life: Results from a literature review. Health Qual. Life Outcomes 2006, 4, 73. [Google Scholar] [CrossRef]
- Njeim, R.; Naouss, B.; Bou-Fakhredin, R.; Haddad, A.; Taher, A. Unmet needs in β-thalassemia and the evolving treatment landscape. Transfus. Clin. Biol. 2023, 30, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Li, H. Gut microbiota and iron: The crucial actors in health and disease. Pharmaceuticals 2018, 11, 98. [Google Scholar] [CrossRef]
- Morgenthau, A.; Pogoutse, A.; Adamiak, P.; Moraes, T.; Schryvers, A. Bacterial receptors for host transferrin and lactoferrin: Molecular mechanisms and role in host-microbe interactions. Future Microbiol. 2013, 8, 1575–1585. [Google Scholar] [CrossRef] [PubMed]
- Mousa, W.; Chehadeh, F.; Husband, S. Microbial dysbiosis in the gut drives systemic autoimmune diseases. Front. Immunol. 2022, 13, 906258. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Kolodziejczyk, A.; Thaiss, C.; Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Weatherall, D.J.; Clegg, J.B. The Thalassaemia Syndromes; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Yang, W.; Cong, Y. Gut microbiota-derived metabolites in the regulation of host immune responses and immune-related inflammatory diseases. Cell Mol. Immunol. 2021, 18, 866–877. [Google Scholar] [CrossRef]
- Vento, S.; Cainelli, F.; Cesario, F. Infections and thalassemia. Lancet Infect. Dis. 2006, 6, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.J.; Zheng, L.; Wang, Y.; Colgan, S.P. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 2015, 17, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Salvi, P.S.; Cowles, R.A. Butyrate and the intestinal epithelium: Modulation of proliferation and inflammation in homeostasis and disease. Cells 2021, 10, 1775. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.R.; Probert, C.S.; Cogan, T.A. Identification and characterisation of an iron-responsive candidate probiotic. PLoS ONE 2011, 6, e26507. [Google Scholar] [CrossRef] [PubMed]
- Arrazuria, R.; Pérez, V.; Molina, E.; Juste, R.; Khafipour, E.; Elguezabal, N. Diet-induced changes in the microbiota and cell composition of rabbit gut-associated lymphoid tissue (GALT). Sci. Rep. 2018, 8, 32484. [Google Scholar] [CrossRef] [PubMed]
- Usuda, H.; Okamoto, T.; Wada, K. Leaky gut: Effect of dietary fiber and fats on microbiome and intestinal barrier. Int. J. Mol. Sci. 2021, 22, 7613. [Google Scholar] [CrossRef] [PubMed]
- Aoki, Y. The risk of severe bacterial infection in non-transfusion-dependent thalassemia. Pediatr. Res. 2021, 92, 360–361. [Google Scholar] [CrossRef]
- Bidell, M.R.; Hobbs, A.L.; Lodise, T.P. Gut microbiome health and dysbiosis: A clinical primer. Pharmacotherapy 2022, 42, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Rusu, I.G.; Suharoschi, R.; Vodnar, D.C.; Pop, C.R.; Socaci, S.A.; Vulturar, R.; Istrati, M.; Moroșan, I.; Fărcaș, A.C.; Kerezsi, A.D.; et al. Iron supplementation influence on the gut microbiota and probiotic intake effect in iron deficiency—A literature-based review. Nutrients 2020, 12, 1993. [Google Scholar] [CrossRef] [PubMed]
- Puga, A.; Samaniego-Vaesken, M.; Montero-Bravo, A.; Ruperto, M.; Partearroyo, T.; Varela-Moreiras, G. Iron supplementation at the crossroads of nutrition and gut microbiota: The state of the art. Nutrients 2022, 14, 1926. [Google Scholar] [CrossRef] [PubMed]
- Alzhrani, F.S.; Algethmi, F.A.; Makin, M.; Barayan, N.A.; Hilal, R.M.; Alnakhli, S. Prevalence and risk factors of severe bacterial infections in thalassemia patients. Curr. Pediatr. Res. 2020, 24, 264–269. [Google Scholar]
- Wang, S.-C.; Lin, K.-H.; Chern, J.P.S.; Lu, M.-Y.; Jou, S.-T.; Lin, D.-T.; Lin, K.-S. Severe bacterial infection in transfusion-dependent patients with thalassemia major. Clin. Infect. Dis. 2003, 37, 984–988. [Google Scholar] [CrossRef]
