Abstract
Background. Knee and hip arthroplasty are two of the most frequently performed procedures in orthopaedic surgery. They are associated with positive patient-reported outcomes and significant improvements in quality of life for patients. Despite this, there may be room for further progress by quantifying functional improvements with gait analysis. Our study therefore aims to characterise the disease-specific gait pattern of participants with knee and hip osteoarthritis undergoing total joint replacement using a single chest-based wearable sensor. Methods. Twenty-nine participants awaiting total hip replacement and 28 participants awaiting total knee replacement underwent three-dimensional motion analysis with inertial wearable sensors. These gait metrics were then compared with 28 healthy controls of similar ages. Differences in gait metrics were evaluated using a T-test. The participants were recruited through a single centre to participate in this cross-sectional observational study. Participants with osteoarthritis severity sufficient to warrant surgical intervention were considered for inclusion in our study. The participants were instructed to walk 15–120 m in a hospital environment while fitted with a chest-based wearable sensor. Results. In total, three domains were evaluated, including spatiotemporal, variability and asymmetry parameters. There were marked variations in the gait asymmetry parameters and step length variation in both the hip and knee osteoarthritis patients compared with the healthy controls. The magnitude of gait deterioration in terms of step length asymmetry was greater on average in the hip osteoarthritis group than the knee group. The hip osteoarthritis (+180%, p < 0.001) and knee osteoarthritis (+129%, p = 0.001) groups demonstrated marked differences in step length asymmetry. Discussion. A single chest-based sensor was found to be capable of detecting pathological gait signatures in osteoarthritis patients when compared with age-matched controls. Future studies should compare pre- and postoperative changes to disease-specific gait impairments to validate the use of wearable sensors as a clinical adjunct.
1. Background
Osteoarthritis (OA) is a disabling condition that affects a significant proportion of the population, causing joint pain, muscle weakness, reduced quality of life and increased risk of falls [1,2]. The global incidence of hip and knee OA was more than 300 million cases in 2017 [3]. Joint replacements are recommended for patients who suffer from end-stage OA and are amongst the most common orthopaedic procedures performed [4,5]. The goal of joint replacement is to improve the locomotive function of the joint, correct joint deformity, reduce gait instability and alleviate pain [1,5,6,7,8]. There are a few methods for quantifying the functional severity of OA, including patient-reported outcome measures such as the Western Ontario McMaster Universities Osteoarthritis (WOMAC) Index questionnaire as well as quantitative biomechanical gait analyses [8]. In this paper, we explore wearable sensor technology as a convenient method of objective gait analysis.
In recent years, there has been a shift of interests from the gold-standard optoelectronic camera with force plates to lightweight, portable, commercial-use wearable sensors [9,10,11]. Furthermore, commercialised and advertised wearable systems such as Nike+ and FitBit have encouraged consumers to embrace the continuous tracking of daily activities [11]. Whilst gold-standard laboratory-based gait analysis is time-consuming and expensive to set up, wearables sensors allow for easier follow-ups with patients’ post joint replacement procedures [4,9]. Wearable sensors often have accelerometers, gyroscopes, magnetometers, inclinometers and many more embedded functions [12,13,14,15]. The different types of motion sensors can mimic camera motion capture systems to provide positional data [16]. Accelerometers provide information on body sway and inclination by measuring the displacement from a reference point along the axes [17]. Gyroscopes estimate the angular velocity by measuring the Coriolis force [12,14]. Meanwhile, the magnetometers provide a sense of orientation by measuring the magnetic field towards the north pole [11]. However, there are also trade-offs when it comes to motion sensors which affect their accuracy, and these include noise, drift errors and distortion from local magnetic fields [11].
Previous research explored the postoperative outcomes of joint replacement in terms of monitoring and assessing rehabilitation progress [18]. There is a need for further research into preoperative gait patterns to understand disease progression and characterise the disease-specific gait profiles for hip and knee osteoarthritis. Moreover, gait analysis data may influence surgical planning, with Lofterod et al. (2007) finding a 13% reduction in the total number of pre-planned surgical procedures in children with cerebral palsy upon examining preoperative gait analysis data [19].
There is limited research that adopted solely wearable sensors as their mainstay of gait analysis. Most previous research has involved wearable sensor placement on the back or shank [9]. Within the studies conducted with wearable sensors, the mean spatiotemporal parameters were most extensively studied [18,20]. Therefore, the purpose of this study is to assess whether a single chest-based wearable sensor is sufficient to detect differences in not only spatiotemporal gait metrics but also asymmetry and variability metrics between a pathological (knee OA or hip OA) and normative gait (healthy age-matched controls). Secondly, we also aim to characterise the preoperative gait profile in participants undergoing total knee and hip replacement as a preliminary exploration of feasibility in the clinical assessment of a pathological gait.
2. Methods
2.1. Objectives
The present study is a single-centre observational (case-control) study of participants with hip and knee osteoarthritis awaiting total hip and total knee replacement, respectively. Gait parameters across the domains of spatial, temporal, asymmetric and variability metrics were collected using an inertial wearable sensor. The gait metrics were quantitatively analysed (against the normative gait of the age-matched control participants) to profile the pattern of gait deterioration.
2.2. Ethics
Approval was obtained from the South Eastern Sydney Local Health District in New South Wales, Australia (HREC 17/184, approved on 16 June 2021). All participants provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
2.3. Study Participants
The participants of this study were a sample of patients presenting to Prince of Wales Hospital in Sydney, Australia between February 2021 and July 2021. During their clinic visits, the study parameters and risks were discussed, and consent was obtained. Patients with diagnoses of either hip osteoarthritis (THR) or knee osteoarthritis (TKR) and surgical candidates for total joint replacement were considered for inclusion. The included patients experienced relapses and prolonged knee or hip pain, warranting surgical intervention. Patients of all severities were included as well as patients with prior arthroplasty (of the contralateral joint). The exclusion criteria included prior replacement, infection, cancer or the presence of other potentially gait-altering pathologies (e.g., spinal disease). The participants completed a questionnaire to obtain demographic and clinical information.
2.4. Controls
Participants of a similar age bracket (50–70) with pain-free gaits were recruited from the community as controls for this study, following a similar semi-structured interview and questionnaire, and they were age-matched at a 1:1 ratio. Population screening was conducted in an identical manner to Natarajan et al., 2022 [10]. Participants were not invited into this study if the presence of other gait-altering pathologies was found. All participants that met the eligibility criteria over the study period were included in our study, accounting for uneven sample sizes (29 versus 28 versus 28). As there are no previous studies on this patient population, there were no expected effect sizes on which to base the sample size power calculations.
