1. Introduction
Patients admitted to neurorehabilitation settings following severe acquired brain injuries—such as post-anoxic encephalopathy, ischaemic or haemorrhagic strokes, or severe traumatic brain injury—are frequently long-term carriers of tracheostomy and often require prolonged enteral nutrition via nasogastric tube (NGT). In this clinically fragile population, the occurrence of tracheo-oesophageal fistulae represents a significant clinical risk, with considerable implications for the rehabilitation course and overall prognosis. The management of such fistulae is particularly challenging due to the coexistence of systemic impairments, and requires careful assessment of the timing of surgical intervention, which must be aligned with the patient’s general condition and the specific goals of the rehabilitation process.
Acquired tracheo-oesophageal fistulae (TEF) occur predominantly in patients undergoing prolonged mechanical ventilation and/or tracheostomy. The incidence of tracheoesophageal fistulas secondary to prolonged intubation ranges between 0.3% and 3%, according to international studies [
1,
2,
3]. Green et al. (2017), in particular, emphasise that incidence of tracheal erosion as a result of an endotracheal tube in mechanically ventilated patients [
1]. Couraud, Ballester and Delaisement (1996) describe TEFs secondary to prolonged ventilation and confirm the same incidence range [
2]. Finally, Stoica (2024) highlights that although iatrogenic TEFs in ICU settings remain rare, they present with an incidence of approximately 0.5% in ventilated patients [
1,
2,
3].
In most non-malignant cases, these fistulae arise secondarily from endotracheal cuff-related trauma, particularly when the endotracheal or tracheostomy tube is maintained for a prolonged period. Indeed, approximately 75% of acquired, non-neoplastic TEFs result from ischaemic lesions caused by excessive pressure from the tube cuff against the posterior tracheal wall—often in the simultaneous presence of a rigid nasogastric tube exerting counterpressure on the oesophagus.
