Changes in Hematologic Lab Measures Observed in Patients with Paroxysmal Nocturnal Hemoglobinuria Treated with C5 Inhibitors, Ravulizumab and Eculizumab: Real-World Evidence from a US Based EMR Network
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Patient Eligibility and Stratification
2.3. Study Outcomes
- LDH of at least 480 U/L (≥2 × ULN) and at least one new symptom or sign of intravascular hemolysis (i.e., fatigue, hemoglobinuria, abdominal pain, dyspnea, anemia (Hb < 10 g/dL), major adverse vascular event (including thrombosis), dysphagia, or erectile dysfunction) within one, three, or seven days of the elevated LDH.
- LDH of at least 480 U/L (≥2 × ULN) alone, regardless of other signs/symptoms.
- Elevated LDH (≥50% increase from baseline) and decreased Hb (Hb ≥ 2 g/dL from baseline) within one week of one another and measured within ≥4 months after index.
2.4. Follow-up and Event Rates
2.5. Treatment Switch
2.6. Statistical Analysis
3. Results
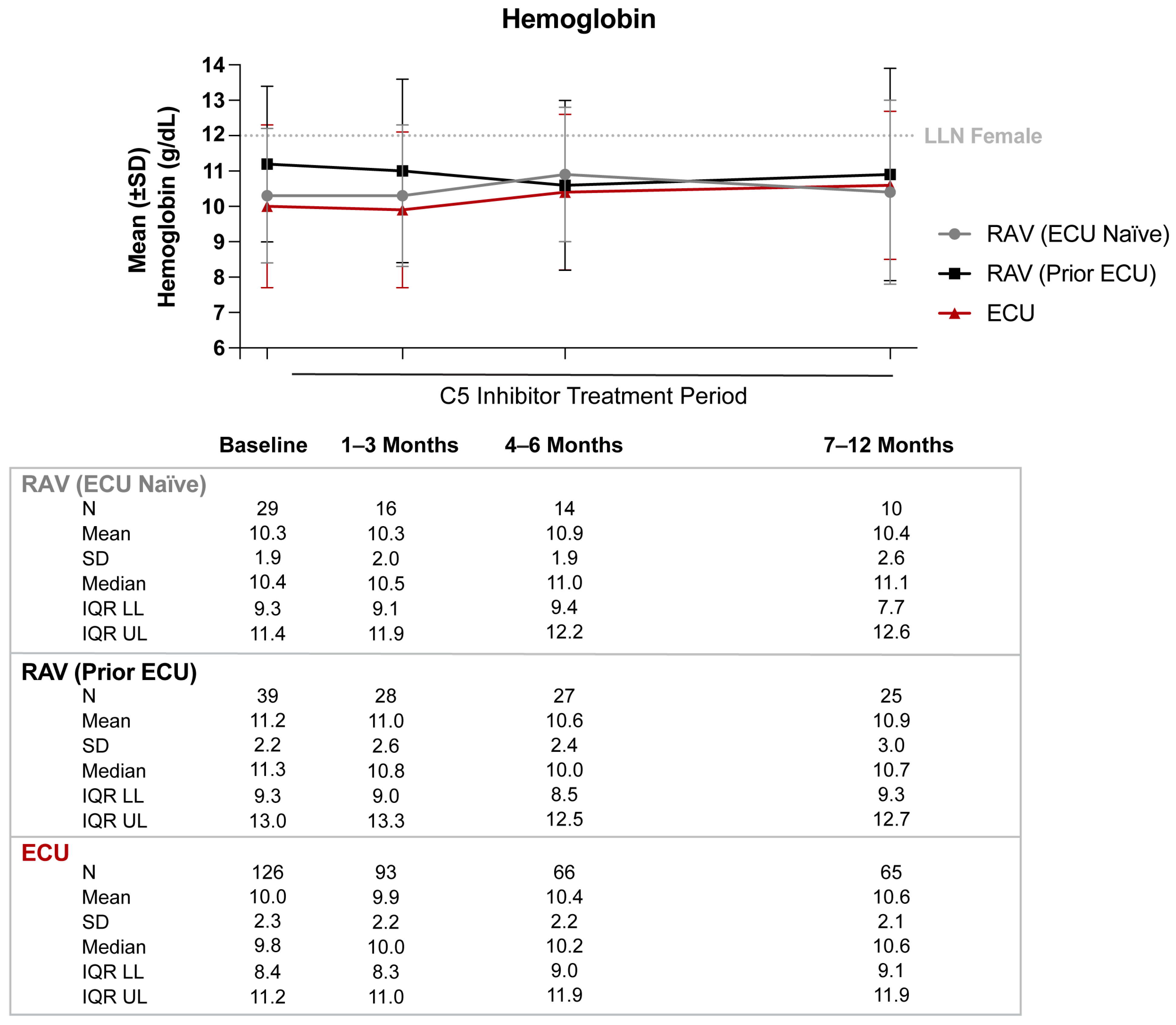
3.1. Hemoglobin
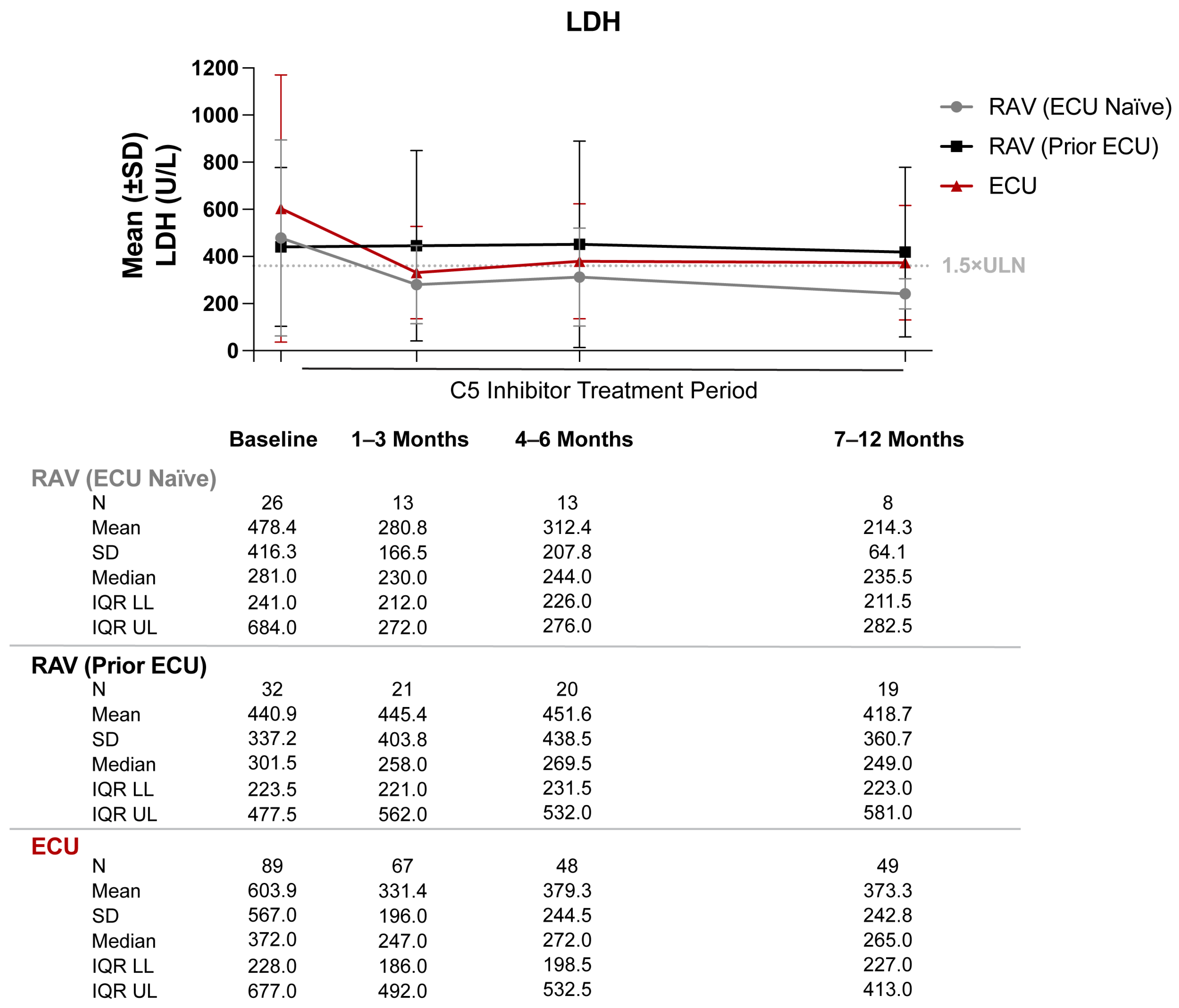
3.2. Lactate Dehydrogenase
3.3. Breakthrough Hemolysis and Complement-Amplifying Conditions
3.4. Absolute Reticulocyte Count
3.5. Long-Term Clinical Outcomes
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takeda, J.; Miyata, T.; Kawagoe, K.; Iida, Y.; Endo, Y.; Fujita, T.; Takahashi, M.; Kitani, T.; Kinoshita, T. Deficiency of the GPI anchor caused by a somatic mutation of the PIG-A gene in paroxysmal nocturnal hemoglobinuria. Cell 1993, 73, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Boccuni, P.; Del Vecchio, L.; Di Noto, R.; Rotoli, B. Glycosyl phosphatidylinositol (GPI)-anchored molecules and the pathogenesis of paroxysmal nocturnal hemoglobinuria. Crit. Rev. Oncol. Hematol. 2000, 33, 25–43. [Google Scholar] [CrossRef]
