Abstract
Salinity is a long-standing global environmental stressor of terrestrial agroecosystems, with important implications for viticulture sustainability, especially in arid and semi-arid environments. Salt-induced physiological and biochemical disruptions to grapevines undermine yield and long-term vineyard sustainability. This review aims to integrate physiological, molecular, and omics-based insights to elucidate how grapevine rootstocks confer salinity tolerance and to identify future breeding directions for sustainable viticulture. This review critically assesses the ecological and molecular processes underlying salt stress adaptation in grapevine (Vitis spp.) rootstocks, with an emphasis on their contribution to modulating scion performance under saline conditions. Core adaptive mechanisms include morphological plasticity, ion compartmentalization, hormonal regulation, antioxidant defense, and activation of responsive genes to stress. Particular emphasis is given to recent integrative biotechnological developments—including transcriptomics, proteomics, metabolomics, and genomics—that reveal the intricate signaling and regulatory networks enabling rootstock-mediated tolerance. By integrating advances across eco-physiological, agronomic, and molecular realms, this review identifies rootstock selection as a promising strategy for bolstering resilience in grapevine production systems confronted by salinization, a phenomenon increasingly exacerbated by anthropogenic land use and climate change. The research highlights the value of stress ecology and adaptive root system strategies for alleviating the environmental consequences of soil salinity for perennial crop systems.
1. Introduction
Soil salinity is one of the major environmental constraints threatening global agricultural productivity, affecting more than 20% of irrigated lands worldwide and expanding steadily due to climate change, poor drainage, and the overuse of saline irrigation water [1]. The accumulation of soluble salts—particularly sodium chloride (NaCl)—in the root zone disrupts soil structure, reduces water uptake by roots, and limits the availability of essential nutrients, resulting in significant yield losses across many crops [2]. In viticulture, salinity has become an increasingly critical issue in major grape-growing regions such as Australia, Spain, China, and the United States, where saline irrigation and rising groundwater tables are common. Grapevines (Vitis vinifera L.) are moderately sensitive to salt stress, and the accumulation of Na+ and Cl− ions in their tissues can impair photosynthesis, alter ionic balance, and reduce berry quality [2,3]. To sustain vineyard productivity under saline conditions, the use of salt-tolerant rootstocks has become an essential adaptive strategy, as these rootstocks can restrict ion uptake, maintain osmotic balance, and protect the scion from ion toxicity. Understanding the physiological and molecular mechanisms underlying rootstock-mediated salt tolerance is therefore crucial for developing resilient viticultural systems in salt-affected environments.
Globally, soil salinization affects over 800 million hectares of agricultural land, with approximately 20–50% of irrigated areas showing moderate to severe salt accumulation [4]. The annual economic loss attributed to salinity-induced yield decline is estimated at over 27 billion USD, highlighting its substantial threat to global food security. Rapid population growth and the corresponding demand for food production—projected to increase by nearly 70% by 2050—further underscore the urgency of developing sustainable management strategies for saline-affected soils [5]. In this context, improving the salt tolerance of existing crops through breeding and rootstock selection offers a more viable alternative than expanding cultivation into new lands.
In viticulture, the problem is particularly acute in arid and semi-arid regions such as California’s Central Valley, the Mediterranean Basin, and northwestern China, where the combined effects of high evaporation, limited rainfall, and irrigation with brackish water have intensified soil salinity [6]. In China alone, approximately one-quarter of agricultural land is affected by salinity, posing a growing challenge to grapevine production in major regions such as Xinjiang and Hebei provinces. Similar trends have been reported in Australia, Spain, and the United States, where high-quality wine-producing areas are increasingly exposed to saline groundwater [5,6,7]. These conditions necessitate a deeper understanding of rootstock physiology and molecular adaptation mechanisms to mitigate salt stress while maintaining productivity and fruit quality in grapevine cultivation.
Salt stress affects grapevine growth and productivity through both osmotic and ionic mechanisms that disrupt cellular homeostasis and metabolic activity. High concentrations of Na+ and Cl− ions in the rhizosphere reduce the plant’s ability to absorb water, leading to osmotic stress, while excessive ion accumulation in tissues causes ionic toxicity, oxidative stress, and nutrient imbalance [5,8]. These effects impair photosynthetic efficiency, enzyme function, and hormonal regulation, ultimately resulting in reduced vigor and yield. At the molecular level, salinity triggers complex signaling cascades involving calcium-dependent protein kinases, mitogen-activated protein kinase (MAPK) pathways, and transcriptional regulators such as WRKY, NAC, MYB, and bHLH families, which coordinate antioxidant defense, ion transport, and osmolyte biosynthesis [9].
In this context, grapevine rootstocks play a pivotal role in mediating tolerance to saline environments. Rootstocks function as the physiological interface between the soil and scion, regulating water uptake, ion exclusion, and hormonal signaling that influence the entire plant’s response to stress [10]. Salt-tolerant rootstocks such as 1103 Paulsen (Vitis berlandieri × V. rupestris), Ramsey (Vitis champinii × V. berlandieri), and SO-4 (Vitis berlandieri × V. riparia) maintain lower Na+ and Cl− accumulation in shoots by enhancing selective ion transport, vacuolar sequestration, and osmotic adjustment [5,8,9]. Understanding the genetic and biochemical mechanisms underlying these processes provides a foundation for developing improved rootstock genotypes that enhance scion performance under saline irrigation.
Despite the considerable progress made in understanding the physiological effects of salt stress in grapevines, the integration of recent molecular discoveries with practical viticultural strategies remains limited. Most earlier studies have examined individual components—such as ion transport, osmotic adjustment, or antioxidant defense—in isolation, without providing a unified perspective linking these mechanisms to the agronomic performance of rootstocks. Moreover, rapid advances in transcriptomics, proteomics, metabolomics, and epigenomics have revealed new layers of regulatory complexity that are yet to be fully synthesized within the context of grapevine salt tolerance.
This review therefore aims to provide a comprehensive and integrative analysis of the mechanisms by which grapevine rootstocks confer tolerance to salt stress. It systematically examines the morphological, physiological, and molecular adaptations that underlie rootstock-mediated resilience; highlights the contributions of recent omics studies in uncovering key genes, pathways, and metabolites; and evaluates how these insights can inform the breeding and management of salt-tolerant rootstocks for sustainable viticulture. By critically linking multi-omics evidence with field-level agronomic outcomes, this review seeks to bridge the gap between molecular biology and vineyard practice, offering a holistic framework for improving grapevine performance in saline environments.
