A Case Report of Ropeginterferon Alfa-2b for Polycythemia Vera during Pregnancy
Abstract
1. Introduction
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Barbui, T.; Thiele, J.; Gisslinger, H.; Kvasnicka, H.M.; Vannucchi, A.M.; Guglielmelli, P.; Orazi, A.; Tefferi, A. The 2016 WHO classification and diagnostic criteria for myeloproliferative neoplasms: Document summary and in-depth discussion. Blood Cancer J. 2018, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A. Myeloproliferative neoplasms: A decade of discoveries and treatment advances. Am. J. Hematol. 2016, 91, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Pardanani, A. Myeloproliferative Neoplasms: A Contemporary Review. JAMA Oncol. 2015, 1, 97–105. [Google Scholar] [CrossRef]
- Maze, D.; Kazi, S.; Gupta, V.; Malinowski, A.K.; Fazelzad, R.; Shah, P.S.; Shehata, N. Association of Treatments for Myeloproliferative Neoplasms During Pregnancy With Birth Rates and Maternal Outcomes: A Systematic Review and Meta-analysis. JAMA Netw. Open 2019, 2, e1912666. [Google Scholar] [CrossRef]
- Greenfield, G.; McMullin, M.F.; Mills, K. Molecular pathogenesis of the myeloproliferative neoplasms. J. Hematol. Oncol. 2021, 14, 103. [Google Scholar] [CrossRef]
- Barbui, T.; Tefferi, A.; Vannucchi, A.M.; Passamonti, F.; Silver, R.T.; Hoffman, R.; Verstovsek, S.; Mesa, R.; Kiladjian, J.J.; Hehlmann, R.; et al. Philadelphia chromosome-negative classical myeloproliferative neoplasms: Revised management recommendations from European LeukemiaNet. Leukemia 2018, 32, 1057–1069. [Google Scholar] [CrossRef]
- Marchetti, M.; Vannucchi, A.M.; Griesshammer, M.; Harrison, C.; Koschmieder, S.; Gisslinger, H.; Álvarez-Larrán, A.; De Stefano, V.; Guglielmelli, P.; Palandri, F.; et al. Appropriate management of polycythaemia vera with cytoreductive drug therapy: European LeukemiaNet 2021 recommendations. Lancet Haematol. 2022, 9, e301–e311. [Google Scholar] [CrossRef]
