Shade as an Agro-Technique to Improve Gas Exchange, Productivity, Bioactive Potential, and Antioxidant Activity of Fruits of Hylocereus costaricensis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growing Conditions
2.2. Experimental Design and Shade Conditions
2.3. Physiological Performance
2.3.1. Nighttime CO2 Assimilation
2.3.2. Total Carotenoids
2.4. Productivity Estimation
2.5. Post-Harvest Evaluation
2.5.1. Total Soluble Sugars
2.5.2. Bioactive Potential and Antioxidant Activity
2.6. Statistical Analysis
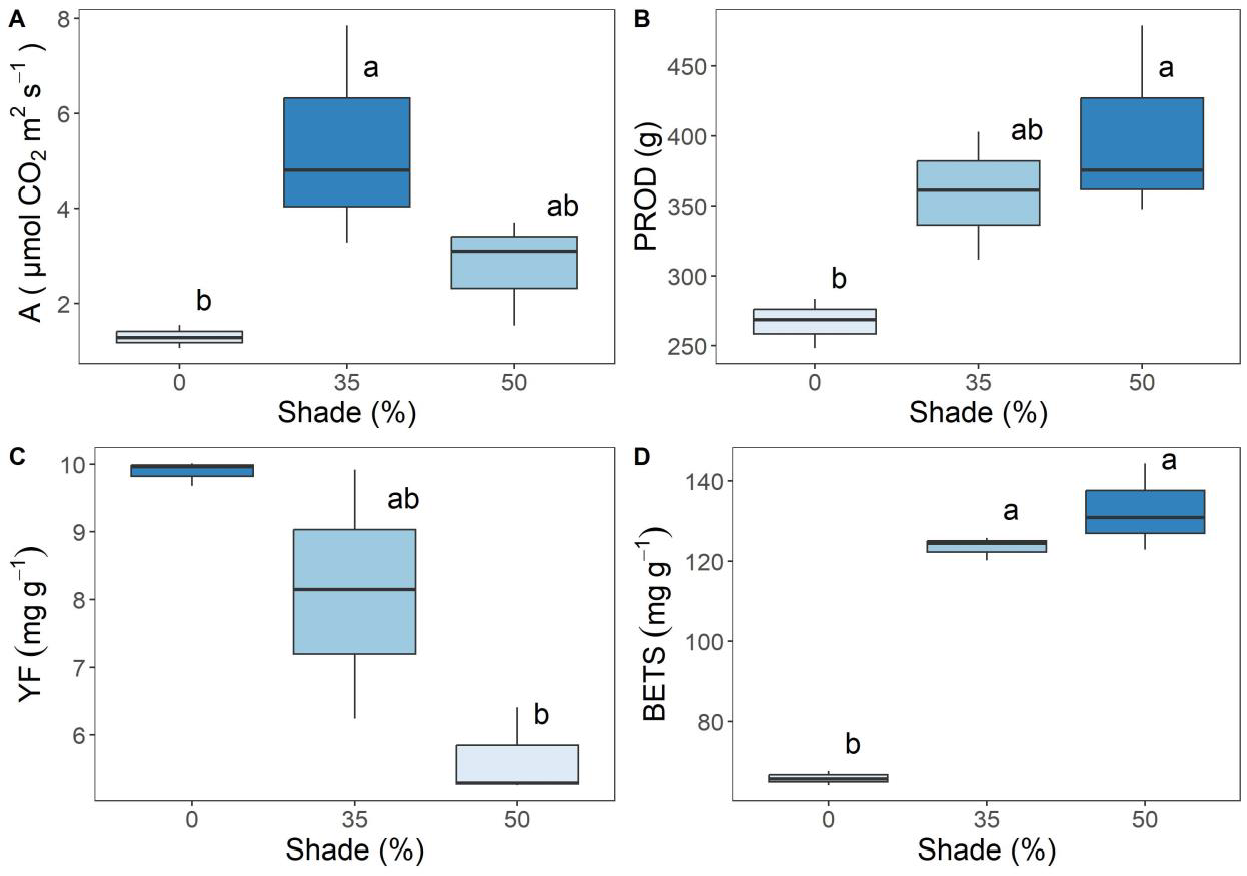
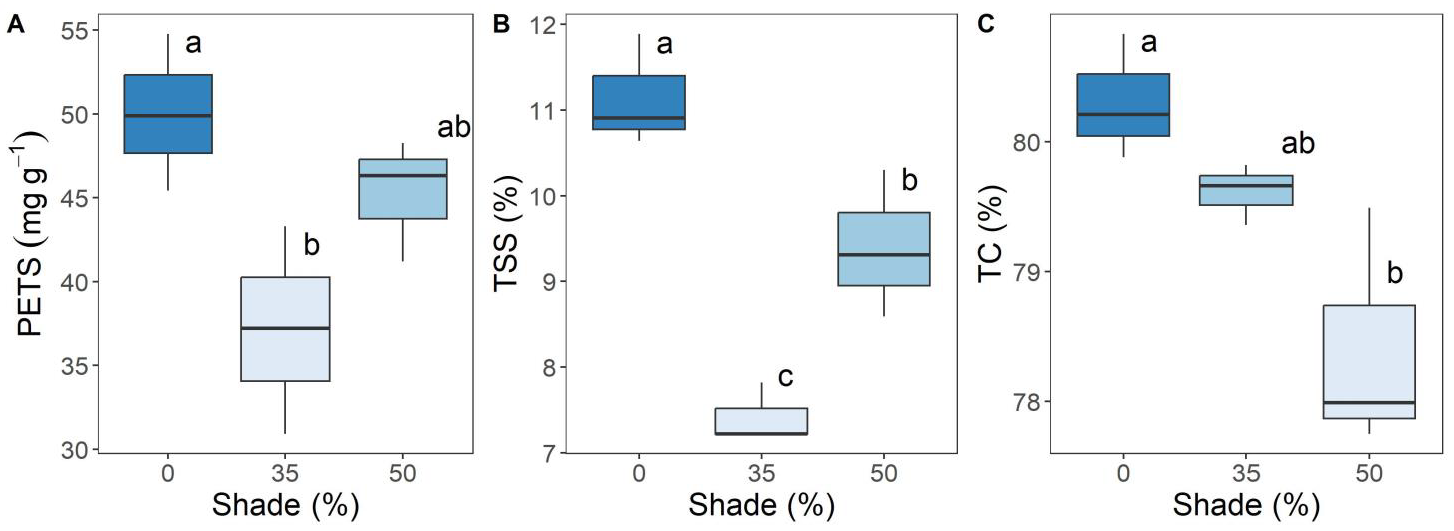
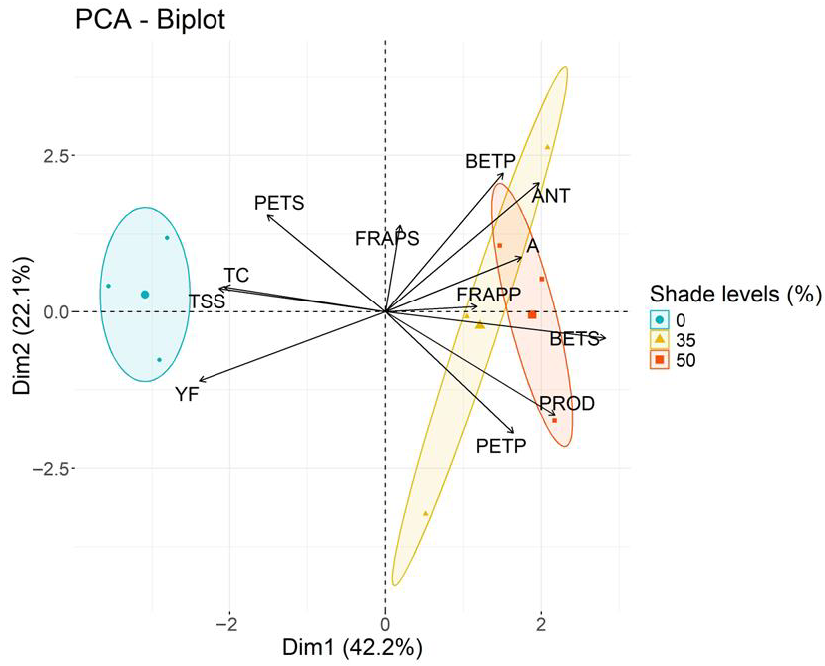
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Oliveira, M.M.T.; Tel-Zur, N. Cactus pear and pitaya: Fruit production and orchard management. Acta Hortic. 2022, 343–354. [Google Scholar] [CrossRef]
- Ferreres, F.; Grosso, C.; Gil-Izquierdo, A.; Valentão, P.; Mota, A.T.; Andrade, P.B. Optimization of the recovery of high-value compounds from pitaya fruit by-products using microwave-assisted extraction. Food Chem. 2017, 230, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Le Bellec, F.; Vaillant, F.; Imbert, E. Pitahaya (Hylocereus spp.): A new fruit crop, a market with a future. Fruits 2006, 61, 237–250. [Google Scholar] [CrossRef]
- Sim Choo, W.; Khing Yong, W. Antioxidant properties of two species of Hylocereus fruits. Adv. Appl. Sci. Res. 2011, 2, 418–425. [Google Scholar]
