Abstract
Background: Autologous fat grafting is increasingly used in daily clinical practice across various surgical fields, including the treatment of chronic wounds, scars, burns, and non-healing perianal fistulas. Recently, some studies have shown that non-enteric cutaneous fistulas can also benefit from adipose tissue injections, but the efficacy remains unclear. This study aims to systematically review the literature on fat grafting in the context of non-enteric cutaneous fistulas and to assess treatment outcomes. Methods: A comprehensive search of the PubMed/Medline database was conducted following Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines up to January 2024 without restrictions on the time period or the language of publication. Results: Seven studies meeting the inclusion criteria were analyzed, encompassing 13 patients with non-healing cutaneous fistulas treated with injections of autologous fat. The mean age of the patients was 58 ± 3 years, of which 85% had comorbidities. Fat grafting resulted in complete healing in 92% of the cases, with a mean fistula persistence of 158 days before treatment. Treatment protocols varied among patients, including preparation of the fistulous tract, fat processing techniques, and suturing of the fistulous orifice. Conclusions: The results highlight the potential of autologous fat grafting in promoting tissue regeneration and healing of non-enteric cutaneous fistulas. Standardized protocols are essential to confirm and optimize treatment efficacy and, eventually, improve patient outcomes. Further research with a larger sample size and standardization is needed to confirm fat graft efficacy.
1. Introduction
Autologous fat grafting, also known as autologous fat transplantation or lipofilling, was first described by Neuber in 1893 [1]. This procedure involves harvesting of the patient’s adipose tissue from the subcutaneous tissue layers, most often of the abdomen or thighs, using liposuction techniques, followed by its reinjection into target sites. This adipose tissue is composed of mature adipocytes, extracellular matrix components, as well as adipose-derived stem cells (ASCs) and microvascular fragments (MVFs) that contribute to its strong regenerative capacity [2,3,4,5]. ASCs can differentiate into various cell types and secrete cytokines, growth factors, and extracellular vesicles that exert anti-inflammatory, antifibrotic, angiogenic, and tissue remodeling effects [6,7,8]. Moreover, MVFs within fat grafts facilitate the establishment of new blood-perfused functional microvascular networks, essential for tissue survival and integration [9].
In recent years, several approaches have been proposed to further enhance the beneficial effects of autologous fat grafting. Specifically, adipose tissue can be processed enzymatically or mechanically [10]. Enzymatic processing involves the destruction of the dense extracellular matrix bonds within adipose tissue and centrifugation obtaining a stromal cell concentrate known as stromal vascular fraction (SVF), which contains a high concentration of ASCs [11]. However, this method is expensive, time-consuming, and subject to regulatory restrictions such as good manufacturing practice (cGMP) and good laboratory practice (cGLP) requirements [12]. In contrast, mechanical processing is not a GMP method, and it is becoming increasingly popular. Since the first definition of nanofat by Tonnard et al. [13], several mechanical processing techniques have been described in the literature [10]. These aim to produce autologous fat derivatives, such as nanofat, characterized by a high content of extracellular matrix, growth factors, ASCs, and MVFs. This advancement serves to enhance the biological properties of lipoaspirate, resulting in improved therapeutic outcomes. Moreover, the beneficial effects of autologous fat grafting can be further enhanced by the enrichment of the adipose tissue or the recipient site with stem cells, MVFs, or biologically active gels. Finally, preconditioning of either the transplanted adipose tissue or the host tissue at the recipient site as well as a combination of both with different stimuli, such as local heat or dietary restriction, has been shown to improve the outcome of autologous fat grafting.
Nowadays, autologous fat grafting finds widespread use in daily clinical practice across surgical specialties. Common applications include aesthetic procedures, aiming at soft tissue augmentation and body contouring or tissue rejuvenation. Furthermore, autologous fat grafting is often used to treat congenital deformities, as well as post-traumatic or post-surgical organ reconstruction, for example, in the breast [14,15,16,17,18,19,20,21,22]. Additionally, the regenerative potential of adipose tissue, attributed to its cellular components and secretory profile, has been used in wound healing to facilitate the repair of chronic or non-healing wounds, adherent or retracted scars, and burns [23,24,25]. Moreover, it has been demonstrated that the direct injection of autologous fat or isolated ASCs can result in the restoration of Crohn’s perianal non-healing fistulas [26]. Similarly, chronic and non-healing cutaneous fistulas could also benefit from treatment with the local administration of autologous fat. They are a consequence of dehiscence of surgical or traumatic wounds that fail to heal but rather progressively undergo intrafistulous fibrosis that hinders healing. Nevertheless, the efficacy of local administration of autologous fat graft to treat non-enteric cutaneous non-healing fistulas remains unclear. Therefore, the aim of this study was to systematically review the current literature on the use of autologous fat grafting in the context of non-enteric cutaneous fistulas.