2.5. Procedure
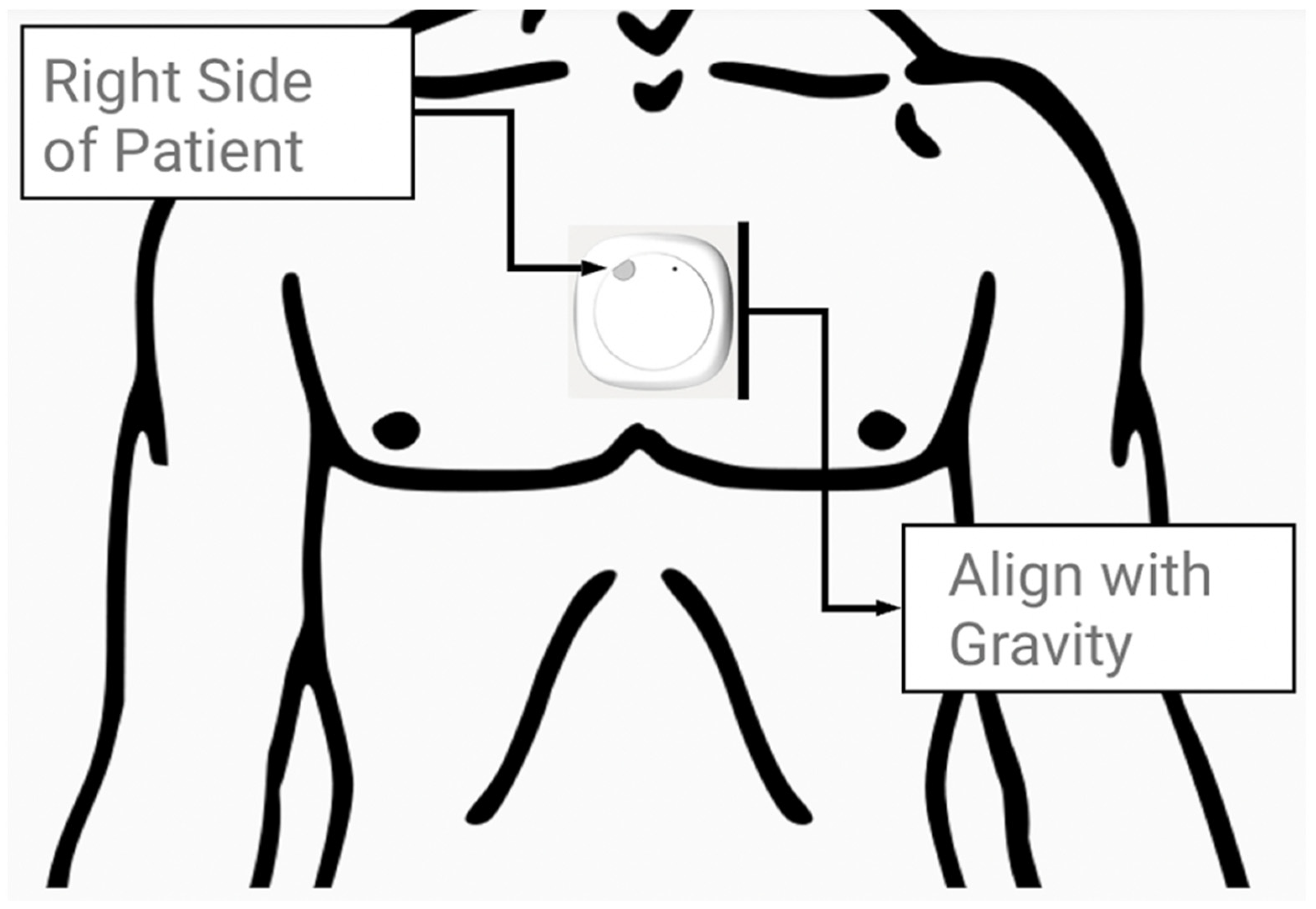
Prior to the walk, the inertial measurement unit, a MetaMotion© (MMC) manufactured by Mbientlab Inc. (San Francisco, CA, USA), was fitted on the skin immediately above the sternal angle of the participants, as shown in Figure 1. The device was aligned vertically with the line of gravity and secured firmly using medical tape. Following a short initial pause to orient the MMC device, the participants walked a self-selected distance (15–120 m) along an unobstructed hospital corridor pathway on level ground. Trials were discarded if the patient did not (or could not) pause to orient the device or required a walking aid during the walking bout. The MMC device’s position was confirmed before and after the procedure by an engineer and assistant on site. Further information on wearable devices and data processing can be found via Betteridge C. et al. (2021) and Natarajan et al. (2022) [10,21]. The MMC device was previously tested for accuracy and reliability in 22 healthy control patients by Betteridge C. et al. (2021) [21].
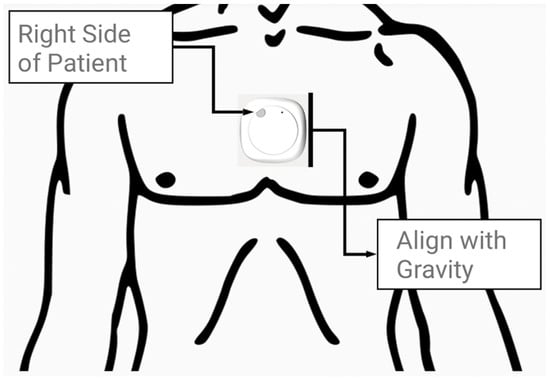
Figure 1.
A MetaMotionC wearable device (27 mm × 8 mm) was placed on the skin immediately superior to the sternal angle prior to the participant’s walking bout (not to scale).
2.6. Wearable Device
The wearable device (MMC) manufactured by Mbientlab Inc. contains a 16-bit 100 Hz triaxial accelerometer, a 16 bit 100 Hz triaxial gyroscope and a 0.3 µT 25 Hz triaxial magnetometer. The signals received was processed with a Kalman filter, and captured data were stored as a matrix of values corresponding to each time point (100 captures per second) for up to 20 min of walking.