2. Morphological Responses to Grapevine Rootstock Under Salt Stress
A holistic understanding of salt tolerance in grapevine rootstocks begins with the characterization of their morphological and physiological responses to salt stress. These traits form the foundation upon which molecular and biochemical mechanisms operate, determining a rootstock’s ability to sustain growth, regulate ion balance, and maintain scion vigor under adverse conditions. Salinity is one of the most significant abiotic stresses limiting grapevine productivity in arid and semi-arid viticultural regions. Excessive accumulation of soluble salts in the rhizosphere alters soil osmotic potential, reduces water availability, and disrupts the balance of essential mineral nutrients [8]. Grapevines are considered moderately sensitive to salt stress; however, the degree of tolerance varies widely among cultivars and rootstocks depending on their genetic background and ion-exclusion efficiency [11]. In saline environments, sodium (Na+) and chloride (Cl−) ions are the primary contributors to ionic toxicity, leading to nutrient imbalances, reduced vegetative growth, and altered berry composition [8,9]. Therefore, understanding how different rootstocks respond and adapt to salinity is critical for maintaining vineyard productivity and fruit quality in salt-affected regions.
Prolonged accumulation of salts in the root zone disrupts grapevine physiology by inducing both osmotic and ionic stress. Elevated Na+ and Cl− concentrations impair nutrient uptake, reduce stomatal conductance, and limit photosynthetic efficiency, ultimately leading to decreased growth and yield [8]. At the root level, high salinity alters membrane permeability, interferes with aquaporin function, and disturbs water potential gradients, thereby reducing hydraulic conductivity. These root-level disturbances extend to the canopy, manifesting as reduced transpiration, lower leaf water potential, and premature leaf senescence. Sensitive grapevine cultivars often display severe growth inhibition and reproductive imbalance under such stress conditions, whereas tolerant rootstocks maintain higher stomatal regulation, antioxidant activity, and osmotic adjustment capacity, supporting physiological stability under saline irrigation [12].
The ability of tolerant rootstocks to mitigate the effects of salinity involves several structural adaptations. These include a deeper and more extensive root system, larger root surface area, and optimized xylem structure that improves water and nutrient transport [3,11]. Morphological adjustments in leaves and petioles, as well as root architecture, also contribute to salt exclusion and the maintenance of plant vigor [5]. Previous studies have shown that rootstocks can reduce the impact of salinity by modifying scion growth patterns, leaf area, and biomass distribution, thereby enhancing yield and quality even under saline irrigation [9,12]. Figure 1 summarizes the key morphological and physiological traits associated with grapevine rootstock responses to salt stress.
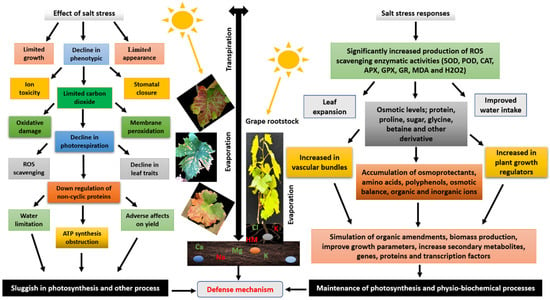
Figure 1.
Different traits of grapevine rootstocks under salt stress. Abbreviations: ROS = reactive oxygen species; APX = ascorbate peroxidase; POD = peroxidase; CAT = catalase; SOD = Superoxide dismutase; MDA = Malondialdehyde; GR = Glutathione Reductase; GPX = Glutathione Peroxidase.
2.1. Shoot System
The shoot system is among the most salt-sensitive components of grapevine plants. Elevated salinity levels markedly impair shoot development, photosynthetic performance, and overall vegetative growth. Under salt stress, grapevine shoots exhibit reduced elongation, smaller leaf area, and stunted growth due to the combined effects of osmotic imbalance and ionic toxicity [13,14]. Characteristic symptoms include chlorosis, leaf tip necrosis, and premature abscission, which collectively decrease canopy efficiency and yield potential [15,16]. Salinity commonly lowers the shoot-to-root ratio because aerial tissues are more sensitive to osmotic and ionic disturbances than root tissues [17]. Excess sodium (Na+) and chloride (Cl−) accumulation interferes with the uptake and translocation of essential minerals such as potassium (K+), calcium (Ca2+), and magnesium (Mg2+), leading to nutrient deficiencies in leaves and petioles [18,19]. Impaired stomatal regulation further restricts CO2 assimilation and water-use efficiency, thereby reducing photosynthetic capacity [20].
Tolerant rootstocks alleviate these adverse effects by maintaining ionic balance and sustaining physiological stability in the scion. They regulate Na+ and Cl− transport, promote osmotic adjustment through compatible solutes such as proline and glycine betaine, and enhance hormone-mediated signaling (particularly abscisic acid and jasmonic acid) that preserves leaf turgor and gas exchange [21,22]. Studies have demonstrated that grafting salt-sensitive cultivars onto tolerant rootstocks—such as 1103 Paulsen (Vitis berlandieri × V. rupestris), Ramsey (V. champinii × V. berlandieri), and SO-4 (V. berlandieri × V. riparia)—significantly improves shoot vigor, leaf water potential, and photosynthetic activity under saline irrigation [23,24,25]. These physiological advantages translate into improved fruit yield, berry composition, and canopy longevity [26].
In addition, biochemical defense systems contribute to shoot resilience. Antioxidant enzymes—superoxide dismutase (SOD), catalase (CAT), and ascorbate peroxidase (APX)—detoxify reactive oxygen species (ROS) produced during salt stress [27,28]. This antioxidant protection limits membrane lipid peroxidation and chlorophyll degradation, maintaining photosynthetic integrity. Consequently, grapevines grafted on tolerant rootstocks display higher chlorophyll retention, greater transpiration rates, and delayed leaf senescence compared with ungrafted or salt-susceptible combinations [29]. Collectively, these morphological, physiological, and biochemical adaptations preserve shoot functionality and ensure sustained biomass accumulation under saline environments. The coordination between rootstock ion regulation and shoot physiological responses is summarized in Figure 2, which illustrates the integrated mechanisms by which tolerant grapevine rootstocks sustain canopy performance during salt exposure [30].
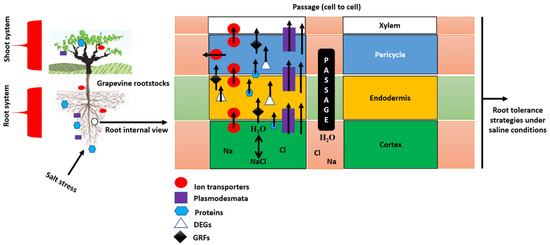
Figure 2.
Mechanistic model of grapevine rootstock responses of the root system under salt stress. Schematic representation showing how tolerant rootstocks mitigate salt injury by reducing Na+ and Cl− uptake, enhancing osmotic adjustment, and maintaining hydraulic conductivity. Key features include thicker root cortex, active SOS1/NHX1/HKT transporters, accumulation of compatible solutes (proline, sugars), and sustained xylem function. Together, these mechanisms support efficient water and nutrient transport to the shoot and contribute to overall salinity tolerance in grafted grapevines. Abbreviations: ABA = abscisic acid; JA = jasmonic acid; SA = salicylic acid; ETH = ethylene; IAA = indole-3-acetic acid; BR = brassinosteroid; MT = melatonin; ROS = reactive oxygen species; APX = ascorbate peroxidase; POD = peroxidase; CAT = catalase; NHX = vacuolar Na+/H+ antiporter; SOS = Salt Overly Sensitive pathway; HKT = high-affinity K+ transporter; CLC = vacuolar Cl− channel; V-ATPase = vacuolar H+-ATPase; V-PPase = vacuolar H+-pyrophosphatase.