- de Latour, R.P.; Mary, J.Y.; Salanoubat, C.; Terriou, L.; Etienne, G.; Mohty, M.; Roth, S.; de Guibert, S.; Maury, S.; Cahn, J.Y.; et al. Paroxysmal nocturnal hemoglobinuria: Natural history of disease subcategories. Blood 2008, 112, 3099–3106. [Google Scholar] [CrossRef] [PubMed]
- Schrezenmeier, H.; Muus, P.; Socié, G.; Szer, J.; Urbano-Ispizua, A.; Maciejewski, J.P.; Brodsky, R.A.; Bessler, M.; Kanakura, Y.; Rosse, W.; et al. Baseline characteristics and disease burden in patients in the International Paroxysmal Nocturnal Hemoglobinuria Registry. Haematologica 2014, 99, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Omine, M.; Richards, S.; Nishimura, J.-I.; Bessler, M.; Ware, R.; Hillmen, P.; Luzzatto, L.; Young, N.; Kinoshita, T.; et al. Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood 2005, 106, 3699–3709. [Google Scholar] [CrossRef]
- Risitano, A.M.; Marotta, S.; Ricci, P.; Marano, L.; Frieri, C.; Cacace, F.; Sica, M.; Kulasekararaj, A.; Calado, R.T.; Scheinberg, P.; et al. Anti-complement Treatment for Paroxysmal Nocturnal Hemoglobinuria: Time for Proximal Complement Inhibition? A Position Paper from the SAAWP of the EBMT. Front. Immunol. 2019, 10, 1157. [Google Scholar] [CrossRef]
- Hillmen, P.; Young, N.S.; Schubert, J.; Brodsky, R.A.; Socié, G.; Muus, P.; Röth, A.; Szer, J.; Elebute, M.O.; Nakamura, R.; et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N. Engl. J. Med. 2006, 355, 1233–1243. [Google Scholar] [CrossRef]
- Drug Approval Package: Soliris (Eculizumab) NDA #125166: U.S. Food & Drug Administration. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/125166s0000toc.cfm (accessed on 10 January 2023).
