Abstract
(1) Introduction and Aim: Surgical navigation has evolved as a vital tool in maxillofacial surgery, offering precise and patient-specific data. This study explores the clinical applications and accuracy of intraoperative tool tracking in maxillofacial surgery. (2) Materials and Methods: The research includes 42 patients with various pathologies who underwent surgeries assisted by a surgical navigation system using tracked instruments. Four representative cases are exhibited in the study: the first case involving coronoid hyperplasia with mouth opening deficit, the second case addressing naso-orbital-ethmoidal-frontal ossifying fibroma resection, the third case showcasing a subapical osteotomy (Köle) for a class III dentoskeletal malocclusion, and the fourth one exposing the treatment of a recurrent ameloblastoma. (3) Results: The results indicate that surgical navigation with tracked instruments provides high precision (<1.5 mm error), reduced surgical time, and a less invasive approach. (4) Conclusions: This study highlights the potential for reproducible outcomes and increased safety, especially in complex cases. Despite some limitations, the synergy between surgical navigation and tracked instruments offers a promising approach in maxillofacial surgery, expanding its applications beyond current practices.
1. Introduction
Surgical navigation can be considered an evolution of stereotaxic surgery. One of the primary limitations of the earlier procedure was its failure to account for variability among individual patients and presence of pathological tissues [1]. Only in the 1980s, with the arrival of CT and MRI technologies, it became possible to provide more precise and patient-specific data [2].
The introduction and implementation of modern techniques, such as software systems for planning, rapid prototyping, intraoperative visualisation and surgical navigation, has revolutionized the preoperative approach to surgical procedures in maxillofacial surgery [3,4,5].
Surgical navigation was firstly introduced in oral and maxillofacial surgery in the early 1990s, increasingly assuming a more prominent role in medical practices within maxillofacial surgery units. This procedure’s advantages are particularly significant in areas associated with challenging and limited exposure. The three-dimensional complexity of facial anatomy needs accurate three-dimensional reconstruction to ensure functional recovery, favourable aesthetic outcomes, and a reduction in the need for repeated procedures [6].
Nowadays, navigation technology plays a crucial role in oral and maxillofacial surgery, with its predominant application in traumatology and also in procedures such as tumour resection and reconstruction, craniomaxillofacial malformation correction, implantology, orthognathic surgery, TMJ arthroplasty, and the removal of supernumerary teeth or foreign bodies [3,7,8,9].
Surgical navigation is particularly valuable in post-traumatic orbital wall reconstruction, representing the primary field of application in maxillofacial surgery. Novelli et al. (2014) emphasise the benefits of adopting this protocol during the pre-surgical phase, which enables a more precise diagnosis and a better understanding of facial defect reconstruction. Moreover, during surgery, it is helpful to verify whether the reconstruction matches with the pre-surgical planning, reducing operating time and standardizing the approach to orbital fracture treatment, thereby ensuring reproducibility [4].
Surgical navigation is also a valuable technique for the accurate reconstruction of the mid-third of the face, which is characterised by non-motile bones. However, its application in the jaw is limited by bone movements that reduce its accuracy [7,10,11,12]. Hirch et al. (2009) and Zhang et al. (2016) observed that real-time guidance offered by surgical navigation in mandibular reconstruction with vascularized fibula flap reduces the margin of error compared to free-hand techniques, decreases surgery duration, and demonstrates high accuracy and applicability [7,13,14]. Sozzi et al. (2022) described in their protocol how the positions of the screw holes for the reconstruction plate are pre-recorded on a 3D model of the patient’s jaw. Subsequently, before performing the resection, the screw holes are created using a recorded drill, whose bite position can be tracked in real-time on CT images to ensure the precise placement of the screws as per the planned location. Nevertheless, they show that this method requires a substantial amount of pre-surgical setup time and a steep learning curve for the surgeon [15].
The craniomaxillofacial area is characterized by numerous delicate vascular and nervous structures that must be preserved during surgery. The widespread use of piezoelectric tools has significantly advanced oral and maxillofacial surgery. In 2000, Vercellotti introduced piezoelectric surgery and developed an angulated, thin, and tapered cutting saw tip that is now widely used in surgical procedures [16]. Piezoelectric surgery offers several advantages, including the prevention or reduction of soft tissue injuries during osteotomies, minimal blood loss, a less invasive approach, and reduced postoperative pain [17]. However, the use of piezosurgical instruments requires a wide open field for precise visualisation of the instrument’s tip position. Bianchi et al. (2015) sought to combine the safety of piezosurgical instruments with the precise three-dimensional tip localisation offered by surgical navigation, enabling not only sequential or indirect navigation, but also direct and continuous navigation with the piezoelectric device [18].
With our department’s extensive 15-year experience in surgical navigation, coupled with the findings from the aforementioned studies, we were strongly inclined to believe that the use of tracked tools in maxillofacial surgery has the potential for broader applications.
According to the existing literature, there are limited publications exploring the possibilities of new tracking tools in oral and craniomaxillofacial surgery.
This study aims to showcase the clinical applications and indications of intraoperative tracking of various tools, such as drills, cutters, saws, chisels, and piezoelectric instruments, and to evaluate the accuracy of tool tracking in millimetres of error. Additionally, we plan to assess the advantages, disadvantages, potential errors, and complications associated with this surgical protocol.
2. Materials and Methods
2.1. Sample
The sample of this study was derived from a pool of cases of craniofacial and oromaxillofacial surgery that underwent operative procedures with the assistance of surgical navigation over a period of 15 years.
The inclusion criteria encompassed the following: we considered from the sample of patients only those cases that underwent surgery under the control of surgical navigation, in which surgical instruments were tracked to enhance the precision when deemed necessary.
A total of 42 patients were included in the study, presenting the following pathologies: 1 case of facial dysmorphism, 24 cases of oncologic surgery, 12 cases of traumatology, and 5 cases of craniofacial malformations. The tracked instruments used were the periosteal elevator, the mini-saw, the chisel, the drill and the piezoelectric device. The surgical treatments and the disease features are illustrated in Table 1.

Table 1.
Characteristics of the cases operated using navigation surgery and tracking instruments technique.
All cases were treated at the Maxillofacial Surgery Unit, Fondazione IRCCS San Gerardo dei Tintori—Monza (Italy). The surgical procedures were performed by various surgeons belonging to the department’s team, each possessing established skills in maxillofacial surgery.
2.2. Surgical Navigation Protocol
To perform the surgical navigation, an upper dental resin bite is created. It is crucial that the bite fits one position only. At least 5 screws with different space vectors are then positioned on it, serving as fiducial markers. In some cases, an osseous screw is placed in the fronto-zygomatic region to enhance precision, and it is removed after surgery. When mandibular navigation is necessary since it is a mobile bone, a surgical bite that immobilises the lower jaw through rigid intermaxillary fixation is then created. This enables us to perform the procedure in the theatre with the mandible in the same position as measured during the preoperative CT scan. A high-definition CT scan, with a thickness of 0.6 mm, is carried out with the maxillary bite. Virtual planning is then performed using iPlan 3.0 software by Brainlab®. First, the fiducial markers are identified. Subsequently, the resection of the lesion is designed, along with the tracing of any necessary osteotomies or the positioning points for anchoring osteosynthesis plates. Once these procedures are completed, the surgery can be performed.
In the operating room, the following steps are undertaken during surgery. Firstly, the dynamic reference frame (DRF) is positioned for navigation in the contralateral parietal region. Next, the maxillary bite is positioned, and the previously marked points are registered. In order to track the tools, it was necessary to place the navigator’s reflective spheres on them.
In the case of performing Virtual Surgical Simulation (VSS), a 3D model based on a CT scan can be realised and used for pre-surgical navigation. Virtual planning is carried out using iPlan 3.0 software by Brainlab®, where well-defined anatomical markers such as the anterior nasal spine and the infraorbital foramen, among others, are positioned. Subsequently, navigated surgery is performed on the 3D model after the placement of the DRF and the registration of the fiducial markers. Once the surgery is completed, the reference points for screw/plate placement or osteotomy tracing are stored in the system for later use in the operating room.
Surgical navigation workflow.
- Maxillary bite realization.
- Definition of virtual fiducial markers.
- Virtual Planning:
- (a)
- Fiducial markers’ identification.
- (b)
- Osteotomies’ tracing/Resection margins’ definition/Mapping of the position of osteosynthesis tools.
- Virtual Surgical Simulation (VSS).
- Operating Room:
- (a)
- Placement of Dynamic Reference Frame (DRF).
- (b)
- Registration of Reference Points (error < 1.5 mm).
- (c)
- Tracked tools’ registration.
2.3. Accuracy Evaluation
We established a baseline with a maximum accuracy error of <2 mm, in line with literature standards for accuracy. This reference value ensures registration accuracy, a crucial aspect in maxillofacial surgery, achieved using dynamic reference frames (DRF) and stable maxillary anchorage markers, occasionally supplemented by mid-third anchorage markers [19].