2.7. Data Processing
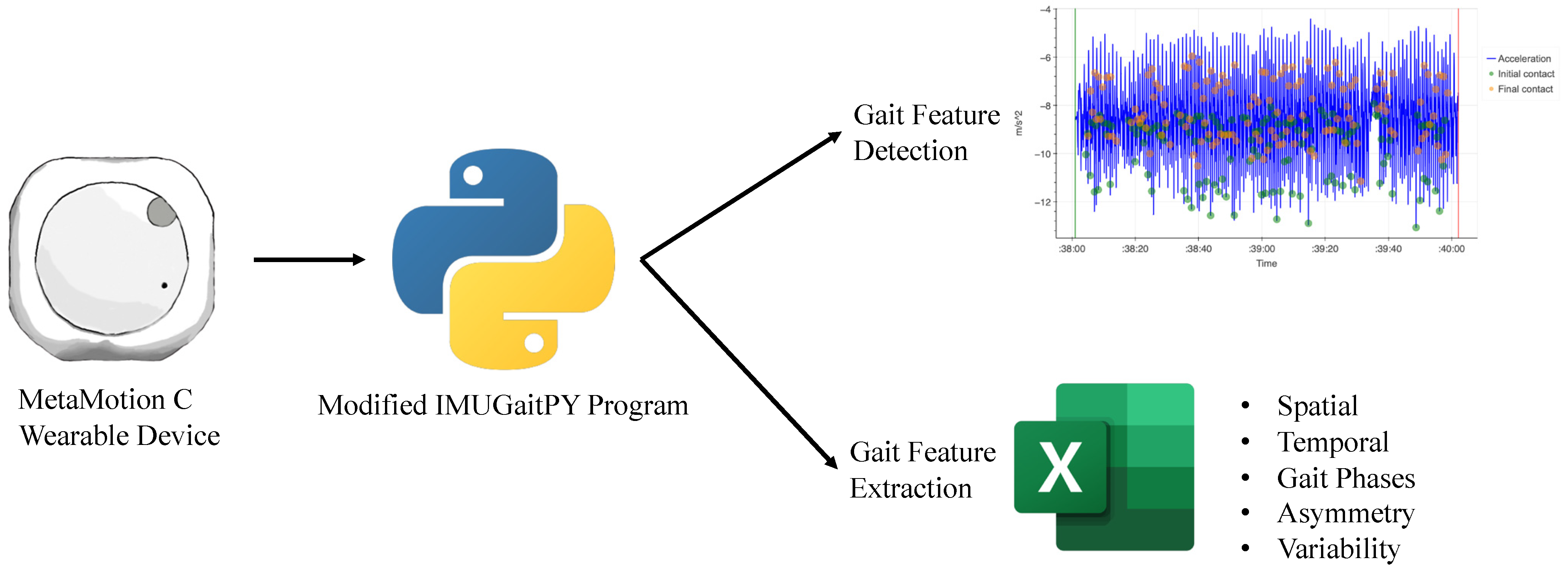
The IMUGait Recorder application (version 1.0) is a piece of software developed for this study as an intermediary between the MMC device and IMUGaitPy program (a modified open-source Python program). The wearable device (MMC) recorded the entire walking bout, and captured data were transmitted via Bluetooth™ to an AndroidTM smartphone running IMUGait Recorder. The IMUGait Recorder application then uploaded the raw data to a centralised database for processing by the modified IMUGaitPy program. The program detected and extracted the gait features across three domains (spatiotemporal, asymmetry and variability) to generate relevant gait metrics (such as gait velocity, step length asymmetry and step length variation). The flow diagram in Figure 2 demonstrates the data capturing process.
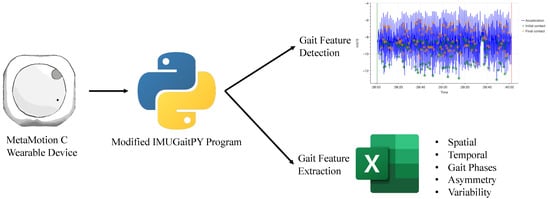
Figure 2.
Flow diagram of data capture and processing. Gait features extracted from detection of initial (green) and final contact (orange) based on acceleration changes (blue).
2.8. Statistical Analysis
Data analyses were performed using Prism 9 (GraphPad Software, Version 9.5.1). Normality was assessed using Shapiro–Wilk tests and inspection of the histograms where necessary, and statistical significance was considered for a p value < 0.05. Descriptive statistics were calculated for demographic variables including age, gender, height, body mass, BMI, presence of diabetes and smoking. The spatiotemporal parameters of the gaits were calculated, and step measurements (rather than stride) were chosen for the calculations of gait asymmetry [22]. Differences in gait metrics between the participants with osteoarthritis and the control participants were calculated using Mann–Whitney U tests or independent sample (two-tailed) t-tests.
3. Results
A total of 29 participants awaiting total hip replacement, 28 awaiting total knee replacement and 28 control participants met the eligibility criteria and consented for inclusion over the five-month study period spanning February–June 2021.
3.1. Participant Characteristics
All participants (THR, TKR and control participants) included were of similar age ((mean +/− standard deviation): 60 +/− 10 versus 63 +/− 15 versus 57 +/− 9.8 years). There were generally no significant differences in the THR and TKR participants’ demographic and clinical characteristics such as age, body mass index, smoking and diabetes when compared with the controls, as seen in Table 1 and Table 2 below.

Table 1.
Characteristics of THR and control participants.

Table 2.
Characteristics of TKR and control participants.
3.2. Pathological Gait Signatures
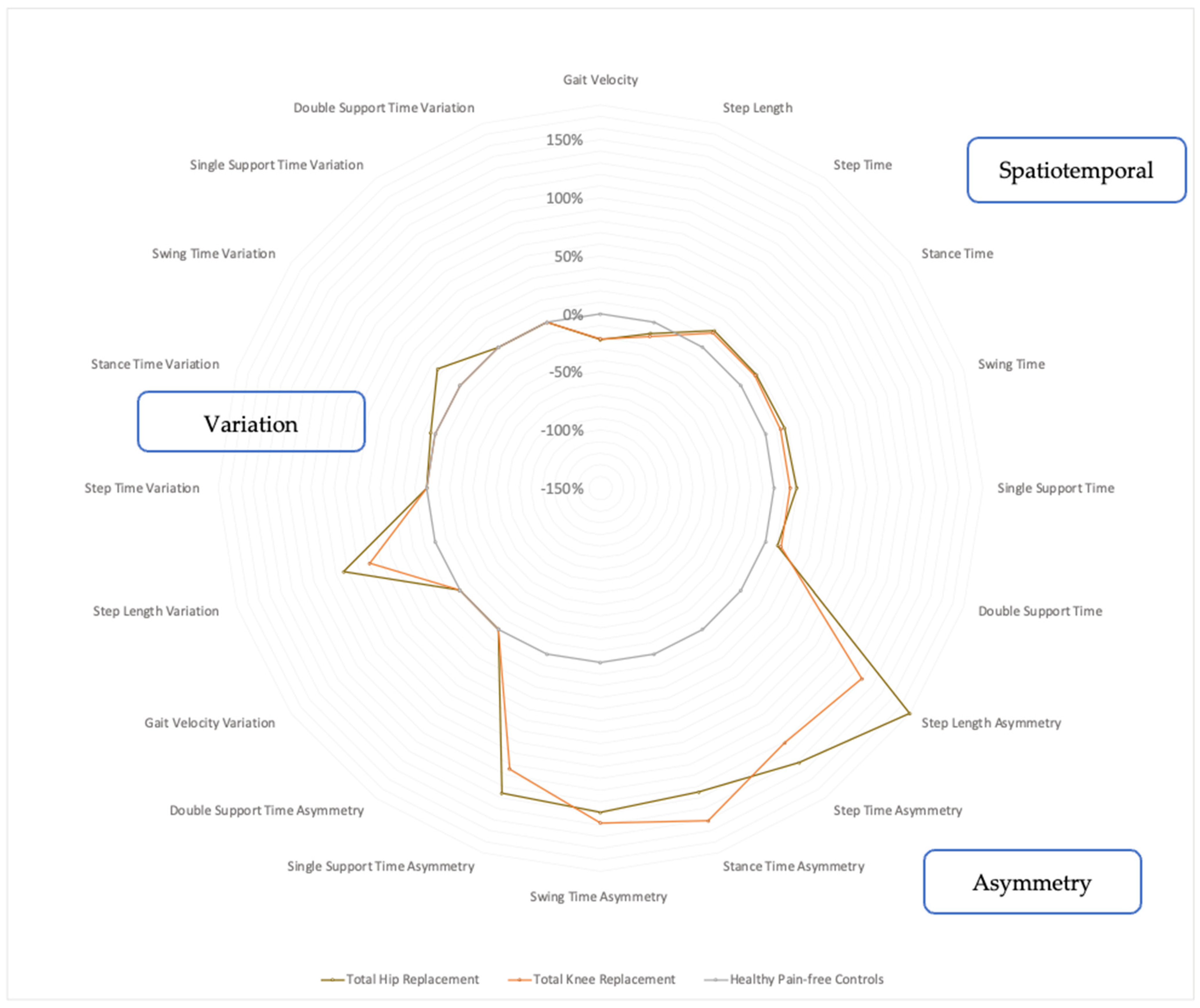
The THR participants demonstrated variations in their temporal and spatial gait metrics along with gait asymmetry, as shown in Figure 3. They typically walked with a lower gait velocity (−22.2%, p < 0.001) and shorter step length (−10.1%, p = 0.0115), whilst temporal parameters such as the step time (+17.3%, p < 0.001), stance time (+16.2, p < 0.001), swing time (+17.2%, p < 0.001), single support time (+19.5%, p < 0.001) and double support time (+10.8%, p = 0.0024) were increased. Notably, the THR participants also had markedly increased gait asymmetry in terms of step length asymmetry (+180%, p < 0.001), step time asymmetry (+142%, p = 0.0011), stance time asymmetry (+125%, p = 0.0018), swing time asymmetry (+129%, p = 0.0021) and single support time asymmetry (+126%, p = 0.0066). The THR group also walked with gait variability in terms of increased step length variability (+83.2, p < 0.001), stance time variability (+4.3%, p = 0.0018) and swing time variability (+24%, p = 0.0021). These gait metrics are presented in Figure 3 (and Appendix A Table A1).