- Belardelli, F.; Gresser, I. The neglected role of type I interferon in the T-cell response: Implications for its clinical use. Immunol. Today 1996, 17, 369–372. [Google Scholar] [CrossRef]
- Hasselbalch, H.C.; Holmstrom, M.O. Perspectives on interferon-alpha in the treatment of polycythemia vera and related myeloproliferative neoplasms: Minimal residual disease and cure? Semin. Immunopathol. 2019, 41, 5–19. [Google Scholar] [CrossRef]
- Vilcek, J. Fifty years of interferon research: Aiming at a moving target. Immunity 2006, 25, 343–348. [Google Scholar] [CrossRef]
- Kiladjian, J.J.; Cassinat, B.; Chevret, S.; Turlure, P.; Cambier, N.; Roussel, M.; Bellucci, S.; Grandchamp, B.; Chomienne, C.; Fenaux, P. Pegylated interferon-alfa-2a induces complete hematologic and molecular responses with low toxicity in polycythemia vera. Blood 2008, 112, 3065–3072. [Google Scholar] [CrossRef]
- Kiladjian, J.J.; Cassinat, B.; Turlure, P.; Cambier, N.; Roussel, M.; Bellucci, S.; Menot, M.L.; Massonnet, G.; Dutel, J.L.; Ghomari, K.; et al. High molecular response rate of polycythemia vera patients treated with pegylated interferon alpha-2a. Blood 2006, 108, 2037–2040. [Google Scholar] [CrossRef]
- Masarova, L.; Patel, K.P.; Newberry, K.J.; Cortes, J.; Borthakur, G.; Konopleva, M.; Estrov, Z.; Kantarjian, H.; Verstovsek, S. Pegylated interferon alfa-2a in patients with essential thrombocythaemia or polycythaemia vera: A post-hoc, median 83 month follow-up of an open-label, phase 2 trial. Lancet Haematol. 2017, 4, e165–e175. [Google Scholar] [CrossRef]
- Quintas-Cardama, A.; Kantarjian, H.; Manshouri, T.; Luthra, R.; Estrov, Z.; Pierce, S.; Richie, M.A.; Borthakur, G.; Konopleva, M.; Cortes, J.; et al. Pegylated interferon alfa-2a yields high rates of hematologic and molecular response in patients with advanced essential thrombocythemia and polycythemia vera. J. Clin. Oncol. 2009, 27, 5418–5424. [Google Scholar] [CrossRef]
- Yazdani Brojeni, P.; Matok, I.; Garcia Bournissen, F.; Koren, G. A systematic review of the fetal safety of interferon alpha. Reprod. Toxicol. 2012, 33, 265–268. [Google Scholar] [CrossRef]