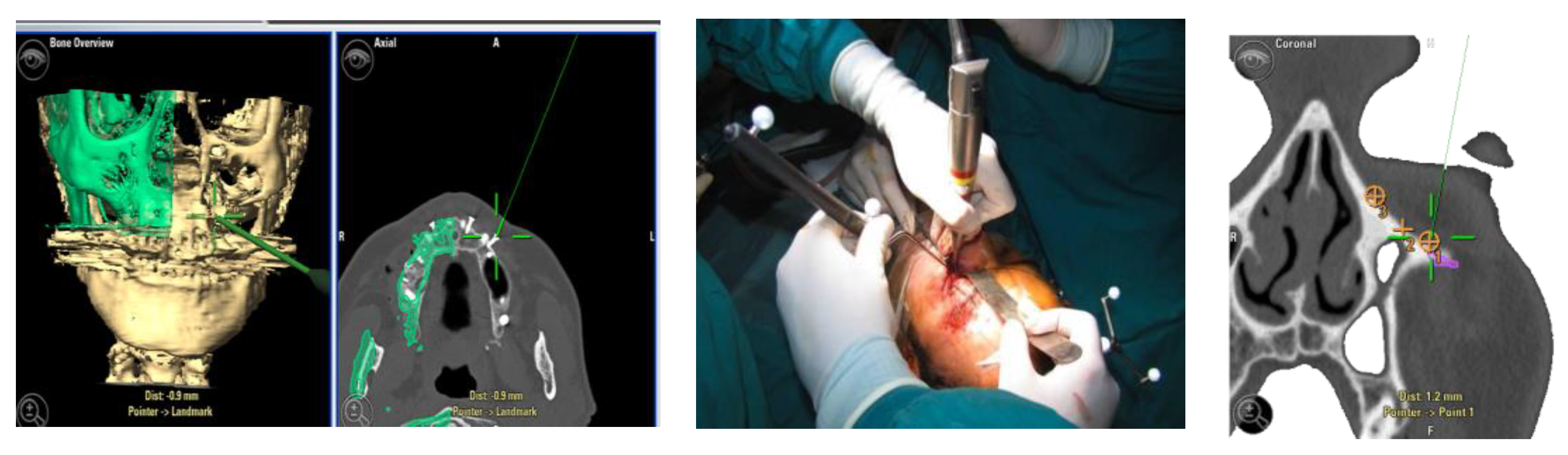
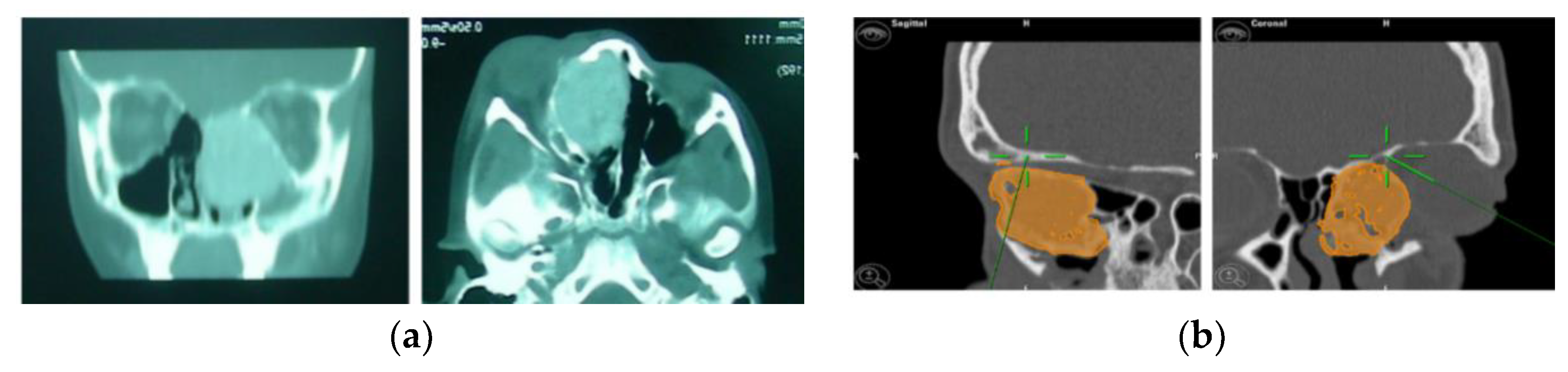
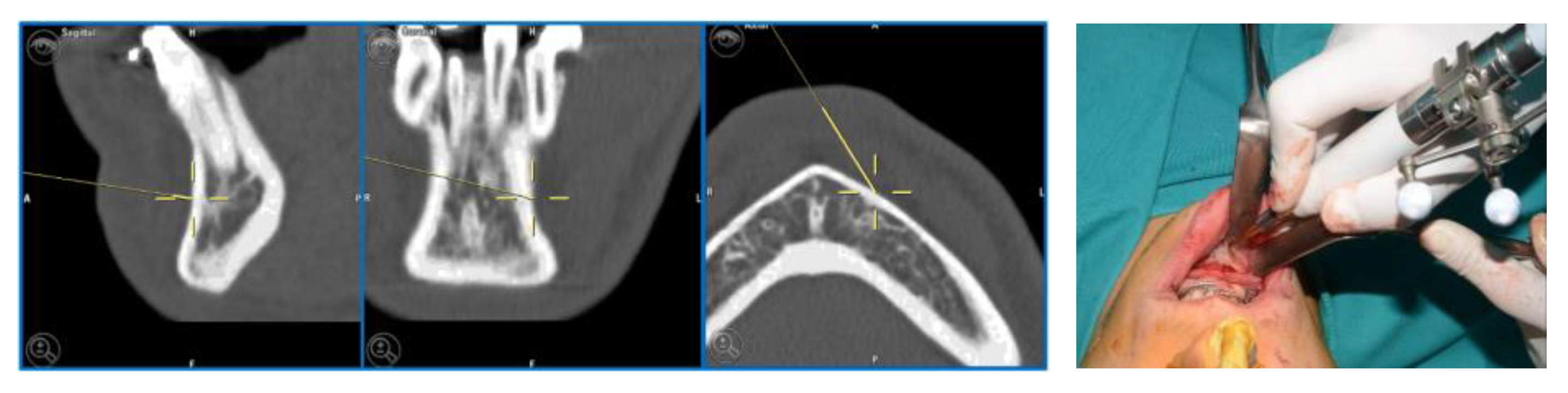
For greater precision, we deemed an error of less than 1.5 mm as acceptable. Accuracy was measured using fiducial markers essential for the tracking process, along with anatomical targets defined during it (Figure 1). To simplify the interpretation of data, we identified the average values detected among different points in individual patients.
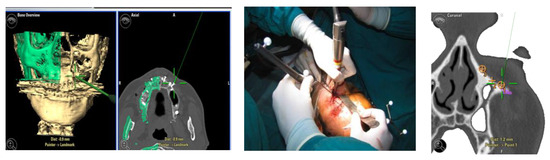
Figure 1.
Example of accuracy control on fiducial markers and preoperative targets.
We showcase four representative cases that illustrate our workflow and demonstrate the capabilities of surgical navigation using tracked surgical tools.
3. CASE 1: Right Coronoid Hyperplasia with Mouth Opening Deficit
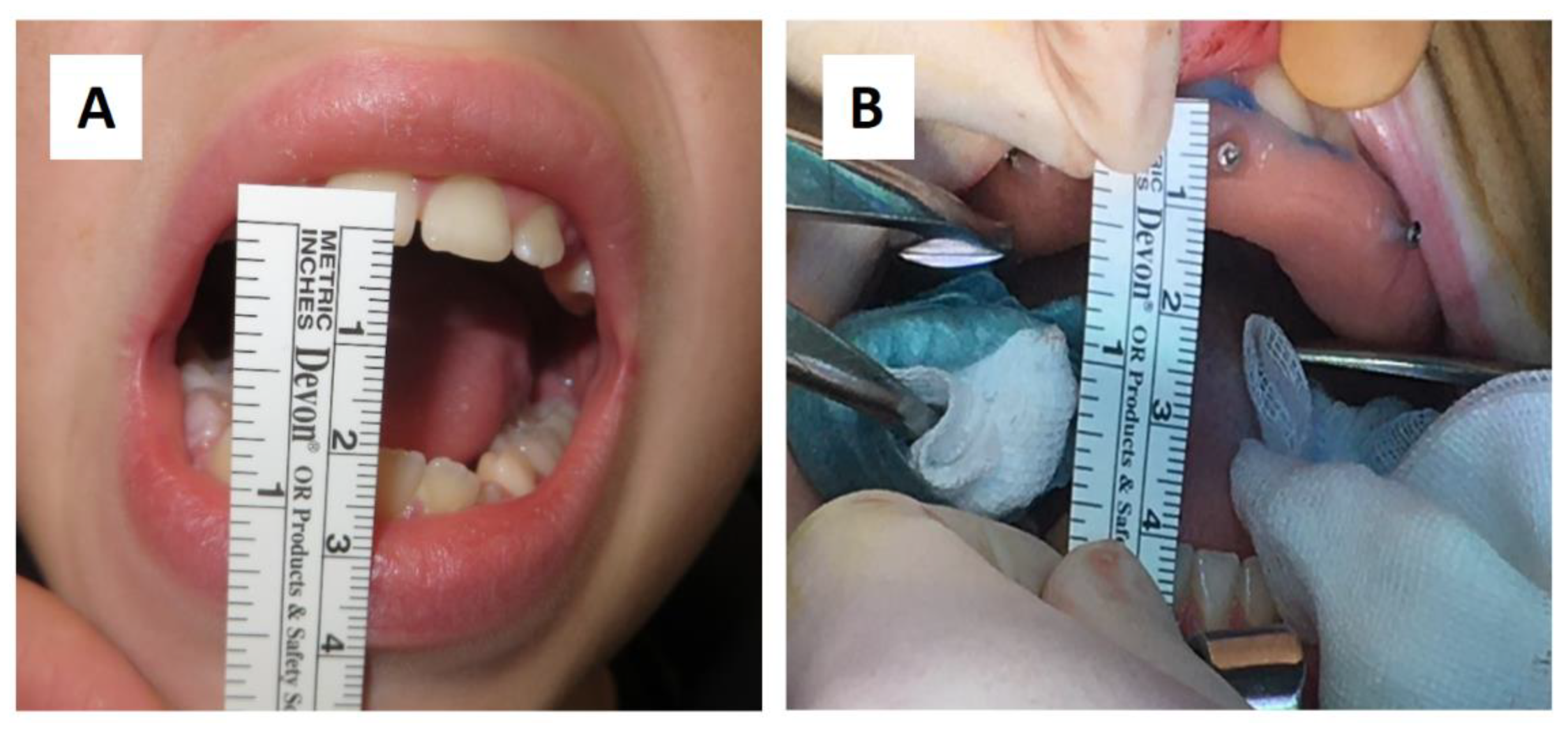
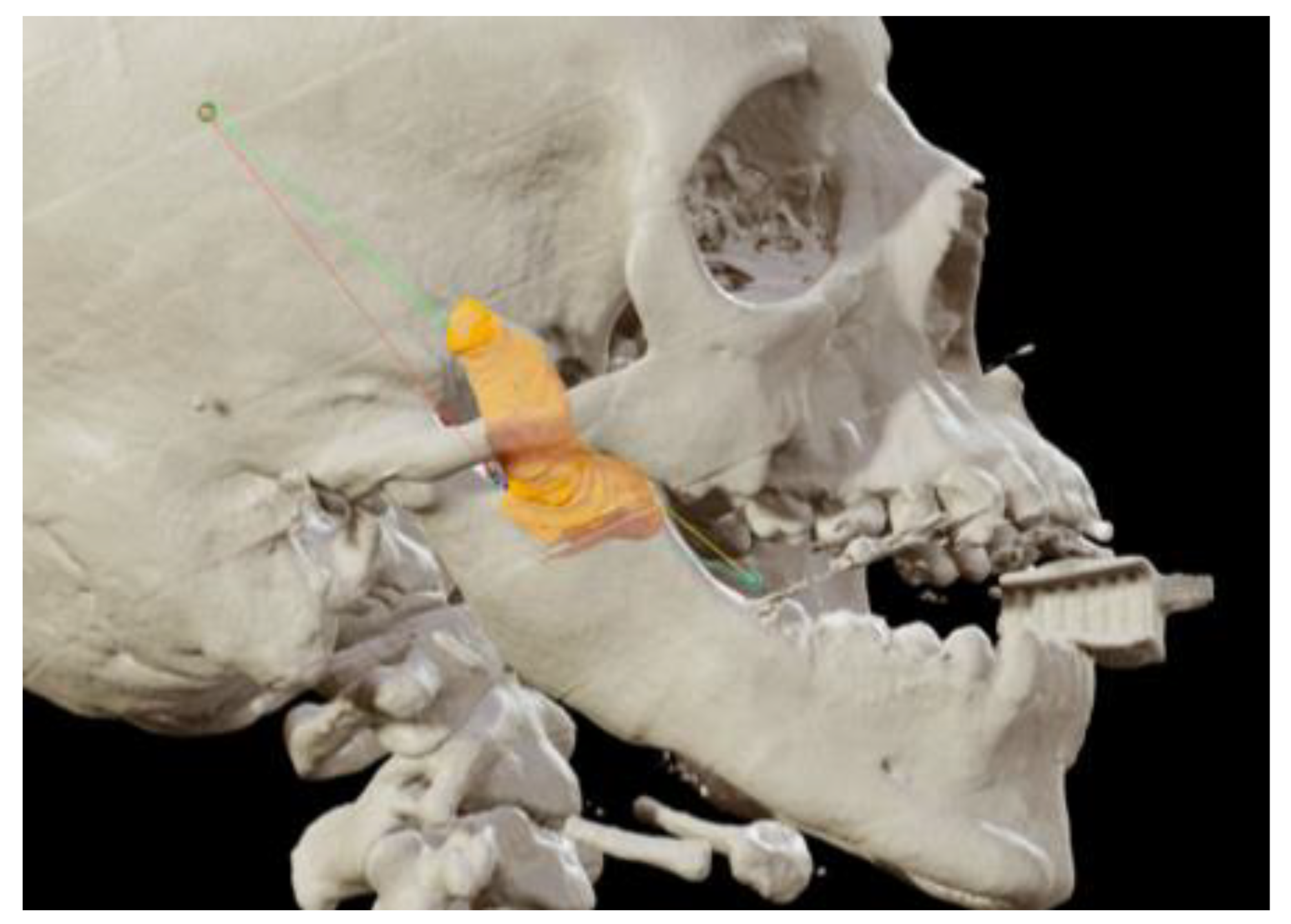
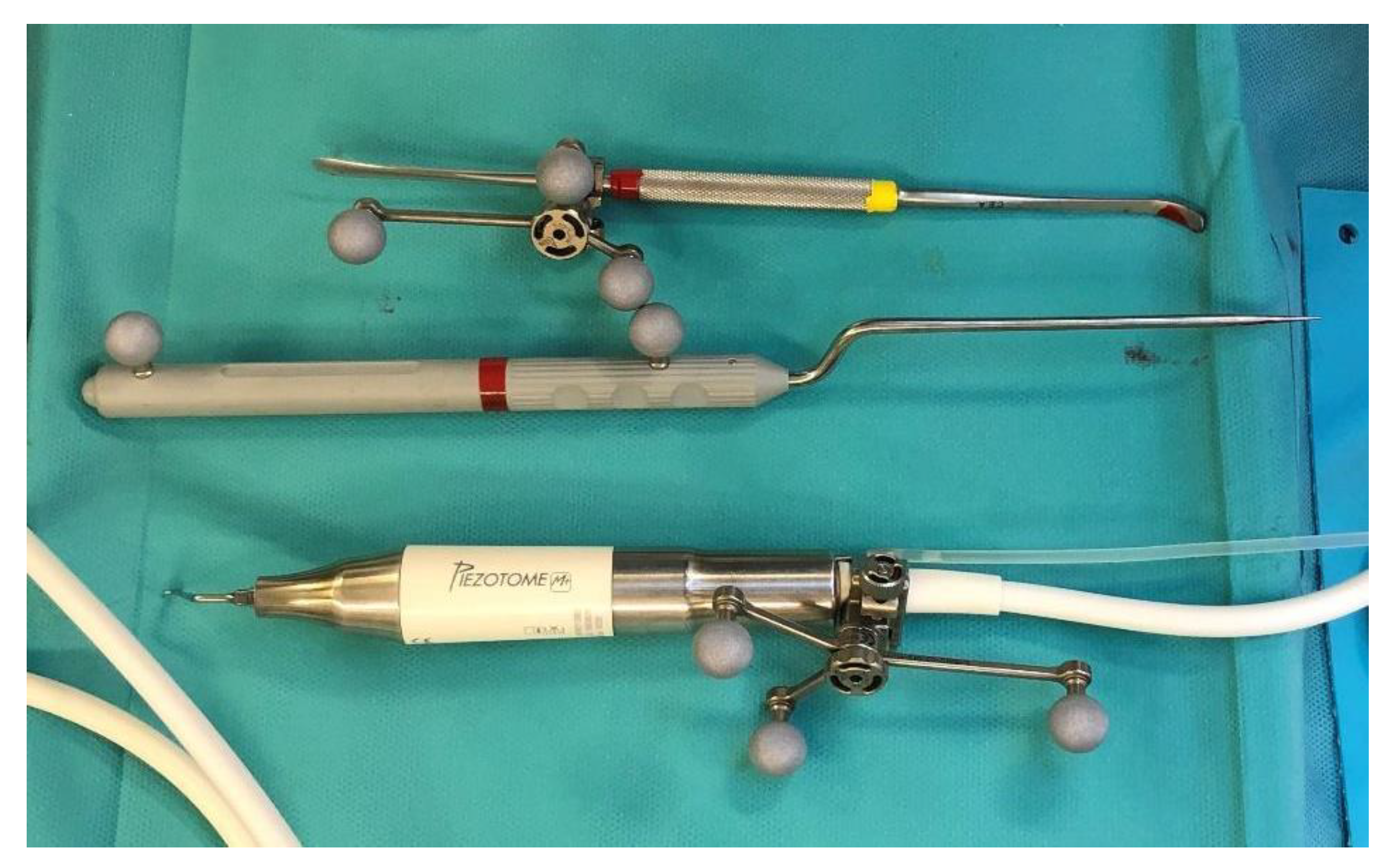
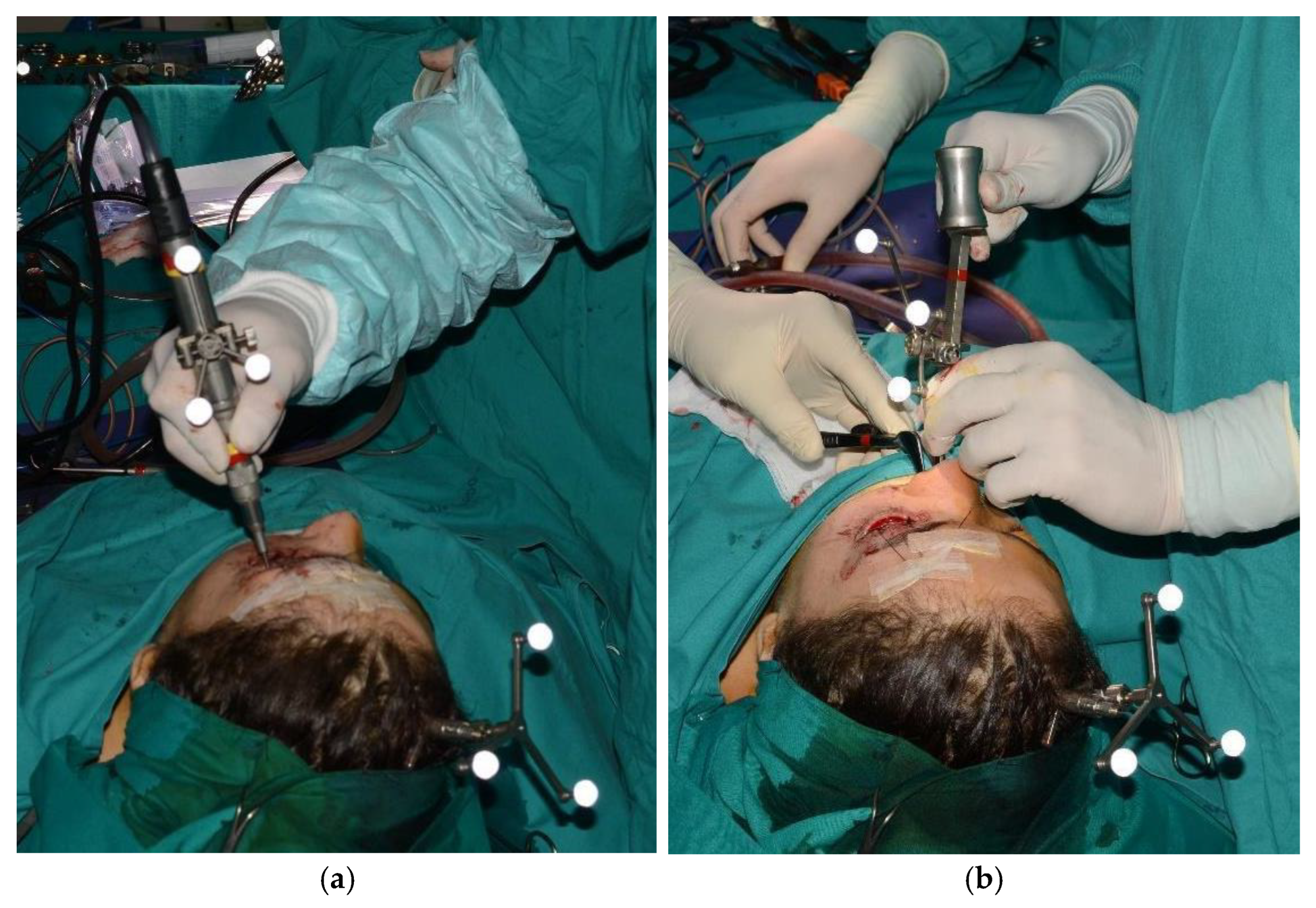
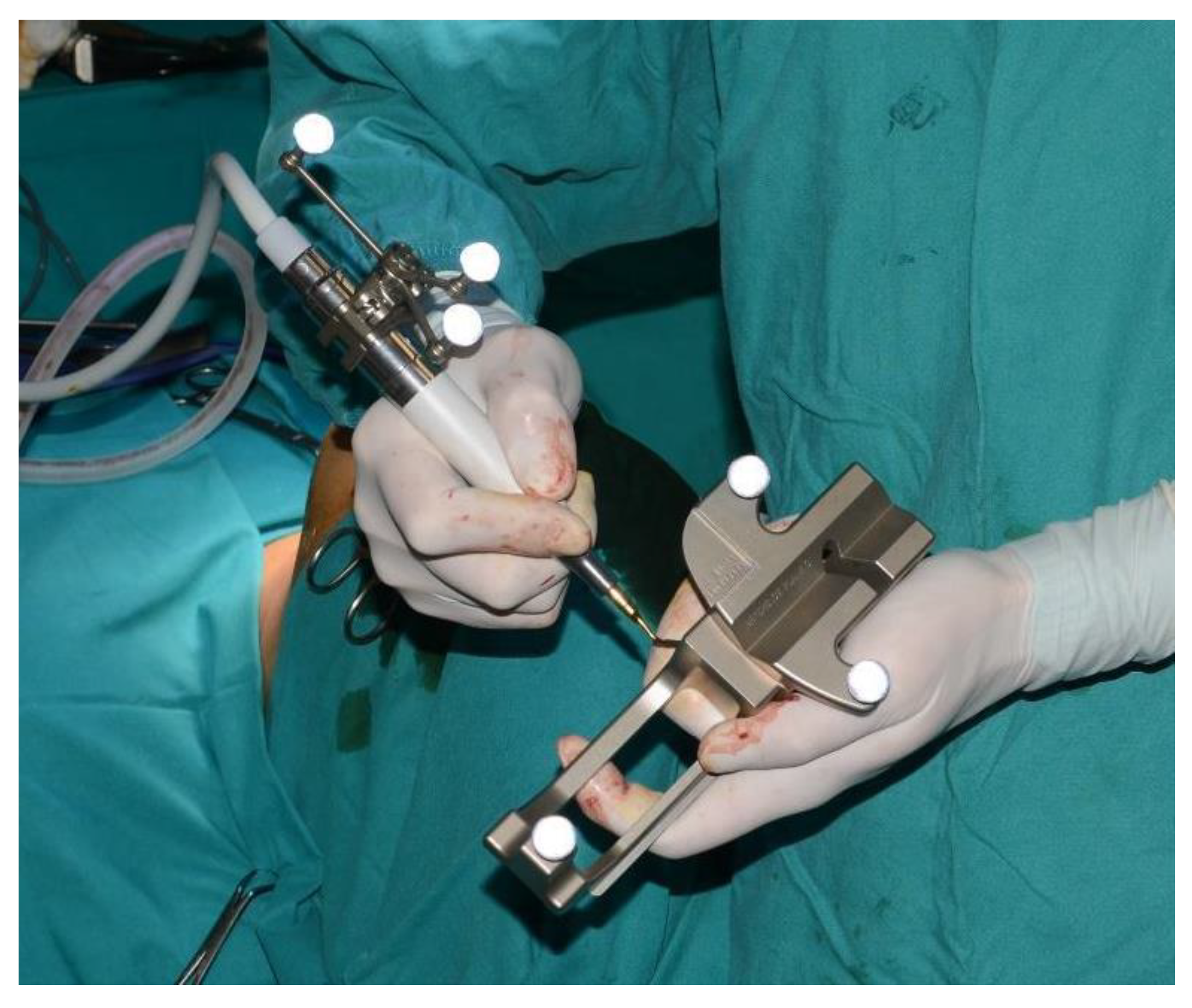
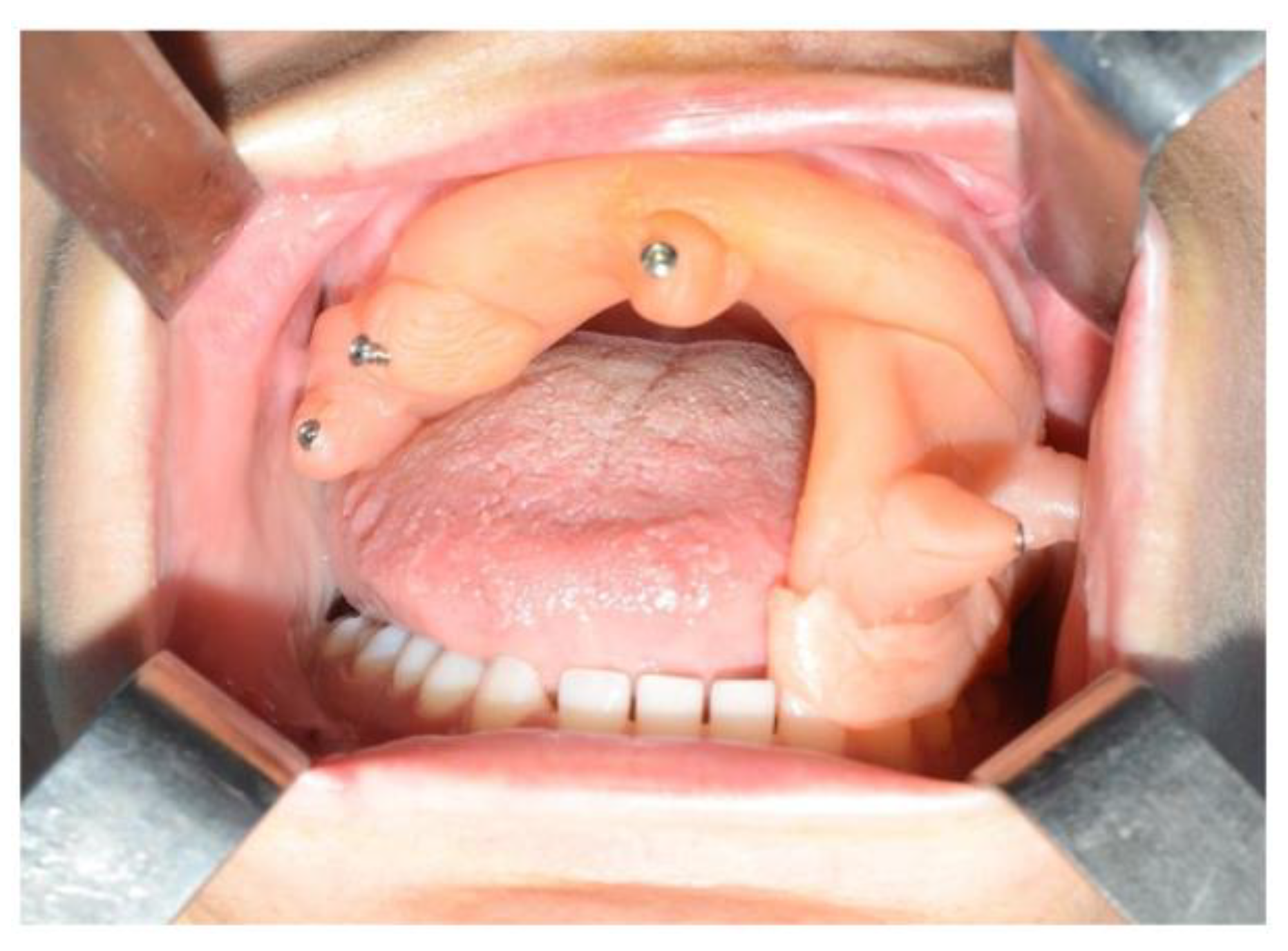
A sixteen-year-old male patient exhibited dento-facial asymmetry due to hypoplasia in the right mandibular region and hyperplasia of the right coronoid. The patient had previously undergone a coronoidectomy at another facility seven years earlier, which subsequently led to restricted mouth opening (17 mm) (Figure 2a). A thick occlusal bite at maximal mouth opening was created, providing enough space for the surgical procedure and maintaining the jaw in the same position during the preoperative CT acquisition. This bite was employed to maintain the mandible in a consistent position