Triple-Negativity Identifies a Subgroup of Patients with Better Overall Survival in Essential Thrombocythemia
Abstract
:1. Introduction
2. Patients and Methods
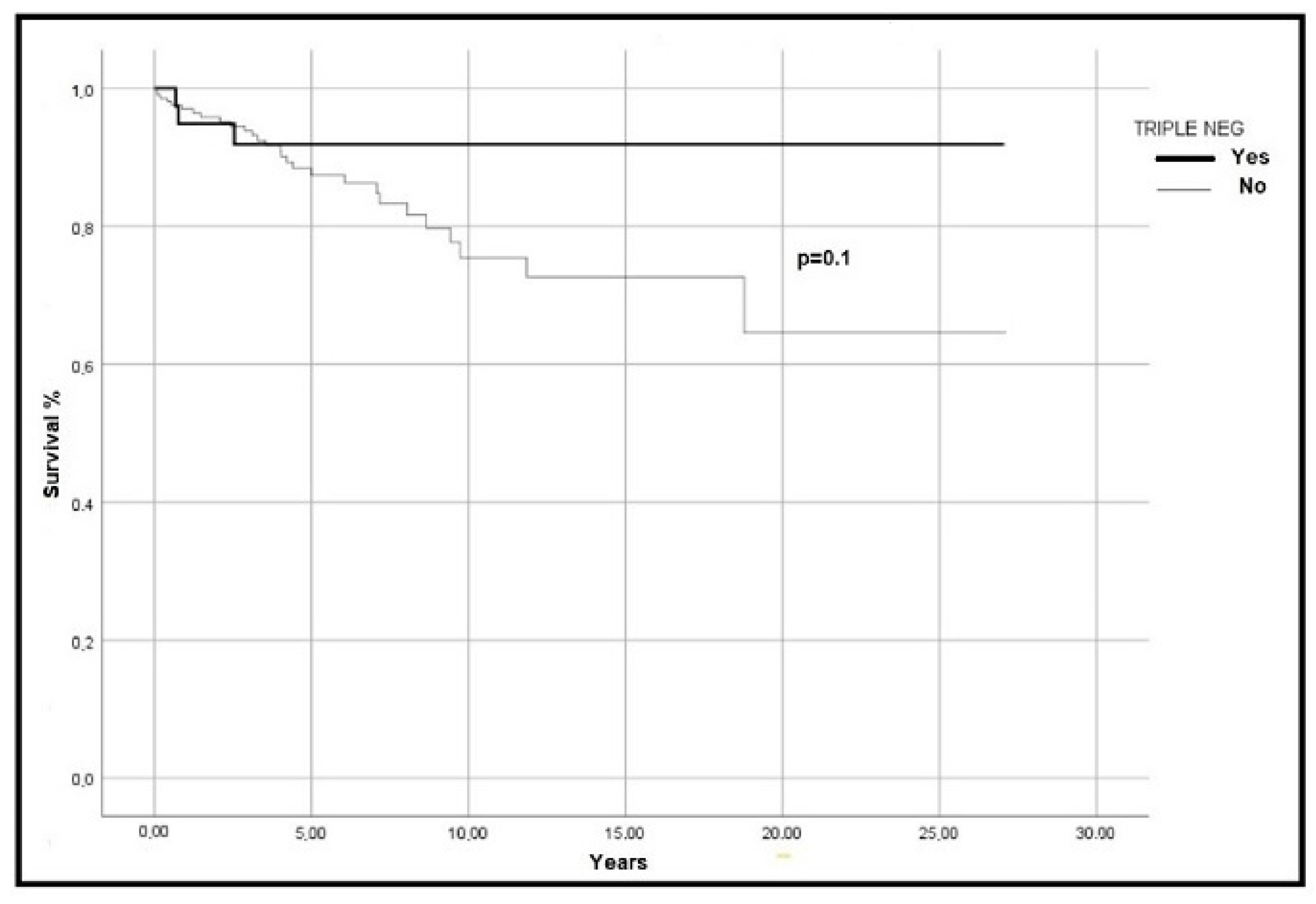
3. Results
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dameshek, W. Some speculations on the myeloproliferative syndromes. Blood 1951, 6, 372–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fritz, A.P.C.; Jack, A.; Shanmugaratnam, K.; Sobin, L.; Parkin, D.M.; Whelan, S. nternational Classification of Diseases for Oncology; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Kralovics, R.; Passamonti, F.; Buser, A.S.; Teo, S.-S.; Tiedt, R.; Passweg, J.R.; Tichelli, A.; Cazzola, M.; Skoda, R.C. A Gain-of-Function Mutation of JAK2 in Myeloproliferative Disorders. N. Engl. J. Med. 2005, 352, 1779–1790. [Google Scholar] [CrossRef] [Green Version]
- Pikman, Y.; Lee, B.H.; Mercher, T.; McDowell, E.; Ebert, B.L.; Gozo, M.; Cuker, A.; Wernig, G.; Moore, S.; Galinsky, I.; et al. MPLW515L Is a Novel Somatic Activating Mutation in Myelofibrosis with Myeloid Metaplasia. PLoS Med. 2006, 3, e270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardanani, A.D.; Levine, R.L.; Lasho, T.; Pikman, Y.; Mesa, R.A.; Wadleigh, M.; Steensma, D.P.; Elliott, M.A.; Wolanskyj, A.P.; Hogan, W.J.; et al. MPL515 mutations in myeloproliferative and other myeloid disorders: A study of 1182 patients. Blood 2006, 108, 3472–3476. [Google Scholar] [CrossRef] [Green Version]
- Klampfl, T.; Gisslinger, H.; Harutyunyan, A.S.; Nivarthi, H.; Rumi, E.; Milosevic, J.D.; Them, N.C.C.; Berg, T.; Gisslinger, B.; Pietra, D.; et al. Somatic Mutations of Calreticulin in Myeloproliferative Neoplasms. N. Engl. J. Med. 2013, 369, 2379–2390. [Google Scholar] [CrossRef] [Green Version]
- Vardiman, J.W.; Thiele, J.; Arber, D.A.; Brunning, R.D.; Borowitz, M.J.; Porwit, A.; Harris, N.L.; Le Beau, M.M.; Hellström-Lindberg, E.; Tefferi, A.; et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: Rationale and important changes. Blood 2009, 114, 937–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- Paz, D.L.; Jouanneau-Courville, R.; Riou, J.; Ianotto, J.-C.; Boyer, F.; Chauveau, A.; Renard, M.; Chomel, J.-C.; Cayssials, E.; Gallego-Hernanz, M.-P.; et al. Leukemic evolution of polycythemia vera and essential thrombocythemia: Genomic profiles predict time to transformation. Blood Adv. 2020, 4, 4887–4897. [Google Scholar] [CrossRef]
- Accurso, V.; Santoro, M.; Mancuso, S.; Napolitano, M.; Carlisi, M.; Mattana, M.; Russo, C.; Di Stefano, A.; Sirocchi, D.; Siragusa, S. The Essential Thrombocythemia in 2020: What We Know and Where We Still Have to Dig Deep. Clin. Med. Insights Blood Disord. 2020, 13, 2634853520978210. [Google Scholar] [CrossRef]