Several decades ago, eminent Italian authors already highlighted the clinical relevance of benign acquired TEFs in a comprehensive case series of ten patients, confirming the possibility of definitive treatment through resection or direct surgical repair. In their experience, three patients developed fistulae following prolonged intubation, and all underwent successful surgery, with direct suture of the tracheal and oesophageal defects. Muscle interposition and, when required, resection of infected pulmonary parenchyma were key elements of the therapeutic strategy [
4].
This pioneering work stands as one of the earliest systematic Italian contributions on the treatment of benign TEFs, laying the foundation for the later formalisation of modern surgical selection criteria. The purpose of this report is to propose a clinical and diagnostic model for the assessment, management, and multidisciplinary referral of patients with suspected tracheo-oesophageal fistula, based on a clinically representative case report. The evaluation process was supported by the expertise of a renowned Italian thoracic surgery team with internationally recognised competence in the management of airway fistulae. This collaboration proved decisive in guiding clinical decisions and strengthened the overall diagnostic and therapeutic pathway. Among other relevant studies, we wish to recall one of their iconic papers [
5], an investigation assessing the feasibility, efficacy, and safety of a conical self-expandable stent for the treatment of post-pneumonectomy bronchopleural fistula (PPBPF), which further highlights the need for innovative and multidisciplinary approaches in complex airway fistula management. Clinicians should be vigilant for signs such as coughing during oral intake, recurrent respiratory infections, unexplained gastric distension, or desaturation during feeding. These symptoms, though subtle in sedated or tracheostomized patients, may be suggestive of TEF and should prompt early endoscopic evaluation. A schematic overview of the pathway is provided (
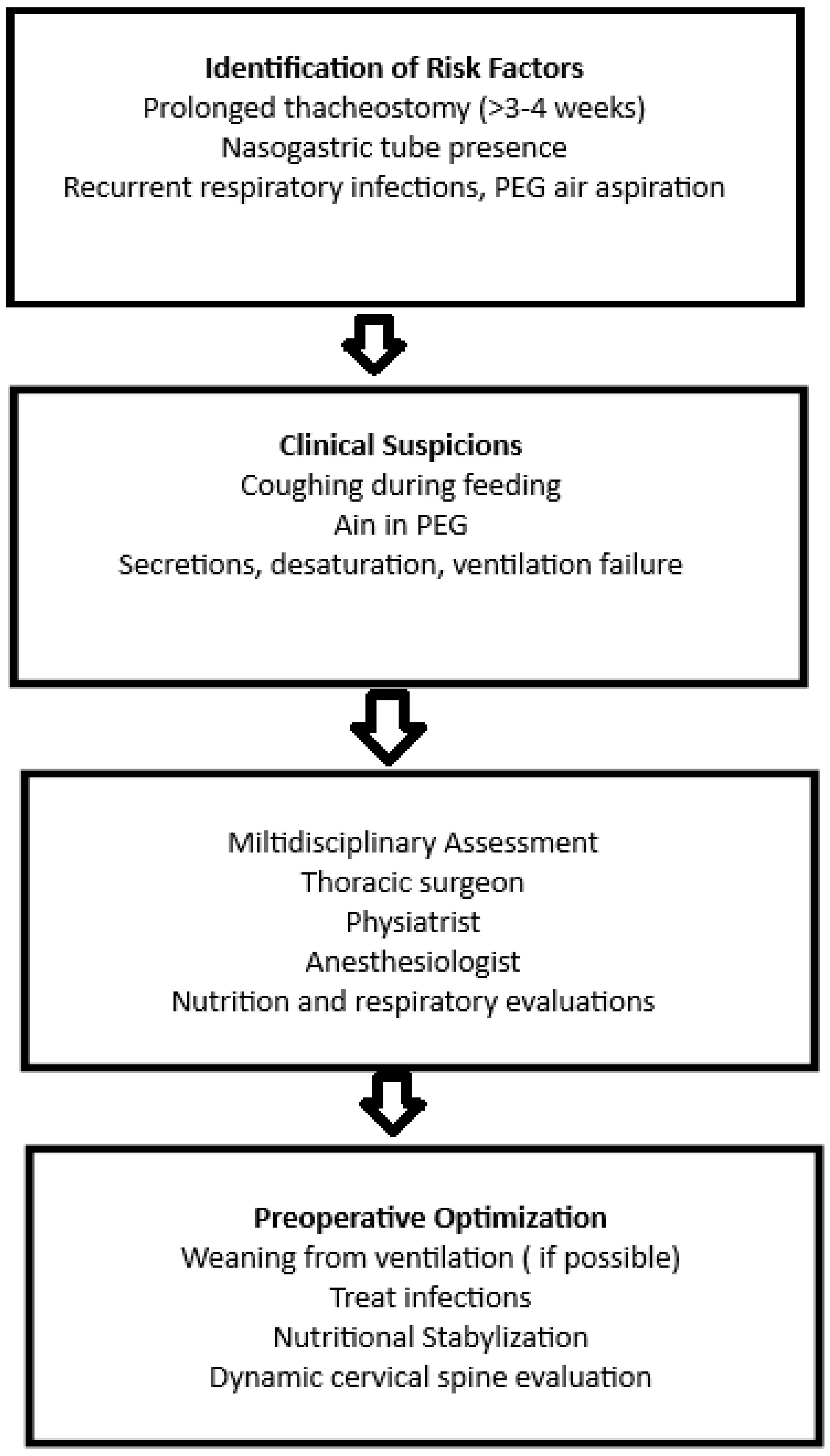
Figure 1).