2. Materials and Methods
A comprehensive review of the entire PubMed/Medline database on the use of injections of autologous fat graft for the treatment of cutaneous fistulas was performed by two authors independently (F.B. and E.L.) in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (http://www.prisma-statement.org, accessed on 16 April 2024). Articles were initially selected by applying the following search algorithm: ((skin fistula) OR (cutaneous fistula) OR (dermal fistula) OR (epidermal fistula) OR (mucocutaneous fistula) OR (orocutaneous fistula) OR (ureterocutaneous fistula) OR (vesiculocutaneous fistula) OR (broncocutaneous fistula) OR (tracheotomic fistula) OR (non-healing fistula) OR (tunneled cutaneous wound) OR (tunneling cutaneous wound) OR (undermined cutaneous wound)) AND ((fat) OR (adipose tissue) OR (adipose graft) OR (nanofat) OR (lipogems) OR (adipose-derived stem cells) OR (adipose mesenchymal stem cells) OR (ASCs) OR (ADSCs)) NOT ((perianal fistula) OR (enterocutaneous fistula) OR (Crohn)). All searches were undertaken up to January 2024. References of all publications were carefully screened to add additional relevant articles not registered by the initial database search. Duplicates were removed. The authors used a protocol for this systematic review, but the protocol was not registered in any public database.
Studies were allowed to involve surgical preparation of the fistula tract before injection of autologous fat graft, as well as additional surgical techniques during fat grafting. Moreover, fat derivatives generated by mechanical or enzymatic processing and the isolation of ASCs from adipose tissue were considered. Studies reporting on enterocutaneous fistulas (such as oro-antral, esophago-cutaneous, perianal), fistulas lacking any cutaneous orifice, and fistulas treated not only with autologous fat grafting but also adipose tissue flaps were excluded. No restrictions on the time period or language of publication were applied. At first, studies resulting from primary research were screened for titles and abstracts, followed by a detailed evaluation of the full text of the remaining references to determine eligibility.
The following information for each article was recorded in a structured table: name of the first author, year of publication, geographic origin of the publication, number of patients included, patient’s age or mean age (if more than one patient), patient’s comorbidities, type and location of the fistula, type of autologous fat graft or fat derivative used, fat donor site, volume of injected adipose tissue, procedure (method of injection, method of preparation of the fistulous tract, or additional surgical procedures), persistence of the fistula before the procedure, and outcome.
Quality assessment of the included studies was conducted by two authors (F.B. and E.L.) according to the NIH tool for Case Series Studies (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools, accessed on 13 March 2025). In case of case reports, the tool was adapted by excluding questions not applicable.
3. Results
The primary database search identified 1042 publications up to January 2024. Two citations were added from external sources and following the analysis of the articles’ bibliography. All 1044 publications were assessed for eligibility based on titles, with a total of 94 publications relevant for manuscript review. After full-text screening, seven studies comprising a total of 13 patients with non-healing cutaneous fistulas treated with injections of autologous fat graft met the inclusion criteria (Table 1). The PRISMA flowchart of the study is shown in Figure 1. Details of all studies included are summarized in alphabetical order in Table 1. The final judgment on study quality according to the NIH tool (i.e., poor/fair/good quality) is presented in Table 1.
