Plasmacytoma in the Maxillary Jaw: A Diagnostic and Therapeutic Challenge
Abstract
1. Introduction
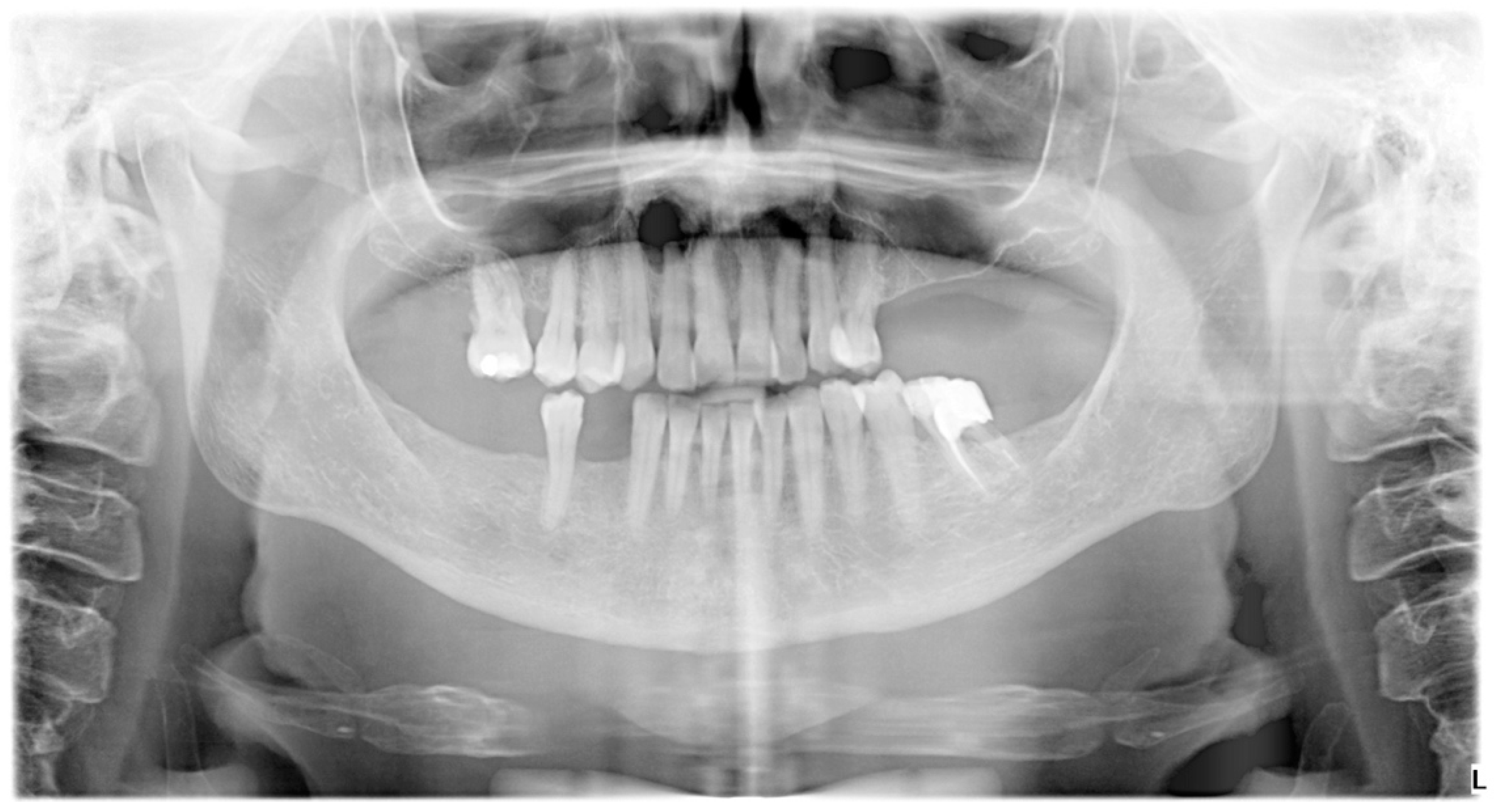
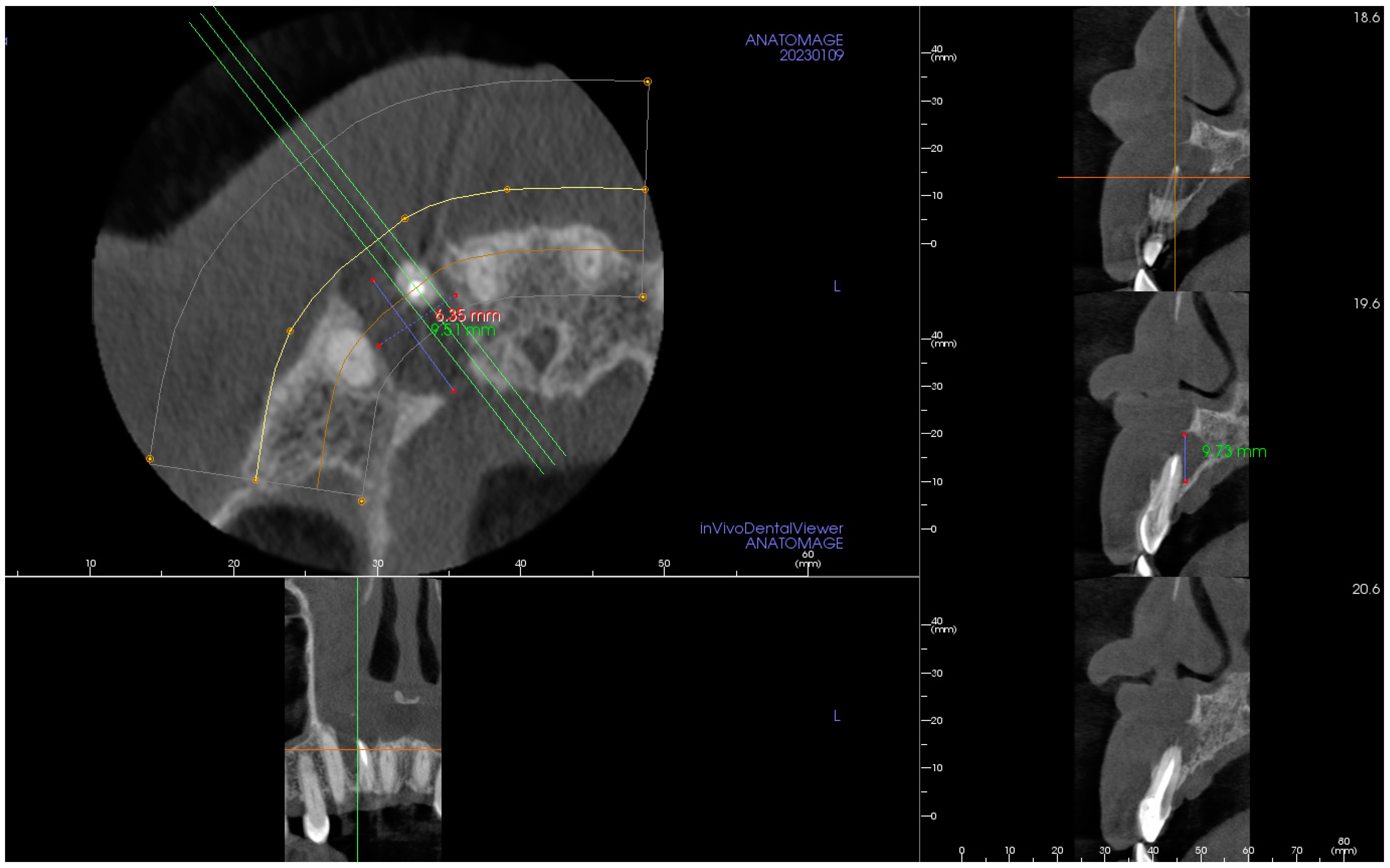
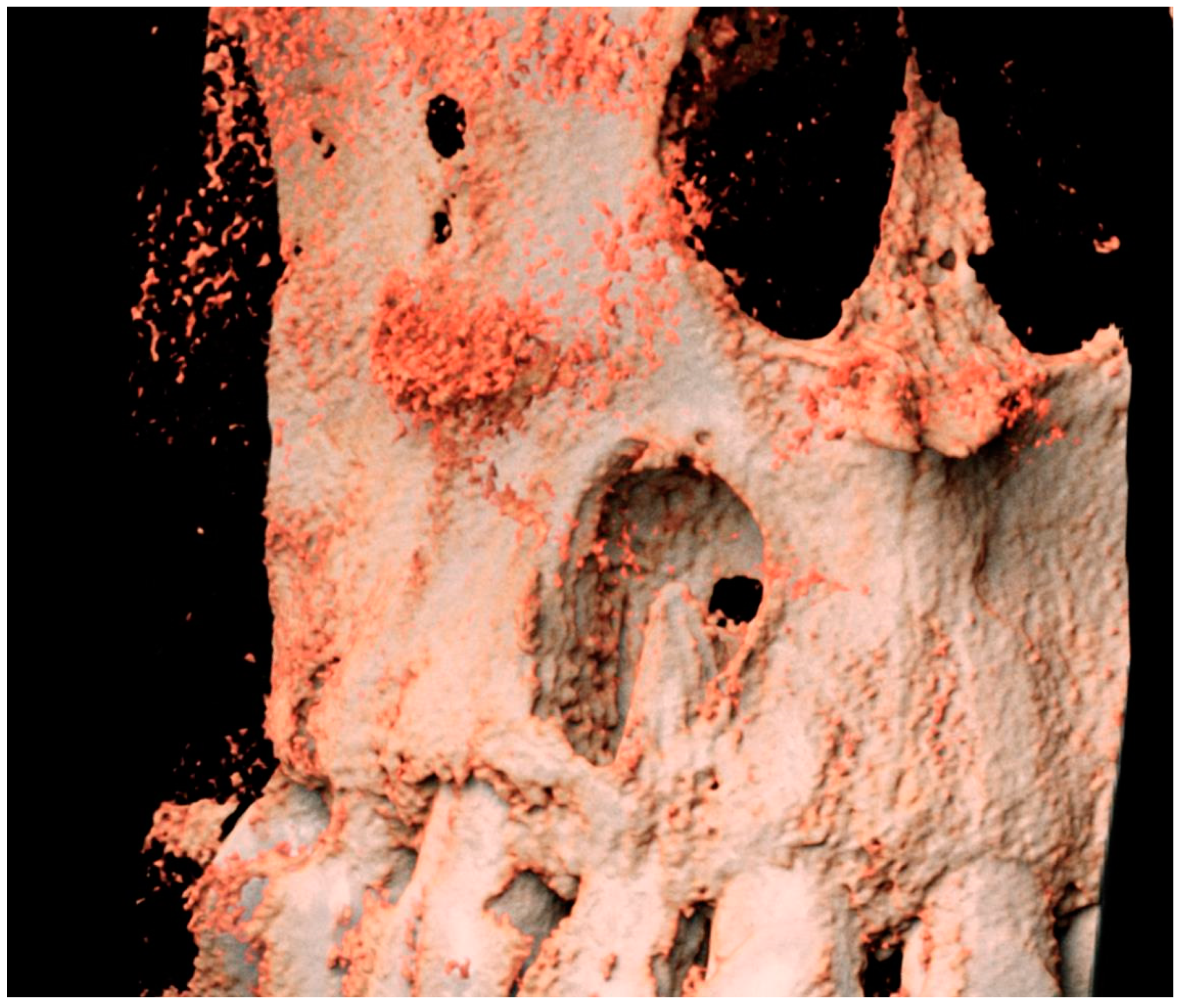
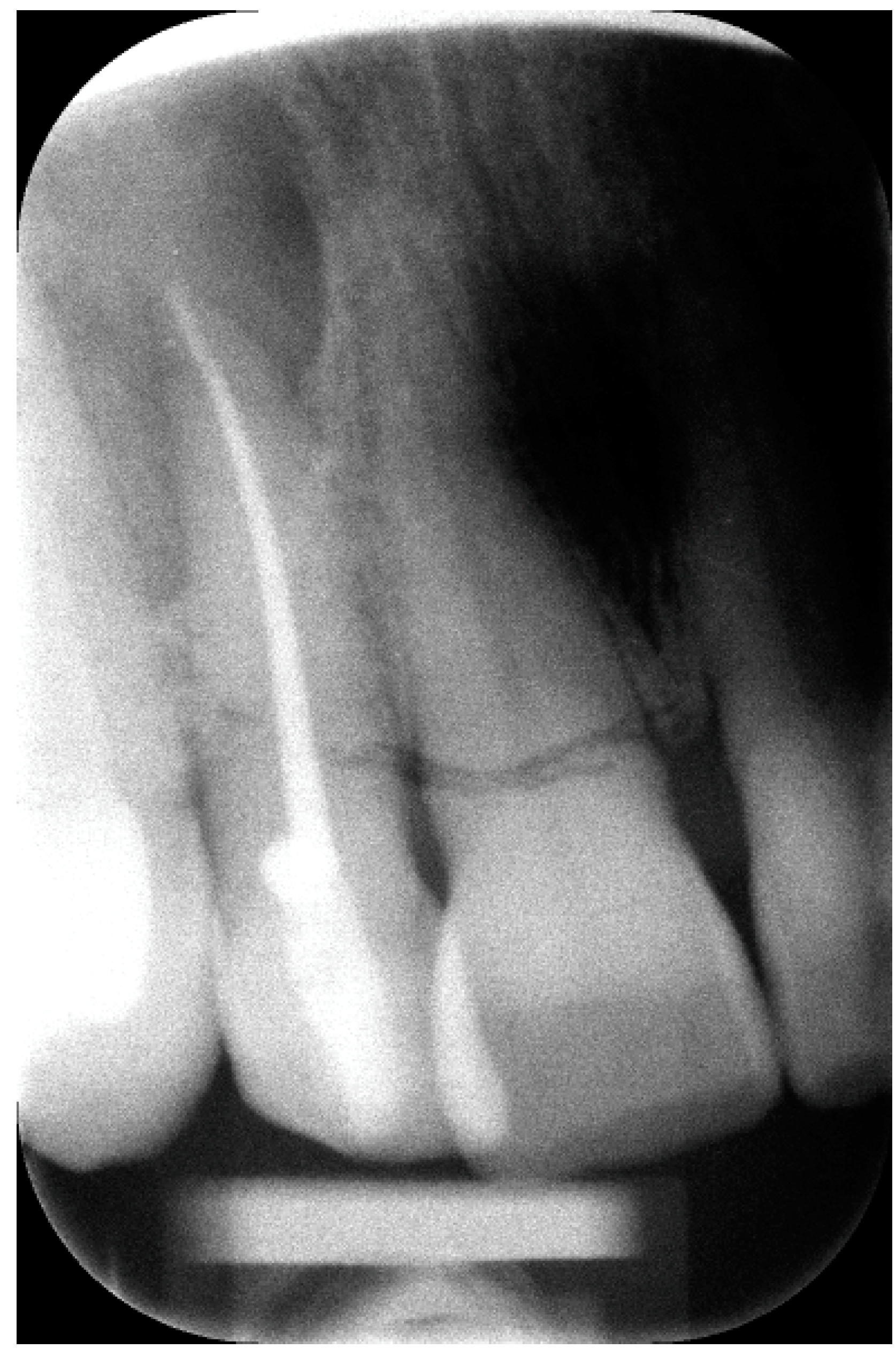
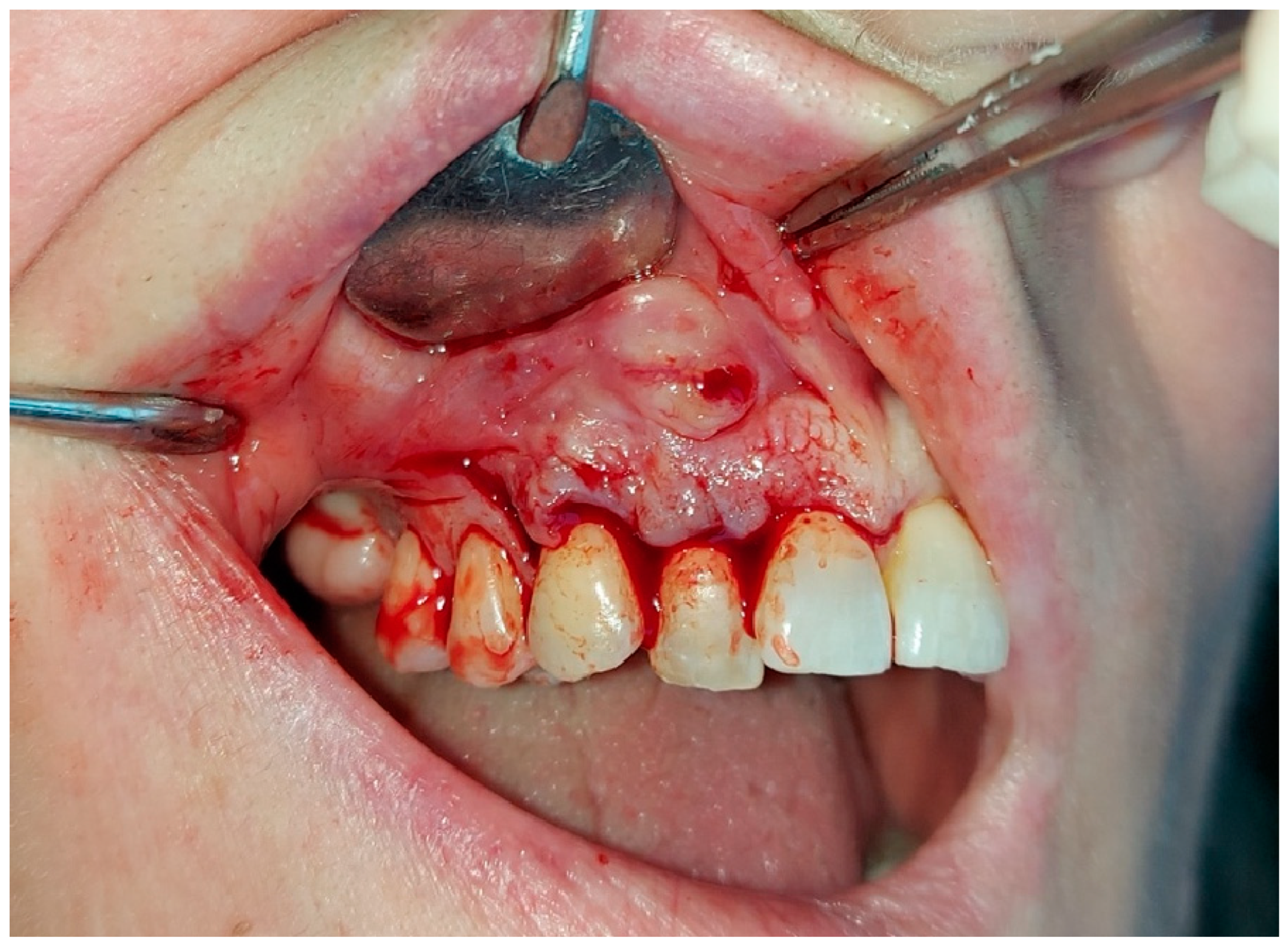
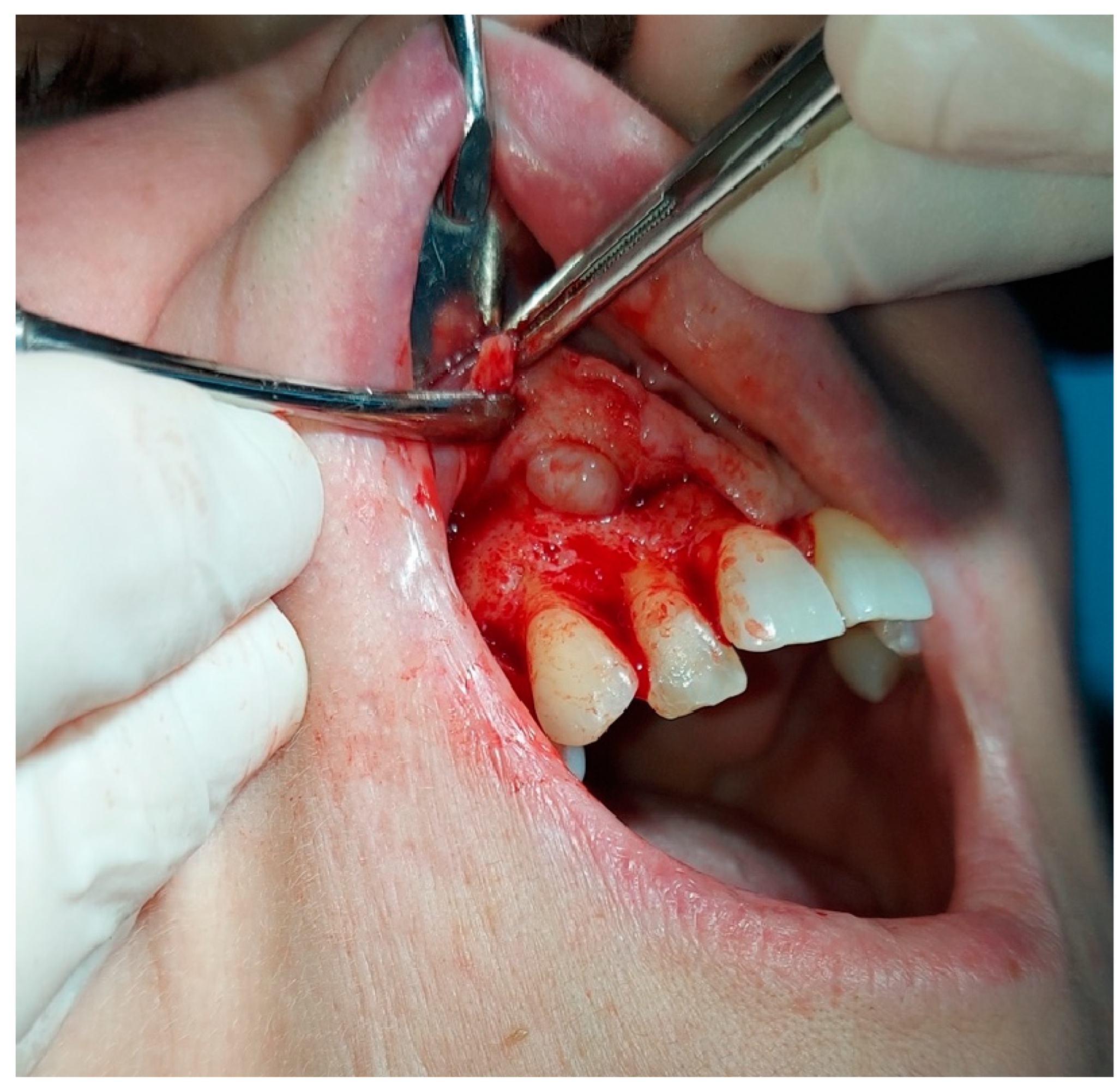
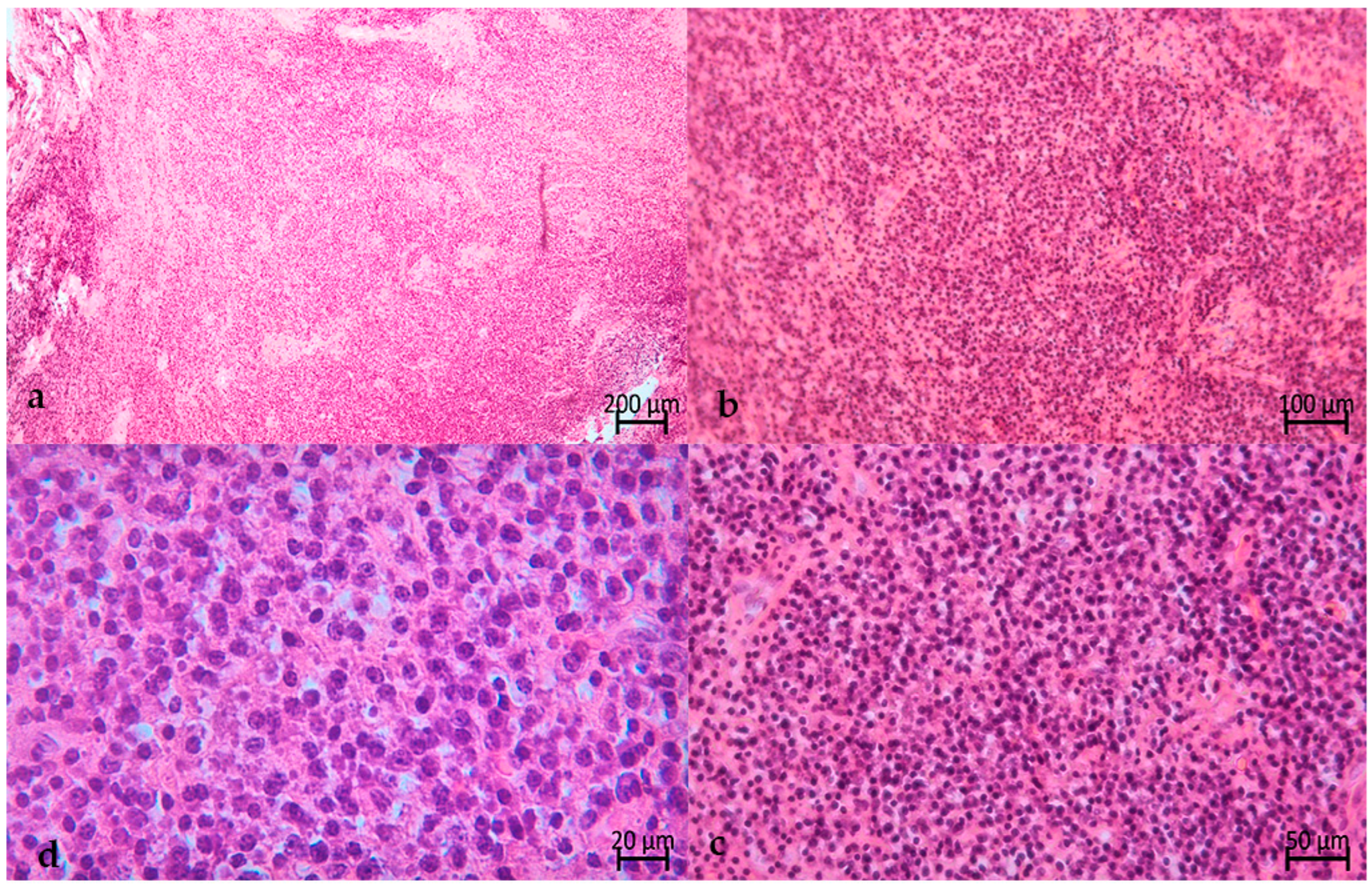
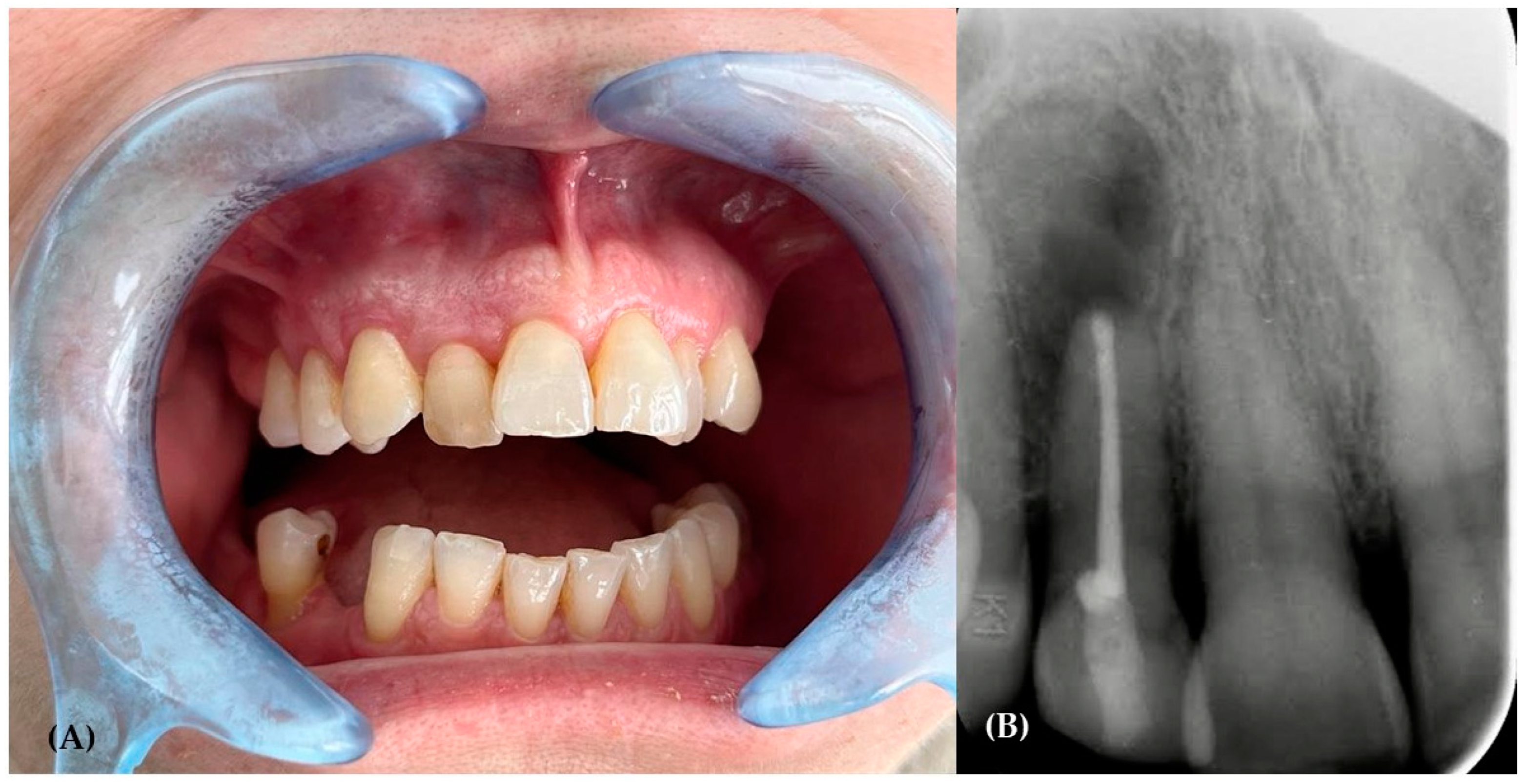
2. The Case
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diebold, J. World Health Organization classification of malignant lymphomas. Exp. Oncol. 