2. Case Report
In the clinical case we observed, a 23-year-old male patient undergoing neurorehabilitation following severe traumatic brain injury (TBI) had been transferred to our facility with a pre-existing tracheostomy, previously performed due to episodes of food aspiration. Ten days after the tracheostomy, the patient exhibited a marked increase in oral secretions and clear signs of aspiration of the enteral feeding administered via NGT, with material leaking through the tracheostomy cannula. Frequent endobronchial suctioning became necessary. Chest auscultation revealed inspiratory crackles and reduced breath sounds at the lung bases. The patient had a tracheostomy with a reinforced no. 8 cannula in place, inserted due to a critical sub-cricoid tracheal stenosis, estimated at 90% (
Figure 2).
The clinical suspicion of an abnormal communication between the oesophagus and the trachea had already emerged following a diagnostic test using dye: the oral instillation of methylene blue resulted in the appearance of the tracer within the trachea, a finding suggestive of a tracheo-oesophageal fistula (TEF). An additional indirect sign supporting the diagnosis was the presence of air within the PEG collection bag, indicative of possible retrograde tracheo-oesophageal passage.
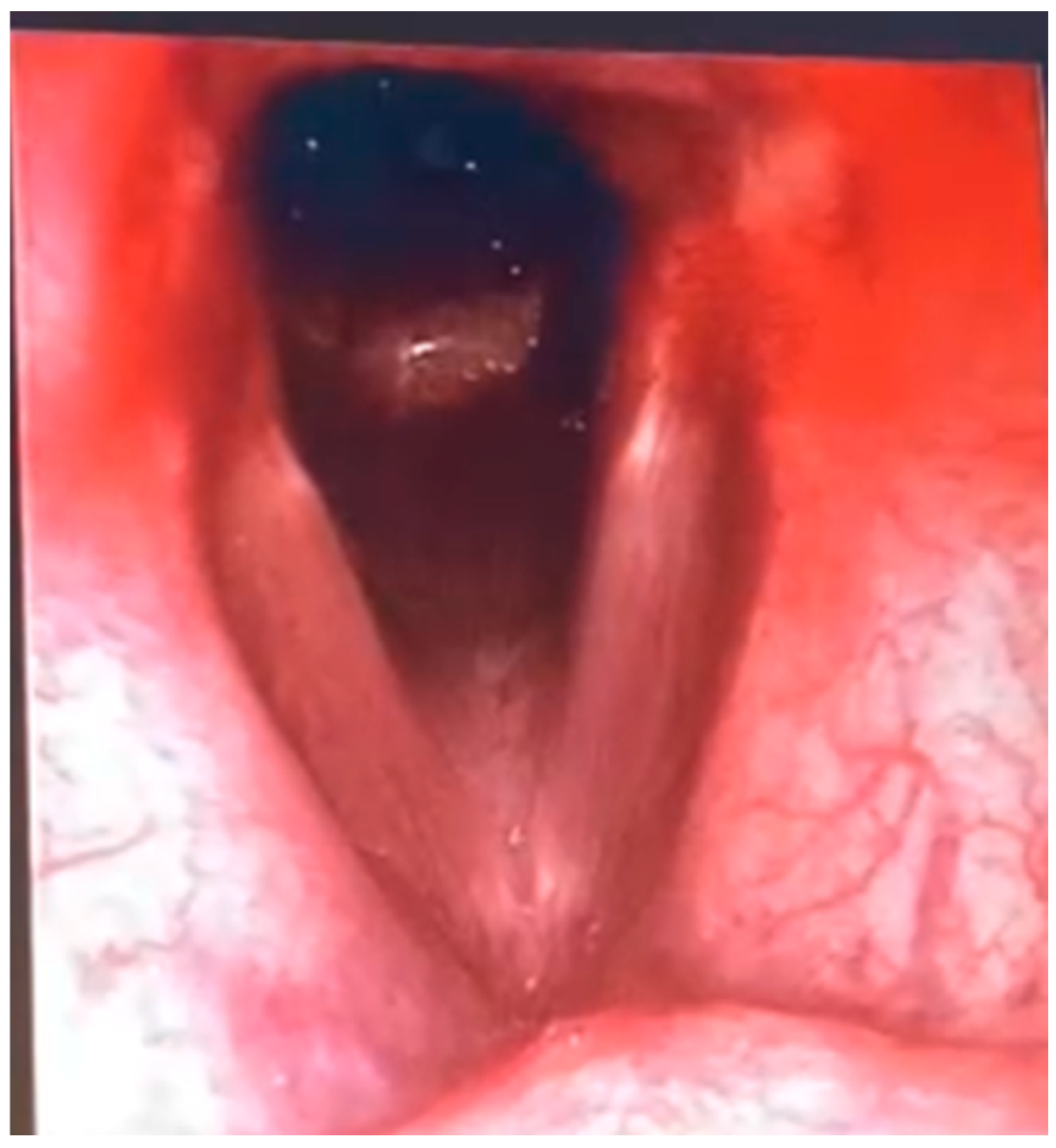
The videofibrolaryngoscopy in endoscopic view revealed a laceration of the posterior tracheal wall compatible with a tracheo-oesophageal fistula (TEF), with normal vocal cord mobility and an area of mucosal discontinuity posteriorly-typical of TEFs acquired due to prolonged pressure from a tracheostomy cuff and nasogastric tube (
Figure 3). A bronchoscopy performed under sedation showed oedematous laryngeal structures with preserved motility, increased tracheal secretions, and a linear laceration of the posterior tracheal wall, confirming the diagnosis of tracheo-oesophageal fistula.
Bronchoscopy confirmed the diagnosis, revealing a linear laceration of the posterior tracheal wall consistent with TEF.
Following multidisciplinary evaluation, the patient was deemed suitable for reconstructive surgery. As indicated by the Thoracic Surgery team, tracheo-oesophageal reconstruction requires specific conditions of general and respiratory stability, with weaning from ventilatory support being a prerequisite. Autonomy from mechanical ventilation, even if partial or supported by intermittent decannulation, is generally considered essential before surgery, as also documented in the literature [