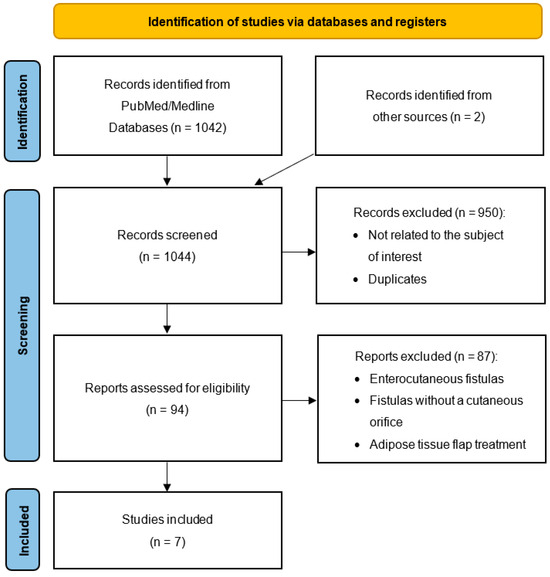
Figure 1.
Flow diagram of the review of the literature.

Table 1.
Summary of all studies included.
Table 1.
Summary of all studies included.
| Author, Year, Origin | Patients (n) | Age (Mean) | Patient’s Comorbidities | Type of Fistula | Donor Site | Type of Fat Graft | Fat Graft Volume (Mean cm3) | Procedure | Persistence of the Fistula Before Procedure (Mean Days) | Outcome | Quality Assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| De Monti, 2022, Switzerland [27] | 7 | 64 | Diabetes (n = 3), hypertension (n = 2), arteriopathy (n = 2), COPD (n = 3), AF (n = 2), ischemic heart disease (n = 1), valvular heart disease (n = 1), asthma (n = 1), sleep apnea syndromes (n = 2), thrombophilic syndrome (n = 1). venous thrombosis (n = 1), previous melanoma in immunotherapy treatment (n = 1), under- or overweight (n = 3), recent surgery with prolonged immobilization (n = 1), rheumatoid arthritis (n = 1) | Tracheocutaneous fistula post-tracheostomy (n = 1), thigh fistula post-hematoma (n = 1), arm lymphatic fistula post-dog bite (n = 1), interdigital fistula (n = 2), foot fistula (n = 2) | Abdomen hips (n = 5), lower abdomen (n = 1), right inner thigh and right hip (n = 1) | MFAT (n = 7) | 23 | Curettage of the fistulous passage, fistular MFAT infiltration, closure of the fistulous orifices with non-resorbable stitches, peri-fistular MFAT infiltration (n = 7) | 165 | Complete healing (n = 6), persistence (n = 1) | Fair |
| Bazarov, 2021, Russia [28] | 1 | NR | Cicatricial tracheal stenosis | Tracheocutaneous fistula after tracheoplasty for cicatricial tracheal stenosis | Abdomen | Emulsified fat graft | NR | Endoscopic fistular fat infiltration and perifistular cutaneous fat infiltration | 90 | Complete healing | Fair |
| Canales-Medina, 2021, Mexico [29] | 1 | 46 | NR | Pharyngocutaneous fistula following deep neck infection | Abdomen | Fat graft | 4 | Endoscopic mucosal perifistular fat infiltration, cauterization of the mucosa, closure of the mucosal opening with 4/0 vycril, closure of the cutaneous opening by planes | 49 | Complete healing | Fair |
| Sapundzhiev, 2019, Bulgaria [30] | 1 | 61 | Laryngeal carcinoma treated | Pharyngocutaneous fistula after chemotherapy, radiotherapy and total laryngectomy with partial resection of tongue base for a laryngeal carcinoma | Abdomen | Morcellated fat graft | 4 | Mucosal fat injection with a Peretti angular injection cannula followed by sutures with a single Z-like fast-absorbing polyglactin | NR | Complete healing | Fair |
| Haubner, 2016, Germany [31] | 1 | 54 | Hypopharyngeal carcinoma | Pharyngocutaneous fistula after balloon pharyngeal dilatations for hypopharyngeal stenosis following laryngectomy, neck dissection and radiochemotherapy for hypopharyngeal carcinoma | Abdomen | Mechanical processed fat graft | 2 | Perifistular fat infiltration | 180 | Complete healing | Fair |
| Hespe, 2013, USA [32] | 1 | 67 | Hypercholesterolemia, obesity, diabetes, previous prostate cancer | Pharyngocutaneous fistula after several surgeries and chemoradiotherapy for squamous cell carcinoma of the right paratracheal groove with overlying skin involvement | Abdomen | Purified fat graft with Telfa rolling | 14 | 2 perifistular fat infiltrations at 3 months distance | 240 | Complete healing | Fair |
| Klinger 2011, Italy [33] | 1 | 55 | NR | Breast fistula and scar contraction after breast augmentation | Lower abdomen | Centrifugated fat graft (Coleman technique) | 2.5 | Perifistular fat infiltration | 180 | Complete healing | Fair |
n: number; COPD: chronic obstructive pulmonary disease; AF: atrial fibrillation; MFAT: micro-fragmented adipose tissue; NR: not reported.