2.2. Mechanism of Root Tolerance
Under saline conditions, the root system serves as the first line of defense against salt-induced stress. Grapevine roots regulate water uptake, ion transport, and overall plant homeostasis, but exposure to excess Na+ and Cl− disrupts these processes, causing osmotic imbalance, nutrient deficiency, and reduced hydraulic conductivity [17,19]. The degree of tolerance depends on structural, physiological, and molecular adaptations unique to each rootstock. Salt-tolerant rootstocks mitigate these adverse effects through several coordinated mechanisms. Morphologically, they develop deeper and more extensive root systems with larger surface areas, allowing for efficient water extraction from the soil [18]. Anatomical features such as thicker root cortex and reinforced xylem vessels enhance water flow and limit ion accumulation in root tissues. Physiologically, tolerant genotypes restrict Na+ and Cl− entry into the xylem while maintaining adequate transport of K+ and Ca2+, preserving ionic balance and minimizing toxicity in aerial organs [20,21,22]. Comparative transcriptomic analyses of resistant (3309-C, 520A, 1103-P) and sensitive (5-BB, 101-14 Mgt, Beta) grapevine rootstocks have revealed differential expression of salt-responsive genes [1]. Under salt stress, Salt Overly Sensitive genes SOS1 and SOS2 are up-regulated in resistant genotypes, whereas SOS3 and SOS5 show a down-regulation trend, suggesting genotype-specific modulation of ion-homeostasis pathways [31]. These molecular responses strengthen the operation of the SOS cascade, improving Na+ extrusion from the cytoplasm and maintaining cytosolic K+/Na+ balance in tolerant rootstocks.
At the cellular level, tolerance is governed by the activation of ion-transport and signaling pathways. The Salt Overly Sensitive (SOS) pathway—including SOS1 (plasma-membrane Na+/H+ antiporter), SOS2 (serine/threonine kinase), and SOS3 (Ca2+-binding protein)—plays a central role in extruding excess Na+ from the cytoplasm [22,25]. Vacuolar Na+/H+ exchangers (NHX1) and high-affinity K+ transporters (HKT1) complement this process by compartmentalizing Na+ into vacuoles and retrieving it from the xylem sap. These combined actions maintain low cytosolic Na+ concentrations and ensure metabolic stability during salt exposure [23,24]. Physiological screening of several rootstocks under saline irrigation further supports these molecular findings. Among five tested rootstocks—Dog Ridge, Salt Creek, RS-19, SO-4, and 1613-C—Dog Ridge displayed the highest root-to-shoot ratio (0.6), indicating superior osmotic adjustment and water uptake efficiency. Conversely, 1613-C exhibited the greatest K+/Na+ ratio, demonstrating effective ion selectivity and compartmentation compared with other genotypes [32]. Such physiological resilience corresponds closely with enhanced expression of SOS and NHX transporters in tolerant rootstocks.
Osmotic adjustment through compatible solutes—such as proline, glycine betaine, and soluble sugars—further stabilizes membranes and proteins, maintaining cellular hydration [26,28]. Enhanced antioxidant activity, including SOD, CAT, and peroxidase (POD), counteracts oxidative stress triggered by reactive oxygen species (ROS) [27]. Collectively, these responses sustain root metabolism and growth in saline environments. Grafting sensitive scions onto tolerant rootstocks amplifies these mechanisms. Rootstocks such as SO-4, Ramsey, and 1103 Paulsen maintain efficient root elongation, vascular conductivity, and selective ion transport, reducing Na+ translocation to the shoot and preserving whole-plant vigor and fruit quality under saline irrigation [29,30].
3. Origin and Global Contribution of Grapevine Rootstocks to Salt Stress
The development and utilization of grapevine rootstocks represent one of the most successful strategies for managing abiotic stresses, particularly salinity, drought, and soil-borne diseases. Rootstocks originated primarily from wild Vitis species native to North America, such as Vitis riparia, V. rupestris, and V. berlandieri, which were originally selected for their resistance to phylloxera and nematodes [33,34]. Over time, these species were hybridized to produce rootstocks with complementary traits—such as salt exclusion, drought tolerance, and lime tolerance—leading to the widely used commercial hybrids seen today [35]. Globally, grapevine rootstocks have become indispensable components of modern viticulture. Their use extends across Europe, the Americas, Africa, Australia, and Asia, enabling viticulture in regions where salinity and alkaline soils limit ungrafted vine performance [36]. The success of grapevine cultivation in Mediterranean, Middle Eastern, and coastal Asian regions is largely attributed to the ability of specific rootstocks to confer tolerance against high soil salinity and water scarcity [31]. For example, rootstocks such as 1103 Paulsen, 140 Ruggeri, Ramsey, and SO-4are extensively planted in saline-prone vineyards of southern Europe, Australia, and China due to their proven capacity to restrict sodium (Na+) and chloride (Cl−) transport to the scion [37,38].
In China, the expansion of viticulture into inland and coastal saline regions has driven extensive breeding and testing of salt-tolerant rootstocks. Hybrids derived from V. amurensis and V. berlandieri show high adaptability to saline and alkaline soils, supporting the rapid growth of the Chinese wine and table grape industries [39]. Similarly, in arid regions of Australia and North Africa, salt-tolerant rootstocks have enabled sustainable vineyard establishment under saline irrigation regimes, where conventional ungrafted vines cannot survive [32,40]. These rootstocks help maintain osmotic balance, sustain vine vigor, and improve fruit composition under high electrical conductivity (EC) conditions.
The role of rootstocks extends beyond stress mitigation—they also influence vineyard management, fruit yield, and quality. Rootstocks modify scion water-use efficiency, nutrient uptake, and hormone signaling, thereby contributing to long-term soil health and plant resilience [41]. Their deployment has transformed viticultural practices in salt-affected areas, making grapevine one of the few high-value crops capable of productive cultivation in moderately saline environments [42]. The global adoption of such rootstocks underscores their agronomic and economic significance in sustaining viticulture under increasing salinization pressure driven by climate change and irrigation with marginal-quality water [43,44]. Figure 3 summarizes the global distribution of major grapevine rootstocks and their adaptation zones in relation to soil salinity severity. The map illustrates the predominant rootstocks used in Mediterranean, coastal, and arid environments and highlights their genetic lineage from salt-tolerant Vitis species.
