Abstract
Introduction: Generally, microcytic anaemia is caused by sideropenia or a genetic gap. The suspicion that microcytic anaemia is caused by a genetic gap must always be considered in the face of an inadequate response to martial therapy. The aim of this paper is to highlight how biochemical diagnosis alone is sometimes not sufficient to understand the cause of microcytic anaemia. For this reason, for a correct genotype–phenotype correlation, it is essential to identify the defective gene underlying the microcytic anaemia. Detailed Case Description: This case concerns a married couple who both have microcytic anaemia. They came to our attention because the lady, pregnant at 12 weeks, underwent screening for chromosomal abnormalities using combined tests in the first trimester of pregnancy. A biochemical screening performed ten years earlier showed that both spouses were healthy carriers of the beta-thalassemia trait. A careful analysis of the biochemical data and an in-depth molecular diagnosis of the alpha and beta globin genes showed that the woman was a healthy carrier of the beta-thalassemia trait while the husband was a healthy carrier of a mutation in the ALAS2 gene. Analysis of the biochemical data of her husband and family members revealed that she had X-linked microcytic sideroblastic anaemia caused by an alteration in the function of the ALAS2 (5′-Aminolevulinate Synthase 2) gene located on the short arm of the X chromosome (Xp11.21). Discussion and Conclusions: This result is very relevant as, during genetic counselling, we explained to the couple that invasive prenatal diagnosis was not necessary as there is no risk of procreating a transfusion-dependent individual.
1. Introduction
Microcytic anaemia (AM) is the most common type of anaemia, characterized by the presence of small hypochromic red blood cells typically marked by a low mean corpuscular volume in the peripheral blood (MCV) [1].
Once common reasons are ruled out, unusual causes of anaemia should be considered; these are primarily genetic in nature, and include anaemia with the ectopic production of hepcidin, sideroblastic anaemias, porphyria, and anaemias resulting from a deficiency of genes related to iron metabolism [2].
Understanding the appropriate framework for a case of anaemia is essential, particularly in the context of preconceptional and prenatal counselling. In fact, this particular type of anaemia is frequently confused with iron deficiency anaemia, which can be caused by malnutrition or the presence of a thalassaemia trait carrier. This can, in some instances, result in an incorrect diagnosis [3].
2. Detailed Case Description
A 28-year-old woman who was pregnant at 12 weeks of gestation (Mrs. La. Ch., Figure 1, family tree III1) came to our clinic to undergo combined testing and screening for major foetal chromosomal abnormalities (trisomy of chromosomes 13, 18 and 21).
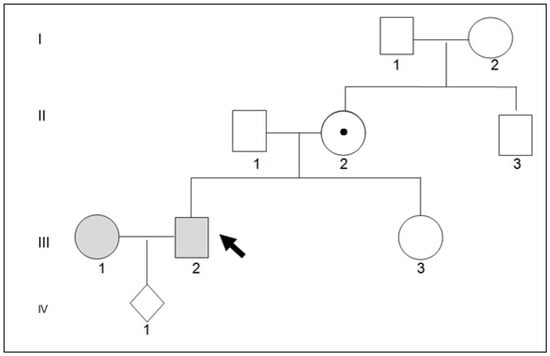
Figure 1.
Pedigree family. Mr. III1 and III2 came to observation because Mrs. III2 was pregnant in the first trimester of pregnancy. Molecular family investigation has shown that II2 and III2 are healthy carriers of a mutation in the ALAS2 gene. The ALAS2 gene is localed on the short arm of the X chromosome (Xp11.21).
A biochemical screening performed ten years earlier showed that both spouses were healthy carriers of the beta thalassaemia trait and had microcytic anaemia.
Mrs La. Ch.’s erythrocyte indices (gene tree III1) are shown in Table 1 and display the features associated with trait beta thalassaemia. Molecular analysis of the globin genes revealed the β039 C→T mutation in heterozygosity. Mrs Pe. Gi.’s erythrocyte indices (family tree III2) are given in Table 1 and show the features associated with trait beta thalassaemia. Molecular analysis of the globin genes did not reveal the presence of pathogenic variants. Therefore, Mrs Pe. Gi. did not appear to carry the thalassaemic trait [4]. Mr. Pe. Gi.’s family history did not show any consanguinity between his parents (Figure 1 family tree: II1 and II2). It was reported that the maternal grandfather (Figure 1, family tree I1) died at the age of 51 and had microcytic anaemia. In addition, the maternal grandfather’s brother was reported to have microcytic anaemia and has undergone hepatotransplantation for unspecified causes. Mr. Pe. Gi. reported that for his microcytic anaemia, he took pyridoxine chlorhydrate (vitamin B6) at a dose of 300 mg/day for ninety days. This therapy has proven to be effective in combating microcytic anaemia. An abdominal ultrasound showed a liver of normal structure and size, a gallbladder with adenomas up to 6 mm in diameter, and a spleen measuring 170 × 65 mm. A comparison of the biochemical data of Mr. Pe. Gi. and his parents (Table 2) showed that the biochemical parameters of his parents were perfectly within the normal range. The microscopic study of the erythrocytes in Mr. Pe. Gi.’s venous blood shows anisocytosis and poikilocytosis. In the bone marrow, there is hyperplasia in the erythroblasts, with there being 60% ring sideroblasts. For these reasons, we thought that Mr. Pe. Gi. had X-linked microcytic anaemia. In addition, a positive response to treatment with pyridoxine poses an indication for the molecular analysis of the ALAS2 gene. This gene indicates an enzyme localised in the mitochondrion and is specific for an erythroid called 5-aminolevulinate synthase. The encoded protein catalyses the first step in the heme biosynthetic pathway. Mutations in this gene are responsible for the X-linked form of sideroblastic anaemia [5]. Next-generation sequencing (NGS) analysis of the ALAS2 gene identified the pathogenic variant c.1354 C > T (p. Arg452Cys) in exon 9 in a heterozygous state [6].