both during the preoperative CT scan acquisition and throughout the entire surgical procedure The surgery was meticulously planned by virtually tracing the coronoid ostectomy on a CT scan. The operation was performed under general anaesthesia, utilising the navigation system. The DRF system was positioned in the left parietal region, ensuring a navigation accuracy of <0.5 mm. An incision was executed from the right inferior vestibular fornix to the right superior vestibular fornix, enabling the visualisation of the base of the coronoid process. Using surgical navigation, the sigmoid notch was precisely located, and the planned osteotomy (Figure 3) was performed in real-time, tracking the position and the direction of the periosteal elevator and piezoelectric tool (Figure 4). The piezoelectric tool and the periosteal elevator were detected by attaching the tracking tool with three reflecting spheres to the handpiece, while the tip of the piezoelectric tool and the edge of the periosteal elevator were marked and utilised as a navigation reference point. Following the procedure, the oral cavity demonstrated a notable increase in opening (36 mm) (Figure 2b).
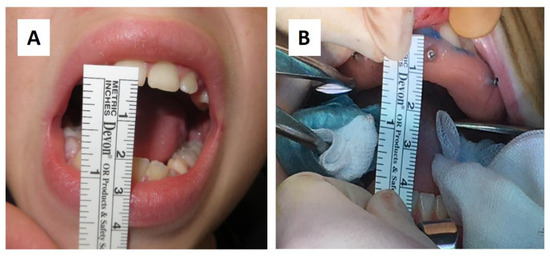
Figure 2.
(A) Preoperative (17 mm) and (B) intraoperative (36 mm) mouth opening.
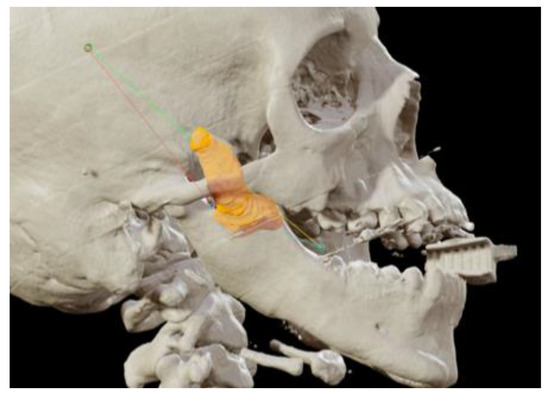
Figure 3.
Coronoidectomy (yellow area) and preoperative planned osteotomy lines.
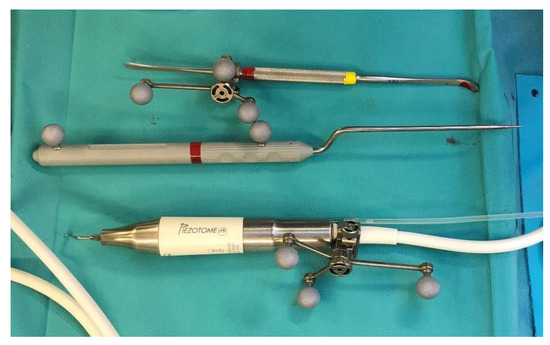
Figure 4.
Tracked tools used during surgery.
4. CASE 2: Nose-Orbital-Ethmoidal-Frontal Ossifying Fibroma Resection