- Taher, A.T.; Saliba, A.N. Iron overload in thalassemia: Different organs at different rates. Hematol. Am. Soc. Hematol. Educ. Program. 2017, 2017, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Price, V.E.; Blanchette, V.S.; Ford-Jones, E.L. The prevention and management of infections in children with asplenia or hyposplenia. Infect. Dis. Clin. N. Am. 2007, 21, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Miri-Aliabad, G.; Rezaeifar, A.; Salarzaei, M. Comparison of immunoglobulins status in splenectomized and non-splenectomized patients with major beta-thalassemia. J. Pediatr. Rev. 2022, 10, 161–166. [Google Scholar] [CrossRef]
- Khan, F.A.; Fisher, M.A.; Khakoo, R.A. Association of hemochromatosis with infectious diseases: Expanding spectrum. Int. J. Infect. Dis. 2007, 11, 482–487. [Google Scholar] [CrossRef]
- Moura, E.; Noordermeer, M.A.; Verhoeven, N.; Verheul, A.F.; Marx, J.J. Iron release from human monocytes after erythrophagocytosis in vitro: An investigation in normal subjects and hereditary hemochromatosis patients. Blood 1998, 92, 2511–2519. [Google Scholar] [CrossRef]
- Visitchanakun, P.; Panpetch, W.; Saisorn, W.; Chatthanathon, P.; Wannigama, D.L.; Thim-Uam, A.; Leelahavanichkul, A. Increased susceptibility to dextran sulfate-induced mucositis of iron-overload β-thalassemia mice, another endogenous cause of septicemia in thalassemia. Clin. Sci. 2021, 135, 1467–1486. [Google Scholar] [CrossRef] [PubMed]
- Sae-Khow, K.; Chantainayarong, A.; Visitchanakun, P.; Saisorn, W.; Svasti, S.; Fucharoen, S.; Leelahavanichkul, A. Pathogen-associated molecules from gut translocation enhance severity of cecal ligation and puncture sepsis in iron-overload β-thalassemia mice. J. Inflamm. Res. 2020, 13, 719–735. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Zhang, W.; Lin, M.; Teymournejad, O.; Budachetri, K.; Lakritz, J.; Rikihisa, Y. Iron robbery by intracellular pathogen via bacterial effector–induced ferritinophagy. Proc. Natl. Acad. Sci. USA 2021, 118, e2026598118. [Google Scholar] [CrossRef] [PubMed]
- Pajuelo, D.; Hernández-Cabanyero, C.; Sanjuán, E.; Lee, C.; Silva-Hernández, F.X.; Hor, L.; Mackenzie, S.; Amaro, C. Iron and Fur in the life cycle of the zoonotic pathogen Vibrio vulnificus. Environ. Microbiol. 2016, 18, 4005–4022. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.H.Y.; Ha, S.Y.; Chan, G.C.F.; Chiang, A.; Lee, T.L.; Ho, H.K.; Lee, C.Y.; Luk, C.W.; Lau, Y.L. Klebsiella infection in patients with thalassemia. Clin. Infect. Dis. 2003, 36, 575–579. [Google Scholar] [CrossRef]
- Hansen, M.G.; Pearl, G.; Lévy, M. Intussusception due to Yersinia enterocolitica enterocolitis in a patient with beta-thalassemia. Arch. Pathol. Lab. Med. 2009, 125, 1486–1488. [Google Scholar] [CrossRef] [PubMed]
- Drevets, D.A.; Leenen, P.J.; Greenfield, R.A. Invasion of the central nervous system by Listeria monocytogenes: Implications for bacterial entry across the blood-brain barrier. Infect. Immun. 2004, 72, 155–167. [Google Scholar]
- Charlier, C.; Perrodeau, E.; Leclercq, A.; Cazenave, B.; Pilmis, B.; Henry, B.; Lopes, A.; Maury, M.M.; Moura, A.; Goffinet, F.; et al. Clinical features and prognostic factors of listeriosis: The MONALISA national prospective cohort study. Lancet. Infect. Dis. 2017, 17, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Arnold, D.M.; Crowther, M.A.; Cook, R.J.; Sigouin, C.S.; Heddle, N.M.; Molnar, L.; Kelton, J.G. Adverse outcomes after splenectomy in adults. JAMA 2008, 299, 2324–2331. [Google Scholar]
- Sinwar, P.D. Overwhelming post-splenectomy infection syndrome—Review study. Int. J. Surg. 2014, 12, 1314–1316. [Google Scholar] [CrossRef]
- Obed, F.A.; Omran, A.M.; Jebur, K.S. Effect of iron overload on prevalence of common bacterial infection in thalassemia patients. Iraqi J. Hematol. 2024, 13, 38–43. [Google Scholar] [CrossRef]
- Spelman, D.; Buttery, J.; Daley, A.; Isaacs, D.; Jennens, I.; Kakakios, A.; Lawrence, R.; Roberts, S.; Torda, A.; Watson, D.A.; et al. Guidelines for the prevention of sepsis in asplenic and hyposplenic patients. Intern. Med. J. 2008, 38, 349–356. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).