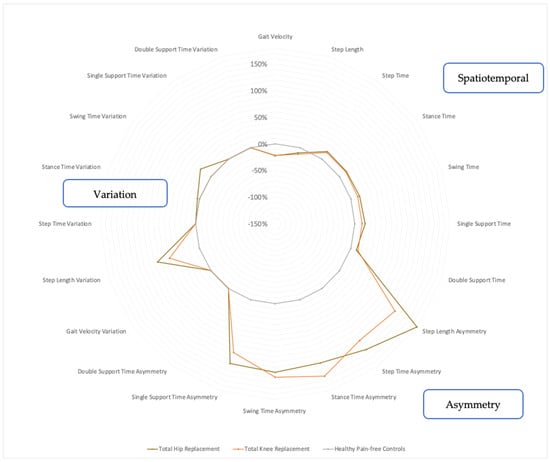
Figure 3.
Radar plot of walking metrics of THR and TKR groups compared with age-matched controls. Percentage values represent magnitude of group difference of walking metrics for THR participants (brown, n = 29) and TKR participants (orange, n = 28) from normative control participant values (grey, n = 28) placed at 0%. Calculated using Student’s independent t-tests or Mann–Whitney U tests, with normality confirmed using Shapiro–Wilk tests and by inspecting histograms. Statistical significance set to p < 0.05.
The TKR group also involved deteriorations in the spatial and temporal metrics of their gaits but to a lesser extent than the THR group. The TKR participants walked with a lower gait velocity (−21.5%, p < 0.001) and a shorter step length (−12.7%, p = 0.006), whilst the step time (+15.2%, p < 0.001), stance time (+14.8%, p < 0.001), swing time (+13.6%, p < 0.001), single support time (+13.9%, p < 0.001) and double support time (+13.8%, p < 0.001) increased, as shown in Figure 3 (and Appendix B Table A2). The TKR participants also demonstrated marked gait asymmetry, which involved step length asymmetry (+129%, p = 0.001), step time asymmetry (+121%, p = 0.003), stance time asymmetry (+151%, p = 0.002), swing time asymmetry (+138%, p = 0.006) and single support time asymmetry (+104%, p = 0.005). In terms of gait variability, the TKR group demonstrated only increased step length variability (+59.5%, p = 0.001), as seen in Figure 3.
The pattern of gait deterioration between the THR and TKR groups were similar when considering the spatial and temporal metrics. However, the THR group was found to be more asymmetric and variable (in step length) overall, as seen in Figure 3. The largest difference between the THR and TKR groups was the step length asymmetry, which was 1.5 times worse in the THR patients.
4. Discussion
The aim of the present study was to investigate the use of a single chest-based wearable sensor in identifying the pathological gait signatures of hip and knee OA. Previous research explored hip OA and knee OA gaits extensively with traditional laboratory-based gait analysis equipment and electromyography (EMG) [23,24,25,26]. Some of the gait parameters obtained from traditional gait analysis are not directly translatable to wearable sensor technology, such as knee adduction moment (KAM) [9]. Our observational study was performed using the minimum number of wearable sensors (one chest-based wearable sensor). These findings validated that wearable sensors can highlight relevant gait parameters for gait differentiation and profiling. In support of utilising a simplified methodology, Kobsar et al. (2017) found that the classification accuracy of two-sensor arrays was not significantly different from that of a three-sensor array while delivering similar results [27].
In terms of methodology, the wrist is one of the most common placement locations for gait analysis due to the ease of attachment. However, for this observational study, the preferred placement location for the researcher was above the sternal angle. Chest-based wearable sensors are more suitable for ambulatory activities such as walking, jumping and running as they provide holistic representations of the body by including upper body balance [28,29,30]. Chest-based wearables are in line with the centre of gravity and can better quantify the energy expenditure of the participants [28,31]. In terms of the accuracy percentage of the results, the hip and waist are generally preferred but tend to have lower wear compliance from participants due to physical discomfort at the wear site and negative feelings about the visibility of the device [31]. Zhang et al. (2016) found that chest-based wearables only performed marginally worse than hip or waist wearables and therefore can be seen as a suitable alternative [31]. Chest-based wearables are also subject to less disruptions from upper limb movements [31].
Our results are suggestive of distinct preoperative gait profiles in patients awaiting TKR and THR surgery (and similarities between the two pathological gaits) when compared with control participants, which can be detected with a single tri-axial wearable sensor [32]. The overall magnitude of the gait deterioration was more significant with the preoperative THR patients. Hip OA patients have weak hip adductor muscles. Therefore, to stabilise the hips, patients will mobilise with exaggerated lateral bending of their bodies [33]. The hallmark of compensatory gait patterns is known as the “Duchenne limp” [33]. Step length asymmetry was previously noted by Roerdink et al. (2011) and Balasubramanian et al. (2007) as being due to weaker propulsive force generated by a paretic limb or a compensation mechanism of greater propulsive force generated by the nonparetic limb [34,35]. It is the sum of the forward foot placement associated with trunk progression, which is well studied in the hemiparesis gaits of stroke patients [34,35]. Higgs et al. highlighted that advanced OA patients demonstrated less peak sagittal hip flexion and extension and lower peak sagittal hip moments on the paretic limb [2].