A 20-year-old female patient came to us due to the persistence of a neoplasm in the left naso-ethmoid-frontal-orbital region. At the age of 7, she reported the development of a left nasal neoplasm, with a histological biopsy confirming a diagnosis of fibrous dysplasia. She had previously undergone multiple surgical procedures across different surgical centres to remove the neoplasm. A re-evaluation of the histological slides was conducted, which yielded a diagnosis consistent with ossifying fibroma.
A preoperative CT scan was performed, and the resection margins of the neoplasm were prototyped with the assistance of the iPlan 3.0 software by Brainlab (Figure 5). The operation was performed under general anaesthesia, utilising the navigation system. The DRF system was positioned in the right parietal region, ensuring a navigation accuracy of <0.5 mm. The surgical intervention was performed with a left transconjunctival retrocaruncular approach. The resection was performed using a “piecemeal” technique with a tracked chisel and a mini-saw, while the resection margins were monitored under the guidance of surgical navigation (Figure 6). The instruments were registered by anchoring the three reflecting spheres of the tracking tool to both its handpiece and the edge of the chisel, while the marked tip of the mini-saw served as a reference for navigation. The reconstruction of the medial wall and the left orbit floor was made with a pre-moulded titanium mesh (Figure 7).
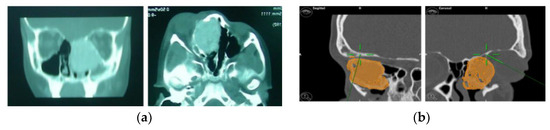
Figure 5.
(a) Preoperative CT scan showing the tumour. (b) Virtual planning of resection margins.
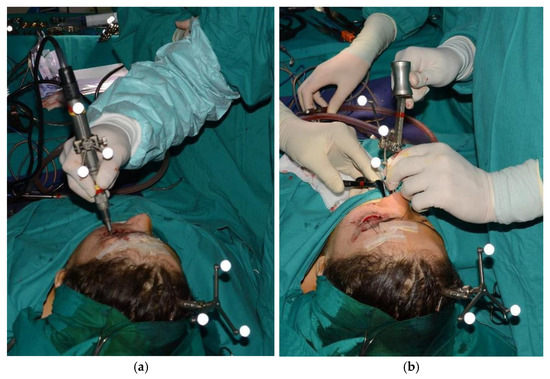
Figure 6.
Tracked mini-saw (a) and chisel (b).