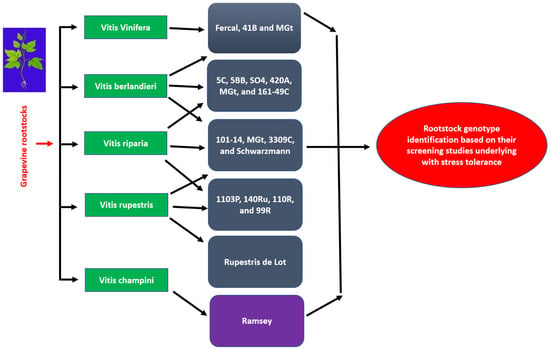
Figure 3.
Grapevine rootstock origin.
Numerous studies have categorized grapevine rootstocks according to their salinity tolerance, highlighting wide genetic variability among commercial hybrids [32,33,39,40]. The majority of globally used rootstocks are derived from three Vitis species—V. berlandieri, V. riparia, and V. rupestris—which collectively provide salt exclusion, drought resistance, and lime tolerance [45]. These hybrids, including 1103 P, 5-BB, 41-B, and Dog Ridge, exhibit contrasting physiological behaviors under saline irrigation, forming the foundation for breeding and regional adaptation. Table 1 and Table 2 summarize the principal rootstocks evaluated in previous studies, their tolerance classification (tolerant, semi-tolerant, moderate, and susceptible), and their morphological, nutritional, and molecular responses to salinity. Figure 3 illustrate the genetic origin of these hybrids and their global distribution across viticultural zones affected by soil salinity [40,41,42,43,44,45,46].

Table 1.
Grapevine rootstocks/cultivars based on their tolerance to various abiotic stresses are used around the globe.

Table 2.
Agronomic traits of grapevine rootstocks in response to abiotic stresses.
3.1. Effects of Rootstock on Grafted Scion
Rootstock on grafted scion cultivars influences grapevine growth, and the impact of the grafted scion and stock relationship mostly depends on the tolerant rootstock, such as 3309-C [2,56]. Rootstocks concerning grafted scion cultivars involve crop vigor, inflorescences, compatibility, softening of flower, maturation, propagation of flower blooming, fruiting body, fruiting color/size, and tolerance to salt stress [56,110]. It was reported that grapevine rootstock and scion cultivar dealings carried a broader impact as compared to grafted scion on crop normal weight, yield, growth, germination, and flowering rate [12,111,112] as well as post-harvest fruit quality under salt stress [113]. Rootstock and grafted scion interplay impacts the superiority of fruit cultivation by alleviating salt stress conditions [32]. Salt Creek rootstock seedling, imparted dwarfing, in contrast to 1103-Paulsen, which was grafted on its seedling, showed high crop vigor [78]. Non-irrigated Seedless grapevine ‘Sultana’ grown vigorously, grafted on three grapevine rootstocks, namely (1) 1103-P, (2) R2, and (3) Ramsey genotypes, increased leaf water potential, yield, and fruit composition [113]. In addition, less tolerant 101-14 Mgt and Schwarzman, while highly tolerant 116-60, 1103-Paulsen, 140-R, K5-BB, and 110 grapevine rootstock were reported [113]. A study found that grapevine rootstock grafted onto ‘Sultana’ recorded the highest morphological traits, including shoot length, dry weight, and reduced stomatal diffusion resistance, when compared to 1103-Paulsen grapevine rootstock under salt stress [78]. Interestingly, thematic investigations on the aspects of rootstocks and grafted scion cultivars are well investigated [113,114,115,116,117].
Analysis of Table 3 highlights that grapevine rootstock choice under saline environments involves balancing salinity tolerance (A–C), scion vigor (A–D), and nutrient-specific sensitivities such as K+ and Mg2+ inadequacy (Y = Yes, N = No). 1103 Paulsen (1103 P) combines high salt tolerance (A) with moderate vigor (B) and limited Mg2+ deficiency (N) but exhibits some K+ inadequacy (Y); if high salinity and moderate vigor are desired, 1103 P is ideal, provided that K+ fertilization is monitored to avoid nutritional imbalance. 140 Ruggeri (140 R) expresses high salt tolerance (A) and strong vigor (A) with no Mg2+ limitation (N) but similar K+ sensitivity (Y); if a site has high salinity and low inherent vigor, 140 R is preferable, though periodic leaf-tissue K+ analysis is advised. Harmony, a hybrid of Vitis champinii, provides excellent salt tolerance (A) but low vigor (C) and potential micronutrient (Mg2+/K+) issues; if root vigor suppression is desired—for example, in fertile or irrigated soils—Harmony performs well, but supplemental Mg2+ application is recommended. Dogridge offers very high tolerance (A) and high vigor (A) with minimal nutrient limitations (0); if the vineyard faces severe salinity and drought together, Dogridge is preferred, although excessive vigor may require pruning or canopy management to maintain fruit quality.

Table 3.
Different grapevine rootstocks based on their salinity tolerance, grafted scion vigor, susceptibility to magnesium “Mg” inadequacy, and potassium “K” agronomic characteristic features were slightly modified [111].
Recent field evaluations confirm that rootstock-mediated salinity tolerance observed in controlled environments is also expressed under commercial vineyard conditions. A field trial was carried out in a young ‘Tempranillo’ vineyard comparing M1, M4, and 1103-Paulsen (1103P) rootstocks under saline irrigation (3.5 dS m−1). Salinity reduced water relations and gas exchange rates equally in all three rootstocks due to osmotic stress, though M1 induced significantly lower net photosynthesis rates than 1103P. Crucially, 1103-Paulsen effectively restricted leaf chloride and sodium accumulation in the scion, a limitation that was not maintained by the M-rootstocks. Additionally, 1103P showed fewer overall transcriptomic changes in the scion compared to M1 and M4. These results suggest that 1103-Paulsen’s superior capacity for ion exclusion and physiological stability may confer greater long-term salinity tolerance compared to the new M-rootstocks, though M4 was the only rootstock to maintain grape phenolic ripeness under saline conditions [117]. Similarly, Zhao et al. [1], reported vineyard-scale evidence from Xinjiang, China, where SO-4 and 140 Ruggeri rootstocks preserved berry firmness, anthocyanin content, and soluble-solids concentration despite elevated NaCl in irrigation water. These long-term results demonstrate that the physiological mechanisms described earlier—selective ion transport, antioxidant activity, and osmolyte regulation—translate directly into improved yield stability and fruit composition in saline vineyards, validating the agronomic importance of salt-tolerant rootstocks.
3.2. Significance of Grapevine Rootstocks
In response to salt stress, grapevine rootstocks are an integral characteristic feature of sustainable agriculture production worldwide. They can improve the vigor, cultivation, and health of the whole plant, as well as the survival rate, and reduce damage from bacterial disease. Grapevine rootstocks can be used for better performance of scion cultivars, and stress-related problems, e.g., salinity, drought, waterlogging, flooding, and inappropriate drainage systems. Grafting rootstocks plays a pivotal role in the grapevine cultivar, such as phenotypic alteration, vine production, maturity of fruits [118], and control of root-knot diseases [119]. Rootstock restricts anthropogenic activities, nematodes, and abiotic and biotic stresses, especially salt stress [120]. Tolerant rootstocks help to improve morphophysiological traits, such as leaf color, shape, and fruit quality [121].