- Song, H.; Zheng, Z.; Wu, J.; Lai, J.; Chu, Q.; Zheng, X. White Pitaya (Hylocereus undatus) Juice Attenuates Insulin Resistance and Hepatic Steatosis in Diet-Induced Obese Mice. PLoS ONE 2016, 11, e0149670. [Google Scholar] [CrossRef]
- Chen, S.Y.; Xu, C.Y.; Mazhar, M.S.; Naiker, M. Nutritional Value and Therapeutic Benefits of Dragon Fruit: A Comprehensive Review with Implications for Establishing Australian Industry Standards. Molecules 2024, 29, 5676. [Google Scholar] [CrossRef]
- Arivalagan, M.; Karunakaran, G.; Roy, T.K.; Dinsha, M.; Sindhu, B.C.; Shilpashree, V.M.; Satisha, G.C.; Shivashankara, K.S. Biochemical and nutritional characterization of dragon fruit (Hylocereus species). Food Chem. 2021, 353, 129426. [Google Scholar] [CrossRef]
- Tarte, I.; Singh, A.; Dar, A.H.; Sharma, A.; Altaf, A.; Sharma, P. Unfolding the potential of dragon fruit (Hylocereus spp.) for value addition: A review. eFood 2023, 4, e76. [Google Scholar] [CrossRef]
- Chen, S.Y.; Islam, M.A.; Johnson, J.B.; Xu, C.Y.; Mazhar, M.S.; Naiker, M. Comparative Analysis of Shelf-Life, Antioxidant Activity, and Phytochemical Contents of Australian-Grown and Imported Dragon Fruit under Ambient Conditions. Horticulturae 2024, 10, 1048. [Google Scholar] [CrossRef]