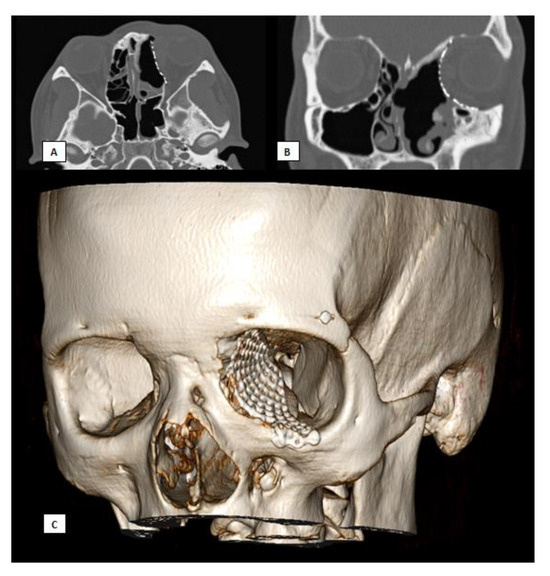
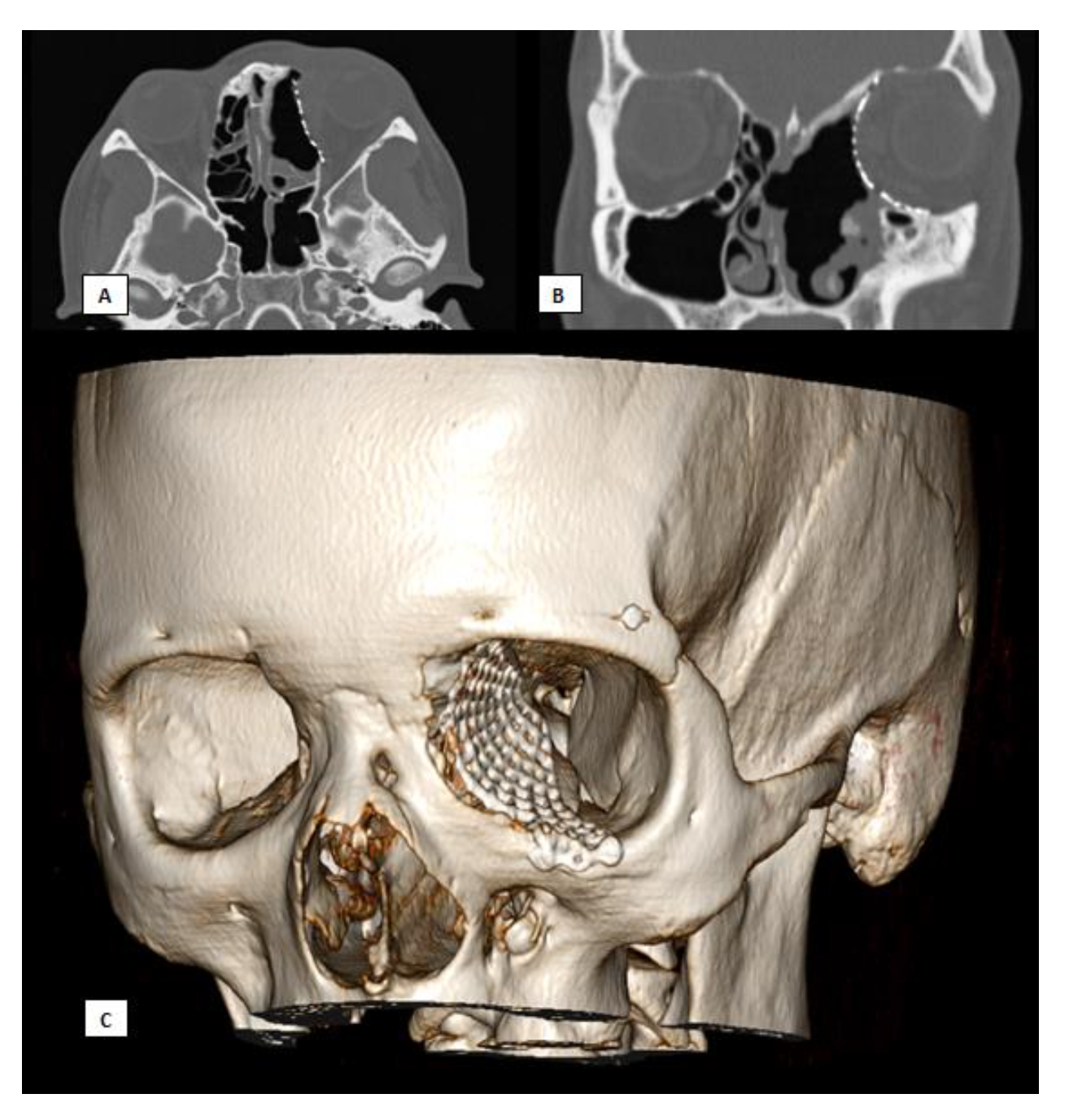
Figure 7.
Postoperative CT scan. (A) Axial view, (B) Coronal view, (C) 3D.
Subsequent follow-up visits over a long-term period of nine years demonstrated excellent aesthetic results, the preservation of extrinsic eye movements, and no neoplasm recurrence.
5. CASE 3: Subapical (Köle) Osteotomy in Dento-Facial Deformity
A 23-year-old patient exhibited a class III dentoskeletal malocclusion, as well as transverse and sagittal hypoplasia of the upper maxilla. Moreover, the patient presented an inclination of the teeth in the anterior mandibular sector that could not be orthodontically treated. Consequently, the following treatment plan was formulated:
- First step: SARPE (Surgical Assisted Rapid Palatal Expansion) and subapical (Köle) mandibular osteotomy, followed by subsequent distraction osteogenesis.
- Second step: LeFort I maxillary osteotomy segmented into two pieces.
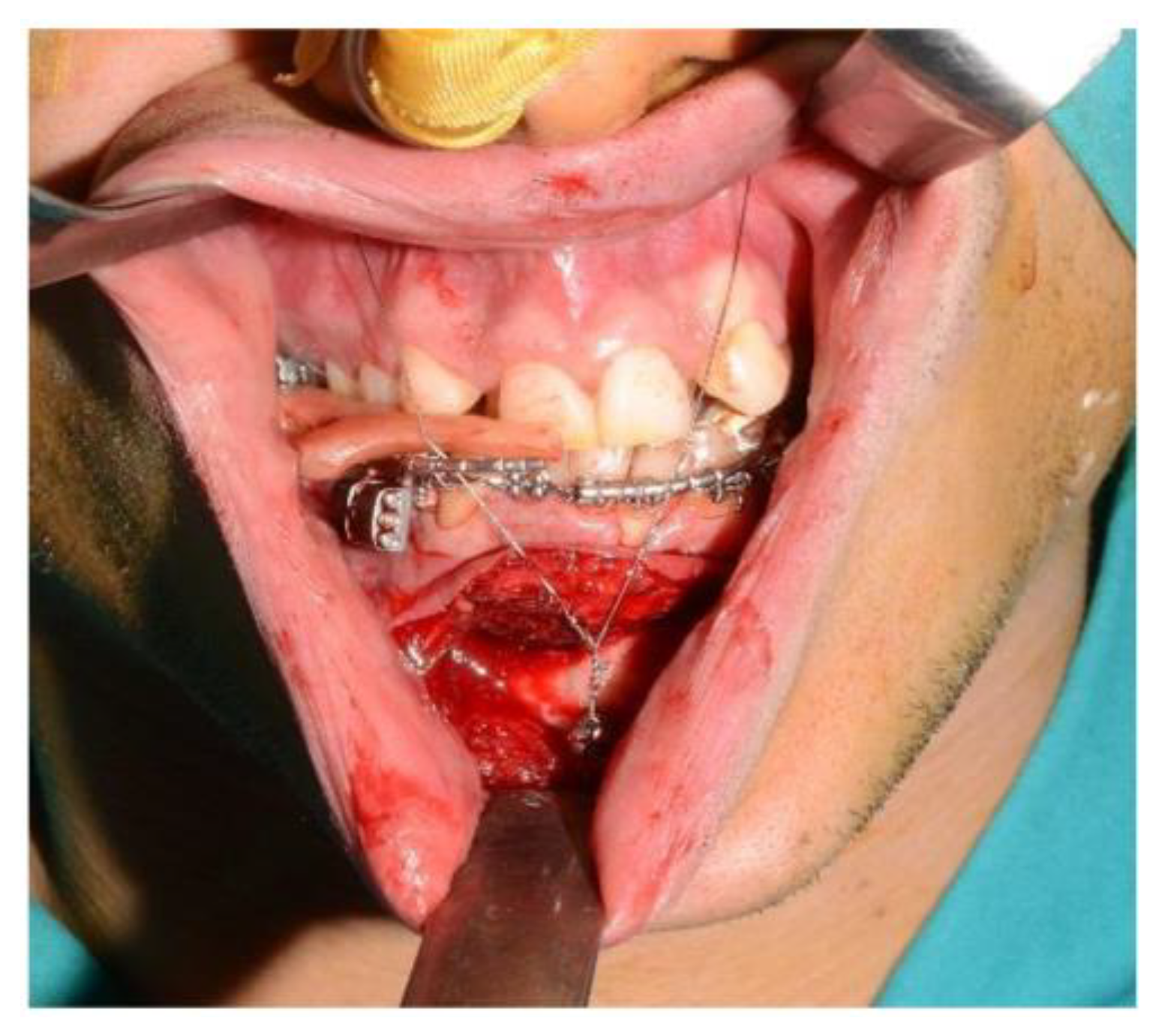
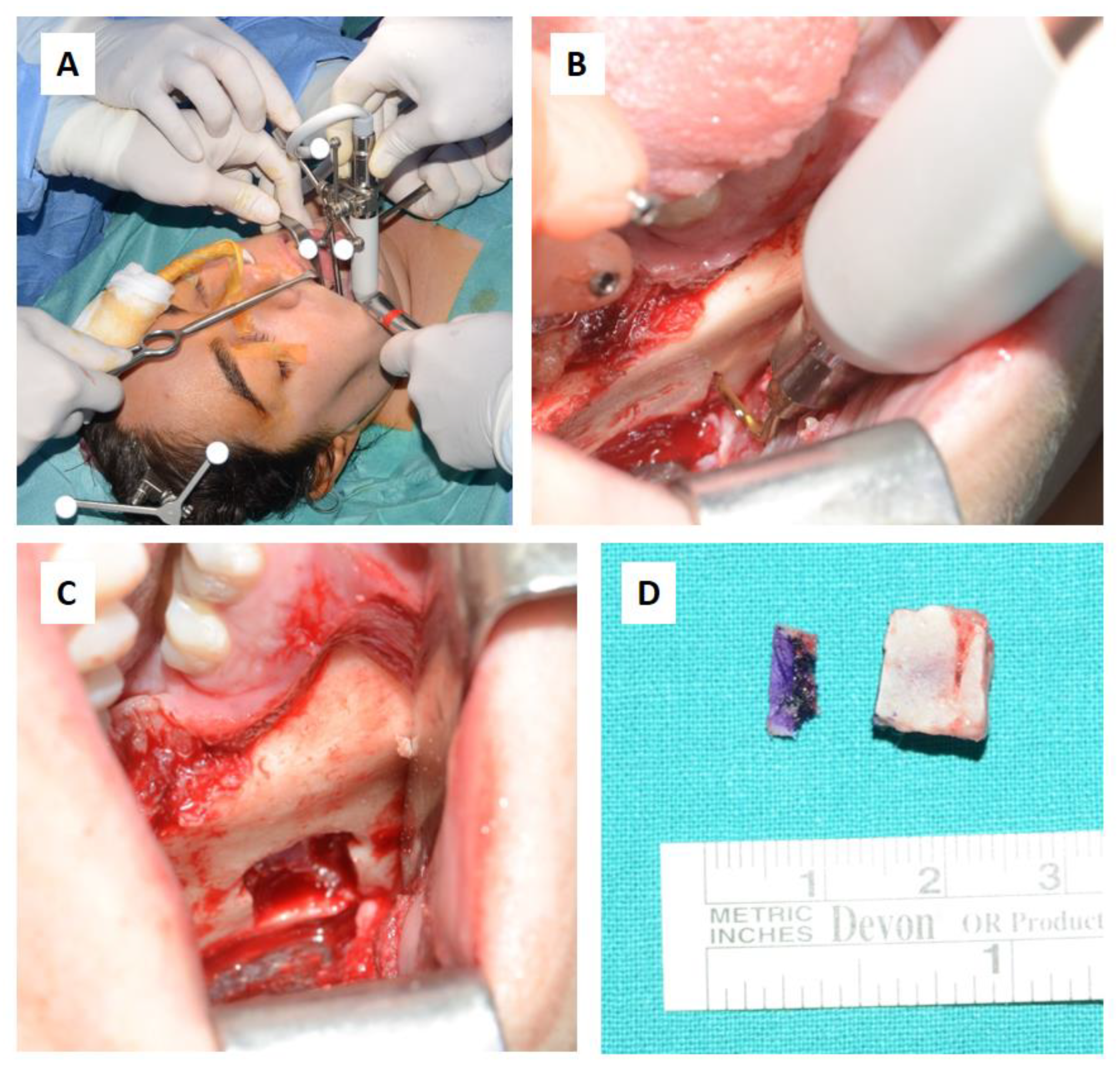
For the subapical osteotomy, it was decided to use the tracked tool technique with a piezoelectric scalpel to perform the procedure and avoid the risk of dental root injury, thereby enhancing surgical precision. A maxillary bite was created, and 5 screws with different space vectors were then placed on it, serving as fiducial markers. The bite was used to ensure that, during the entire surgical procedure, the mandible maintained the same position as in the preoperative CT scan. Virtual planning was performed with careful consideration of dental root preservation in the osteotomy tracing. During the surgical procedure, the Dynamic Reference Frame (DRF) was positioned in the left parietal bone, and the screws located on the bite were used as fiducial markers. The same bite used during the acquisition of CT scan images was used. Two screws were secured onto the maxillary bone, with an additional one in the mandibular symphyseal region. Simultaneously, an intermaxillary fixation was used to replicate as accurately as possible the mandible’s position maintained during the CT scan image acquisition (Figure 8).