- Kelly, R.J.; Hill, A.; Arnold, L.M.; Brooksbank, G.L.; Richards, S.; Cullen, M.; Mitchell, L.D.; Cohen, D.R.; Gregory, W.M.; Hillmen, P. Long-term treatment with eculizumab in paroxysmal nocturnal hemoglobinuria: Sustained efficacy and improved survival. Blood 2011, 117, 6786–6792. [Google Scholar] [CrossRef]
- Kulasekararaj, A.G.; Hill, A.; Rottinghaus, S.T.; Langemeijer, S.; Wells, R.; Gonzalez-Fernandez, F.A.; Gaya, A.; Lee, J.W.; Gutierrez, E.O.; Piatek, C.I.; et al. Ravulizumab (ALXN1210) vs eculizumab in C5-inhibitor-experienced adult patients with PNH: The 302 study. Blood 2019, 133, 540–549. [Google Scholar] [CrossRef]
- Lee, J.W.; de Fontbrune, F.S.; Lee, L.W.L.; Pessoa, V.; Gualandro, S.; Füreder, W.; Ptushkin, V.; Rottinghaus, S.T.; Volles, L.; Shafner, L.; et al. Ravulizumab (ALXN1210) vs eculizumab in adult patients with PNH naive to complement inhibitors: The 301 study. Blood 2019, 133, 530–539. [Google Scholar] [CrossRef]
- Socié, G.; Mary, J.-Y.; de Gramont, A.; Rio, B.; Leporrier, M.; Rose, C.; Heudier, P.; Rochant, H.; Cahn, J.-Y.; Gluckman, E. Paroxysmal nocturnal haemoglobinuria: Long-term follow-up and prognostic factors. French Society of Haematology. Lancet 1996, 348, 573–577. [Google Scholar] [CrossRef]
- Hillmen, P.; Muus, P.; Röth, A.; Elebute, M.O.; Risitano, A.M.; Schrezenmeier, H.; Szer, J.; Browne, P.; Maciejewski, J.P.; Schubert, J.; et al. Long-term safety and efficacy of sustained eculizumab treatment in patients with paroxysmal nocturnal haemoglobinuria. Br. J. Haematol. 2013, 162, 62–73. [Google Scholar] [CrossRef]
- McKinley, C.E.; Richards, S.J.; Munir, T.; Griffin, M.; Mitchell, L.D.; Arnold, L.; Riley, K.; Copeland, N.; Newton, D.J.; Hill, A.; et al. Extravascular Hemolysis due to C3-Loading in Patients with PNH Treated with Eculizumab: Defining the Clinical Syndrome. Blood 2017, 130, 3741. [Google Scholar]
- Debureaux, P.-E.; Kulasekararaj, A.G.; Cacace, F.; Silva, B.G.P.; Calado, R.T.; Barone, F.; de Fontbrune, F.S.; Prata, P.H.; Soret, J.; Sica, M.; et al. Categorizing hematological response to eculizumab in paroxysmal nocturnal hemoglobinuria: A multicenter real-life study. Bone Marrow Transplant. 2021, 56, 2600–2602. [Google Scholar] [CrossRef]
- Brookhart, M.A. Counterpoint: The treatment decision design. Am. J. Epidemiol. 2015, 182, 840–845. [Google Scholar] [CrossRef]
- Jenicek, M. Foundations of Evidence-Based Medicine, 2nd ed.; The Parthenon Publishing Group Limited: London, UK, 2003. [Google Scholar]
- Sardella, M.; Belcher, G. Pharmacovigilance of medicines for rare and ultrarare diseases. Ther. Adv. Drug Saf. 2018, 9, 631–638. [Google Scholar] [CrossRef]
- Song, J.W.; Chung, K.C. Observational studies: Cohort and case-control studies. Plast. Reconstr. Surg. 2010, 126, 2234–2242. [Google Scholar] [CrossRef]
- Taquet, M.; Geddes, J.R.; Husain, M.; Luciano, S.; Harrison, P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: A retrospective cohort study using electronic health records. Lancet Psychiatry 2021, 8, 416–427. [Google Scholar] [CrossRef]