- Bishoyi, A.K.; Saeed, F.; Shehzadi, U.; Shankar, A.; Balaji, J.; Kaur, J.; Afzaal, M.; Imran, A.; Rasheed, M.; Hussain, B.; et al. Nutritional composition, phytochemical profile, and health benefits of Hylocereus Undatus (pitaya): A comprehensive review. eFood 2024, 5, e70017. [Google Scholar] [CrossRef]
- Nobel, P.S.; De la Barrera, E. High Temperatures and Net CO2 Uptake, Growth, and Stem Damage for the Hemiepiphytic Cactus Hylocereus undatus1. Biotropica 2002, 34, 225–231. [Google Scholar] [CrossRef]
- Nobel, P.S. Cacti: Biology and Uses; University of California Press: London, UK, 2002; ISBN 0520231570. Available online: https://books.google.co.il/books/about/Cacti.html?id=DISMqbY-igwC&redir_esc=y (accessed on 19 September 2019).
- de Oliveira, M.M.T.; Shuhua, L.; Kumbha, D.S.; Zurgil, U.; Raveh, E.; Tel-Zur, N. Performance of Hylocereus (Cactaceae) species and interspecific hybrids under high-temperature stress. Plant Physiol. Biochem. 2020, 153, 30–39. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.M.T.; Albano-Machado, F.G.; Penha, D.M.; Pinho, M.M.; Natale, W.; de Miranda, M.R.A.; Moura, C.F.H.; Alves, R.E.; de Medeiros Corrêa, M.C. Shade improves growth, photosynthetic performance, production and postharvest quality in red pitahaya (Hylocereus costaricensis). Sci. Hortic. 2021, 286, 110217. [Google Scholar] [CrossRef]
- Larcher, W. Ecofisiologia Vegetal; RiMa Editora: São Paulo, Brazil, 2006; Available online: https://www.martinsfontespaulista.com.br/ecofisiologia-vegetal-206202/p (accessed on 30 June 2025).