The average age of patients was 58 ± 3 years. A total of 11 patients (85%) had known comorbidities, including obesity, diabetes, arterial hypertension, hypercholesterolemia, arteriopathy, chronic obstructive pulmonary disease, atrial fibrillation, conditions requiring long-term steroid treatment, obstructive sleep apnea, or oncological disease of the neck treated with radiotherapy and chemotherapy. The included studies described four pharyngo-cutaneous fistulas (31%), two tracheocutaneous fistulas (15%), two interdigital fistulas (15%), two foot fistulas (15%) as well as a fistula on the thigh following hematoma (8%), a lymphatic fistula at the arm after a dog bite (8%), and one fistula in the breast (8%). These fistulas were present for an average duration of 158 days (49–243) before treatment with autologous fat was initiated. Seven patients (54%) underwent curettage, i.e., surgical debridement of the fistulous tract before fat grafting, while no kind of surgical preparation was described in the remaining six patients (46%). The adipose tissue was harvested by liposuction from the abdomen in 12 patients (92%) and from the inner thigh and hip region in one patient (8%). Processing and injection techniques of the adipose tissue, as well as the volume of injected tissue components and the number of injections varied across the different studies (Table 1). In nine patients (69%), the fistulous orifice was sutured to facilitate closure, and postoperative antibiotic therapy was prescribed in eight patients (61%). Complete healing was observed in 12 patients (92%).
4. Discussion
A fistula is defined as a pathological communication between two structures or cavities of the body or between a structure and the external environment [28]. Almost all of the regions in the body can be affected by this condition, which develops through different pathogenetic mechanisms [34,35,36,37]. Classifications of fistulas are based on the involved organs and tissues, their location (internal or external), and their anatomical structure (simple or multiple). Non-enteric cutaneous fistulas are a consequence of soft-tissue dehiscence originating from surgical or traumatic wounds that fail to heal properly, resulting in intra-fistulous and perilesional fibrosis [27]. Current treatment options such as curettage, advanced dressings, or negative wound pressure therapy often yield limited efficacy to heal the fistulas and are associated with high costs or patient discomfort.
In this context, autologous fat grafting emerges as a promising solution due to the almost ubiquitous availability of adipose tissue resource in the body, as well as its rather easy harvesting and applicability. Moreover, grafted fat bears the advantage of being not only a filler for volume correction, but also a biologically active tissue with regenerative properties.
The review of the current literature on this topic suggests possible beneficial effects of local administration of autologous fat grafting in non-enteric cutaneous fistulas. In fact, 92% of treated fistulas, which had been present for almost 160 days, showed uncomplicated postoperative healing. One out of thirteen described cases failed to heal despite autologous fat, probably due to an incorrect indication on a severely arteriopathic diabetic foot [27]. The herein presented results are in line with previous studies showing successful treatment of enteric fistulas originating from Crohn’s disease with local application of autologous fat [26]. Of interest, autologous fat grafting bears the major advantage of not only promoting fistula healing but also improving soft tissue quality or the final aesthetic outcome, as experienced in four patients [27,33].
To further optimize the healing potential of locally applied autologous fat, the fistulous tracts were prepared or treated using curettage in 55% of the included cases before the procedure. Moreover, in 92% of the treated cases, the harvested fat was infiltrated not only into the fistulous wound but also the adjacent, relatively healthy tissues in order to stimulate tissue regeneration in a three-dimensional way. Since these fistulas represent a rather chronic and resistant condition offering a hostile environment, we believe that the use of preconditioned and/or mechanically processed adipose tissue can further improve the biological properties of the graft and eventually the treatment outcomes [10,38]. Finally, in 69% of the analyzed cases, the fistulous orifice was closed using sutures to better retain the adipose tissue graft within the lesion.