6].
Another crucial aspect in evaluating surgical eligibility is the precise timing of the procedure. Inappropriate timing—such as in the presence of clinical instability or active respiratory infections—could severely compromise the surgical outcome. Equally fundamental are the diagnostic–functional assessments requested by the thoracic team. For surgical planning, the thoracic team required a series of targeted investigations to define both the fistula’s anatomical features and the patient’s overall condition. These included a computed tomography (CT) scan of the neck and chest (
Figure 2), a dynamic video of the patient in full cervical extension (
Figure 4), videolaryngoscopy (
Figure 3), as well as assessments of nutritional, neurological, and general medical status.
The CT scan (
Figure 2) allowed clear visualization of tracheal narrowing below the cricoid and identified indirect signs of TEF, providing critical preoperative information (
Figure 2). The dynamic video in maximal cervical extension is especially useful in patients with potential post-coma rigidity or dystonia, allowing the surgical team to pre-empt challenges in field exposure, especially with anterior tracheal approaches (
Figure 4). Nutritional assessment included confirming the correct positioning of the PEG, which is essential to ensure appropriate nutritional support both before and after the operation. Lastly, neurological and general clinical evaluations were required to exclude haemodynamic instability, ongoing airway infections, or other contraindications to major thoracic surgery. Even partial neurological cooperation was considered a favourable factor.
3. Discussion
Acquired tracheoesophageal fistulas (TEFs) represent a rare but severe complication in patients undergoing prolonged mechanical ventilation and tracheostomy, with an estimated incidence ranging between 0.3% and 3% among critically ill individuals. Their management constitutes a multidisciplinary challenge, particularly within neurorehabilitation settings, where patients are often clinically fragile, with neurological comorbidities and impaired swallowing function.
In some cases, suspicion of a TEF may arise from the immediate inefficacy of ventilation through a tracheostomy cannula—an occurrence that should alert the clinician even in the absence of classic respiratory symptoms [
7].