2001, 23, 101–103. [Google Scholar]
- Longo, D.L. Plasma cell disorders. In Harrison’s Principles of Internal Medicine, 15th ed.; Fauci, A.S., Braunwald, E., Kasper, D.L., Hauser, S., Longo, D., Jameson, L.J., Eds.; McGraw Hill, Inc.: New York, NY, USA, 2001; pp. 727–733. [Google Scholar]
- Pisano, J.J.; Coupland, R.; Chen, S.Y.; Miller, A.S. Plasmacytoma of the oral cavity and jaws: A clinicopathological study of 13 cases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1997, 83, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Marotta, S.; Di Micco, P. Solitary plasmacytoma of the jaw. J. Blood Med. 2010, 22, 33–36. [Google Scholar] [CrossRef][Green Version]
- Bachar, G.; Goldstein, D.; Brown, D.; Tsang, R.; Lockwood, G.; Perez-Ordonez, B.; Irish, J. Solitary extramedullary plasmacytoma of the head and neck—Long-term outcome analysis of 68 cases. Head Neck 2008, 30, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, S.; Koo, R.M.; Harrison, S.J. A Wolf in Sheep’s clothing: A case report series of oral manifestations of multiple myeloma. Aust. Dent. J. 2021, 66, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Skerget, M.; Dovsak, T.; Kos, G.; Zver, S. Surgery results in low relapse and progression rates in extramedullary plasmacytoma of the head and neck: A case cohort and review of the literature. Hematol. Rep. 2020, 12, 8396. [Google Scholar] [CrossRef] [PubMed]
- Riley, D.S.; Barber, M.S.; Kienle, G.S.; Aronson, J.K.; von Schoen-Angerer, T.; Tugwell, P.; Kiene, H.; Helfand, M.; Altman, D.G.; Sox, H.; et