Despite the interesting findings of this study, several limitations must be mentioned. Firstly, this systematic review of the literature is limited to a single database and mainly summarizes case reports and case series that may have a low Level of Evidence (LoE). Furthermore, the small number of included studies, and thus patients, affects the generalizability of the findings described. Additional multicenter clinical trials should be conducted to further confirm the results. Moreover, there was variability in baseline patient characteristics, fistula etiology, and treatment protocols across the different studies, including fat processing techniques, preparation of the fistulous tract before fat grafting and post-infiltration management of the orifice of the fistula. Notably, most studies did not report treatment approaches prior to fat grafting. Only one study explicitly described multiple previous unsuccessful surgical interventions [31]. This represents a potential bias as the effectiveness of fat grafting in treating non-healing cutaneous fistulas cannot be fully interpreted independently of the surgical procedure. Finally, the absence of long-term follow-up data necessitates caution, and future research should extend follow-up periods to validate the durability of outcomes.
In conclusion, autologous fat grafting may represent a promising therapeutic approach for the treatment of non-enteric cutaneous fistulas by promoting tissue regeneration and eventually healing. However, further research with a larger sample size, higher LoE, and standardization is needed to confirm its efficacy. Moreover, to optimize treatment effectiveness, considerations such as preoperative fat preconditioning, fat harvesting and processing, fistula tract preparation, fat infiltration, as well as management of the fistula’s orifice should further be evaluated to define clear treatment protocols.
Author Contributions
Conceptualization: F.B. and M.D.M.; Data curation: F.B. and E.L.; Formal analysis: F.B. and E.L.; Investigation: F.B.; Methodology: F.B.; Project administration: M.D.M.; Resources: Y.H., K.G. and M.D.M.; Supervision: Y.H., K.G. and M.D.M.; Validation: Y.H., K.G. and M.D.M.; Visualization: F.B., E.L., Y.H., K.G. and M.D.M.; Writing—original draft: F.B.; Writing—editing & review: F.B., E.L., Y.H., K.G. and M.D.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original data presented in the study are openly available in the PubMed/Medline databases, and further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors have no financial conflicts of interest.
References
- Neuber, G. Über die Wiederanheilung vollständig vom Körper getrennter, die ganze Fettschicht enthaltender Hautstücke. Zentralbl Chir. 1893, 30, 16. [Google Scholar]
- Buschmann, J.; Gao, S.; Härter, L.; Hemmi, S.; Welti, M.; Werner, C.M.; Calcagni, M.; Cinelli, P.; Wanner, G.A. Yield and proliferation rate of adipose-derived stromal cells as a function of age, body mass index and harvest site-increasing the yield by use of adherent and supernatant fractions? Cytotherapy 2013, 15, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.I.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef]
- Laschke, M.W.; Menger, M.D. Adipose tissue-derived microvascular fragments: Natural vascularization units for regenerative medicine. Trends Biotechnol. 2015, 33, 442–448. [Google Scholar] [CrossRef]