- Victório, C.P.; Kuster, R.M.; Lage, C.L.S. Qualidade de Luz e Produção de Pigmentos Fotossintéticos em Plantas In Vitro de Phyllanthus tenellus Roxb. Rev. Bras. Biociências 2007, 5, 213–215. [Google Scholar]
- Streit, N.M.; Canterle, L.P.; do Canto, M.W.; Hecktheuer, L.H.R. The chlorophylls. Ciência Rural 2005, 35, 748–755. [Google Scholar] [CrossRef]
- Almeida, E.I.B.; Corrêa, M.C.M.; Mesquita, R.O.; Queiroz, R.F.; Cajazeira, J.P.; Amorim, F.F.V. Growth and gas exchanges of red pitaya under different shading conditions. Rev. Bras. Ciências Agrárias-Brazilian J. Agric. Sci. 2018, 13, 1–8. [Google Scholar] [CrossRef]
- Tel-Zur, N. Vine cacti (Hylocereus species): An emerging fruit crop. Italus Hortus 2017, 24, 19–24. [Google Scholar] [CrossRef]
- Mizrahi, Y. Vine-cacti pitayas: The new crops of the world. Rev. Bras. Frutic. 2014, 36, 124–138. [Google Scholar] [CrossRef]
- Raveh, E.; Nerd, A.; Mizrahi, Y. Responses of two hemiepiphytic fruit crop cacti to different degrees of shade. Sci. Hortic. 1998, 73, 151–164. [Google Scholar] [CrossRef]
- Raveh, E.; Gersani, M.; Nobel, P.S. CO2 uptake and fluorescence responses for a shade-tolerant cactus Hylocereus undatus under current and doubled CO2 concentrations. Physiol. Plant. 1995, 93, 505–511. [Google Scholar] [CrossRef]
- Köppen, W. Grundriss der Klimakunde: Outline of Climate Science; Walter de Gruyter: Berlin, Germany, 1931. [Google Scholar]
- Almeida, E.I.B.; Corrêa, M.C.d.M.; Crisostomo, L.A.; de Araújo, N.A.; do Vale Silva, J.C. Nitrogênio e potássio no crescimento de mudas de pitaia [Hylocereus undatus (Haw.) Britton & Rose]. Rev. Bras. Frutic. 2014, 36, 1018–1027. [Google Scholar] [CrossRef][Green Version]
- Corrêa, M.C.d.M.; Almeida, E.I.B.; Marques, V.B.; do Vale Silva, J.C.; de Aquino, B.F. Crescimento inicial de pitaia em função de combinações de doses de fósforo-zinco. Rev. Bras. Frutic. 2014, 36, 261–270. [Google Scholar] [CrossRef][Green Version]
- Cajazeira, J.P.; Corrêa, M.C.d.M.; Almeida, E.I.B.; Queiroz, R.F.; Mesquita, R.O. Growth and gas exchange in white pitaya under different concentrations of potassium and calcium. Rev. Ciência Agronômica 2018, 49. [Google Scholar] [CrossRef]
- Mizrahi, Y.; Nerd, A. Climbing and Columnar Cacti: New Arid Land Fruit Crops. Perspect New Crop New Uses 1999, 1, 358–366. [Google Scholar]
- Wintermans, J.F.G.M.; De Mots, A. Spectrophotometric characteristics of chlorophylls a and b and their phenophytins in ethanol. BBA-Biophys. Incl. Photosynth. 1965, 109, 448–453. [Google Scholar] [CrossRef]
- Yem, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef]
- Francis, J.F. Analysis of Anthocyanins. In Anthocyanins. In Anthocyanins as Food Colors; Elsevier Science: Amsterdam, The Netherlands, 1982; p. 280. ISBN 9780323157902. [Google Scholar]
- Lim, S.D.; Yusof, Y.A.; Chin, N.L.; Talib, R.A.; Endan, J.; Aziz, M.G. Effect of extraction parameters on the yield of betacyanins from pitaya fruit (Hylocereus polyrhizus) pulps. J. Food Agric. Environ. 2011, 9, 158–162. [Google Scholar]
- Larrauri, J.A.; Rupérez, P.; Saura-Calixto, F. Effect of Drying Temperature on the Stability of Polyphenols and Antioxidant Activity of Red Grape Pomace Peels. J. Agric. Food Chem. 1997, 45, 1390–1393. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- R Core Team. The R Project for Statistical Computing. Available online: https://www.r-project.org/?utm_source=chatgpt.com. (accessed on 30 June 2025).