- Gisslinger, H.; Zagrijtschuk, O.; Buxhofer-Ausch, V.; Thaler, J.; Schloegl, E.; Gastl, G.A.; Wolf, D.; Kralovics, R.; Gisslinger, B.; Strecker, K.; et al. Ropeginterferon alfa-2b, a novel IFNalpha-2b, induces high response rates with low toxicity in patients with polycythemia vera. Blood 2015, 126, 1762–1769. [Google Scholar] [CrossRef]
- Gisslinger, H.; Klade, C.; Georgiev, P.; Krochmalczyk, D.; Gercheva-Kyuchukova, L.; Egyed, M.; Rossiev, V.; Dulicek, P.; Illes, A.; Pylypenko, H.; et al. Ropeginterferon alfa-2b versus standard therapy for polycythaemia vera (PROUD-PV and CONTINUATION-PV): A randomised, non-inferiority, phase 3 trial and its extension study. Lancet Haematol. 2020, 7, e196–e208. [Google Scholar] [CrossRef]
- Tefferi, A.; Barbui, T. Polycythemia vera and essential thrombocythemia: 2017 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2017, 92, 94–108. [Google Scholar] [CrossRef]
- Huang, C.E.; Wu, Y.Y.; Hsu, C.C.; Chen, Y.J.; Tsou, H.Y.; Li, C.P.; Lai, Y.H.; Lu, C.H.; Chen, P.T.; Chen, C.C. Real-world experience with Ropeginterferon-alpha 2b (Besremi) in Philadelphia-negative myeloproliferative neoplasms. J. Formos Med. Assoc. 2021, 120, 863–873. [Google Scholar] [CrossRef]
- Hultcrantz, M.; Ravn Landtblom, A.; Andreasson, B.; Samuelsson, J.; Dickman, P.W.; Kristinsson, S.Y.; Bjorkholm, M.; Andersson, T.M. Incidence of myeloproliferative neoplasms - trends by subgroup and age in a population-based study in Sweden. J. Intern. Med. 2020, 287, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.; Lee, J.O.; Bang, S.M. Incidence, Survival and Prevalence Statistics of Classical Myeloproliferative Neoplasm in Korea. J. Korean Med. Sci. 2016, 31, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Hong, J.; Hwang, S.M.; Lee, J.H.; Ma, Y.; Kim, S.A.; Lee, J.Y.; Lee, J.O.; Bang, S.M. Evaluation of the need for cytoreduction and its potential carcinogenicity in children and young adults with myeloproliferative neoplasms. Ann. Hematol. 2021, 100, 2567–2574. [Google Scholar] [CrossRef] [PubMed]