Table 1.
Blood indices of Mrs Pe. Gi. (pedigree III 2).

Table 2.
Blood indices. ↑: higher than normal value; ↓: lower than normal value.
3. Discussion and Conclusions
This report demonstrates how biochemical parameters are useful for diagnosing microcytic non-sideropenic anaemia but, in some cases, are insufficient to understand the origin of the defect in erythropoiesis. For this reason, a family and genetic study must go hand in hand with the biochemical picture to rule out or confirm potential genetic disorders. The family and genetic study allowed us to identify with extreme precision the factors that led to the occurrence of microcytic anaemia in both spouses. The biochemical screening performed ten years earlier only suspected that the presence of a possible trait, beta thalassemia, was the cause in both spouses.
The molecular study of the beta globin genes carried out on Mrs La. Ch. revealed the presence of the pathogenic β039 C→T variant in heterozygosity. She is therefore a healthy carrier of the beta thalassaemic trait. The molecular analysis of the globin genes performed on Mr Pe. Gi. did not reveal the presence of pathogenic variants, but a molecular investigation of the ALAS2 gene revealed the pathogenic variant ALAS2 c.1354 C > T (p. Arg452Cys) in the heterozygote in exon 9. As a result, Mr Pe.Gi. was found to have X-linked sideroblastic anaemia [7,8].
Identifying these specific cases has significant implications for how invasive prenatal diagnosis is approached. An accurate description of the healthy carrier followed by a thorough examination of the partner’s condition is essential. Studying the gene defect molecularly helped determine that the spouses are not at risk of having children who are dependent on transfusions. The couple received detailed information during genetic counselling, leading them to understand the pointlessness of undergoing an invasive prenatal diagnosis, as they had been informed a decade ago.
Author Contributions
Conceptualization, D.D. and A.A.; methodology, B.P.; validation, D.D., A.A. and F.L.R.; writing—original draft preparation, D.D.; writing—review and editing, C.C.; visualization, D.D.; supervision, D.D.; project administration, D.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was approved by the Clinical Trial Center of Azienda Sanitaria di Matera (April 2023) and all patients provided informed consent according to the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Acknowledgments
We thank the Volunteer Organizations (ODV) Gian Franco Lupo and AnimaMundi.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ekpe, A.C.; Adefemi, S.A.; Pemi, M.D. Predictors of Anaemia among Pregnant Women Booking for Antenatal Care at Federal Medical Centre, Bida, Niger State, Nigeria. West Afr. J. Med. 2023, 40, 831–837. [Google Scholar] [PubMed]
- Ducamp, S.; Fleming, M.D. The molecular genetics of sideroblastic anemia. Blood 2019, 133, 59–69. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, F.; Sheane, R.; Reynaud, N.; McAuliffe, F.M.; Walsh, J.M. Screening and treatment of iron deficiency anemia in pregnancy: A review and appraisal of current international guidelines. Int. J. Gynaecol. Obstet. 2023, 9, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Dell’Edera, D.; Pacella, E.; Epifania, A.A.; Benedetto, M.; Tinelli, A.; Mazzone, E.; Laterza, F.; Malvasi, A. Importance of molecular biology in the characterization of beta-thalassemia carriers. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 79–86. [Google Scholar] [PubMed]
- Dell’Edera, D.; Vitucci, A.; Andrisani, G.; Epifania, A.A. Limitations of the biochemical research in the identification of the thalassemic trait carrier. Minerva Ginecol. 2016, 68, 478–479. [Google Scholar] [PubMed]
- Ono, K.; Fujiwara, T.; Saito, K.; Nishizawa, H.; Takahashi, N.; Suzuki, C.; Ochi, T.; Kato, H.; Ishii, Y.; Onodera, K.; et al. Congenital sideroblastic anemia model due to ALAS2 mutation is susceptible to ferroptosis. Sci. Rep. 2022, 12, 9024. [Google Scholar] [CrossRef] [PubMed]
- Rollón, N.; Fernández-Jiménez, M.C.; Moreno-Carralero, M.I.; Murga-Fernández, M.J.; Morán-Jiménez, M.J. Microcytic anemia in a pregnant woman: Beyond iron deficiency. Int. J. Hematol. 2015, 101, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Matthes, T.; Rustin, P.; Trachsel, H.; Darbellay, R.; Costaridou, S.; Xaidara, A.; Rideau, A.; Beris, P. Different pathophysiological mechanisms of intramitochondrial iron accumulation in acquired and congenital sideroblastic anemia caused by mitochondrial DNA deletion. Eur. J. Haematol. 2006, 77, 169–174. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).