- Topaloglu, U.; Palchuk, M.B. Using a Federated Network of Real-World Data to Optimize Clinical Trials Operations. JCO Clin. Cancer Inform. 2018, 2, 1–10. [Google Scholar] [CrossRef]
- Public Policy Committee, International Society of Pharmacoepidemiology. Guidelines for Good Pharmacoepidemiology Practice (GPP). Pharmacoepidemiol. Drug Saf. 2016, 25, 2–10. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. PLoS Med. 2007, 4, e296. [Google Scholar] [CrossRef] [PubMed]
- Hillmen, P.; Szer, J.; Weitz, I.; Röth, A.; Höchsmann, B.; Panse, J.; Usuki, K.; Griffin, M.; Kiladjian, J.-J.; de Castro, C.; et al. Pegcetacoplan versus Eculizumab in Paroxysmal Nocturnal Hemoglobinuria. N. Engl. J. Med. 2021, 384, 1028–1037. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, R.A.; de Latour, R.P.; Rottinghaus, S.T.; Röth, A.; Risitano, A.M.; Weitz, I.C.; Hillmen, P.; Maciejewski, J.P.; Szer, J.; Lee, J.W.; et al. Characterization of breakthrough hemolysis events observed in the phase 3 randomized studies of ravulizumab versus eculizumab in adults with paroxysmal nocturnal hemoglobinuria. Haematologica 2021, 106, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, R.A. How I treat paroxysmal nocturnal hemoglobinuria. Blood 2021, 137, 1304–1309. [Google Scholar] [CrossRef]
- Dickman, P.W.; Sloggett, A.; Hills, M.; Hakulinen, T. Regression models for relative survival. Stat. Med. 2004, 23, 51–64. [Google Scholar] [CrossRef]
- Rostgaard, K. Methods for stratification of person-time and events—A prerequisite for Poisson regression and SIR estimation. Epidemiol. Perspect. Innov. 2008, 5, 7. [Google Scholar] [CrossRef]
- Latimer, N.R.; Abrams, K.R. NICE Decision Support Unit Technical Support Documents; NICE DSU Technical Support Document 16: Adjusting Survival Time Estimates in the Presence of Treatment Switching; National Institute for Health and Care Excellence (NICE): London, UK, 2014. [Google Scholar]
- Latimer, N.R.; Abrams, K.; Lambert, P.; Crowther, M.; Wailoo, A.; Morden, J.; Akehurst, R.; Campbell, M. Adjusting for treatment switching in randomised controlled trials—A simulation study and a simplified two-stage method. Stat. Methods Med. Res. 2017, 26, 724–751. [Google Scholar] [CrossRef]
- Versmold, K.; Alashkar, F.; Raiser, C.; Ofori-Asenso, R.; Xu, T.; Liu, Y.; Katz, P.; Shang, A.; Roeth, A. Clinical Profile and Long-Term Outcomes of Patients with Paroxysmal Nocturnal Hemoglobinuria Treated with Eculizumab in a Real-World Setting: High Frequency of Anemia Despite Decreased Intravascular Hemolysis. Blood 2021, 138, 4314. [Google Scholar] [CrossRef]
- Lee, J.W.; De Latour, R.P.; Brodsky, R.A.; Jang, J.H.; Hill, A.; Röth, A.; Schrezenmeier, H.; Wilson, A.; Marantz, J.L.; Maciejewski, J.P. Effectiveness of eculizumab in patients with paroxysmal nocturnal hemoglobinuria (PNH) with or without aplastic anemia in the International PNH Registry. Am. J. Hematol. 2019, 94, E37–E41. [Google Scholar] [CrossRef]