- Lavi, N.; Brenner, B.; Avivi, I. Management of pregnant women with myeloproliferative neoplasms. Thromb. Res. 2013, 131 (Suppl. 1), S11–S13. [Google Scholar] [CrossRef]
- Alimam, S.; Bewley, S.; Chappell, L.C.; Knight, M.; Seed, P.; Gray, G.; Harrison, C.; Robinson, S. Pregnancy outcomes in myeloproliferative neoplasms: UK prospective cohort study. Br. J. Haematol. 2016, 175, 31–36. [Google Scholar] [CrossRef]
- Robinson, S.E.; Harrison, C.N. How we manage Philadelphia-negative myeloproliferative neoplasms in pregnancy. Br. J. Haematol. 2020, 189, 625–634. [Google Scholar] [CrossRef]
- Tefferi, A.; Barbui, T. Polycythemia vera and essential thrombocythemia: 2021 update on diagnosis, risk-stratification and management. Am. J. Hematol. 2020, 95, 1599–1613. [Google Scholar] [CrossRef]
- Barbui, T.; Barosi, G.; Grossi, A.; Gugliotta, L.; Liberato, L.N.; Marchetti, M.; Mazzucconi, M.G.; Rodeghiero, F.; Tura, S. Practice guidelines for the therapy of essential thrombocythemia. A statement from the Italian Society of Hematology, the Italian Society of Experimental Hematology and the Italian Group for Bone Marrow Transplantation. Haematologica 2004, 89, 215–232. [Google Scholar]
- Gangat, N.; Tefferi, A. Myeloproliferative neoplasms and pregnancy: Overview and practice recommendations. Am. J. Hematol. 2021, 96, 354–366. [Google Scholar] [CrossRef]
- How, J.; Leiva, O.; Bogue, T.; Fell, G.G.; Bustoros, M.W.; Connell, N.T.; Connors, J.M.; Ghobrial, I.M.; Kuter, D.J.; Mullally, A.; et al. Pregnancy outcomes, risk factors, and cell count trends in pregnant women with essential thrombocythemia. Leuk. Res. 2020, 98, 106459. [Google Scholar] [CrossRef]
- Landtblom, A.R.; Andersson, T.M.; Johansson, A.L.V.; Wendel, S.B.; Lundberg, F.E.; Samuelsson, J.; Bjorkholm, M.; Hultcrantz, M. Pregnancy and childbirth outcomes in women with myeloproliferative neoplasms-a nationwide population-based study of 342 pregnancies in Sweden. Leukemia 2022, 36, 2461–2467. [Google Scholar] [CrossRef]
