The First Case of Haemophagocytic Lymphohistiocytosis Triggered by the Booster Dose of Anti-SARS-CoV-2 Vaccine in a Patient with β-Thalassemia
Abstract
:1. Introduction
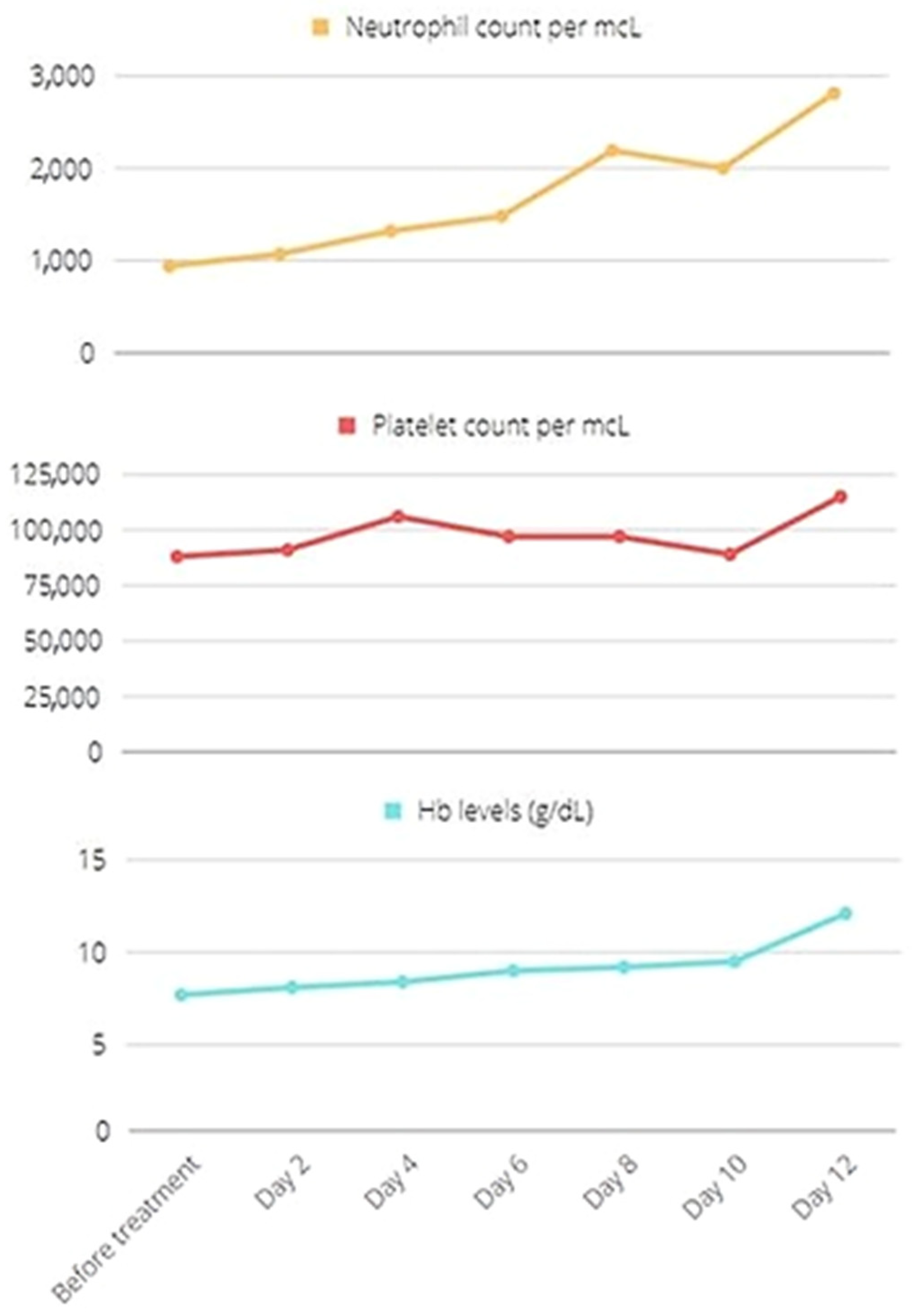
2. Clinical Case
3. Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Debaugnies, F.; Mahadeb, B.; Ferster, A.; Meuleman, N.; Rozen, L.; Demulder, A.; Corazza, F. Performances of the H-Score for Diagnosis of Hemophagocytic Lymphohistiocytosis in Adult and Pediatric Patients. Am. J. Clin. Pathol. 2016, 145, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Henter, J.I.; Horne, A.; Aricò, M.; Egeler, R.M.; Filipovich, A.H.; Imashuku, S.; Ladisch, S.; McClain, K.; Webb, D.; Winiarski, J.; et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr. Blood Cancer 2007, 48, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Henter, J.I.; Aricò, M.; Egeler, R.M.; Elinder, G.; Favara, B.E.; Filipovich, A.H.; Gadner, H.; Imashuku, S.; Janka-Schaub, G.; Komp, D.; et al. HLH-94: A treatment protocol for hemophagocytic lymphohistiocytosis. HLH study Group of the Histiocyte Society. Med. Pediatr. Oncol. 1997, 28, 342–347. [Google Scholar] [CrossRef]
- La Rosée, P.; Horne, A.C.; Hines, M.; von Bahr Greenwood, T.; Machowicz, R.; Berliner, N.; Birndt, S.; Gil-Herrera, J.; Girschikofsky, M.; Jordan, M.B.; et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood 2019, 133, 2465–2477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, L.V.; Hu, Y. Hemophagocytic lymphohistiocytosis after COVID-19 vaccination. J. Hematol. Oncol. 2021, 14, 87. [Google Scholar] [CrossRef] [PubMed]
- Caocci, G.; Fanni, D.; Porru, M.; Greco, M.; Nemolato, S.; Firinu, D.; Faa, G.; Scuteri, A.; La Nasa, G. Kikuchi-Fujimoto disease associated with hemophagocytic lymphohistiocytosis following the BNT162b2 mRNA COVID-19 vaccination. Haematologica 2022, 107, 1222–1225. [Google Scholar] [CrossRef] [PubMed]
- Baek, D.W.; Hwang, S.; Kim, J.; Lee, J.M.; Cho, H.J.; Moon, J.H.; Hwang, N.; Jeong, J.Y.; Lee, S.W.; Sohn, S.K. Patients presenting high fever with lymphadenopathy after COVID-19 vaccination were diagnosed with hemophagocytic lymphohistiocytosis. Infect. Dis. 2022, 54, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Attwell, L.; Zaw, T.; McCormick, J.; Marks, J.; McCarthy, H. Haemophagocytic lymphohistiocytosis after ChAdOx1 nCoV-19 vaccination. J. Clin. Pathol. 2022, 75, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Ai, S.; Awford, A.; Roncolato, F. Hemophagocytic lymphohistiocytosis following ChAdOx1 nCov-19 vaccination. J. Med. Virol. 2022, 94, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Rocco, J.M.; Mallarino-Haeger, C.; Randolph, A.H.; Ray, S.M.; Schechter, M.C.; Zerbe, C.S.; Holland, S.M.; Sereti, I. Hyperinflammatory syndromes after SARS-CoV-2 mRNA vaccination in individuals with underlying immune dysregulation. Clin. Infect. Dis. 2021, ciab1024. [Google Scholar] [CrossRef] [PubMed]