For knee OA, Kobsar et al. (2020) and Mills et al. (2013) found that the stride time is the single most consistent gait parameter in severe disease progression [9,36]. Pertaining to spatial gait metrics, some studies reported lower gait speeds due to a decreased cadence and decreased stride length [33,37]. Tadano et al. (2016) did not comment on the gait speed but did highlight shorter gait cycles in severe OA patients [38]. This is further supported by Boyer et al. (2019) and Tadano et al. (2016), as there was less knee extension (heel strike), ankle flexion (toe off) and abduction motion (toeing out) in severe knee OA patients [37,38]. Moreover, Sagawa et al. (2013) suggested that the gait speed may vary with OA of various hip knee angles (HKAs) [8]. Much research has explored the KAM, KFA and KFM as the parameters for identifying the severity of knee OA [23,24]. Changes in these parameters can indicate increase cartilage loss and more severe disease progression [24]. Favre et al. (2016) proposed that healthy patients with larger heel strike KFAs might contribute to the initiation of idiopathic OA [24]. Some studies discovered that pain relief medications can lead to rapid disease progression, as there have been higher joint loading and peak adduction moments demonstrated [39,40]. In the present study, those parameters were not taken into account as that would require specialised equipment (such as motion-cameras and force plates) for measurements of the ground reaction forces and angles (such as the toe-in angle and toe-out angle) [23,41].
4.1. Strengths
The present study captured the pathological gait signatures of hip and knee OA patients as a basis for future longitudinal studies involving gait rehabilitation and monitoring. Our novel finding of step length asymmetry as an identifiable feature separating knee and hip OA patients would require further reaffirmation in a larger cohort of patients of different disease severities. This study further strengthens the existing notable features of a lower gait speed and decreased stride length. Gait modification training is theorised to allow for offloading pressure on affected joints (i.e., decrease the HAM impulse), allowing for slower disease progression [26].
The external hip adductor moment impulse (HAM), much like the KAM, is mentioned in many studies as a measurable outcome for increased hip loading and progression of disease [26]. Moreover, there is radiographic evidence of progression of disease in patients with larger HAM impulses and mean numbers of steps per day [26]. Thus, identifying the gait parameters is critical for rehabilitation in mild-to-moderate OA patients. This study differs from mainstream wearables research as it has a different wearable placement location and seeks to use the minimal number of wearables (more discreet and having better wearer compliance) [28,30]. Some studies differ in terms of the number of wearables. The reason for this would be that more wearables would provide additional compensatory joint motions that are difficult to discriminate from a single wearable sensor [27]. However, this increases the complexity in interpreting the high-dimensional data to provide clear, distinguishable gait signatures [27]. Another strength of the present study is that the recruited participants had similar background characteristics (i.e., age, body mass and BMI) that could influence compensatory gait movements (such as trunk leaning). Furthermore, as the study was conducted in the same laboratory setting, the results are not subject to operator-dependent bias.
4.2. Limitations
There are a few limitations that merit consideration when interpreting the results. This study only recruited patients of a severity and functional impact significant enough to warrant surgical interventions. We also did not explore single or bilateral joint OA, as patients typically present with some degree of OA in both joints. Moreover, this study did not exclude patients who had previous joint replacements. One notable limitation is that the study did not include the “free-living” gait and was conducted in a well-lit, obstacle-free hospital environment. Therefore, the results are not generalisable to real-world conditions. One of the reasons for this is that the MetaMotionC wearable sensor has a battery life of 20 min, which limited the ability to capture day-to-day gaits and activities. Another limitation of this study is the lack of knee joint loading data to estimate the knee flexion angle (KFA) and KAM. This observational study did not incorporate force plates or pressure-sensitive shoes to measure the ground reaction force, which is required to calculate the knee flexion moments (KFMs) and KFAs. The KFM and KFA have previously been demonstrated to correlate with disease progression. Stetter et al. (2020) proved that with the use of machine learning technology (artificial neural networks) with wearable sensors, the KFM and KFA can be estimated [42]. Furthermore, the recruitment process of the participants was limited to a single-center and single-surgeon practice. It is important to note that the participants in this study were scheduled for joint replacement and therefore might not represent patients with less severe osteoarthritis. Despite the chest-based location, upper body motions were not evaluated in this study, and potentially important discriminatory parameters were not studied [43].
4.3. Future Research
Future avenues of research should seek to explore wearable sensors’ diagnostic capabilities of different degrees of osteoarthritis severity according to radiographic evidence and clinical correlation. Additionally, reassessing the changes of disease-specific gait impairments postoperatively may provide insights into optimising rehabilitation towards a normative gait, in addition to incorporating machine learning technology with the use of wearable sensors for diagnosing, disease monitoring or rehabilitation in patients with osteoarthritis [42]. Gait analysis using machine learning technology has seen increased utilisation in diseases involving balance (such as Parkinson’s disease and cerebellar ataxia) and upper limb ataxic movements [44]. Despite the higher computational requirements, machine learning has shown great promise in accurately differentiating different types of pathological gaits. Future studies should conduct a multi-center recruitment process with a longer pre- and post-op study period inclusive of the “free-living gait” [10]. A larger cohort size or multi-centre recruitment allows for subgroup analysis of the participants, easier accommodation of participants in case of dropouts and more generalisable results. Future studies can consider venturing into correlating the clinical findings (e.g., WOMAC score) with individual gait profiles as well as considering postoperative gaits during rehabilitation. In addition to utilising an objective scoring system, future studies could consider incorporating upper body motion, as Boekesteijn et al. (2021) highlighted the possibility of compensatory action with an increased trunk range of motion (RoM) [43].
5. Conclusions
Our study found similar changes in the spatiotemporal gait parameters of OA patients, as previously identified in other studies. In addition to that, this present study captured another domain of gaits: step length asymmetry as a discriminatory feature between hip and knee OA patients. Our present study identified that hip and knee OA patients have unique pathological signatures of gait impairment. The hip OA patients had the overall larger percentage values for domains of asymmetry and variation in gait parameters compared with the knee OA patients. This present study validates that single chest-based wearables can capture and examine disease-specific gait parameters. Future steps should explore incorporating the use of wearable technology in the community as a simplistic and cost-effective clinical adjunct in the assessment and identification of gait disorders, as well as utilising wearable sensor technology for a variety of patient populations in various natural environments and in continuous use.
Author Contributions
Conception and design, R.J.M. and M.M.; administrative support, R.J.M., M.M., K.R. and L.K.; provision of study materials or patients, R.J.M. and K.R.; collection and assembly of data, R.J.M. and K.R.; data analysis and interpretation, P.N. and R.D.F.; manuscript writing, P.N., A.L.C.Y., R.D.F., D.A.-H., K.R., L.S., M.M., D.B., L.K. and R.J.M.; final approval of manuscript, P.N., A.L.C.Y., R.D.F., D.A.-H., K.R., L.S., M.M., D.B., L.K. and R.J.M. All authors have read and agreed to the published version of the manuscript.
Funding
The authors declare that they received no funding.
Institutional Review Board Statement
Ethics approval for this study was obtained from the South Eastern Sydney Local Health District Ethics Board under reference code HREC: 17/184, approved on 16 June 2021.