4. Physiological Responses of Grapevine Rootstocks to Salt Stress
Grapevine plants exhibit complex physiological and biochemical mechanisms that collectively determine their tolerance to salt stress. Salinity affects multiple functions—photosynthesis, transpiration, stomatal regulation, chlorophyll stability, antioxidant metabolism, hormone signaling, and carbohydrate turnover—culminating in reduced growth and productivity [122,123,124]. Under saline irrigation, osmotic stress initially restricts water uptake, followed by ionic toxicity as sodium (Na+) and chloride (Cl−) accumulate in tissues. These conditions cause stomatal closure, impaired CO2 assimilation, and a decline in chlorophyll fluorescence efficiency [125,126]. Tolerant rootstocks moderate these adverse effects through ion exclusion, osmotic regulation, and redox control. They restrict Na+ and Cl− movement to the shoot via selective transporters at the root–xylem interface and sustain K+ and Ca2+ uptake, maintaining favorable K+/Na+ ratios. Such regulation preserves membrane potential and photosynthetic capacity [31,80]. The gas-exchange response—stomatal conductance and transpiration rate—is closely linked to abscisic-acid (ABA)-mediated signaling that enables rapid adjustment of guard-cell turgor under saline conditions [9,88].
Biochemically, salt tolerance is reinforced by enhanced antioxidant defense. Activities of superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), and ascorbate peroxidase (APX) increase markedly in tolerant rootstocks, scavenging reactive-oxygen species (ROS) produced during ionic imbalance [68,69]. Rootstocks such as Dog Ridge and 1103 Paulsen maintain higher antioxidant enzyme activity and proline accumulation than sensitive ones, reflecting stronger detoxification and osmoprotection [68,80]. In addition, salinity triggers the synthesis of compatible solutes—proline, glycine betaine, soluble sugars, and polyols—that stabilize proteins, protect cellular membranes, and maintain osmotic equilibrium [122,123]. Hormonal and metabolic adjustments further underpin salt resilience. Elevated ABA, salicylic acid (SA), and jasmonic acid (JA) concentrations in tolerant rootstocks coordinate stress-responsive gene activation and ion-homeostasis regulation [127,128]. Enhanced ethylene (ETH) and brassinosteroid (BR) signaling promotes cell-wall remodeling and xylem differentiation, facilitating water movement under stress [81,82]. In parallel, cytoplasmic Ca2+ fluxes activate calcium-dependent protein kinases (CDPKs) and calmodulin pathways that modulate ion-transporter expression [129].
Comparative studies of grafted vines confirm that tolerant rootstocks sustain greater photosynthetic efficiency, chlorophyll retention, and transpiration stability than sensitive combinations [125,126]. Pinot Noir and Chardonnay scions grafted on 1103 P exhibited higher photosynthetic rates and less chlorophyll degradation than those grafted on SO-4 [125]. Similarly, vines grafted on Fercal maintained photochemical quenching and stomatal conductance under salinity stress [126]. Figure 4 summarizes these integrated mechanisms by which tolerant rootstocks alleviate salt stress in grapevine. It illustrates the coordination between ion transport, osmotic adjustment, and antioxidant defense that collectively maintain ionic balance, cellular homeostasis, and vine productivity under saline conditions.
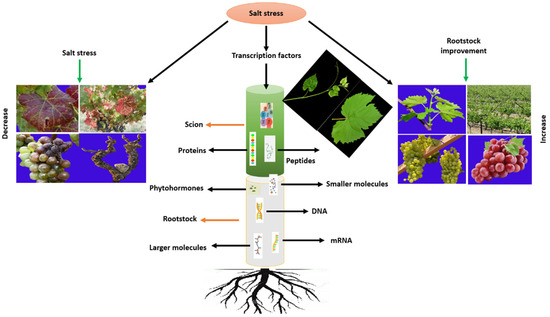
Figure 4.
Mechanistic overview of rootstock-mediated salinity tolerance in grapevine. Schematic illustration showing how tolerant rootstocks regulate salt stress through (1) ion exclusion via SOS1, NHX1, and HKT1 transporters; (2) osmotic adjustment through the synthesis of compatible solutes such as proline and sugars; and (3) oxidative protection via antioxidant enzymes (SOD, CAT, APX).
4.1. Photosynthesis
Photosynthesis is one of the primary physiological processes inhibited under salt stress, as salinity disrupts both stomatal function and chloroplast metabolism. High concentrations of NaCl in the soil create osmotic and ionic imbalances that restrict water uptake, reduce stomatal aperture, and impair cellular expansion, ultimately suppressing photosynthetic activity in grapevines [128]. However, the extent of this inhibition varies among rootstocks. For instance, two grapevine rootstocks—1103 Paulsen (Vitis berlandieri × Vitis rupestris) and SO-4 (V. berlandieri × V. riparia)—showed improved photosynthetic performance under 30 mM NaCl stress, despite limited changes in stomatal conductance [129]. Similarly, comparative analysis of IAC 313 (a salt-excluding rootstock) and SO-4 under 0, 50, and 100 mM NaCl demonstrated that SO-4 maintained higher photosynthetic rates and stomatal conductance, suggesting a more efficient photosynthetic apparatus under salinity [88]. These findings indicate that tolerant rootstocks can mitigate salt-induced constraints on photosynthesis by preserving stomatal regulation and sustaining carbon assimilation, thereby supporting vine growth and productivity under saline conditions.
4.2. Fiber Quality
Grapevine tissues contain approximately 56.8–83.6% dietary fiber, an important structural component influencing fruit quality and postharvest properties [130]. Fiber traits—such as fineness, length, and tensile strength—are strongly affected by environmental stress factors, including salinity, waterlogging, drought, and low temperature [130,131,132,133,134,135,136]. Salt stress alters fiber biosynthesis by interfering with cellulose and lignin deposition, leading to shortened and less mature fibers. Although fiber fineness may increase, overall fiber length and structural integrity often decline as sodium ion concentration rises in plant tissues, negatively affecting fiber bundle strength and elasticity [137,138]. From a viticultural standpoint, selecting appropriate rootstocks can enhance fiber integrity and vascular development even under saline irrigation. Rootstocks with higher salt exclusion capacity, such as 1103 Paulsen and SO-4, help maintain favorable tissue turgor and nutrient transport, indirectly supporting better fiber formation and mechanical resilience [31].