- Hill, A.; Rother, R.P.; Arnold, L.; Kelly, R.; Cullen, M.J.; Richards, S.; Hillmen, P. Eculizumab prevents intravascular hemolysis in patients with paroxysmal nocturnal hemoglobinuria and unmasks low-level extravascular hemolysis occurring through C3 opsonization. Haematologica 2010, 95, 567–573. [Google Scholar] [CrossRef]
- Risitano, A.M.; Notaro, R.; Marando, L.; Serio, B.; Ranaldi, D.; Seneca, E.; Ricci, P.; Alfinito, F.; Camera, A.; Gianfaldoni, G.; et al. Complement fraction 3 binding on erythrocytes as additional mechanism of disease in paroxysmal nocturnal hemoglobinuria patients treated by eculizumab. Blood 2009, 113, 4094–4100. [Google Scholar] [CrossRef]
- Rondelli, T.; Risitano, A.M.; de Latour, R.P.; Sica, M.; Peruzzi, B.; Ricci, P.; Barcellini, W.; Iori, A.P.; Boschetti, C.; Valle, V.; et al. Polymorphism of the complement receptor 1 gene correlates with the hematologic response to eculizumab in patients with paroxysmal nocturnal hemoglobinuria. Haematologica 2014, 99, 262–266. [Google Scholar] [CrossRef]
- Cheng, W.Y.; Sarda, S.P.; Mody-Patel, N.; Krishnan, S.; Yenikomshian, M.; Mahendran, M.; Lejeune, D.; Yu, L.H.; Duh, M.S. Real-World Healthcare Resource Utilization (HRU) and Costs of Patients with Paroxysmal Nocturnal Hemoglobinuria (PNH) Receiving Eculizumab in a US Population. Adv. Ther. 2021, 38, 4461–4479. [Google Scholar] [CrossRef]
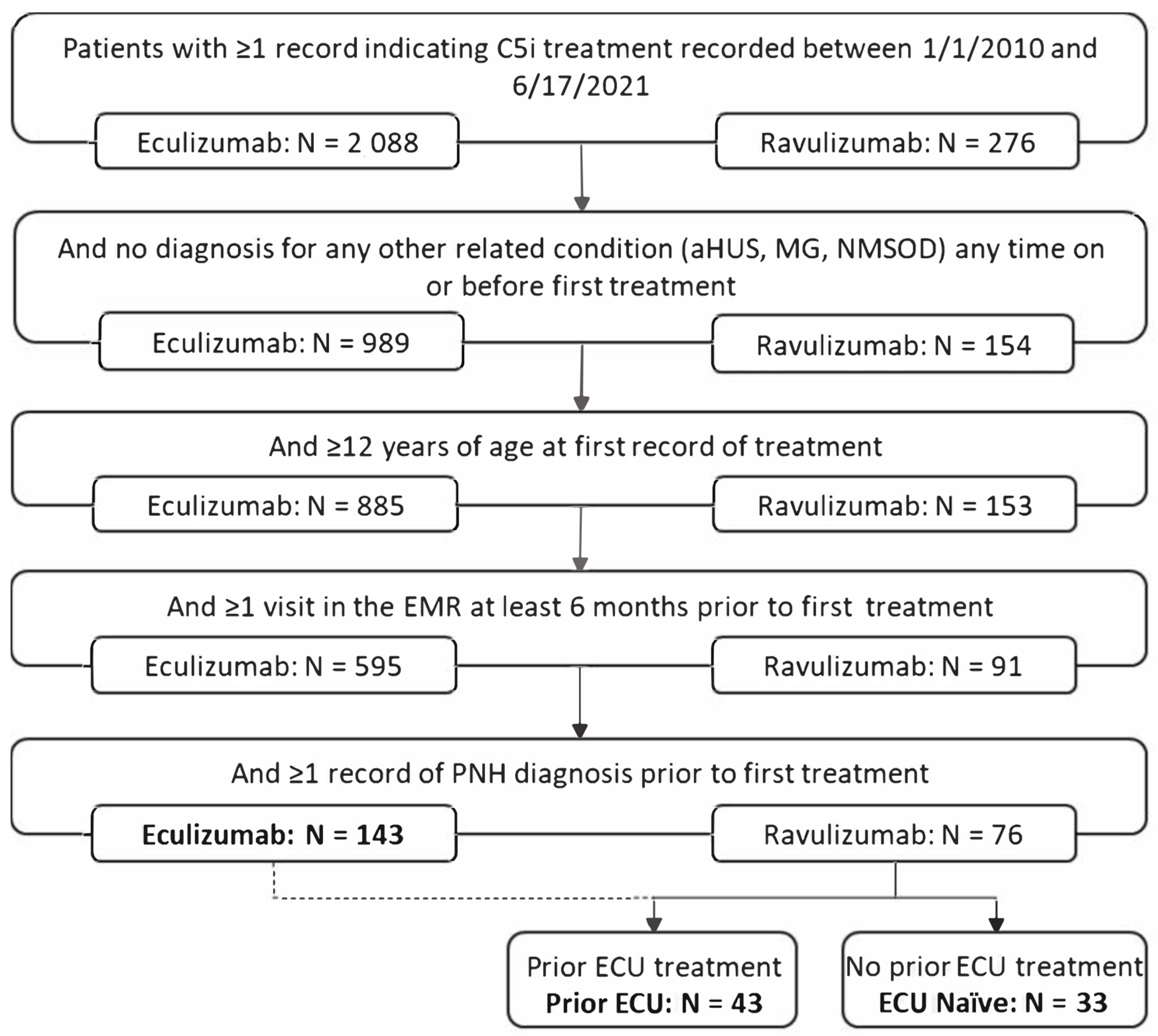

| RAV (ECU Naïve) N = 33 | RAV (Prior ECU) N = 43 | ECU N = 143 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n/ mean | %/ SD | LL 95% CI | UL 95% CI | n/ mean | %/ SD | LL 95% CI | UL 95% CI | n/ mean | %/ SD | LL 95% CI | UL 95% CI | |
| Age at index (mean, SD) | 51 | 16.7 | 45.3 | 56.7 | 44.2 | 15.6 | 39.5 | 48.9 | 42.6 | 17.2 | 39.8 | 45.4 |
| Age group at index (n, %) | ||||||||||||
| 12–17 | 0 | 0.0% | - | - | 0 | 0.0% | - | - | 5 | 3.5% | - | - |
| 18–34 | 7 | 21.2% | 7.3% | 35.2% | 11 | 25.6% | 12.5% | 38.6% | 48 | 33.6% | 25.8% | 41.3% |
| 35–64 | 20 | 60.6% | 43.9% | 77.3% | 26 | 60.5% | 45.9% | 75.1% | 70 | 49.0% | 40.8% | 57.1% |
| 65+ | 6 | 18.2% | 5.0% | 31.3% | 6 | 14.0% | 3.6% | 24.3% | 20 | 14.0% | 8.3% | 19.7% |
| Sex (n, %) | ||||||||||||
| Female | 18 | 54.5% | 37.6% | 71.5% | 26 | 60.5% | 45.9% | 75.1% | 79 | 55.2% | 47.1% | 63.4% |
| Male | 15 | 45.5% | 28.5% | 62.4% | 16 | 37.2% | 22.8% | 51.7% | 64 | 44.8% | 36.6% | 52.9% |
| Race (n, %) | ||||||||||||
| White | 24 | 72.7% | 57.5% | 87.9% | 27 | 62.8% | 48.3% | 77.2% | 93 | 65.0% | 57.2% | 72.9% |
| Black or African American | 5 | 15.2% | 2.9% | 27.4% | 6 | 14.0% | 3.6% | 24.3% | 23 | 16.1% | 10.1% | 22.1% |
| Other a | 1 | 3.0% | - | - | 2 | 4.7% | - | - | 5 | 3.5% | - | - |
| Unknown | 3 | 9.1% | −0.7% | 18.9% | 8 | 18.6% | 7.0% | 30.2% | 22 | 15.4% | 9.5% | 21.3% |
| US region (n, %) | ||||||||||||
| Northeast | 4 | 12.1% | 1.0% | 23.3% | 7 | 16.3% | 5.2% | 27.3% | 23 | 16.1% | 10.1% | 22.1% |
| Midwest | 5 | 15.2% | 2.9% | 27.4% | 7 | 16.3% | 5.2% | 27.3% | 20 | 14.0% | 8.3% | 19.7% |
| South | 19 | 57.6% | 40.7% | 74.4% | 13 | 30.2% | 16.5% | 44.0% | 62 | 43.4% | 35.2% | 51.5% |