- Al-Ghadban, S.; Bunnell, B.A. Adipose Tissue-Derived Stem Cells: Immunomodulatory Effects and Therapeutic Potential. Physiology 2020, 35, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Casado-Díaz, A.; Quesada-Gómez, J.M.; Dorado, G. Extracellular Vesicles Derived From Mesenchymal Stem Cells (MSC) in Regenerative Medicine: Applications in Skin Wound Healing. Front. Bioeng. Biotechnol. 2020, 8, 146. [Google Scholar] [CrossRef]
- Alonso-Alonso, M.L.; García-Posadas, L.; Diebold, Y. Extracellular Vesicles from Human Adipose-Derived Mesenchymal Stem Cells: A Review of Common Cargos. Stem Cell Rev. Rep. 2022, 18, 854–901. [Google Scholar] [CrossRef]
- Park, G.T.; Lim, J.K.; Choi, E.-B.; Lim, M.-J.; Yun, B.-Y.; Kim, D.K.; Yoon, J.W.; Hong, Y.G.; Chang, J.H.; Bae, S.H.; et al. Transplantation of adipose tissue-derived microvascular fragments promotes therapy of critical limb ischemia. Biomater. Res. 2023, 27, 70. [Google Scholar] [CrossRef]
- Copcu, H.E.; Oztan, S. Not Stromal Vascular Fraction (SVF) or Nanofat, but Total Stromal-Cells (TOST): A New Definition. Systemic Review of Mechanical Stromal-Cell Extraction Techniques. Tissue Eng. Regen. Med. 2021, 18, 25–36. [Google Scholar] [CrossRef]
- Aronowitz, J.A.; Lockhart, R.A.; Hakakian, C.S. Mechanical versus enzymatic isolation of stromal vascular fraction cells from adipose tissue. Springerplus 2015, 4, 713. [Google Scholar] [CrossRef]
- Rohrich, R.J.; Wan, D. Making sense of stem cells and fat grafting in plastic surgery: The hype, evidence, and evolving U.S. Food and Drug Administration Regulations. Plast Reconstr Surg 2019, 143, 417e–424e. [Google Scholar] [CrossRef] [PubMed]
- Tonnard, P.; Verpaele, A.; Peeters, G.; Hamdi, M.; Cornelissen, M.; Declercq, H. Nanofat grafting: Basic research and clinical applications. Plast. Reconstr. Surg. 2013, 132, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Hsu, V.M.; Stransky, C.A.; Bucky, L.P.; Percec, I. Fat grafting’s past, present, and future: Why adipose tissue is emerging as a critical link to the advancement of regenerative medicine. Aesthet. Surg. J. 2012, 32, 892–899. [Google Scholar] [CrossRef]
- Mazzola, R.F.; Mazzola, I.C. History of fat grafting: From ram fat to stem cells. Clin. Plast. Surg. 2015, 42, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Khouri, R.K., Jr. Current Clinical Applications of Fat Grafting. Plast Reconstr Surg 2017, 140, 466e–486e. [Google Scholar] [CrossRef]
- Davis, M.J.; Perdanasari, A.T.; Abu-Ghname, A.; Gonzalez, S.R.; Chamata, E.; Rammos, C.K.; Winocour, S.J. Application of Fat Grafting in Cosmetic Breast Surgery. Semin. Plast. Surg. 2020, 34, 24–29. [Google Scholar] [CrossRef]
- Hanson, S.E.; Kapur, S.K.; Hwang, R.F.; Dryden, M.S. Autologous fat grafting in breast reconstruction: Implications for follow-up and surveillance. Gland. Surg. 2021, 10, 487–493. [Google Scholar] [CrossRef]
- Glasgold, M.; Lam, S.M.; Glasgold, R. Autologous fat grafting for cosmetic enhancement of the perioral region. Facial Plast. Surg. Clin. North Am 2007, 15, 461–470, vi. [Google Scholar] [CrossRef]
- Glasgold, M.; Glasgold, R.; Lam, S. Autologous fat grafting for midface rejuvenation. Clin. Plast. Surg. 2015, 42, 115–121. [Google Scholar] [CrossRef]
- Schultz, K.P.; Raghuram, A.; Davis, M.J.; Abu-Ghname, A.; Chamata, E.; Rohrich, R.J. Fat Grafting for Facial Rejuvenation. Semin. Plast. Surg. 2020, 34, 30–37. [Google Scholar] [CrossRef]
- Crowley, J.S.; Kream, E.; Fabi, S.; Cohen, S.R. Facial Rejuvenation With Fat Grafting and Fillers. Aesthet. Surg. J. 2021, 41, S31–S38. [Google Scholar] [CrossRef]
- Piccolo, N.S.; Piccolo, M.T.S. Fat grafting for treatment of burns, burn scars, and other difficult wounds. Clin. Plast. Surg. 2015, 42, 263–283. [Google Scholar] [CrossRef]