- Lishner, M.; Avivi, I.; Apperley, J.F.; Dierickx, D.; Evens, A.M.; Fumagalli, M.; Nulman, I.; Oduncu, F.S.; Peccatori, F.A.; Robinson, S.; et al. Hematologic Malignancies in Pregnancy: Management Guidelines From an International Consensus Meeting. J. Clin. Oncol. 2016, 34, 501–508. [Google Scholar] [CrossRef]
- Barbui, T.; Barosi, G.; Birgegard, G.; Cervantes, F.; Finazzi, G.; Griesshammer, M.; Harrison, C.; Hasselbalch, H.C.; Hehlmann, R.; Hoffman, R.; et al. Philadelphia-negative classical myeloproliferative neoplasms: Critical concepts and management recommendations from European LeukemiaNet. J. Clin. Oncol. 2011, 29, 761–770. [Google Scholar] [CrossRef]
- Sanchez, I.; Woodley, C.; Durgam, G.; Curto-Garcia, N.; Sullivan, J.O.; Robinson, S.E.; Ali, S.; McLornan, D.; Harrison, C. A case of polycythaemia vera treated with ropeginterferon-2b during pregnancy. Br. J. Haematol. 2021, 193, 118–119. [Google Scholar]
- Shechter-Maor, G.; Sadeh-Mestechkin, D.; Ganor Paz, Y.; Sukenik Halevy, R.; Markovitch, O.; Biron-Shental, T. Does parity affect pregnancy outcomes in the elderly gravida? Arch. Gynecol. Obstet. 2020, 301, 85–91. [Google Scholar] [CrossRef]
- Reese, J.A.; Peck, J.D.; Deschamps, D.R.; McIntosh, J.J.; Knudtson, E.J.; Terrell, D.R.; Vesely, S.K.; George, J.N. Platelet Counts during Pregnancy. N. Engl. J. Med. 2018, 379, 32–43. [Google Scholar] [CrossRef]
- Bertozzi, I.; Rumi, E.; Cavalloni, C.; Cazzola, M.; Fabris, F.; Randi, M.L. Pregnancy outcome and management of 25 pregnancies in women with polycythemia vera. Am. J. Hematol. 2018, 93, E234–E235. [Google Scholar] [CrossRef]
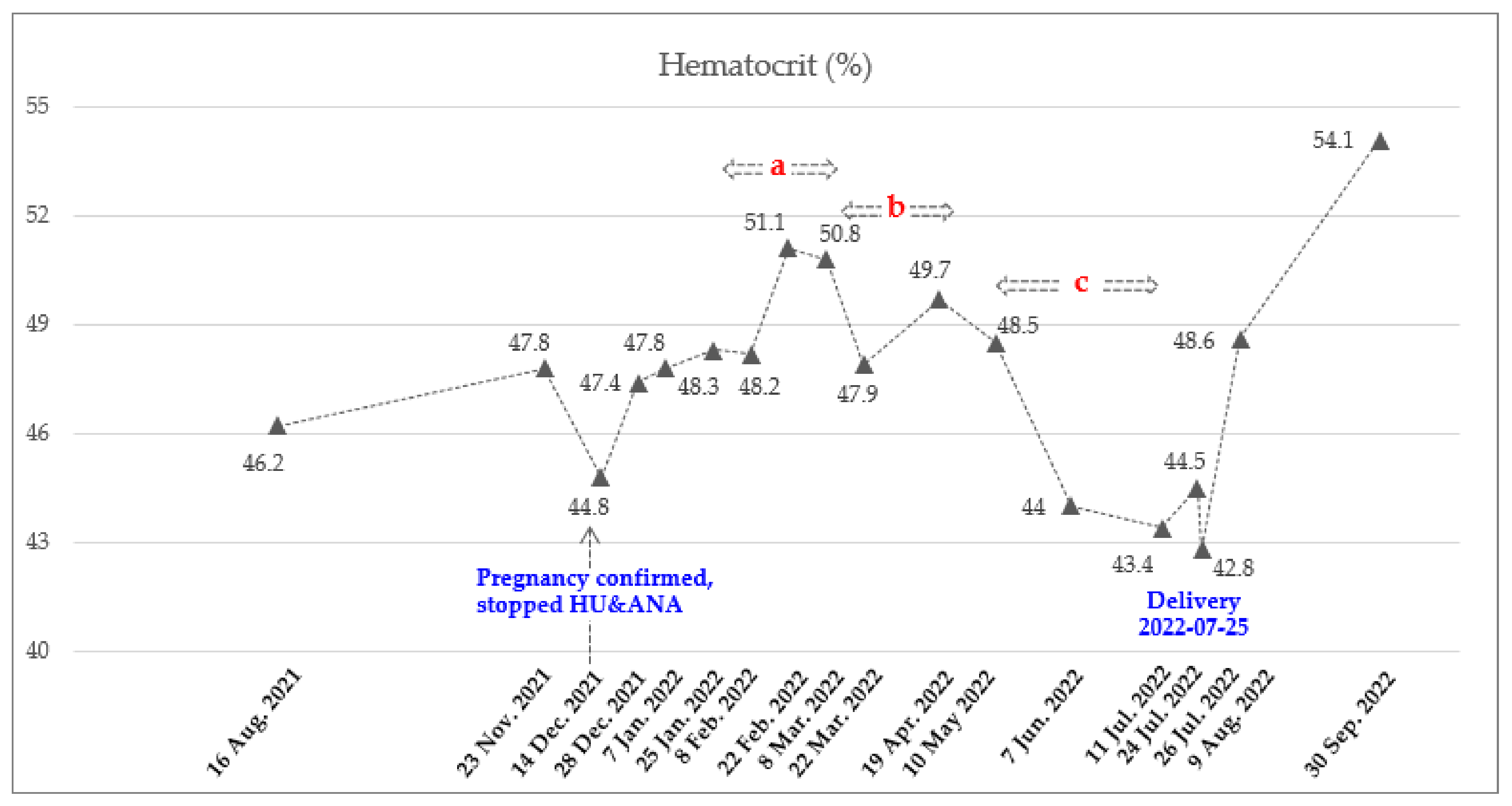
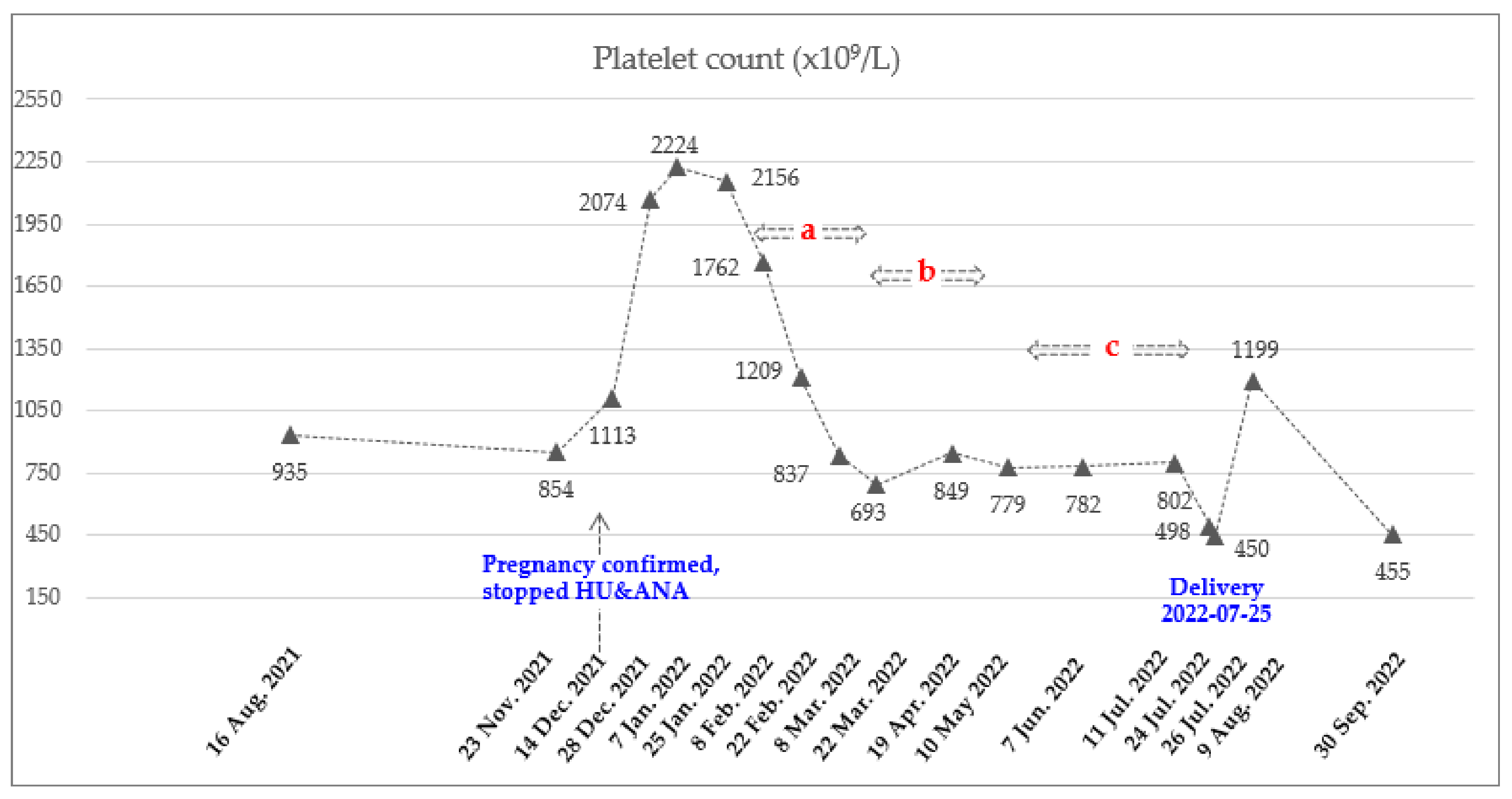
| Date | Hct (%) | PLT (×109/L) | Hb (g/dL) | WBC (×109/L) | ANC (×109/L) | Treatment Status |
|---|---|---|---|---|---|---|
| 18 December 2017 | 55.6 | 821 | 18.2 | 13.87 | At the time of diagnosis | |
| December 2020~November 2021 (Mean ± SD) | 46.3 ± 2.2 | 835 ± 130 | 13.9 ± 0.7 | 18.65 ± 0.58 | 13.88 ± 0.71 | Phlebotomy and HU, ANA |