| West | 5 | 15.2% | 2.9% | 27.4% | 15 | 34.9% | 20.6% | 49.1% | 38 | 26.6% | 19.3% | 33.8% |
| Clinical characteristics b (n, %) | ||||||||||||
| Any anemia | 20 | 60.6% | 43.9% | 77.3% | 28 | 65.1% | 50.9% | 79.4% | 88 | 61.5% | 53.6% | 69.5% |
| Aplastic anemia | 11 | 33.3% | 17.2% | 49.4% | 16 | 37.2% | 22.8% | 51.7% | 57 | 39.9% | 31.8% | 47.9% |
| Myelodysplastic syndrome | 2 | 6.1% | - | - | 1 | 2.3% | - | - | 11 | 7.7% | - | - |
| Hypertension | 7 | 21.2% | 7.3% | 35.2% | 7 | 16.3% | 5.2% | 27.3% | 21 | 14.7% | 8.9% | 20.5% |
| Thrombocytopenia | 11 | 33.3% | 17.2% | 49.4% | 15 | 34.9% | 20.6% | 49.1% | 47 | 32.9% | 25.2% | 40.6% |
| GERD | 4 | 12.1% | - | - | 3 | 7.0% | - | - | 15 | 10.5% | - | - |
| Venous embolism | 3 | 9.1% | −0.7% | 18.9% | 11 | 25.6% | 12.5% | 38.6% | 20 | 14.0% | 8.3% | 19.7% |
| Arterial embolism | 0 | 0.0% | - | - | 0 | 0.0% | - | - | 0 | 0.0% | - | - |
| Follow-up time (mean days, SD) | ||||||||||||
| Total available follow-up time | 690 | 971 | 358.7 | 1021.3 | 420 | 274 | 338.1 | 501.9 | 224 | 201 | 191.1 | 256.9 |
| Follow-up capped at 12 months | 201 | 161 | 146.1 | 255.9 | 270 | 133 | 230.2 | 309.8 | 184 | 135 | 161.9 | 206.1 |
| Treatment duration c | ||||||||||||
| Mean days, SD | 642 | 958 | 315.1 | 968.9 | 382 | 273 | 300.4 | 463.6 | 184 | 198 | 151.5 | 216.5 |
| Median days, IQR | 166 | 0–931 | - | - | 404 | 41–619 | - | - | 104 | 27–363 | - | - |
| RAV (ECU Naïve) N = 33 | RAV (Prior ECU) N = 43 | ECU N = 143 | ||||
|---|---|---|---|---|---|---|
| Hb performance 91–180 days post-index a | ||||||
| Patients with ≥1 Hb lab value, N | 14 | 27 | 63 | |||
| Average person-time until last Hb value in the time frame (months), mean (SD) | 5.1 | 0.8 | 5.0 | 0.7 | 5.2 | 0.8 |
| Patients with ≥1 transfusion, N (%) | 2 | 14.3% | 2 | 7.4% | 17 | 27.0% |
| Hb stabilization within 2 g/dL, N (%) | 10 | 71.4% | 20 | 74.1% | 42 | 66.7% |
| Hb stabilization within 1 g/dL, N (%) | 7 | 50.0% | 16 | 59.3% | 29 | 46.0% |
| Hb response; Hb ≥1 g/dL increase from baseline, N (%) | 3 | 21.4% | 5 | 18.5% | 19 | 30.2% |
| Hb normalization; Hb ≥12 g/dL, N (%) | 5 | 35.7% | 7 | 25.9% | 14 | 22.2% |
| Hb performance 181–365 days post-index b | ||||||
| Patients with ≥1 Hb lab value, N | 10 | 25 | 62 | |||
| Average person-time until last Hb value in the time frame (months), mean (SD) | 10.9 | 1.7 | 10.6 | 1.1 | 10.7 | 1.6 |
| Patients with ≥1 transfusion, N (%) | 3 | 30.0% | 2 | 8.0% | 20 | 32.3% |
| Hb stabilization within 2 g/dL, N (%) | 5 | 50.0% | 17 | 68.0% | 33 | 53.2% |
| Hb stabilization within 1 g/dL, N (%) | 3 | 30.0% | 12 | 48.0% | 15 | 24.2% |
| Hb response; Hb ≥1 g/dL increase from baseline, N (%) | 3 | 30.0% | 7 | 28.0% | 18 | 29.0% |
| Hb normalization; Hb ≥12 g/dL, N (%) | 5 | 50.0% | 11 | 44.0% | 11 | 17.7% |
| Patients with BTH | Person-Time (months) a | ||||
|---|---|---|---|---|---|
| PNH Expert Consensus Definitions of BTH [24] | N b | n | % [N/n] | Mean | SD |
| BTH defined as symptoms ±7 days c from elevated LDH d | |||||