4.3. Oxidative Stress
Salt stress induces profound oxidative disturbances in grapevines, disrupting ion balance, redox homeostasis, and eco-physiological stability. Excessive accumulation of Na+ and Cl− in cells triggers the overproduction of reactive oxygen species (ROS)—including superoxide anion (O2−), hydrogen peroxide (H2O2), and hydroxyl radicals (•OH)—in chloroplasts, mitochondria, and peroxisomes [1,139]. While basal ROS levels play essential signaling roles in plant growth and defense [140,141,142], uncontrolled ROS accumulation leads to oxidative damage of lipids, proteins, and nucleic acids, causing membrane peroxidation and photosynthetic inhibition [121]. A dynamic balance between ROS generation and scavenging determines the oxidative state of grapevine cells. Enzymatic antioxidants such as superoxide dismutase (SOD), catalase (CAT), and ascorbate peroxidase (APX), together with non-enzymatic molecules including ascorbate, glutathione, and flavonoids, maintain redox equilibrium [141,143]. Recent transcriptomic studies, however, highlight that ROS are not merely toxic by-products but also critical secondary messengers regulating stress-responsive gene expression. Controlled ROS bursts activate mitogen-activated protein kinase (MAPK) cascades and transcriptional regulators, linking oxidative cues to broader adaptation pathways (Figure 5) [144].
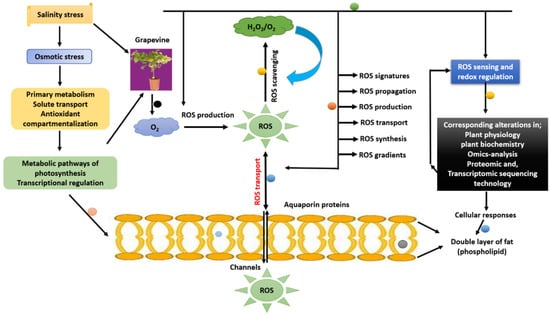
Figure 5.
Mechanism of reactive oxygen species (ROS) signaling pathways to grapevine plants under salt stress. Abbreviations: ABA = abscisic acid; JA = jasmonic acid; SA = salicylic acid; ETH = ethylene; IAA = indole-3-acetic acid; BR = brassinosteroid; MT = melatonin; ROS = reactive oxygen species; APX = ascorbate peroxidase; POD = peroxidase; CAT = catalase; NHX = vacuolar Na+/H+ antiporter; SOS = Salt Overly Sensitive pathway; HKT = high-affinity K+ transporter; CLC = vacuolar Cl− channel; V-ATPase = vacuolar H+-ATPase; V-PPase = vacuolar H+-pyrophosphatase.
In Vitis vinifera cultivars such as ‘Thompson Seedless’ and ‘Cabernet Sauvignon,’ ROS-mediated signaling directly intersects with major transcription-factor families—WRKY, NAC, MYB, and bHLH—that integrate redox perception with ion-transport and hormonal-response genes. For instance, VvWRKY46 and VvNAC17 are rapidly induced by H2O2 accumulation and activate downstream SOS1, NHX1, and APX genes, restoring ionic and oxidative balance [65]. Similarly, VvMYB108A and VvbHLH104 participate in ROS-dependent regulation of antioxidant enzymes, reinforcing stress-mitigation networks [145]. These responses confirm that ROS function as both signals and effectors, mediating cross-talk among ion homeostasis, hormone signaling, and transcriptional control. Salt-tolerant rootstocks exhibit stronger ROS perception and detoxification capacity, allowing them to maintain redox stability during prolonged exposure. The model presented in Figure 6 integrates these molecular and physiological interactions, showing how ion transporters, channels, and signaling cascades sustain mineral equilibrium under salinity. Specifically, Na+ is expelled via SOS1 antiporters at the plasma membrane and sequestered into vacuoles through NHX1, while HKT1 retrieves Na+ from the xylem to prevent leaf toxicity. CLC transporters regulate Cl− compartmentation, and CAX and ACA transporters maintain Ca2+ signaling. Concurrently, K+ channels (AKT, HAK) stabilize osmotic potential and enzyme activation. Collectively, these mechanisms preserve nutrient balance, protect photosynthetic metabolism, and enhance salinity tolerance in grafted grapevines.
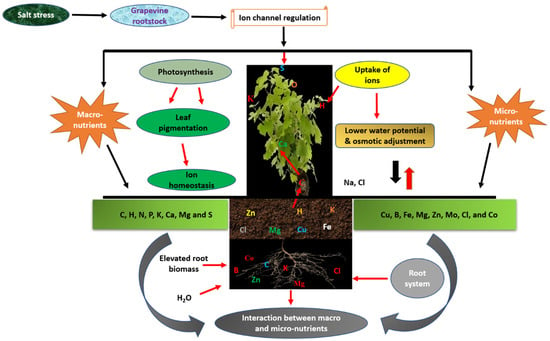
Figure 6.
Regulation of mineral elements in grapevine rootstock under salt stress.
4.4. Impact and Mechanistic Regulation of Mineral Ions in Grapevine Rootstocks Under Salt Stress
Ion-transport regulation represents a central adaptive mechanism underlying salt tolerance in grapevine rootstocks, as illustrated in Figure 6. Under saline conditions, ionic homeostasis is disturbed by excessive uptake and accumulation of sodium (Na+) and chloride (Cl−) ions, which impair photosynthesis, enzyme activity, and membrane stability. Salt-tolerant rootstocks counter these effects by precisely modulating ion uptake, exclusion, and compartmentalization, thereby maintaining optimal cellular ionic balance [25,146]. A key determinant of salt tolerance is the ability to restrict Na+ and Cl− translocation from roots to shoots, ensuring a favorable potassium-to-sodium (K+/Na+) ratio and safeguarding photosynthetically active tissues [147]. The Salt Overly Sensitive (SOS) signaling pathway remains a cornerstone of Na+ homeostasis in plants [17,148]. In grapevine, the plasma membrane Na+/H+ antiporter SOS1, activated by the SOS2–SOS3 kinase complex, facilitates the extrusion of excess Na+ from the cytosol into the apoplast or external medium. This process prevents intracellular Na+ accumulation and preserves cellular metabolism under salt stress.