- Hieber, M.L.; Sprute, R.; Eichenauer, D.A.; Hallek, M.; Jachimowicz, R.D. Hemophagocytic lymphohistiocytosis after SARS-CoV-2 vaccination. Infection 2022, 26, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wu, V.; Lopez, C.A.; Hines, A.M.; Barrientos, J.C. Haemophagocytic lymphohistiocytosis following COVID-19 mRNA vaccination. BMJ Case Rep. 2022, 15, e247022. [Google Scholar] [CrossRef] [PubMed]
| Test | Results | Normal Values |
|---|---|---|
| Hb | 10.1 g/dL | 12–16 g/dL |
| MCV | 81 fL | 78–100 fL |
| WBC | 2.8 × 103/µL | 4–9 × 103/µL |
| Neutrophils, lymphocytes | 1.12 × 103/µL, 1.09 × 103/µL | 2–8 × 103/µL, 1–5 × 103/µL |
| PLT | 107 × 103/µL | 150–450 × 103/µL |
| Reticulocytes | 13.4 × 103/µL, 0.46% | 25–75 × 103/µL, 1–2% |
| Direct and indirect Coombs test | Positive | Negative |
| Bilirubin total/indirect | 2.3/1.6 mg/dL | <1.2/<0.7 mg/dL |
| AST/ALT | 35/24 U/L | <35 U/L |
| LDH | 287 U/L | 125–220 U/L |
| Haptoglobin | ˂8 mg/dL | >25 mg/dL |
| Hs-CRP | 1.3 mg/dL | <0.5 mg/dL |
| Ferritin | 1590 ng/mL | 15–150 ng/mL |
| Triglycerides | 146 mg/dL | <150 mg/dL |
| AP, INR | 82%, 1.14 | 80–120% |
| PTT | 56″ | 22–34″ |
| Fibrinogen | 276 mg/dL | 150–450 mg/dL |
| D-dimer | 327 ng/mL | <250 ng/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvaruso, G.; Chiavetta, M.; Renda, D.; Raso, S.; Dieli, F.; Lentini, V.L.; Gentile, M.; Carroccio, A.; Maggio, A. The First Case of Haemophagocytic Lymphohistiocytosis Triggered by the Booster Dose of Anti-SARS-CoV-2 Vaccine in a Patient with β-Thalassemia. Thalass. Rep. 2022, 12, 46-50. https://doi.org/10.3390/thalassrep12020009
Calvaruso G, Chiavetta M, Renda D, Raso S, Dieli F, Lentini VL, Gentile M, Carroccio A, Maggio A. The First Case of Haemophagocytic Lymphohistiocytosis Triggered by the Booster Dose of Anti-SARS-CoV-2 Vaccine in a Patient with β-Thalassemia. Thalassemia Reports. 2022; 12(2):46-50. https://doi.org/10.3390/thalassrep12020009
Chicago/Turabian StyleCalvaruso, Giuseppina, Marta Chiavetta, Disma Renda, Simona Raso, Francesco Dieli, Vincenzo Luca Lentini, Massimo Gentile, Antonio Carroccio, and Aurelio Maggio. 2022. "The First Case of Haemophagocytic Lymphohistiocytosis Triggered by the Booster Dose of Anti-SARS-CoV-2 Vaccine in a Patient with β-Thalassemia" Thalassemia Reports 12, no. 2: 46-50. https://doi.org/10.3390/thalassrep12020009
APA StyleCalvaruso, G., Chiavetta, M., Renda, D., Raso, S., Dieli, F., Lentini, V. L., Gentile, M., Carroccio, A., & Maggio, A. (2022). The First Case of Haemophagocytic Lymphohistiocytosis Triggered by the Booster Dose of Anti-SARS-CoV-2 Vaccine in a Patient with β-Thalassemia. Thalassemia Reports, 12(2), 46-50. https://doi.org/10.3390/thalassrep12020009