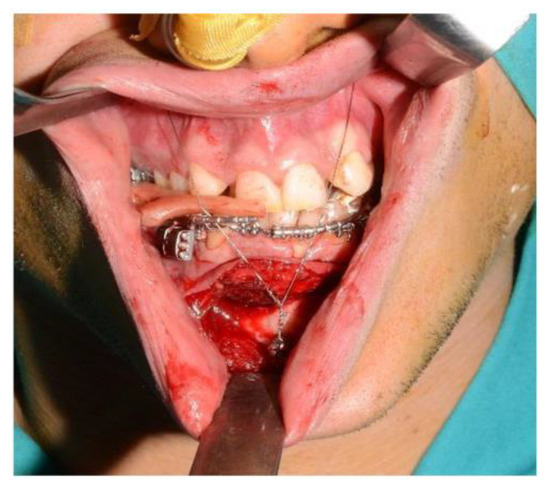
Figure 8.
Intraoperative placement of the bite and setup of the intermaxillary block.
The piezoelectric tool was registered by anchoring the three reflective spheres to the tool’s handpiece. At the same time, the tip of the piezoelectric device was marked and used as a reference for navigation (Figure 9).
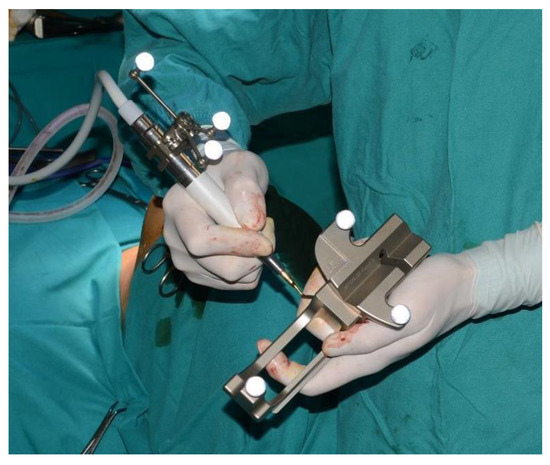
Figure 9.
Registration of the piezoelectric tip and connecting it to the navigator with a calibration matrix.
The navigation accuracy was less than 1 mm. The subapical osteotomy was performed as previously planned (Figure 10).
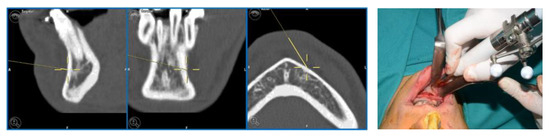
Figure 10.
Surgical navigation and Subapical osteotomy (Köle) with tracked piezoelectric device.
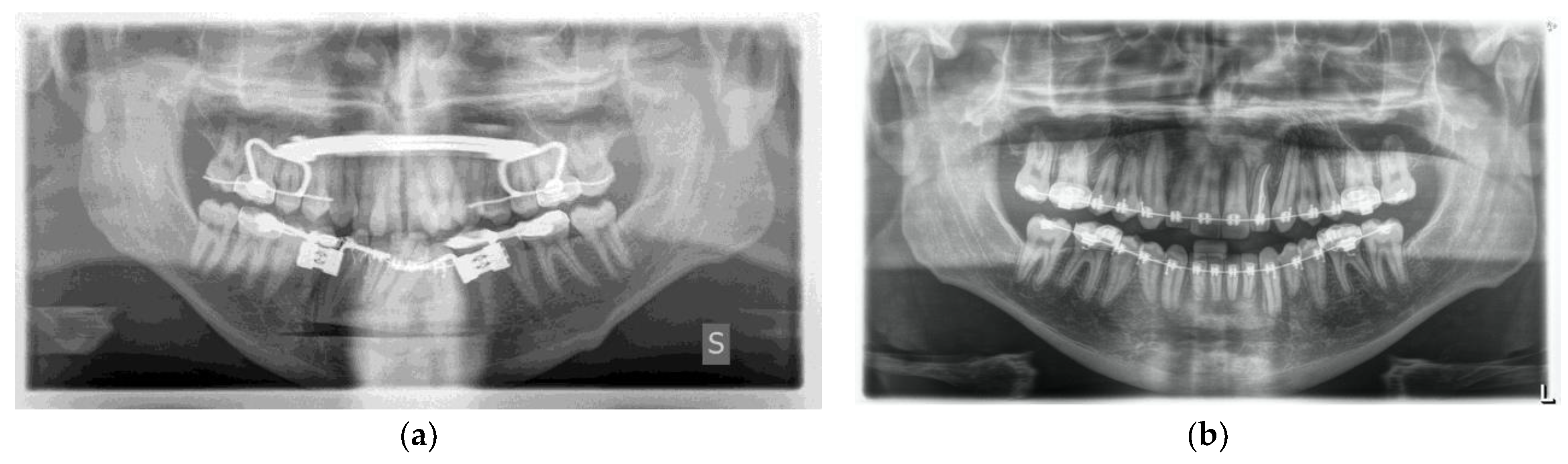
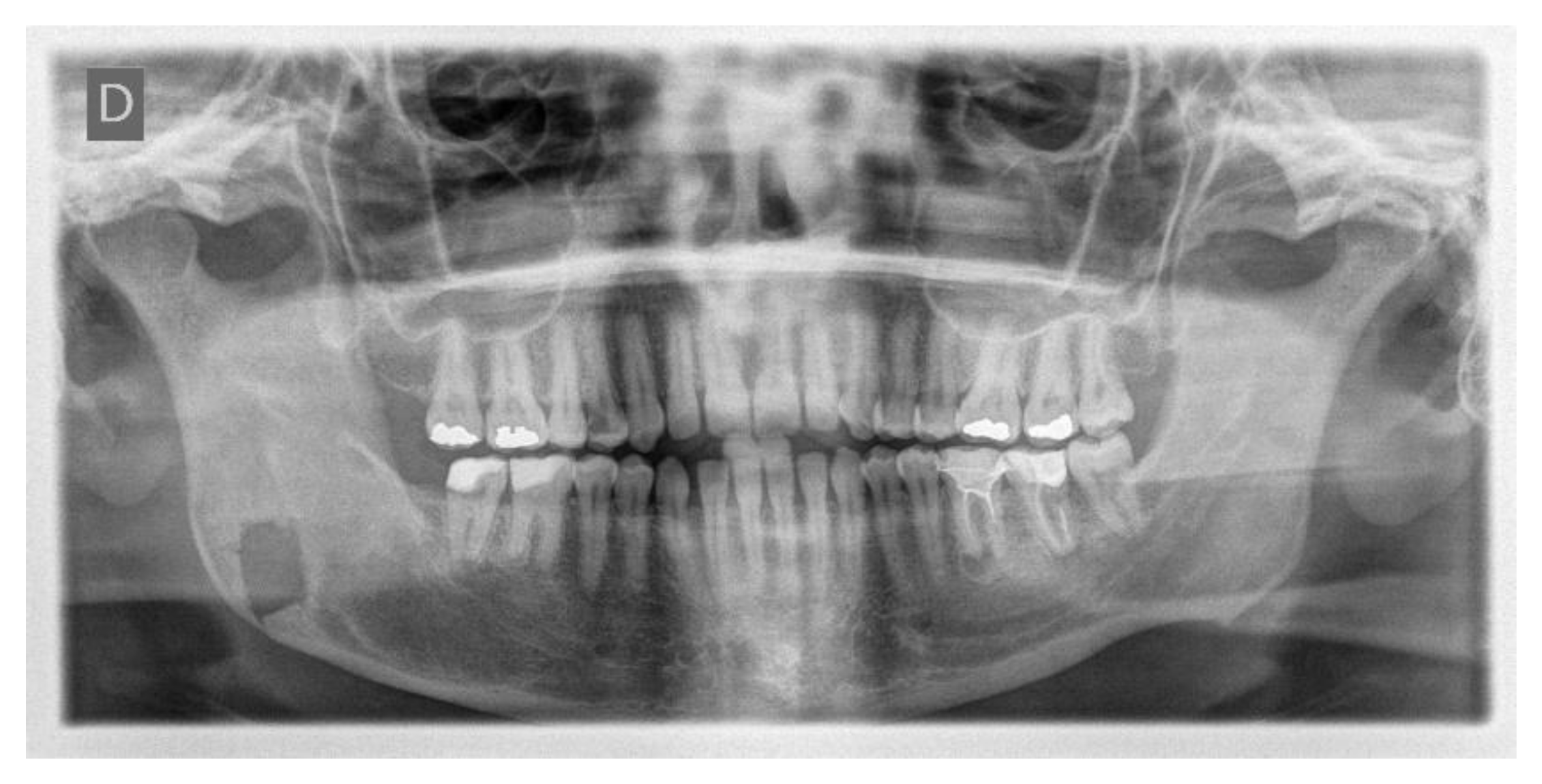
One week post-op, an OPT (Orthopantomography) X-ray was performed, which showed the adequacy of the osteotomy and the preservation of dental roots. Three years later, at the end of orthodontic alignment, the second surgical step was carried out (Figure 11a,b).
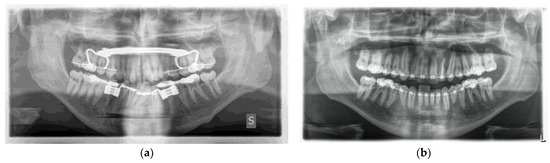
Figure 11.
(a) Postoperative Orthopantomography X-ray; (b) post orthodontic alignment.