Informed Consent Statement
Written consent was obtained from included participants prior to participation in this study.
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| OA | Osteoarthritis |
| BMI | Body mass index |
| SD | Standard deviation |
| CI | Confidence interval |
| IMU | Inertial measurement unit |
| MMC | MetaMotion© wearable sensor |
| IMUPY | Modified IMUGaitPy program |
| WOMAC | Western Ontario McMaster Universities Osteoarthritis Index |
| KFM | Knee flexion moment |
| KFA | Knee flexion angle |
| KAM | Knee adduction moment |
| HAM | Hip adductor moment impulse |
| HKA | Hip-knee angle |
| RoM | Range of motion |
Appendix A
For the quantitative gait signatures of the THR participants (sensor-derived), we must note that the values are presented as the mean ± SD or as the median (range) for metrics with normal and non-normal distributions. The p value represents the statistical significance of the difference between the groups, derived from independent sample two-tailed t-tests (with Welch’s correction applied if unequal in variance) or Mann–Whitney U tests (if they had non-normal distributions). Here, CoV is the coefficient of variance, m stands for metres, s stands for seconds, and ms stands for milliseconds.

Table A1.
Quantitative Gait Signature of THR participants (sensor-derived).
Table A1.
Quantitative Gait Signature of THR participants (sensor-derived).
| THR (n = 28) | Controls (n = 33) | Group Difference (Controls—THR) | |||
|---|---|---|---|---|---|
| Mean ± SD | 95% CI | % | p | ||
| Spatial Gait Metrics | |||||
| Gait Velocity (m/s) | 1.05 ± 0.212 | 1.35 ± 0.177 | −0.400; −0.189 | 22.2 | <0.001 |
| Step Length (m) | 0.624 ± 0.0990 | 0.694 ± 0.694 | −0.123; −0.0163 | 10.1 | 0.0115 |
| Temporal Gait Metrics | |||||
| Step Time (s) | 0.609 ± 0.0965 | 0.519 ± 0.519 | 0.0513; 0.128 | 17.3 | <0.001 |
| Stance Time (s) | 0.754 ± 0.116 | 0.649 ± 0.0323 | 0.0595; 0.152 | 16.2 | <0.001 |
| Swing Time (s) | 0.456 ± 0.0667 | 0.389 ± 0.0200 | 0.0405; 0.0942 | 17.2 | <0.001 |
| Single Support Time (s) | 0.472 ± 0.102 | 0.395 ± 0.0223 | 0.0364; 0.117 | 19.5 | <0001 |
| Double Support Time (s) | 0.292 ± 0.0510 | 0.260 ± 0.0130 | 0.0119; 0.0524 | 10.8 | 0.0024 |
| Gait Asymmetry | |||||
| Step Length Asymmetry (m) | 0.148 ± 0.101 | 0.0529 ± 0.0168 | 0.0556; 0.135 | 180 | <0.001 |
| Step Time Asymmetry (s) | 0.0906 ± 0.0785 | 0.0374 ± 0.0166 | 0.0223; 0.0841 | 142 | 0.0011 |
| Stance Time Asymmetry (s) | 0.0742 ± 0.0635 | 0.0330 ± 0.0152 | 0.0160; 0.0663 | 125 | 0.0018 |
| Swing Time Asymmetry (s) | 0.0764 ± 0.0671 | 0.0334 ± 0.0169 | 0.0163; 0.0696 | 129 | 0.0021 |
| Single Support Time Asymmetry (s) | 0.0864 ± 0.0869 | 0.0383 ± 0.0175 | 0.0140; 0.0823 | 126 | 0.0066 |
| Double Support Time Asymmetry (s) | 0.0272 ± 0.0519 | 0.0116 ± 0.00414 | −0.00440; 0.0357 | 134 | 0.123 |
| Gait Variability | |||||
| Gait Velocity Variability (CoV) | 9.62 ± 2.91 | 10.5 ± 3.08 | −2.46; 0.783 | 9.15 | 0.305 |
| Step Length Variability (CoV) | 17.0 ± 9.75 | 9.28 ± 2.26 | 3.86; 11.6 | 83.2 | <0.001 |
| Step Time Variability (CoV) | 12.77 ± 7.31 | 11.03 ± 4.44 | −1.54; 5.02 | 15.8 | 0.293 |
| Stance Time Variation (CoV) | 8.74 ± 4.74 | 8.38 ± 3.15 | −1.81; 2.55 | 4.30 | 0.0018 |
| Swing Time Variation (CoV) | 17.21 ± 16.7 | 13.88 ± 7.16 | −3.67; 10.3 | 24.0 | 0.0021 |
| Single Support Time Variation (CoV) | 25.5 ± 22.9 | 27.7 ± 19.3 | −13.7; 9.19 | 7.94 | 0.694 |
| Double Support Time Variation (CoV) | 14.3 ± 19.3 | 12.0 ± 7.32 | −5.59; 10.3 | 19.2 | 0.553 |
Appendix B
For the quantitative gait signatures of the TKR participants (sensor-derived), we must note that the values are presented as the mean ± SD or as the median (range) for the metrics with normal and non-normal distributions. Here, the p value represents the statistical significance of the difference between groups derived from independent sample two-tailed t-tests (with Welch’s correction applied if unequal in variance), or Mann–Whitney U tests (if they had non-normal distributions). Here, CoV is the coefficient of variance, m stands for metres, s stands for seconds, and ms stands for milliseconds.

Table A2.
Quantitative Gait Signature of TKR participants (sensor-derived).
Table A2.
Quantitative Gait Signature of TKR participants (sensor-derived).