al. CARE guidelines for case reports: Explanation and elaboration document. J. Clin. Epidemiol. 2017, 89, 218–235. [Google Scholar] [CrossRef]
- Goyal, G.; Bartley, A.C.; Funni, S.; Inselman, J.; Shah, N.D.; Marshall, A.L.; Ashrani, A.A.; Kapoor, P.; Durani, U.; Hashmi, S.K.; et al. Treatment approaches and outcomes in plasmacytomas: Analysis using a national dataset. Leukemia 2018, 32, 1414–1420. [Google Scholar] [CrossRef] [PubMed]
- Agostini, T.; Sacco, R.; Bertolai, R.; Acocella, A.; Lazzeri, D. Solitary plasmacytoma of the jaw. J. Craniofac. Surg. 2011, 22, e2–e10. [Google Scholar] [CrossRef]
- Liu, Y.; Li, K. Solitary plasmacytoma of maxillofacial bones: Correlation of CT features with pathological findings. Dentomaxillofac. Radiol. 2020, 49, 20190277. [Google Scholar] [CrossRef] [PubMed]
- Kucukkurt, S.; Karan, N.B.; Senguven, B.; Kahraman, S. Solitary plasmacytoma of the mandible: Report of two cases. BMJ Case Rep. 2016, 2016, bcr2015214255. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Khalifa, H.M.; Bayoumi, A.; AlMazrooa, S.; Bin Madi, N.O.; Akeel, S.; Sindi, A.M. Osteolytic Lesion of the Maxilla in an Undiagnosed Multiple Myeloma Patient Identified Incidentally by Cone Beam Computed Tomography. Am. J. Case Rep. 2022, 23, e936585. [Google Scholar] [CrossRef] [PubMed]
- Stanford School of Medicine (Surgical Pathology Criteria). Plasmacytoma. [Last Updated 2010 April 15; Cited 2022 February 1]. Available online: https://surgpathcriteria.stanford.edu/plasma_cell/plasmacytoma/printable.html (accessed on 15 May 2023).