Recent transcriptomic and functional studies, however, reveal that grapevine rootstock tolerance involves a broader network of transporters and ion channels beyond the canonical SOS pathway. Members of the NHX family of vacuolar Na+/H+ antiporters—particularly VvNHX1 and VvNHX2—sequester Na+ into vacuoles, preventing cytosolic toxicity while contributing to osmotic adjustment [141,142]. Proton pumps, such as H+-ATPases and H+-PPases, generate the proton motive force required to energize Na+ transport via these exchangers, thereby sustaining intracellular ion equilibrium and turgor maintenance. Chloride exclusion is equally critical in conferring salt tolerance. Comparative analyses between tolerant and sensitive rootstocks, such as Ramsey vs. 101-14 Mgt and 1103 Paulsen vs. 101-14, indicate that tolerant genotypes exhibit significantly lower Cl− translocation to aerial tissues [147]. This trait is associated with upregulated expression of chloride channels (CLCs) and the tonoplast-localized transporter GRET1, which facilitate Cl− sequestration within vacuoles and restrict its xylem-mediated movement to leaves. By reducing Cl− accumulation in the photosynthetic organs, tolerant rootstocks sustain chlorophyll stability and enzymatic activity during prolonged salt exposure. Potassium (K+) retention plays a complementary and synergistic role in salt tolerance. High-affinity K+ transporters (HAKs) and inward-rectifying K+ channels (AKTs) maintain K+ uptake and distribution, even under competitive Na+ influx [147]. The ability of tolerant rootstocks to preserve elevated K+ levels in leaves and berries supports optimal enzyme activation, stomatal regulation, and photosynthetic efficiency under saline irrigation. Collectively, these integrated mechanisms—Na+ exclusion, Cl− sequestration, and K+ retention—enable grapevine rootstocks to sustain ion homeostasis and physiological resilience in saline environments. Their coordination through transcriptional, post-transcriptional, and post-translational control represents a finely tuned adaptive framework that underpins growth, yield stability, and fruit quality in salt-affected vineyards.
4.5. Antioxidant Enzymes
Rootstocks enhance the antioxidant defense system of grapevines exposed to salt stress through both enzymatic and non-enzymatic mechanisms. The enzymatic components include superoxide dismutase (SOD), peroxidase (POD), ascorbate peroxidase (APX), and catalase (CAT), while the non-enzymatic antioxidants primarily involve ascorbate (AsA) and glutathione (GSH). Together, these systems detoxify reactive oxygen species (ROS) and maintain cellular redox equilibrium [149,150]. Among these enzymes, SOD catalyzes the dismutation of the superoxide radical (O2−) into hydrogen peroxide (H2O2) and molecular oxygen. Subsequently, CAT and APX eliminate H2O2 via distinct but complementary pathways: CAT decomposes H2O2 into water and oxygen, whereas APX reduces it through the AsA–GSH cycle, coupling ROS detoxification with redox signaling [151]. These enzymes operate synergistically to prevent oxidative injury to membranes, proteins, and nucleic acids, thereby preserving metabolic stability during salt exposure.
Comparative studies of four grapevine rootstocks—Chawga, Laale Sefid, Khalili, and Shirazi—demonstrated that Chawga exhibited the highest antioxidant activity and most effective enzymatic defense under salt stress, highlighting genotype-dependent variability in oxidative protection [149]. Beyond enzymatic detoxification, molecular signaling cascades further reinforce stress tolerance. The Salt Overly Sensitive (SOS) pathway constitutes a central mechanism regulating Na+ efflux and maintaining ion homeostasis under salinity [140,152,153]. This pathway involves the coordinated action of three core proteins: SOS3, a calcium-binding sensor that detects salinity-induced cytosolic Ca2+ fluctuations; SOS2, a serine/threonine protein kinase activated by SOS3; and SOS1, a plasma-membrane Na+/H+ antiporter that extrudes Na+ from the cytoplasm. Upon salt stress, increased cytosolic Ca2+ concentrations are sensed by SOS3, which binds to and activates SOS2. The resulting SOS2–SOS3 kinase complex phosphorylates and activates SOS1, driving Na+ extrusion from the cytosol to the apoplast. This dynamic coordination prevents toxic Na+ accumulation and sustains intracellular ionic and oxidative balance [154]. Figure 7 illustrates the integration of antioxidant enzymes with SOS-mediated ion-homeostasis pathways in grapevine rootstocks under salinity stress. Together, these enzymatic and signaling systems form an interconnected defense network that protects cells from ROS toxicity while stabilizing ionic and metabolic functions.
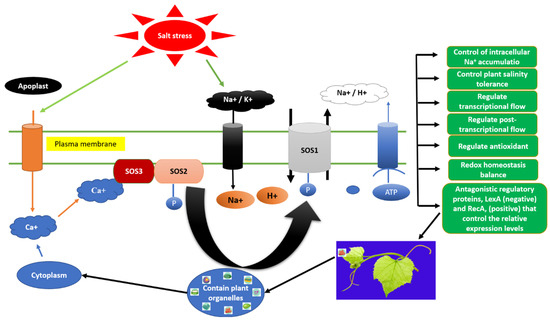
Figure 7.
Trajectory salt overly sensitive (SOS) principle in grapevine followed by [150] with minor modifications. Abbreviations: ABA = abscisic acid; JA = jasmonic acid; SA = salicylic acid; ETH = ethylene; IAA = indole-3-acetic acid; BR = brassinosteroid; MT = melatonin; ROS = reactive oxygen species; APX = ascorbate peroxidase; POD = peroxidase; CAT = catalase; NHX = vacuolar Na+/H+ antiporter; SOS = Salt Overly Sensitive pathway; HKT = high-affinity K+ transporter; CLC = vacuolar Cl− channel; V-ATPase = vacuolar H+-ATPase; V-PPase = vacuolar H+-pyrophosphatase.
5. Molecular Tools for the Improvement of Grapevine Under Salt Stress
Advances in sequencing and omics technologies have transformed the understanding of salt-tolerance mechanisms in grapevine rootstocks. High-resolution genomic, transcriptomic, proteomic, and metabolomic analyses have enabled the identification of stress-responsive genes, proteins, and transcription factors that connect genotypic regulation with morphological and physiological adaptation (Figure 8). Screening studies using global omics platforms have revealed extensive insights into chromatin dynamics, genome assemblies, genetic diversity, DNA marker mapping, and the defense-signaling networks underlying grapevine responses to salinity [140,151]. The following subsections summarize the major omics-based tools applied to dissect and improve salt tolerance in grapevine rootstocks. A comprehensive summary of the differentially expressed genes identified across transcriptomic, proteomic, and metabolomic studies in grapevine rootstocks under salt stress—including those involved in osmolyte metabolism, ion transport, and transcriptional regulation—is presented in Table 4.
5.1. Transcriptomics
Recent developments in transcriptomic approaches—such as RNA sequencing (RNA-Seq), microarray profiling, genotyping-by-sequencing, and single-cell RNA-Seq—have provided powerful resources for exploring salinity responses at the gene-expression level [17,155]. Transcriptomic studies have identified multiple salt-responsive pathways, including Salt Overly Sensitive (SOS), heat-shock proteins (HSPs), and transcription-factor (TF) families such as WRKY, NAC, MYB, and bHLH, which regulate ionic balance and oxidative defense. These studies also highlight differential expression of growth-regulatory factors (GRFs), miRNAs, and hormone-related genes, which together orchestrate stress signaling and acclimation [1,154,155]. Integrating transcriptomic data with physiological phenotyping helps identify tolerant genotypes and candidate biomarkers for use in marker-assisted breeding.