| TKR (n = 28) | Controls (n = 33) | Group Difference (Controls—TKR) | |||
|---|---|---|---|---|---|
| Mean ± SD | 95% CI | % | p | ||
| Spatial Gait Metrics | |||||
| Gait Velocity (m/s) | 1.06 ± 0.264 | 1.35 ± 0.180 | 0.163; 0.405 | 21.5 | <0.001 |
| Step Length (m) | 0.616 ± 0.106 | 0.694 ± 0.101 | 0.0231; 0.134 | 12.7 | 0.006 |
| Temporal Gait Metrics | |||||
| Step Time (s) | 0.598 ± 0.0867 | 0.519 ± 0.0266 | −0.114; −0.0443 | 15.2 | <0.001 |
| Stance Time (s) | 0.745 ± 0.105 | 0.649 ± 0.0329 | −0.139; −0.0541 | 14.8 | <0.001 |
| Swing Time (s) | 0.450 ± 0.0683 | 0.389 ± 0.0203 | −0.0885; −0.0336 | 13.6 | <0.001 |
| Single Support Time (s) | 0.459 ±0.0797 | 0.395 ± 0.0227 | −0.0958; −0.0320 | 13.9 | <0.001 |
| Double Support Time (s) | 0.296 ± 0.0387 | 0.260 ± 0.0132 | −0.0517; −0.0203 | 13.8 | <0.001 |
| Gait Asymmetry | |||||
| Step Length Asymmetry (m) | 0.121 ± 0.0936 | 0.0529 ± 0.0171 | −0.105; −0.0318 | 129 | 0.001 |
| Step Time Asymmetry (s) | 0.0828 ± 0.0738 | 0.0374 ± 0.0167 | −0.0746; −0.0162 | 121 | 0.003 |
| Stance Time Asymmetry (s) | 0.0710 ± 0.0583 | 0.0330 ± 0.0155 | −0.0613; −0.0147 | 151 | 0.002 |
| Swing Time Asymmetry (s) | 0.0714 ± 0.0660 | 0.0334 ± 0.0172 | −0.0643; −0.0117 | 138 | 0.006 |
| Single Support Time Asymmetry (s) | 0.0781 ± 0.0679 | 0.0383 ± 0.0178 | −0.0668; −0.0127 | 104 | 0.005 |
| Double Support Time Asymmetry (s) | 0.0189 ± 0.0202 | 0.0116 ± 0.00421 | −0.0153; 0.00700 | 62.9 | 0.072 |
| Gait Variability | |||||
| Gait Velocity Variability (CoV) | 10.2 ± 3.02 | 10.5 ± 3.14 | −1.41; 1.90 | 2.86 | 0.766 |
| Step Length Variability (CoV) | 14.8 ± 7.54 | 9.28 ± 2.30 | −8.53; −2.46 | 59.5 | 0.001 |
| Step Time Variability (CoV) | 12.0 ± 7.21 | 11.0 ± 4.52 | −4.19; 2.28 | 9.09 | 0.554 |
| Stance Time Variation (CoV) | 8.59 ± 4.51 | 8.37 ± 3.20 | −2.31; 1.88 | 2.63 | 0.835 |
| Swing Time Variation (CoV) | 14.2 ± 10.4 | 13.9 ± 7.29 | −5.17; 4.47 | 2.16 | 0.885 |
| Single Support Time Variation (CoV) | 24.1 ± 21.7 | 27.7 ± 19.7 | −7.51; 14.7 | 13.0 | 0.519 |
| Double Support Time Variation (CoV) | 12.6 ± 11.4 | 12.0 ± 7.45 | −5.82; 4.55 | 5.00 | 0.807 |
References
- Mandeville, D.; Osternig, L.R.; Chou, L.-S. The effect of total knee replacement surgery on gait stability. Gait Posture 2008, 27, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Higgs, J.P.; Diamond, L.E.; Saxby, D.J.; Constantinou, M.; Barrett, R.S. Individuals with Unilateral Mild-to-Moderate Hip Osteo-arthritis Exhibit Lower Limb Kinematic Asymmetry during Walking But Not Sit-to-Stand. Symmetry 2021, 13, 768. [Google Scholar] [CrossRef]
- Safiri, S.; Kolahi, A.-A.; Smith, E.; Hill, C.; Bettampadi, D.; Mansournia, M.A.; Hoy, D.; Ashrafi-Asgarabad, A.; Sepidarkish, M.; Almasi-Hashiani, A.; et al. Global, regional and national burden of osteoarthritis 1990-2017: A systematic analysis of the Global Burden of Disease Study 2017. Ann. Rheum. Dis. 2020, 79, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Rahman, J.; Tang, Q.; Monda, M.; Miles, J.; McCarthy, I. Gait assessment as a functional outcome measure in total knee arthroplasty: A cross-sectional study. BMC Musculoskelet. Disord. 2015, 16, 66. [Google Scholar] [CrossRef] [PubMed]
- Hyodo, K.; Kanamori, A.; Kadone, H.; Takahashi, T.; Kajiwara, M.; Yamazaki, M. Gait Analysis Comparing Kinematic, Kinetic, and Muscle Activation Data of Modern and Conventional Total Knee Arthroplasty. Arthroplast. Today 2020, 6, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Mizner, R.L.; Snyder-Mackler, L. Association between long-term quadriceps weakness and early walking muscle co-contraction after total knee arthroplasty. Knee 2013, 20, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Biggs, P.R.; Whatling, G.M.; Wilson, C.; Metcalfe, A.J.; Holt, C.A. Which osteoarthritic gait features recover following total knee re-placement surgery? PLoS ONE 2019, 14, e0203417. [Google Scholar] [CrossRef] [PubMed]
- Sagawa, Y.; Armand, S.; Lubbeke, A.; Hoffmeyer, P.; Fritschy, D.; Suva, D.; Turcot, K. Associations between gait and clinical parameters in patients with severe knee osteoarthritis: A multiple correspondence analysis. Clin. Biomech. 2013, 28, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Kobsar, D.; Masood, Z.; Khan, H.; Khalil, N.; Kiwan, M.Y.; Ridd, S.; Tobis, M. Wearable Inertial Sensors for Gait Analysis in Adults with Osteoarthritis—A Scoping Review. Sensors 2020, 20, 7143. [Google Scholar] [CrossRef]
- Natarajan, P.; Fonseka, R.D.; Sy, L.; Maharaj, M.; Mobbs, R. Analysing gait patterns in degenerative lumbar spine disease using inertial wearable sensors—An observational study. World Neurosurg. 2022, 163, e501–e515. [Google Scholar] [CrossRef]
- Shull, P.B.; Jirattigalachote, W.; Hunt, M.A.; Cutkosky, M.R.; Delp, S.L. Quantified self and human movement: A review on the clinical impact of wearable sensing and feedback for gait analysis and intervention. Gait Posture 2014, 40, 11–19. [Google Scholar] [CrossRef]
- Tao, W.; Liu, T.; Zheng, R.; Feng, H. Gait Analysis Using Wearable Sensors. Sensors 2012, 12, 2255–2283. [Google Scholar] [CrossRef] [PubMed]
- Tarniţă, D. Wearable sensors used for human gait analysis. Rom. J. Morphol. Embryol. 2016, 57, 373–382. [Google Scholar] [PubMed]
- Caldas, R.; Mundt, M.; Potthast, W.; de Lima Neto, F.B.; Markert, B. A systematic review of gait analysis methods based on inertial sensors and adaptive algorithms. Gait Posture 2017, 57, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.C.Y.; Natarajan, P.; Fonseka, R.D.; Maharaj, M.; Mobbs, R.J. The application of artificial intelligence and custom algorithms with inertial wearable devices for gait analysis and detection of gait-altering pathologies in adults: A scoping review of literature. Digit. Health 2022, 8, 20552076221074128. [Google Scholar] [CrossRef] [PubMed]
- Takeda, R.; Tadano, S.; Todoh, M.; Morikawa, M.; Nakayasu, M.; Yoshinari, S. Gait analysis using gravitational acceleration measured by wearable sensors. J. Biomech. 2009, 42, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-H.; Chen, P.-C.; Liu, K.-C.; Chan, C.-T. Wearable sensor-based rehabilitation exercise assessment for knee osteoarthritis. Sensors 2015, 15, 4193–4211. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-P.; Liu, Y.-Y.; Hsu, W.-H.; Lai, L.-J.; Lee, M.S. Monitoring and Assessment of Rehabilitation Progress on Range of Motion After Total Knee Replacement by Sensor-Based System. Sensors 2020, 20, 1703. [Google Scholar] [CrossRef] [PubMed]