| 14 December 2021 | 44.8 | 1113 | 13.8 | 21.93 | 17.98 | Pregnancy confirmed, stopped HU and ANA |
| 28 December 2021~25 January 2022 (Mean ± SD) | 47.8 ± 0.5 | 2151 ± 75 | 14.8 ± 0.5 | 28.24 ± 6.73 | 23.05 ± 6.62 | |
| 8 February 2022 | 48.2 | 1762 | 14.9 | 22.06 | 18.53 | Ropeginterferon alfa-2b 250 mcg |
| 22 February 2022 | 51.1 | 1209 | 15.9 | 14.23 | 10.67 | Ropeginterferon alfa-2b 350 mcg |
| 8 March 2022 | 50.8 | 837 | 15.8 | 10.17 | 7.42 | Ropeginterferon alfa-2b 500 mcg |
| 22 March 2022 | 47.9 | 693 | 15.2 | 10.51 | 8.09 | Ropeginterferon alfa-2b 500 mcg every 3 weeks (2 times) |
| 19 April 2022 | 49.7 | 849 | 15.9 | 14.2 | 11.79 | |
| 10 May 2022 ~11 July 2022 (Mean ± SD) | 45.3 ± 2.8 | 788 ± 13 | 14.7 ± 0.9 | 16.46 ± 2.83 | 13.54 ± 2.73 | Ropeginterferon alfa 2b 500 mcg every 4 weeks (3 times) Final date of injection 11 July 2022 |
| 24 July 2022 | 44.5 | 498 | 14.3 | 15.93 | 12.82 | LMWH (11 July 2022~23 July 2022) |
| 26 July 2022 | 42.8 | 450 | 14.1 | 14.46 | 11.96 | Delivery (25 July 2022) |
| 9 August 2022 | 48.6 | 1199 | 15.9 | 21.53 | 18.09 | Phlebotomy and HU, ANA |
| 30 September 2022 | 54.1 | 455 | 17.2 | 15.68 | 12.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bang, S.-Y.; Lee, S.-E. A Case Report of Ropeginterferon Alfa-2b for Polycythemia Vera during Pregnancy. Hematol. Rep. 2023, 15, 172-179. https://doi.org/10.3390/hematolrep15010018
Bang S-Y, Lee S-E. A Case Report of Ropeginterferon Alfa-2b for Polycythemia Vera during Pregnancy. Hematology Reports. 2023; 15(1):172-179. https://doi.org/10.3390/hematolrep15010018
Chicago/Turabian StyleBang, Su-Yeon, and Sung-Eun Lee. 2023. "A Case Report of Ropeginterferon Alfa-2b for Polycythemia Vera during Pregnancy" Hematology Reports 15, no. 1: 172-179. https://doi.org/10.3390/hematolrep15010018
APA StyleBang, S.-Y., & Lee, S.-E. (2023). A Case Report of Ropeginterferon Alfa-2b for Polycythemia Vera during Pregnancy. Hematology Reports, 15(1), 172-179. https://doi.org/10.3390/hematolrep15010018