| RAV (overall) e | |||||
| BTH after 6 months of treatment | 32 | 1 | 3.1% | 10.6 | 6.4 |
| BTH after 12 months of treatment | 23 | 1 | 4.3% | 7.1 | 4.8 |
| RAV (prior ECU) | |||||
| BTH 6 months after switch | 24 | 1 | 4.2% | 11.6 | 6.6 |
| BTH 12 months after switch | 18 | 1 | 5.6% | 8.1 | 4.8 |
| ECU | |||||
| BTH after 6 months of treatment | 55 | 14 | 25.5% | 28.4 | 31.8 |
| BTH after 12 months of treatment | 41 | 10 | 24.4% | 30.6 | 31.6 |
| BTH defined only as elevated LDH f | |||||
| RAV (overall) e | |||||
| BTH after 6 months of treatment | 32 | 5 | 15.6% | 9.8 | 6.7 |
| BTH after 12 months of treatment | 23 | 3 | 13.0% | 6.6 | 5.0 |
| RAV (prior ECU) | |||||
| BTH 6 months after switch | 24 | 5 | 20.8% | 10.5 | 7.2 |
| BTH 12 months after switch | 18 | 3 | 16.7% | 7.4 | 5.3 |
| ECU | |||||
| BTH after 6 months of treatment | 55 | 34 | 61.8% | 23.2 | 30.2 |
| BTH after 12 months of treatment | 41 | 24 | 58.5% | 26.6 | 29.6 |
| BTH defined as elevated LDH and decrease in Hb g | |||||
| RAV (overall) e | |||||
| BTH after 4 months of treatment | 32 | 3 | 9.4% | 11.3 | 7.0 |
| RAV (prior ECU) | |||||
| BTH 4 months after switch | 20 | 2 | 10.0% | 13.3 | 6.7 |
| ECU | |||||
| BTH after 4 months of treatment | 44 | 12 | 27.3% | 32.0 | 32.9 |
| Ravulizumab-Treated Patients a N = 76 | Eculizumab-Treated Patients N = 143 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 6-Month BTH | 12-Month BTH | 4-Month BTH | 6-Month BTH | 12-Month BTH | 4-Month BTH | |||||
| Number of patients eligible for BTH assessment | N = 32 | N = 23 | N = 32 | N = 55 | N = 41 | N = 44 | ||||
| Symptoms ± 7 days from elevated LDH b | Elevated LDH c | Symptoms ± 7 days from elevated LDH b | Elevated LDH c | Elevated LDH and Hb decrease d | Symptoms ± 7 days from elevated LDH b | Elevated LDH c | Symptoms ± 7 days from elevated LDH b | Elevated LDH c | Elevated LDH and Hb decrease d | |
| Number of patients with BTH | n = 1 | n = 5 | n = 1 | n = 3 | n = 3 | n = 14 | n = 34 | n = 10 | n = 24 | n = 12 |
| Average person-time among those experiencing BTH (months) e, mean SD | 7.3 (-) | 7.0 (1.0) | 13.0 (-) | 12.8 (0.7) | 8.2 (3.4) | 20.3 (19.4) | 19.4 (20.0) | 26.1 (20.4) | 28.6 (19.7) | 17.7 (12.8) |
| Any CAC within 15 days of BTH f, n (%) | 1 (100.0%) | 3 (60.0%) | 1 (100.0%) | 1 (33.3%) | 2 (66.7%) | 11 (78.6%) | 21 (61.8%) | 6 (60.0%) | 13 (54.2%) | 8 (66.7%) |
| Infection g | 1 (100.0%) | 2 (40.0%) | 0 (0.0%) | 0 (0.0%) | 1 (33.3%) | 6 (42.9%) | 10 (29.4%) | 4 (40.0%) | 8 (33.3%) | 4 (33.3%) |
| Upper respiratory tract infection | 1 (100.0%) | 2 (40.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 3 (21.4%) | 3 (8.8%) | 2 (20.0%) | 3 (12.5%) | 1 (8.3%) |
| Surgery h | 1 (100.0%) | 2 (40.0%) | 1 (100.0%) | 1 (33.3%) | 2 (66.7%) | 9 (64.3%) | 16 (47.1%) | 4 (40.0%) | 9 (37.5%) | 6 (50.0%) |
| Trauma i | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (7.1%) | 0 (0.0%) | 1 (10.0%) | 0 (0.0%) | 0 (0.0%) |
| Pregnancy j | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (2.9%) | 0 (0.0%) | 1 (4.2%) | 0 (0.0%) |