- Lofterød, B.; Terjesen, T.; Skaaret, I.; Huse, A.-B.; Jahnsen, R. Preoperative gait analysis has a substantial effect on orthopedic decision making in children with cerebral palsy: Comparison between clinical evaluation and gait analysis in 60 patients. Acta Orthop. 2007, 78, 74–80. [Google Scholar] [CrossRef]
- Small, S.R.; Bullock, G.S.; Khalid, S.; Barker, K.; Trivella, M.; Price, A.J. Current clinical utilisation of wearable motion sensors for the assessment of outcome following knee arthroplasty: A scoping review. BMJ Open 2019, 9, e033832. [Google Scholar] [CrossRef]
- Betteridge, C.; Mobbs, R.J.; Fonseka, R.D.; Natarajan, P.; Ho, D.; Choy, W.J.; Sy, L.W.; Pell, N. Objectifying clinical gait assessment: Using a sin-gle-point wearable sensor to quantify the spatiotemporal gait metrics of people with lumbar spinal stenosis. J. Spine Surg. 2021, 7, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Galna, B.; Lord, S.; Rochester, L. Is gait variability reliable in older adults and Parkinson’s disease? Towards an optimal testing protocol. Gait Posture 2013, 37, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Duffell, L.D.; Southgate, D.F.; Gulati, V.; McGregor, A.H. Balance and gait adaptations in patients with early knee osteoarthritis. Gait Posture 2014, 39, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Favre, J.; Jolles, B.M. Gait analysis of patients with knee osteoarthritis highlights a pathological mechanical pathway and provides a basis for therapeutic interventions. EFORT Open Rev. 2016, 1, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Foucher, K.C.; Freels, S. Preoperative factors associated with postoperative gait kinematics and kinetics after total hip arthroplasty. Osteoarthr. Cartil. 2015, 23, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Tateuchi, H.; Akiyama, H.; Goto, K.; So, K.; Kuroda, Y.; Ichihashi, N. Gait kinematics of the hip, pelvis, and trunk associated with external hip adduction moment in patients with secondary hip osteoarthritis: Toward determination of the key point in gait modification. BMC Musculoskelet. Disord. 2020, 21, 8. [Google Scholar] [CrossRef] [PubMed]
- Kobsar, D.; Osis, S.T.; Boyd, J.E.; Hettinga, B.A.; Ferber, R. Wearable sensors to predict improvement following an exercise intervention in patients with knee osteoarthritis. J. Neuroeng. Rehabil. 2017, 14, 94. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, M.H.; Berkvens, R.; Weyn, M. Chest-Worn Inertial Sensors: A Survey of Applications and Methods. Sensors 2021, 21, 2875. [Google Scholar] [CrossRef] [PubMed]
- Nazarahari, M.; Rouhani, H. Detection of daily postures and walking modalities using a single chest-mounted tri-axial accel-erometer. Med. Eng. Phys. 2018, 57, 75–81. [Google Scholar] [CrossRef]
- Schwenk, M.; Mohler, J.; Wendel, C.; D’’Huyvetter, K.; Fain, M.; Taylor-Piliae, R.; Najafi, B. Wearable sensor-based in-home assessment of gait, balance, and physical activity for discrimination of frailty status: Baseline results of the arizona frailty cohort study. Gerontology 2014, 61, 258–267. [Google Scholar] [CrossRef]
- Zhang, J.H.; Macfarlane, D.J.; Sobko, T. Feasibility of a Chest-worn accelerometer for physical activity measurement. J. Sci. Med. Sport 2016, 19, 1015–1019. [Google Scholar] [CrossRef] [PubMed]
- Mc Ardle, R.; Galna, B.; Donaghy, P.; Thomas, A.; Rochester, L. Do Alzheimer’s and Lewy body disease have discrete pathological signatures of gait? Alzheimers Dement. 2019, 15, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Reininga, I.H.; Stevens, M.; Wagenmakers, R.; Bulstra, S.K.; Groothoff, J.W.; Zijlstra, W. Subjects with hip osteoarthritis show distinctive patterns of trunk movements during gait-a body-fixed-sensor based analysis. J. Neuroeng. Rehabil. 2012, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, C.K.; Bowden, M.G.; Neptune, R.R.; Kautz, S.A. Relationship between step length asymmetry and walking per-formance in subjects with chronic hemiparesis. Arch. Phys. Med. Rehabil. 2007, 88, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Roerdink, M.; Beek, P.J. Understanding inconsistent step-length asymmetries across hemiplegic stroke patients: Impairments and compensatory gait. Neurorehabil. Neural. Repair. 2011, 25, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.; Hunt, M.A.; Ferber, R. Biomechanical deviations during level walking associated with knee osteoarthritis: A systematic review and meta-analysis. Arthritis Rheum. 2013, 65, 1643–1665. [Google Scholar] [CrossRef] [PubMed]
- Boyer, K.A.; Hafer, J.F. Gait mechanics contribute to exercise induced pain flares in knee osteoarthritis. BMC Musculoskelet. Disord. 2019, 20, 107. [Google Scholar] [CrossRef]
- Tadano, S.; Takeda, R.; Sasaki, K.; Fujisawa, T.; Tohyama, H. Gait characterization for osteoarthritis patients using wearable gait sensors (H-Gait systems). J. Biomech. 2016, 49, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, M.; Graven-Nielsen, T.; Aaboe, J.; Andriacchi, T.P.; Bliddal, H. Gait changes in patients with knee osteoarthritis are rep-licated by experimental knee pain. Arthritis Care Res. 2010, 62, 501–509. [Google Scholar] [CrossRef]
- Miyazaki, T.; Wada, M.; Kawahara, H.; Sato, M.; Baba, H.; Shimada, S. Dynamic load at baseline can predict radiographic disease progression in medial compartment knee osteoarthritis. Ann. Rheum. Dis. 2002, 61, 617–622. [Google Scholar] [CrossRef]
- Thorstensson, C.; Henriksson, M.; von Porat, A.; Sjödahl, C.; Roos, E. The effect of eight weeks of exercise on knee adduction moment in early knee osteoarthritis—A pilot study. Osteoarthr. Cartil. 2007, 15, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Stetter, B.J.; Krafft, F.C.; Ringhof, S.; Stein, T.; Sell, S. A Machine Learning and Wearable Sensor Based Approach to Estimate External Knee Flexion and Adduction Moments During Various Locomotion Tasks. Front. Bioeng. Biotechnol. 2020, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Boekesteijn, R.J.; Smolders, J.M.H.; Busch, V.J.J.F.; Geurts, A.C.H.; Smulders, K. Independent and sensitive gait parameters for objective evaluation in knee and hip osteoarthritis using wearable sensors. BMC Musculoskelet. Disord. 2021, 22, 242. [Google Scholar] [CrossRef] [PubMed]
- Rovini, E.; Maremmani, C.; Moschetti, A.; Esposito, D.; Cavallo, F. Comparative Motor Pre-clinical Assessment in Parkinson’s Disease Using Supervised Machine Learning Approaches. Ann. Biomed. Eng. 2018, 46, 2057–2068. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).