- Shallis, R.M.; Zeidan, A.M.; Wang, R.; Podoltsev, N.A. Epidemiology of the Philadelphia Chromosome-Negative Classical Myeloproliferative Neoplasms. Hematol. Oncol. Clin. N. Am. 2021, 35, 177–189. [Google Scholar] [CrossRef]
- Cervantes, F.; Tàssies, D.; Salgado, C.; Rovira, M.; Pereira, A.; Rozman, C. Acute Transformation in Nonleukemic Chronic Myeloproliferative Disorders: Actuarial Probability and Main Characteristics in a Series of 218 Patients. Acta Haematol. 1991, 85, 124–127. [Google Scholar] [CrossRef]
- Tefferi, A.; Barbui, T. Polycytemia vera and essential thrombocythemia: 2019 update on diagnosis, risk stratification and management. Am. J. Hematol. 2019, 94, 133–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesa, R.A.; Niblack, J.; Wadleigh, M.; Verstovsek, S.; Camoriano, J.K.; Barnes, S.A.; Tan, A.D.; Atherton, P.J.; Sloan, J.A.; Tefferi, A. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): An international Internet-based survey of 1179 MPD patients. Cancer. Cancer 2007, 109, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Andriani, A.; Latagliata, R.; Anaclerico, B.; Spadea, A.; Rago, A.; Di Veroli, A.; Spirito, F.; Porrini, R.; De Muro, M.; Leonetti, S.C.; et al. Spleen enlargement is a risk factor for thrombosis in essential thrombocythemia: Evaluation on 1297 patients. Am. J. Hematol. 2016, 91, 318–321. [Google Scholar] [CrossRef] [Green Version]
- Haider, M.; Gangat, N.; Lasho, T.; Hussein, A.K.A.; Elala, Y.C.; Hanson, C.; Tefferi, A. Validation of the revised international prognostic score of thrombosis for essential thrombocythemia (IPSET-thrombosis) in 585 Mayo clinic patients. Am. J. Hematol. 2016, 91, 390–394. [Google Scholar] [CrossRef] [Green Version]
- Tefferi, A.; Barbui, T. Polycythemia vera and essential thrombocythemia: 2021 update on diagnosis, risk-stratification and management. Am. J. Hematol. 2020, 95, 1599–1613. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Guglielmelli, P.; Larson, D.R.; Finke, C.; Wassie, E.A.; Pieri, L.; Gangat, N.; Fjerza, R.; Belachew, A.A.; Lasho, T.L.; et al. Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood 2014, 124, 2507–2513. [Google Scholar] [CrossRef]
- Emanuel, R.M.; Dueck, A.C.; Geyer, H.L.; Kiladjian, J.-J.; Slot, S.; Zweegman, S.; Te Boekhorst, P.A.W.; Commandeur, S.; Schouten, H.C.; Sackmann, F.; et al. Myeloproliferative Neoplasm (MPN) Symptom Assessment Form Total Symptom Score: Prospective International Assessment of an Abbreviated Symptom Burden Scoring System Among Patients With MPNs. J. Clin. Oncol. 2012, 30, 4098–4103. [Google Scholar] [CrossRef] [Green Version]
- Tremblay, D.; Yacoub, A.; Hoffman, R. Overview of Myeloproliferative Neoplasms. Hematol. Clin. N. Am. 2021, 35, 159–176. [Google Scholar] [CrossRef]
- Akcan, T.; Strati, P.; Yan, M.; Idowu, M. A Rare Case of Triple-Negative Essential Thrombocythemia in a Young Postsplenectomy Patient: A Diagnostic Challenge. Case Rep. Hematol. 2018, 2018, 9079462. [Google Scholar] [CrossRef]