| Organ or hematopoietic stem cell transplantation k | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (7.1%) | 2 (5.9%) | 1 (10.0%) | 1 (4.2%) | 0 (0.0%) |
| Acute transfusion reaction l | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (14.3%) | 1 (2.9%) | 1 (10.0%) | 0 (0.0%) | 1 (8.3%) |
| RAV-Treated Patients a N = 76 | ECU-Treated Patients N = 143 | |||||
|---|---|---|---|---|---|---|
| ≥1 Event 30 Days after Index b | ≥2 Events 30 Days after Index b,c | ≥1 Event 30 Days after Index b | ≥2 Events 30 Days after Index b,c | |||
| n (%) | Person-Time, Months (SD) | n (%) | n (%) | Person-Time, Months (SD) | n (%) | |
| Thrombosis d | 10 (13.2%) | 11.9 (9.0) | 7 (70.0%) | 22 (15.4%) | 25.9 (28.0) | 17 (77.3%) |
| Cardiovascular e | 2 (2.6%) | 14.0 (8.8) | 1 (50.0%) | 4 (2.8%) | 28.2 (29.6) | 3 (75.0%) |
| Persistent anemia f | 17 (22.4%) | 11.2 (8.7) | 17 (100%) | 38 (26.6%) | 25.5 (27.7) | 38 (100%) |
| Kidney disease g | 7 (9.2%) | 13.1 (9.0) | 5 (71.4%) | 27 (18.9%) | 20.7 (27.4) | 19 (70.4%) |
| Kidney injury h | 2 (2.6%) | 13.9 (8.7) | 1 (50.0%) | 15 (10.5%) | 25.6 (29.3) | 8 (53.3%) |
| Iron overload i | 4 (5.3%) | 13.3 (8.7) | 4 (100.0%) | 8 (5.6%) | 28.4 (29.6) | 8 (100.0%) |
| Infection j | 15 (19.7%) | 10.8 (8.9) | 6 (40.0%) | 35 (24.5%) | 22.1 (25.6) | 23 (65.7%) |
| All-cause mortality k | 1 (1.3%) | 8.6 (-) | -- | 10 (7.0%) | 15.1 (19.3) | -- |
| Infusion reaction l | 17 (22.4%) | 11.1 (8.7) | 5 (29.4%) | 34 (23.8%) | 23.3 (25.8) | 26 (76.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fishman, J.; Kuranz, S.; Yeh, M.M.; Brzozowski, K.; Chen, H. Changes in Hematologic Lab Measures Observed in Patients with Paroxysmal Nocturnal Hemoglobinuria Treated with C5 Inhibitors, Ravulizumab and Eculizumab: Real-World Evidence from a US Based EMR Network. Hematol. Rep. 2023, 15, 266-282. https://doi.org/10.3390/hematolrep15020027
Fishman J, Kuranz S, Yeh MM, Brzozowski K, Chen H. Changes in Hematologic Lab Measures Observed in Patients with Paroxysmal Nocturnal Hemoglobinuria Treated with C5 Inhibitors, Ravulizumab and Eculizumab: Real-World Evidence from a US Based EMR Network. Hematology Reports. 2023; 15(2):266-282. https://doi.org/10.3390/hematolrep15020027
Chicago/Turabian StyleFishman, Jesse, Seth Kuranz, Michael M. Yeh, Kaylen Brzozowski, and Herman Chen. 2023. "Changes in Hematologic Lab Measures Observed in Patients with Paroxysmal Nocturnal Hemoglobinuria Treated with C5 Inhibitors, Ravulizumab and Eculizumab: Real-World Evidence from a US Based EMR Network" Hematology Reports 15, no. 2: 266-282. https://doi.org/10.3390/hematolrep15020027
APA StyleFishman, J., Kuranz, S., Yeh, M. M., Brzozowski, K., & Chen, H. (2023). Changes in Hematologic Lab Measures Observed in Patients with Paroxysmal Nocturnal Hemoglobinuria Treated with C5 Inhibitors, Ravulizumab and Eculizumab: Real-World Evidence from a US Based EMR Network. Hematology Reports, 15(2), 266-282. https://doi.org/10.3390/hematolrep15020027