- Hadley, W.; Chang, W.; Henry, L.; Pedersen, T.L.; Wilk, C.; Woo, K.; Yutani, H.; Dunnington, D.; Brand, T.V.D. Create Elegant Data Visualisations Using the Grammar of Graphics. Available online: https://ggplot2.tidyverse.org (accessed on 30 June 2025).
- Graves, S.; Piepho, H.P.; Selzer, P.; Dorai-Raj, S. multcompView: Visualizations of Paired Comparisons. Available online: https://CRAN.R-project.org/package=multcompView (accessed on 30 June 2025).
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. A Grammar of Data Manipulation. Available online: https://github.com/tidyverse/dplyr (accessed on 30 June 2025).
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. factoextra: Extract and Visualize the Results of Multivariate Data Analyses. Available online: https://cran.r-project.org/package=factoextra (accessed on 30 June 2025).
- Wakchaure, G.C.; Minhas, P.S.; Kumar, S.; Mane, P.; Suresh Kumar, P.; Rane, J.; Pathak, H. Long-term response of dragon fruit (Hylocereus undatus) to transformed rooting zone of a shallow soil improving yield, storage quality and profitability in a drought prone semi-arid agro-ecosystem. Saudi J. Biol. Sci. 2022, 30, 103497. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, Í.H.L.; Martins, A.B.G.; Da Silva JúNior, G.B.; Da Rocha, L.F.; Raimundo, F.N.; Lourival, F.C. Adubação orgânica e intensidade luminosa no crescimento e desenvolvimento inicial da Pitaya em Bom Jesus-PI. Rev. Bras. Frutic. 2011, 33, 970–983. [Google Scholar] [CrossRef][Green Version]
- Nobel, P.S.; De la Barrera, E. CO2 uptake by the cultivated hemiepiphytic cactus, Hylocereus undatus. Ann. Appl. Biol. 2004, 144, 1–8. [Google Scholar] [CrossRef]
- Andrade, J.L.; Rengifo, E.; Ricalde, M.F.; Simá, J.L.; Cervera, J.C.; Soto, G.V. Microambientes de luz, crecimiento y fotosíntesis de la pitahaya (Hylocereus undatus) en un agrosistema de Yucatán, México. Agrociencia 2006, 40, 687–697. [Google Scholar]
- Jutamanee, K.; Onnom, S. Improving photosynthetic performance and some fruit quality traits in mango trees by shading. Photosynthetica 2016, 54, 542–550. [Google Scholar] [CrossRef]
- La Mantia, T.; Barbera, G.; Inglese, P. Effect of cladode shading on growth and ripening of fruits of cactus pear (Opuntia ficus-indica L. Miller). J. Hortic. Sci. 1997, 72, 299–304. [Google Scholar] [CrossRef]
- Inglese, P.; Inglese, G.; Liguori, G. Fruit productivity and carbon gain of Opuntia ficus-indica (L.) Mill. trees. Isr. J. Plant Sci. 2012, 60, 283–289. [Google Scholar]
- Cohen, S.D.; Kennedy, J.A. Plant metabolism and the environment: Implications for managing phenolics. Crit. Rev. Food Sci. Nutr. 2010, 50, 620–643. [Google Scholar] [CrossRef]
- de Lima, C.A.; Faleiro, F.G.; Junqueira, N.T.V.; de Oliveira Cohen, K.; Guimarães, T.G. Physico-chemical characteristics, polyphenols and yellow flavonoids in fruits of commercial and wild pitaya species from the Brazilian Savannas. Rev. Bras. Frutic. 2013, 35, 565–570. [Google Scholar] [CrossRef]
- Chang, P.-T.; Hsieh, C.-C.; Jiang, Y.-L. Responses of ‘Shih Huo Chuan’ pitaya (Hylocereus polyrhizus (Weber) Britt. & Rose) to different degrees of shading nets. Sci. Hortic. 2016, 198, 154–162. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Chen, W.; Xiao, T.; Zhao, X.; Ma, Y.; Huang, X. Shading Reduced the Injury Caused by Winter Chill on Pitaya Plant. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 47, 470–477. [Google Scholar] [CrossRef]