- Accurso, V.; Santoro, M.; Raso, S.; Di Contrino, A.; Casimiro, P.; Di Piazza, F.; Perez, A.; Russo, A.; Siragusa, S. Splenomegaly impacts prognosis in essential thrombocythemia and polycythemia vera: A single center study. Hematol. Rep. 2019, 11, 8281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaidi, U.; Shahid, S.; Fatima, N.; Ahmed, S.; Sufaida, G.; Nadeem, M.; Shamsi, T. Genomic profile of a patient with triple negative essential thrombocythemia, unresponsive to therapy: A case report and literature review. J. Adv. Res. 2017, 8, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Ju, M.; Fu, R.; Li, H.; Liu, X.; Xue, F.; Chen, Y.; Liu, W.; Huang, Y.; Zhang, L.; Yang, R.; et al. Mutation profiling by targeted sequencing of “triple-negative” essential thrombocythaemia patients. Br. J. Haematol. 2018, 181, 857–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| All # (%) | Driver-Mut #(%) | Triple-Neg # (%) | p | |
|---|---|---|---|---|
| Patients | 266 (100) | 221 (83.1) | 45 (16.9) | - |
| Female sex | 180 (63.9) | 146 (60.1) | 34 (75.7) | 0.21 |
| Median age, years (range) | 60 (14.3–90.7) | 66.6 (18–90.7) | 52.3 (14.3–89.7) | 0.00028 |
| Median MPN10-TSS *, score (range) | 17 (4–23) | 19 (8–23) | 13 (4–19) | 0.001 |
| Thrombosis | 71 (26.69) | 59 (26.7) | 12 (26.66) | 0.52 |
| Splenomegaly | 21 (7.8) | 14 (6.7) | 7 (15.8) | 0.05 |
| MF evolution | 5 (1.8) | 4 (1.8) | 1 (2.2) | 0.45 |
| Death all cases | 31 (11.6) | 28 (12.7) | 3 (6.7) | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santoro, M.; Accurso, V.; Mancuso, S.; Napolitano, M.; Mattana, M.; Vajana, G.; Russello, F.; Siragusa, S. Triple-Negativity Identifies a Subgroup of Patients with Better Overall Survival in Essential Thrombocythemia. Hematol. Rep. 2022, 14, 265-269. https://doi.org/10.3390/hematolrep14030037
Santoro M, Accurso V, Mancuso S, Napolitano M, Mattana M, Vajana G, Russello F, Siragusa S. Triple-Negativity Identifies a Subgroup of Patients with Better Overall Survival in Essential Thrombocythemia. Hematology Reports. 2022; 14(3):265-269. https://doi.org/10.3390/hematolrep14030037
Chicago/Turabian StyleSantoro, Marco, Vincenzo Accurso, Salvatrice Mancuso, Mariasanta Napolitano, Marta Mattana, Giorgia Vajana, Federica Russello, and Sergio Siragusa. 2022. "Triple-Negativity Identifies a Subgroup of Patients with Better Overall Survival in Essential Thrombocythemia" Hematology Reports 14, no. 3: 265-269. https://doi.org/10.3390/hematolrep14030037
APA StyleSantoro, M., Accurso, V., Mancuso, S., Napolitano, M., Mattana, M., Vajana, G., Russello, F., & Siragusa, S. (2022). Triple-Negativity Identifies a Subgroup of Patients with Better Overall Survival in Essential Thrombocythemia. Hematology Reports, 14(3), 265-269. https://doi.org/10.3390/hematolrep14030037