6. Case 4: Removal of Recurrent Ameloblastoma at the Mandibular Angle
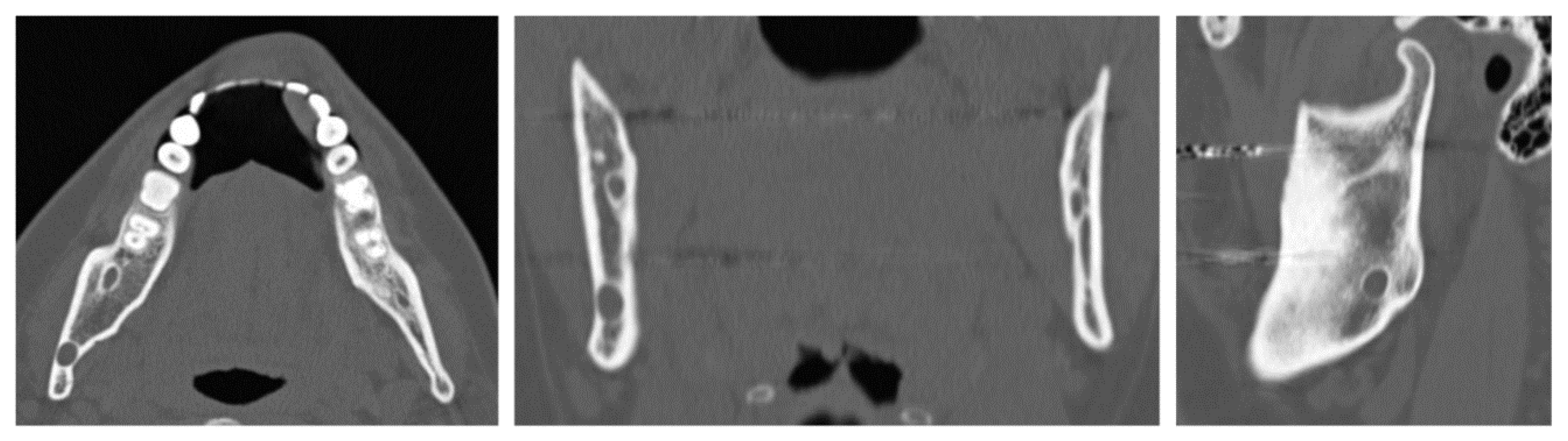
A 32-year-old patient came to our attention during an oncological follow-up, which included the execution of an OPT (Orthopantomography) X-ray, revealing a new osteolytic lesion at the right mandibular angle. In 1996, the patient underwent surgical removal of a unicystic ameloblastoma in a different hospital. We reviewed the patient’s most recent radiological documentation, which was an OPT X-ray taken 12 years earlier (8 years after the initial surgery) showing no mandibular lesions. A mandibular CT scan was requested to better define the lesion (Figure 12).
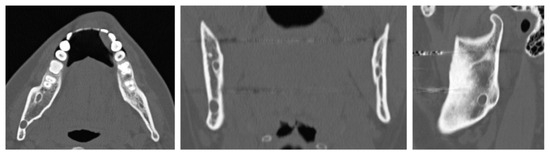
Figure 12.
Evidence on the mandibular CT scan of the appearance of a new osteolytic lesion, visible on axial, coronal, and sagittal views.
In light of the suspicion of an ameloblastoma recurrence, surgical removal was recommended, opting for the use of tracked instruments with surgical navigation. A maxillary bite was created, followed by the attachment of 5 screws with varying spatial orientations that would function as fiducial markers. This bite was employed to maintain the mandible in a consistent position both during the preoperative CT scan acquisition and throughout the entire surgical procedure (Figure 13).
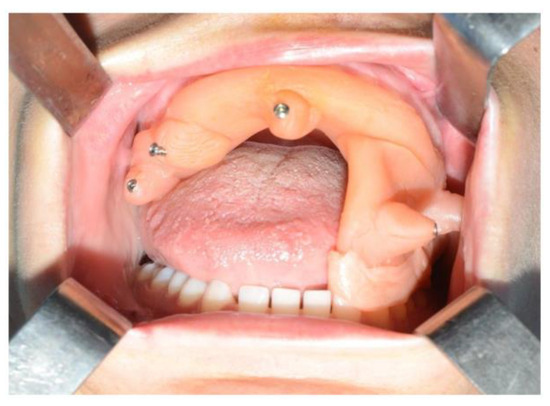
Figure 13.
Intraoperative placement of the same bite used for acquiring mandibular CT scan images. The bite ensures the mandible remains in the same position as during the virtual surgical planning.
During the surgical procedure, the Dynamic Reference Frame (DRF) was positioned in the right parietal bone, and the positions of the screws on the bite were recorded as fiducial markers. The same bite used during the CT image acquisition was utilised for this purpose. The navigation accuracy was less than 0.5 mm. The piezoelectric was registered by attaching a tracking tool with three reflective spheres to the handpiece, and the tip of the piezoelectric was marked and utilised as a reference point for navigation. The navigation accuracy was found to be less than 1 mm.
Under the guidance of the tracked piezoelectric device, the resection of the lesion was carried out as per the preoperative plan, with macroscopically preserved healthy bone margins, including the lower and posterior mandibular border (Figure 14).
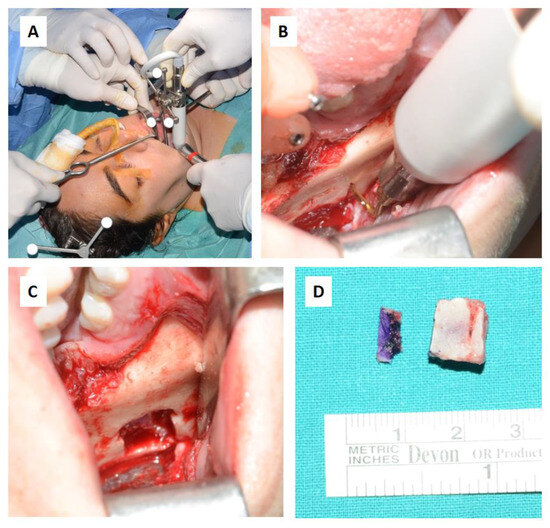
Figure 14.
Surgical steps: (A,B) Resection of the intraosseous osteolytic lesion under the guidance of the tracked piezoelectric device. (C) Complete removal of the mandibular segment containing the osteolytic lesion with macroscopically healthy bone margins preserved, including the lower and posterior mandibular border. (D) The excised surgical resection measured 10 × 10 × 7 mm.
The day following the surgery, a follow-up OPT X-ray was requested, which displayed the resection area and the integrity of the lower and upper borders of the mandible, in accordance with the preoperative virtual planning (Figure 15).
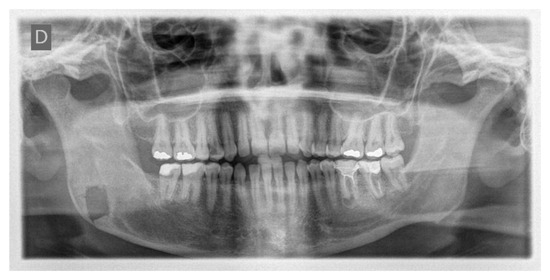
Figure 15.
Postoperative OPT X-ray showing the resection area and the integrity of the lower and upper borders of the mandible.
The histological examination of the surgical resection confirmed the diagnosis of a recurrent unicystic ameloblastoma.
To date, the patient undergoes annual consultations in our department with OPT X-rays, with a follow-up duration of 9 years, and no new recurrence has been detected.
7. Results
All procedures were successfully performed with no reported complications related to the improper tracking of instruments. Standard surgical techniques were employed, and the use of triangulation did not result in any modification of the surgical approach or introduce a different level of risk for the patients. Table 2 displays the results in terms of accuracy.

Table 2.
Accuracy of surgical navigation registration and tracked instrument for patient and surgical disease area.
Among all procedures performed with tracked instruments, the most frequently registered instrument was the drill (31 times), followed by the piezoelectric device (7 times), the periosteal elevator (6 times), the mini-saw (2 times), and, lastly, the chisel (1 time). Among the oncology cases, the recorded accuracy was <0.5 mm in 9 instances, <1 mm in 11 instances, and <1.5 mm in 4 instances, with a mean accuracy of >0.89 mm and a standard deviation of 0.36 mm. Within the traumatology cases, the recorded accuracy was <0.5 mm in 6 instances, <1 mm in 5 instances, and <1.5 mm in 1 instance, with a mean accuracy of <0.79 mm and a standard deviation of 0.33 mm. The only procedure performed in the surgical treatment of dento-facial dysmorphism with tracked instruments had a recorded accuracy of <1 mm. In craniofacial malformation cases, the recorded accuracy was <0.5 mm in 2 instances, <1 mm in 2 instances, and <1.5 mm in 1 instance, with a mean accuracy of <0.9 mm, and a standard deviation of 0.42 mm. (Table 3).

Table 3.
Accuracy average and standard deviation per surgical disease area.
Navigation accuracy measured was as follows: <0.5 mm in 40.47% (17) of the cases, <1 mm in 45.23% (19) of the cases and <1.5 mm in 14.28% (6) of the cases. The recorded mean accuracy was <0.87 mm, with a standard deviation of 0.5 mm.
Despite various surgeons adopting the tracking tools procedure, no complications were observed in terms of the accuracy and quality of detection and, consequently, surgical precision. Since it is a purely technical step, this procedure could be only influenced by an error during the various stages of planning, registration, or recording.
8. Discussion
Navigational surgery is extensively documented in the maxillofacial literature. There are few articles discussing the use of surgical navigation with tracked instruments. This study showcases our experience in the last 15 years using surgical navigation and surgical tracked tools, achieving satisfactory results with an error < 1.5 mm, which we consider acceptable.
In our study sample, the calculated average error was <0.87 mm, consistently below the established baseline of 2 mm. This reference value represents the acceptable mean maximum error of inaccuracy documented in the literature [19].
The osteotomy tracing was performed while confirming anatomical localisation, concurrently adhering to the pre-planned virtual trajectory. In oncological cases, resections were performed with a macroscopically clear margin thanks to concurrent navigation. Reconstructions using the free fibula flap closely matched the preoperatively programmed plan. Tracked instruments during navigation implied a reduction in surgical time, the use of a less invasive surgical approach, and a decrease in the risk of complications. In the treatment of endo-orbital pathology, where surgical approaches are minimal, and there is a high density of anatomical noble structures, the tracking of the periosteal elevator enabled the execution of a precise subperiosteal dissection. This allowed us to verify or shape the titanium mesh in real-time, generating outcomes closely aligned with the pre-planned ones.
The use of surgical navigation with different tracked instruments enabled us to perform surgery under the constant real-time monitoring of anatomical positions in reduced surgical fields. This allowed us to avoid damaging critical structures such as nerves, while also adhering to the osteotomy tracings and surgical resection boundaries, as well as accurately placing reconstructive materials in alignment with the pre-planned virtual program. We were, therefore, able to perform less invasive surgical approaches, thereby reducing postoperative complications. In those cases in which the resection was carried out using a “piecemeal” technique, and lesion margins were hard to identify during surgery, such as in Case 2, navigation allowed us to locate margins in real-time, ensuring confidence in the surgical approach, as previously highlighted by Novelli et al. (2016) in their protocol [20,21]. The navigation system’s precision proves to be particularly useful in complex areas, such as the maxillofacial region. Indeed, in this confined space, numerous delicate anatomical structures are closely located, making the tool indispensable, especially when aesthetic preservation is aimed.
One of the advantages of virtual planning is the reproducibility of the results, potentially allowing novice surgeons to achieve the same outcomes as more experienced surgeons in case of accurate preoperative planning [22,23].
Nowadays, CAD–CAM cutting guides enable the practical application in the operating room of virtual surgical planning despite displaying several challenges related to their precise placement. Notably, positioning these surgical templates requires the execution of wider surgical accesses, leading to increased disruption of surrounding healthy tissues, and resulting in a more challenging postoperative period with an increased risk of complications. The adoption of intraoperative navigation has emerged as a valuable alternative, offering an effective cost-benefit balance for translating virtual surgical plans into practical procedures, and yielding favourable outcomes in terms of precision and safety [24,25].