- de Almeida, A.S.; dos Santos, A.F. Flavonoides do Gênero Annona. Divers. J. 2018, 3, 475–485. [Google Scholar] [CrossRef]
- Haminiuk, C.W.I.; Maciel, G.M.; Plata-Oviedo, M.S.V.; Peralta, R.M. Phenolic compounds in fruits—An overview. Int. J. Food Sci. Technol. 2012, 47, 2023–2044. [Google Scholar] [CrossRef]
- da Silva, L.R.; de Figueiredo, A.R.; da Cunha Junior, P.C.; Barbosa, M.I.M.J.; de Oliveira, M.M.T.; Rosa, R.C.C.; de Morais, L.A.S. Postharvest characterization of purple passion fruits cultivated in conventional and organic system. Nativa 2021, 9, 551–557. [Google Scholar]
- Nishizawa, A.; Yabuta, Y.; Shigeoka, S. Galactinol and Raffinose Constitute a Novel Function to Protect Plants from Oxidative Damage. Plant Physiol. 2008, 147, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
| Traits | DF | F | p-Value |
|---|---|---|---|
| Physiological performance | |||
| A (µmol m−2 s−1) | 2 | 5.517 | 0.0437 * |
| TC (%) | 2 | 7.046 | 0.0266 * |
| Productivity | |||
| PROD (g) | 2 | 5.859 | 0.0388 * |
| Physico-chemical evaluation | |||
| TSS (%) | 2 | 24.26 | 0.0013 ** |
| Bioactive compounds | |||
| ANT (mg 100 g−1 fw) | 2 | 1.586 | 0.2800 ns |
| YF (mg 100 g−1 fw) | 2 | 10.55 | 0.0109 * |
| BETP (mg 100 g−1 fw) | 2 | 0.705 | 0.5310 ns |
| BETS (mg 100 g−1 fw) | 2 | 91.25 | 0.0001 *** |
| PETP (mg 100 g−1 fw) | 2 | 2.861 | 0.1340 ns |
| PETS (mg 100 g−1 fw) | 2 | 5.196 | 0.049 * |
| Antioxidant activity | |||
| FRAPP (μmol of Fe2SO4 g−1 fw) | 2 | 0.556 | 0.6000 ns |
| FRAPS (μmol of Fe2SO4 g−1 fw) | 2 | 0.302 | 0.7500 ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomaz de Oliveira, M.M.; Tel-Zur, N.; Albano-Machado, F.G.; Melo Penha, D.; Pinho, M.M.; Bezerra, M.; Alcântara de Miranda, M.R.; Herbster Moura, C.F.; Elesbão Alves, R.; Natale, W.; et al. Shade as an Agro-Technique to Improve Gas Exchange, Productivity, Bioactive Potential, and Antioxidant Activity of Fruits of Hylocereus costaricensis. Int. J. Plant Biol. 2025, 16, 128. https://doi.org/10.3390/ijpb16040128
Tomaz de Oliveira MM, Tel-Zur N, Albano-Machado FG, Melo Penha D, Pinho MM, Bezerra M, Alcântara de Miranda MR, Herbster Moura CF, Elesbão Alves R, Natale W, et al. Shade as an Agro-Technique to Improve Gas Exchange, Productivity, Bioactive Potential, and Antioxidant Activity of Fruits of Hylocereus costaricensis. International Journal of Plant Biology. 2025; 16(4):128. https://doi.org/10.3390/ijpb16040128
Chicago/Turabian StyleTomaz de Oliveira, Milena Maria, Noemi Tel-Zur, Francisca Gislene Albano-Machado, Daniela Melo Penha, Monique Mourão Pinho, Marlos Bezerra, Maria Raquel Alcântara de Miranda, Carlos Farley Herbster Moura, Ricardo Elesbão Alves, William Natale, and et al. 2025. "Shade as an Agro-Technique to Improve Gas Exchange, Productivity, Bioactive Potential, and Antioxidant Activity of Fruits of Hylocereus costaricensis" International Journal of Plant Biology 16, no. 4: 128. https://doi.org/10.3390/ijpb16040128
APA StyleTomaz de Oliveira, M. M., Tel-Zur, N., Albano-Machado, F. G., Melo Penha, D., Pinho, M. M., Bezerra, M., Alcântara de Miranda, M. R., Herbster Moura, C. F., Elesbão Alves, R., Natale, W., & de Medeiros Corrêa, M. C. (2025). Shade as an Agro-Technique to Improve Gas Exchange, Productivity, Bioactive Potential, and Antioxidant Activity of Fruits of Hylocereus costaricensis. International Journal of Plant Biology, 16(4), 128. https://doi.org/10.3390/ijpb16040128