- Fredman, R.; Katz, A.J.; Hultman, C.S. Fat Grafting for Burn, Traumatic, and Surgical Scars. Clin. Plast. Surg. 2017, 44, 781–791. [Google Scholar] [CrossRef]
- Limido, E.; Weinzierl, A.; Ampofo, E.; Harder, Y.; Menger, M.D.; Laschke, M.W. Nanofat Accelerates and Improves the Vascularization, Lymphatic Drainage and Healing of Full-Thickness Murine Skin Wounds. Int. J. Mol. Sci. 2024, 25, 851. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, M.; Tiang, T.; Lee, T.; Stellingwerf, M.E.; Singh, S.; Thompson, A.J.; D’Souza, B.; Ding, N.S. SVHM IBD Collaborative Group. Autologous fat graft injections for the treatment of perianal fistulas in Crohn’s disease: A systematic review and single-arm meta-analysis. ANZ J. Surg. 2023, 93, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- De Monti, M.; De Pellegrin, L.; Galetti, K. Microfractured Adipose Tissue Graft for the Advanced Treatment of Non-Healing Cutaneous Fistulas. Int. J. Regen. Med. 2022, 5, 2–8. [Google Scholar] [CrossRef]
- Bazarov, D.V.; Khrustaleva, M.V.; Bulganina, N.A.; Titova, I.V.; Vasiliev, V.S.; Kotenko, K.V.; Povolotskaya, O.B. Sovremennye tekhnologii regenerativnoi reabilitatsii patsientov. Opyt uspeshnogo ispol’zovaniya autologichnoi zhirovoi tkani u patsienta s kozhno-trakheal’nym svishchom [Advanced regenerative rehabilitation technologies. Successful use of autologous adipose tissue in a patient with cutaneous tracheal fistula]. Vopr. Kurortol. Fizioter. Lech. Fiz. Kult. 2021, 98, 72–74. [Google Scholar]
- Canales-Medina, M. Endoscopic closure of pharyngocutaneous fistula with fat injections: How i do it (with video). Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2021, 138, 101–102. [Google Scholar] [CrossRef]
- Sapundzhiev, N.R.; Nikiforova, L.T.; Spasova, B.H.; Ivanova, D.; Balev, B. Endoscopic Repair of Pharyngocutaneous Fistula Following Laryngectomy. Cureus 2019, 11, e5871. [Google Scholar] [CrossRef]
- Haubner, F.; Gassner, H.G.; Pérez Álvarez, J.C. Injektion von Bauchfett bei radiogenen Wundheilungsstörungen [Injection of Lipotransplants for Wound Healing Complications after Radiotherapy]. Laryngorhinootologie 2016, 95, 242–244. [Google Scholar]
- Hespe, G.E.; Albornoz, C.R.; Mehrara, B.J.; Kraus, D.; Matros, E. CASE REPORT Pharyngocutaneous Fistula Closure Using Autologous Fat Grafting. Eplasty 2013, 13, e23. [Google Scholar]
- Klinger, F.M.; Caviggioli, F.; Forcellini, D.; Vinci, V.; Maione, L.; Pajardi, G.; Klinger, M. Breast fistula repair after autologous fat graft: A case report. Case Rep. Med. 2011, 2011, 547387. [Google Scholar] [CrossRef]
- Das, A.; Lewandoski, P.; Laganosky, D.; Walton, J.; Shenot, P. Ureteroarterial fistula: A review of the literature. Vascular 2016, 24, 203–207. [Google Scholar] [CrossRef]
- Sugrue, J.; Nordenstam, J.; Abcarian, H.; Bartholomew, A.; Schwartz, J.L.; Mellgren, A.; Tozer, P.J. Pathogenesis and persistence of cryptoglandular anal fistula: A systematic review. Tech. Coloproctol. 2017, 21, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.V.; Cabrera, R.; Kondo, W.; Zomer, M.T.; Ferreira, H. Vesico-Vaginal Fistula: Nature and Evidence-Based Minimally Invasive Surgical Treatment. Surg. Technol. Int. 2019, 35, 189–198. [Google Scholar] [PubMed]
- Colley, E.; Simmons, A.; Varcoe, R.; Thomas, S.; Barber, T. Arteriovenous fistula maturation and the influence of fluid dynamics. Proc. Inst. Mech. Eng. H 2020, 234, 1197–1208. [Google Scholar] [CrossRef] [PubMed]
- Bonomi, F.; Limido, E.; Weinzierl, A.; Harder, Y.; Menger, M.D.; Laschke, M.W. Preconditioning Strategies for Improving the Outcome of Fat Grafting. Tissue Eng. Part. B Rev. 2024, 31, 94–108. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).