The tracking of surgical instruments was previously described by Bianchi et al. (2015), who performed craniofacial osteotomies for oncological reasons or in cases of orthognathic surgery on 18 patients using tracked drills, confirming the lower invasiveness and greater precision of the surgery. In certain instances, the surgical duration exceeded the typical time frame for a comparable procedure [18]. Robiony et al. (2019) performed nasal bone osteotomies using piezoelectric drill tracking [26]. More recently, Alice Dean et al. (2022) reported 32 patients who underwent surgery using surgical navigation with tracked piezoelectric devices. The treatments encompassed a wide range of disciplines, including oncology, traumatology, and orthognathic surgery. The results confirmed a decrease in surgical time and improvements in procedural safety, allowing three-dimensional control over tumour resection or osteotomy depth [27].
The limitations of surgical navigation techniques include the economic cost of the navigation device and software, the additional time required for pre-surgical planning, a steep learning curve for the surgeon, and the necessity for an extra preoperative CT scan image acquisition [28,29]. The study has some limitations, such as sample size, and the considerable heterogeneity in pathologies and procedures, along with the absence of a control group.
Further potential limitations of this procedure may include the time required for the planning, registration, and recording of surgical instruments and the learning curve. However, these are relative disadvantages, as the apparent increased preoperative time needed is largely offset by the time saved during surgery, both in terms of speed of execution and accuracy of intervention. Regarding the learning curve, it should be encompassed within the learning curve necessary for using surgical navigation, and this procedure does not impact time or learning complexity.
In conclusion, the true limitation of this procedure is represented by the cost of the navigation system. However, if used more widely across various fields of oral and maxillofacial surgery and shared with other surgical departments such as neurosurgery, otolaryngology, etc., the investment could be thoroughly justified. Such collaboration would enable the delivery of more predictable and often reproducible surgical outcomes for patients, even when performed by different operators.
In the existing literature, there are no studies that have monitored surgical instruments other than the piezoelectric device. Based on our experience, using different instruments enables the surgeon to better adapt to each anatomical region, and to select the most suitable instrument for the type of surgery required. In addition to the latter advantages, there are also the aforementioned benefits resulting from the synergy of surgical navigation and tracking tools.
9. Conclusions
Surgical navigation using tracked instruments allows the synergistic implementation of the benefits of both techniques. This approach enables the surgeon to perform with greater precision and less invasive surgical access, thereby reducing morbidity and complications. Furthermore, pre-planned virtual surgery facilitates the realisation of potential osteotomy pathways, tumour resections, and placement of reconstructive materials, maintaining real-time control over anatomy throughout.
In terms of reproducibility and predictability of technical outcomes, the methodology of tracking tools is not operator dependent. Due to its purely technical nature, errors during the various stages of planning, registration, or recording are the only factors that could influence this procedure.
The key is to follow the designed “guidelines” and to adhere to all technical procedures.
The main advantage of surgical navigation is represented by its capacity to ensure reproducibility and predictability within surgical procedures. This fundamental attribute guarantees a consistent and standardized outcome, not depending on the performing surgeon. This is particularly crucial in the context of complex oral–maxillofacial surgeries, where precision is of utmost importance such as in orbital reconstruction or bone resection.. Surgical navigation, as a revolutionary paradigm, reshapes the approach to these delicate surgical procedures. However, it is imperative to acknowledge that despite the advancements, the indispensable expertise of the surgeon remains an irreplaceable essential component. The symbiosis between the surgeon’s competency and the appropriate use of navigation technology is the keystone for achieving optimal and replicable results in these procedures.
Author Contributions
Conceptualization, G.N.; methodology, G.N.; software, A.J.P.M.; validation, G.N., D.S. and G.C.; formal analysis, F.S.; investigation, F.S.; resources, F.V. and A.F.; data curation, F.V.; writing—original draft preparation, F.S.; writing—review and editing, A.J.P.M. and G.N.; visualisation, A.J.P.M.; supervision, G.N.; project administration, G.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Given the retrospective feature of the study, ethical approval was not considered necessary to perform the study.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Collyer, J. Stereotactic navigation in oral and maxillofacial surgery. Br. J. Oral Maxillofac. Surg. 2010, 48, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Leksell, L.; Jernberg, B. Stereotaxis and tomography a technical note. Acta Neurochir. 1980, 52, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Novelli, G.; Moretti, M.; Meazzini, M.C.; Cassé, C.M.A.; Mazzoleni, F.; Sozzi, D. Introduction to surgical navigation in oral surgery: A case-series. Oral 2023, 3, 146–154. [Google Scholar] [CrossRef]
- Novelli, G.; Tonellini, G.; Mazzoleni, F.; Bozzetti, A.; Sozzi, D. Virtual surgery simulation in orbital wall reconstruction: Integration of surgical navigation and stereolithographic models. J. Cranio-Maxillofac. Surg. 2014, 42, 2025–2034. [Google Scholar] [CrossRef] [PubMed]
- Lübbers, H.-T.; Jacobsen, C.; Könü, D.; Matthews, F.; Grätz, K.W.; Obwegeser, J.A. Surgical navigation in cranio-maxillofacial surgery: An evaluation on a child with a cranio-facio-orbital tumour. Br. J. Oral Maxillofac. Surg. 2011, 49, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.B. Computer planning and intraoperative navigation in cranio-maxillofacial surgery. Oral Maxillofac. Surg. Clin. N. Am. 2010, 22, 135–156. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Sun, J.; Shen, S.G.; Xu, B.; Li, J.; Zhang, S. Computer-assisted navigation: Its role in intraoperatively accurate mandibular reconstruction. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Gui, H.; Wu, J.; Shen, S.G.; Bautista, J.S.; Voss, P.J.; Zhang, S. Navigation-guided lateral gap arthroplasty as the treatment of temporomandibular joint ankylosis. J. Oral Maxillofac. Surg. 2014, 72, 128–138. [Google Scholar] [CrossRef]
- He, Y.; Huang, T.; Zhang, Y.; An, J.; He, L. Application of a computer-assisted surgical navigation system in temporomandibular joint ankylosis surgery: A retrospective study. Int. J. Oral Maxillofac. Surg. 2017, 46, 189–197. [Google Scholar] [CrossRef]
- Abbate, V.; Orabona, G.D.A.; Solari, D.; Bonavolontà, P.; Iaconetta, G.; Califano, L. Mandibular Surgical Navigation: An Innovative Guiding Method. J. Craniofacial Surg. 2017, 28, 2122–2126. [Google Scholar] [CrossRef]
- Hohlweg-Majert, B.; Schön, R.; Schmelzeisen, R.; Gellrich, N.C.; Schramm, A. Navigational maxillofacial surgery using virtual models. World J. Surg. 2005, 29, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Shen, S.G.; Wang, X.; Zhang, L.; Zhang, S. The indication and application of computer-assisted navigation in oral and maxillofacial surgery—Shanghai’s experience based on 104 cases. J. Cranio-Maxillofac. Surg. 2013, 41, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, D.L.; Garfein, E.S.; Christensen, A.M.; Weimer, K.A.; Saddeh, P.B.; Levine, J.P. Use of computer-aided design and computer-aided manufacturing to produce orthognathically ideal surgical outcomes: A paradigm shift in head and neck reconstruction. J. Oral Maxillofac. Surg. 2009, 67, 2115–2122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Z.; Li, B.; Yu, H.; Shen, S.G.; Wang, X. Evaluation of computer-assisted mandibular reconstruction with vascularized fibular flap compared to conventional surgery. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 121, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Sozzi, D.; Filippi, A.; Canzi, G.; De Ponti, E.; Bozzetti, A.; Novelli, G. Surgical Navigation in Mandibular Reconstruction: Accuracy Evaluation of an Innovative Protocol. J. Clin. Med. 2022, 11, 2060. [Google Scholar] [CrossRef] [PubMed]
- Vercellotti, T. Piezoelectric surgery in implantology: A case report—A new piezoelectric ridge expansion technique. Int. J. Periodontics Restor. Dent. 2000, 20, 359–365. [Google Scholar]
- Spinelli, G.; Lazzeri, D.; Conti, M.; Agostini, T.; Mannelli, G. Comparison of piezosurgery and traditional saw in bimaxillary orthognathic surgery. J. Cranio-Maxillofac. Surg. 2014, 42, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, A.; Badiali, G.; Piersanti, L.; Marchetti, C. Computer-assisted piezoelectric surgery: A navigated approach toward performance of craniomaxillofacial osteotomies. J. Craniofac. Surg. 2015, 26, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Pivazyan, G.; Sandhu, F.A.; Beaufort, A.R.; Cunningham, B.W. Basis for error in stereotactic and computer-assisted surgery in neurosurgical applications: Literature review. Neurosurg. Rev. 2022, 46, 20. [Google Scholar] [CrossRef]
- Novelli, G.; Gramegna, M.; Tonellini, G.; Valente, G.; Boni, P.; Bozzetti, A.; Sozzi, D. Orbital Osteoblastoma: Technical Innovations in Resection and Reconstruction Using Virtual Surgery Simulation. Craniomaxillofac. Trauma Reconstr. 2016, 9, 271–276. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, G.; Lu, M.; Hu, Q. Surgical management of maxillofacial fibrous dysplasia under navigational guidance. Br. J. Oral Maxillofac. Surg. 2015, 53, 336–341. [Google Scholar] [CrossRef]
- Ceccariglia, F.; Cercenelli, L.; Badiali, G.; Marcelli, E.; Tarsitano, A. Application of Augmented Reality to Maxillary Resections: A Three-Dimensional Approach to Maxillofacial Oncologic Surgery. J. Pers. Med. 2022, 12, 2047. [Google Scholar] [CrossRef]
- Velarde, K.; Cafino, R.; Isla, A., Jr.; Ty, K.M.; Palmer, X.-L.; Potter, L.; Nadorra, L.; Pueblos, L.V.; Velasco, L.C. Virtual surgical planning in craniomaxillofacial surgery: A structured review. Comput. Assist. Surg. 2023, 28, 2271160. [Google Scholar] [CrossRef] [PubMed]
- Sukegawa, S.; Kanno, T.; Furuki, Y. Application of computer-assisted navigation systems in oral and maxillofacial surgery. Jpn. Dent. Sci. Rev. 2018, 54, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-B.; Yu, Y.; Wang, Y.; Mao, C.; Liu, X.-J.; Guo, C.-B.; Yu, G.-Y.; Peng, X. Improving the accuracy of mandibular reconstruction with vascularized iliac crest flap: Role of computer-assisted techniques. J. Cranio-Maxillofac. Surg. 2016, 44, 1819–1827. [Google Scholar] [CrossRef] [PubMed]
- Robiony, M.; Toro, C.; Costa, F.; Sembronio, S.; Polini, F.; Politi, M. Piezosurgery: A new method for osteotomies in rhinoplasty. J. Craniofac. Surg. 2007, 18, 1098–1100. [Google Scholar] [CrossRef]
- Dean, A.; Heredero-Jung, S.; Solivera, J.; Sanjuan, A.; Alamillos-Granados, F.J. Computer-assisted and navigated piezoelectric surgery: A new technology to improve precision and surgical safety in craniomaxillofacial surgery. Laryngoscope Investig. Otolaryngol. 2022, 7, 684–691. [Google Scholar] [CrossRef]
- Anand, M.; Panwar, S. Role of Navigation in Oral and Maxillofacial Surgery: A Surgeon’s Perspectives. Clin. Cosmet. Investig. Dent. 2021, 13, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Emery, R.W.; Korj, O.; Agarwal, R. A Review of In-Office Dynamic Image Navigation for Extraction of Complex Mandibular ThirdMolars. J. Oral Maxillofac. Surg. 2017, 75, 1591–1600. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).