The management of TEFs poses a highly complex clinical challenge, requiring a multidisciplinary assessment and individualized therapeutic approach based on the etiology (benign or malignant), the patient’s overall condition, and the fistula’s anatomical location. Santosham et al. highlight that, for benign fistulas, successful single-stage surgical repair depends on optimal preoperative preparation, meticulous surgical dissection, and dedicated postoperative care, underscoring the critical role of the perioperative phase [
8].
In this context, the recent review by Kim et al. [
6] makes a crucial contribution, surveying a wide range of diagnostic and therapeutic strategies—from single or dual stenting to the most innovative endoscopic techniques such as tissue matrix applications, over-the-scope clips, and atrial septal closure devices. The article, which analyzes more than 200 references, emphasizes the importance of individualized patient assessment, even in benign cases, suggesting differentiated management algorithms and stressing the prognostic value of early and targeted intervention.
Shen (2017), on the other hand, reinforces the value of certain “surgical pearls”: single-stage unilateral primary repair with vascularized flap interposition, early extubation, and avoidance of postoperative positive pressure ventilation are critical to favorable outcomes [
9]. This approach demonstrates that not only the technical choice, but also the timing and clinical optimization of the patient (nutrition, infection control, ventilation) are decisive factors.
In line with this evidence, our experience confirms that coordination between the rehabilitation team and thoracic surgery, early progression toward ventilator weaning, and a structured diagnostic-therapeutic strategy allow for improved survival rates and reduced complications. Central to an evidence-based perspective is the implementation of a systematic protocol that first involves the early identification of at-risk patients, particularly those with both nasogastric tubes and tracheostomy in place for more than 3–4 weeks.
It is therefore essential to promptly activate a standardized diagnostic pathway including bronchoscopy and imaging of the neck and mediastinum, to confirm the diagnosis and precisely define the fistula’s characteristics. An indispensable phase is the optimization of preoperative clinical conditions, with particular focus on weaning from mechanical ventilation, improving nutritional status, and controlling any respiratory or systemic infections.
Finally, surgical planning must occur within an appropriate timeframe and follow techniques validated in the literature, prioritizing primary repair of the trachea and esophagus with muscular flap interposition, followed by structured, multidisciplinary follow-up aimed at preventing recurrence and monitoring respiratory and nutritional functional outcomes.
In this framework, the converging literature supports the preferential use of single-stage primary repair with vascularized flaps, avoiding delays or multiple interventions that expose the patient to higher risks of recurrence or mortality. The systematic adoption of these practices—within a replicable model suited to the neurorehabilitation setting—may represent a solid foundation for future operational guidelines.
In this regard, the recent systematic review conducted by Zeng et al. (2024) represents a major contribution, both in terms of the breadth of its literature search and the coherence of its analysis [
10]. The authors selected and evaluated 54 articles from an initial pool of more than 1700 indexed publications between 1975 and 2023, focusing exclusively on acquired benign TEFs and their surgical management [
10]. One of the key innovations proposed is a systematic classification of TEFs based on size and anatomical location, which provides guidance in selecting the most appropriate surgical intervention according to the fistula’s severity and anatomical–functional complexity.