- Cavo, M.; Terpos, E.; Nanni, C.; Moreau, P.; Lentzsch, S.; Zweegman, S.; Hillengass, J.; Engelhardt, M.; Usmani, S.Z.; Vesole, D.H.; et al. Role of 18F-FDG PET/CT in the diagnosis and management of multiple myeloma and other plasma cell disorders: A consensus statement by the International Myeloma Working Group. Lancet Oncol. 2017, 18, e206–e217. [Google Scholar] [CrossRef] [PubMed]
- Hillengass, J.; Usmani, S.; Rajkumar, S.V.; Durie, B.G.M.; Mateos, M.V.; Lonial, S.; Joao, C.; Anderson, K.C.; García-Sanz, R.; Riva, E.; et al. International myeloma working group consensus recommendations on imaging in monoclonal plasma cell disorders. Lancet Oncol. 2019, 20, e302–e312, Erratum in: Lancet Oncol. 2019, 20, e346. [Google Scholar] [CrossRef] [PubMed]
- Caers, J.; Paiva, B.; Zamagni, E.; Leleu, X.; Bladé, J.; Kristinsson, S.Y.; Touzeau, C.; Abildgaard, N.; Terpos, E.; Heusschen, R.; et al. Diagnosis, treatment, and response assessment in solitary plasmacytoma: Updated recommendations from a European Expert Panel. J. Hematol. Oncol. 2018, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Kikuta, S.; Todoroki, K.; Iwanaga, J.; Nakamura, M.; Kusukawa, J. Extramedullary plasmacytoma of the sublingual gland. J. Oral Maxillofac. Surg. Med. Pathol. 2022, 34, 788–790. [Google Scholar] [CrossRef]
- Basavaiah, S.H.; Lobo, F.D.; Philipose, C.S.; Suresh, P.K.; Sreeram, S.; Kini, H.; Sahu, K.K.; Prasad, K. Clinicopathological spectr of solitary Plasmacytoma: A single center experience from coastal India. BMC Cancer 2019, 19, 801. [Google Scholar] [CrossRef] [PubMed]
- Tsang, R.W.; Campbell, B.A.; Goda, J.S.; Kelsey, C.R.; Kirova, Y.M.; Parikh, R.R.; Ng, A.K.; Ricardi, U.; Suh, C.O.; Mauch, P.M.; et al. Radiation Therapy for Solitary Plasmacytoma and Multiple Myeloma: Guidelines From the International Lymphoma Radiation Oncology Group. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 794–808, Erratum in Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 1602. [Google Scholar] [CrossRef] [PubMed]
- Mheidly, A.; Lamy, T.; Escoffre, M.; Hunault, M.; Benboubker, L.; Esvan, M.; Benchalal, M.; Moreau, P.; Decaux, O. Adjuvant Chemotherapy in the Treatment of Solitary Bone Plasmacytoma. Blood 2016, 128, 4514. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Z.; Qu, X. Advances in the treatment of extramedullary disease in multiple myeloma. Transl. Oncol. 2022, 22, 101465. [Google Scholar] [CrossRef] [PubMed]

| Laboratory Test | Resulting Value | Unit of Measurement | Reference Value |
|---|---|---|---|
| White blood cell: total leucocytes (hydrodynamic focusing and flow cytometry) Neutrophils Lymphocytes Monocytes Eosinophils Basophils | 6.10 47.8 43.1 7.4 1.5 0.2 | 103/μL % % % % % | 4.00–10.00 103/μL 40.0–68.0% 20.0–45.0% 2.0–10.0% 0.5–5.0% 0.0–2.0% |
| Erythrocyte count | 4.35 | 106/μL | 4.00–5.10 106/μL |
| Platelet count | 161 | 103/μL | 130–450 103/μL |
| Total plasma protein values (protein electrophoresis) Albumin Alpha 1 globulin Alpha 2 globulin Beta globulin Gamma globulin | 6.89 59.1 2.1 10.2 10.6 18.0 | % % % % % % | 5.20–8.50% 52.0–68.0% 1.4–4.5% 6.5–13.5% 8.0–15.0% 10.5–20.5% |
| C-reactive protein (CRP) | 0.70 | mg/L | Up to 5.0 mg/L |
| Erythrocyte sedimentation rate (ESR) (immunoturbidimetric method) | 20 | mm/h | <15 mm/h |
| Protein in urine | 0.00 | mg/dL | 0–10 mg/dL |
| Kappa free light chains Lambda free light chains Ratio of kappa/lambda | 4.33 6.7 0.63 | mg/L mg/L | 3.3 to 19.4 mg/L 5.71 to 26.3 mg/L 0.26 to 1.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernardi, S.; Bianchi, S.; Lupi, E.; Gerardi, D.; Macchiarelli, G.; Varvara, G. Plasmacytoma in the Maxillary Jaw: A Diagnostic and Therapeutic Challenge. Hematol. Rep. 2024, 16, 22-31. https://doi.org/10.3390/hematolrep16010003
Bernardi S, Bianchi S, Lupi E, Gerardi D, Macchiarelli G, Varvara G. Plasmacytoma in the Maxillary Jaw: A Diagnostic and Therapeutic Challenge. Hematology Reports. 2024; 16(1):22-31. https://doi.org/10.3390/hematolrep16010003
Chicago/Turabian StyleBernardi, Sara, Serena Bianchi, Ettore Lupi, Davide Gerardi, Guido Macchiarelli, and Giuseppe Varvara. 2024. "Plasmacytoma in the Maxillary Jaw: A Diagnostic and Therapeutic Challenge" Hematology Reports 16, no. 1: 22-31. https://doi.org/10.3390/hematolrep16010003
APA StyleBernardi, S., Bianchi, S., Lupi, E., Gerardi, D., Macchiarelli, G., & Varvara, G. (2024). Plasmacytoma in the Maxillary Jaw: A Diagnostic and Therapeutic Challenge. Hematology Reports, 16(1), 22-31. https://doi.org/10.3390/hematolrep16010003