The surgical options described include two-layer direct closure with muscular interposition (e.g., pedicled sternocleidomastoid muscle), segmental tracheal resection with primary anastomosis, tracheoplasty using autologous flaps, and the use of biological or synthetic patches in cases of large defects or recurrences. The article also emphasizes the importance of surgical timing, which must be tightly coordinated with the stabilization of the patient’s general clinical condition (nutrition, infection status, and weaning from mechanical ventilation). It also highlights the necessity of a multidisciplinary approach and case-by-case evaluation, given the lack of validated protocols and the considerable clinical heterogeneity. Finally, Zeng et al. conclude that despite the various techniques described, long-term outcomes are strongly influenced by the surgical center’s experience, pre- and postoperative multidisciplinary support, and proper patient selection. The article reiterates the urgent need to collect prospective multicenter data to build robust evidence and shared guidelines. In light of these considerations, the review serves as a valuable theoretical basis for standardizing, where possible, the management of acquired TEFs, even in complex settings such as neurorehabilitation. In patients who are clinically unstable or present with contraindications to immediate surgery, temporary palliative strategies—such as esophageal or tracheal stenting—may be considered. These bridge-to-surgery approaches aim to stabilize respiratory and nutritional conditions while preparing the patient for definitive surgical repair under safer conditions. Our clinical case aligns with this context, illustrating a shared approach between the rehabilitation team and thoracic surgery in the identification and surgical treatment of TEF.
Shen et al. report that surgical series involving non-malignant TEFs show a healing probability of over 87–95% [
11]. In this series, benign tracheoesophageal fistulas showed a complete healing rate ranging from 87% to 95%, confirming the effectiveness of primary surgical repair. However, mortality in patients who undergo surgery while still ventilated may reach as high as 60%, as highlighted in recent case series [
11].
In a 13-patient series, Foroulis et al. described repair of post-intubation TEF through a left pre-sternocleidomastoid approach, with two-layer esophageal closure, single-layer tracheal closure and interposition of a pedicled sternocleidomastoid muscle flap; the mean time to diagnosis was ~35 days [
12]. Overall postoperative mortality was 23%, rising to 60% in patients still ventilated at surgery, whereas weaned patients generally had favorable courses. Reported complications included left recurrent laryngeal nerve palsy (~10%) and transient swallowing difficulties (~20%). These data reinforce preoperative optimization and ventilator weaning, alongside standardized single-stage repair with flap interposition, to reduce risk and recurrence.
Overall, current literature—though based on limited series and expert opinion—suggests that single-stage repair with muscle interposition is an effective and reproducible strategy, even in critically ill patients. Nevertheless, optimal timing selection and multidisciplinary surgical risk assessment remain essential to reduce mortality and associated complications. As also highlighted by Kim et al. in their study on acquired TEFs [
6], the multidisciplinary approach may in fact be the "key factor" in preventing recurrences and improving postoperative outcomes. Collaboration among physiatrists, pulmonologists, endoscopists, radiologists, and surgeons enables early diagnosis, accurate staging, and appropriate patient selection for definitive intervention. In this sense, our neurorehabilitation experience shows that early identification of at-risk patients—based on sentinel symptoms such as the presence of air in the PEG bag, recurrent respiratory infections, or aspiration—should rapidly activate a shared diagnostic pathway involving a physiatrist, thoracic surgeon, and anesthesiologist.
The availability of diagnostic tools such as bronchoscopy, videofluoroscopy, and sagittal neck CT allows for fast and accurate assessment. Additionally, weaning from mechanical ventilation should be considered a fundamental prerequisite for selecting surgical candidates, as evidenced by better clinical outcomes in non-ventilated patients. As demonstrated in our patient (
Figure 2 and
Figure 3), the integration of sagittal CT imaging with endoscopic evaluation enhances early detection of subtle posterior tracheal wall defects that may be missed on axial-only views. This combined anatomical–functional approach has also been emphasized in recent reviews [
6,
10]. Our case further confirms that structuring a standardized evaluative pathway centered on interdisciplinary collaboration not only improves surgical outcomes but also enables a more timely and targeted rehabilitative approach. Although mechanical ventilation is not an absolute contraindication, partial or complete weaning remains a key factor in surgical eligibility, reducing the risk of perioperative complications and enhancing tissue healing.
When complete weaning is not feasible, protective ventilation strategies—such as pressure-controlled modes with low tidal volumes—should be preferred. Avoiding PEEP and permitting mild hypercapnia can help limit pressure on the posterior tracheal wall and reduce the risk of fistula worsening.