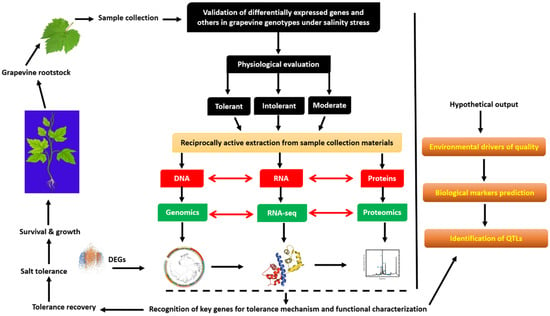
Figure 8.
Multi-omics study provides insights into gaining stress tolerance for grapevine rootstock under salt stress.

Table 4.
Differentially expressed genes (DEGs) involved in osmolyte metabolism, ion transport, and transcriptional regulation of salt tolerance in grapevine rootstocks.
Table 4.
Differentially expressed genes (DEGs) involved in osmolyte metabolism, ion transport, and transcriptional regulation of salt tolerance in grapevine rootstocks.
| Name of Cultivars | NaCl (mM) | Gene Names | Techniques | Functions | References |
|---|---|---|---|---|---|
| Grapevine | 130 | VvSOS1 VvSOS2 | RNA-Seq | Salt overly sensitive genes upregulated the sodium–proton antiporter Protein kinase Phenylalanine pathway Phytohormones | [1] |
| Tunisian | 100 | VvPR10 VvPR10.2 | Proteomics | Displaying a differential expression pattern between proteins Proteins are attached to the different plant organs Regulation of pathogen-related proteins Proteins degradations | [156] |
| Table grape | − | RD22 GTG2 MAPK4 | Genomics | Regulate hormonal signal transduction Modification of cell wall organizations Reactive oxygen species Osmotic stress | [157] |
| Crimson Seedless | − | VvPAL VvCHS VvDOX | Metabolomics | Promoted anthocyanin biosynthesis Regulation of anthocyanin-related derivatives Pigments responsible for coloration in berries | [158] |
| Grapevine | 150 | VvP5CS VvP5CR VvAOT VvPDH | RT-qPCR and PCA analysis | Upregulated the proline synthesis Proline degradation Maintain a high K+ level | [159] |
| Vitis amurensis, Vitis berlandieri × Vitis riparia | 100 | VvLSM2 VvSRSF1 | RNA-Seq | Possible roles in the regulation of alternative splicing in response to abiotic stress | [160] |
| Grapevine | 50 and 100 | VaCPK | RT-qPCR | Acclimation to environmental stress | [161] |
| V. solonis × V. riparia | 400 | VvER | RNA-Seq | Endoplasmic reticulum coding transcripts | [74] |
| Cabernet Sauvignon | 200 | VvAKT1 VvHKT1 VvNHX1 VvNHX2 VvTPC1A VvTPC1B | RNA-Seq and metabolomics | Upregulated ABA and MAPK signaling pathways Involvement of ion transportation Regulate rubisco activity Photosynthetic pathways | [162] |
| Thompson Seedless | 150 | VvWRKY VvEREB VvMYB VvNAC VvbHLH | RNA-Seq | Differentially expressed genes related to stress resistance Transcription factors responsible for metabolic pathways Identification of molecular markers Development of salt resistance grape breeding | [148] |
| Vitis vinifera | 100 | VvNHXs VvNHX1 VvNHX2 VvNHX3 VvNHX4 VvNHX5 VvNHX6 | RT-qPCR | Classified into I vacuolar and group II endosomal groups Growth of berry development Seed maturation Flower development Plant hormone signaling interactions Phylogenetic classification | [163] |
| Summer black | 0.8% | VvHSPs VvTFs VvPRs | RNA-Seq | Salt stress-induced heat shock proteins Encoding of transcription factors Regulatory pathogenesis-related proteins Hormonal regulations | [90] |
| Cabernet Sauvignon | 25, 50 and 100 | GLRaV-3 | In vitro | The virus-infected plants were more tolerant to NaCl for sustainable production and growth | [164] |
| Malbec vines | 0, 50 and 100 | VvLA | Grafting | Leaf area plays a key role in Na+ exclusion for long-term for salt tolerance levels Reduce growth performance and transpiration rate | [165] |
| Qarah Shani and Thompson Seedless | 0, 25, 50, 75 and 100 | Unknown | Cutting and hydroponic | The evaluation of harmful ions and improvement for mineral substances in grape crops under salt tolerance | [166] |
Not available (unknown), Bold font indicates genes discussed in Section 5.5.
5.2. Proteomics
Proteomics focuses on quantitative and qualitative characterization of proteins that respond to environmental stress. Shotgun proteomics and two-dimensional gel electrophoresis coupled with mass spectrometry have identified proteins involved in photosynthesis, protein folding, post-translational modification, and defense in salt-stressed grapevine rootstocks [167]. Proteomic analyses reveal altered expression of structural and regulatory proteins, such as LEA (Late-Embryogenesis Abundant) proteins, HSPs, and enzymes involved in antioxidant defense, confirming their central roles in salt adaptation [156,168]. These datasets help uncover protein–protein interactions and molecular markers linked to salt tolerance, providing essential targets for functional validation and genetic improvement.
5.3. Genomics
Genomics-assisted breeding integrates DNA-based markers and quantitative trait locus (QTL) mapping to identify genomic regions controlling salt-tolerance traits. Mapping studies have demonstrated that salt tolerance is a polygenic and heritable trait influenced by multiple QTLs distributed across grapevine chromosomes [157,169]. Molecular markers—including simple sequence repeats (SSRs), single-nucleotide polymorphisms (SNPs), restriction fragment length polymorphisms (RFLPs), and randomly amplified microsatellite polymorphisms (RAMPs)—facilitate high-resolution genetic mapping. In addition, marker-assisted selection (MAS) and association mapping approaches enable precise tracking of alleles conferring salt exclusion, osmotic adjustment, and antioxidant regulation in rootstocks [170,171,172]. These genomic tools collectively accelerate the introgression of salt-tolerance traits into elite cultivars through targeted breeding and genome editing.
5.4. Metabolomics
Salt stress alters grapevine metabolism at multiple molecular levels, influencing proteins, nucleic acids, metabolites, and cellular architecture. Metabolomics—the comprehensive profiling of small molecules—provides critical insights into these biochemical shifts. Under salt exposure, grapevine genotypes display significant variation in amino-acid synthesis, carbohydrate metabolism, and antioxidant pathways, revealing how tolerant rootstocks remodel metabolism to maintain homeostasis [55,173]. In a recent study, six rootstocks (1103-P, 5C, SO-4, 3309-C, 140-Ru, and control) grafted onto Cabernet Sauvignon were analyzed using partial least-squares discriminant analysis (PLS-DA). The results identified approximately 30 differentially accumulated metabolites and 22 metabolic pathways, including those related to proline biosynthesis, glycolysis, and phenylpropanoid metabolism [174,175]. By integrating metabolomic data with transcriptomic and proteomic evidence, researchers have uncovered networks of differentially expressed genes (DEGs), differentially accumulated metabolites (DAMs), and miRNAs associated with abiotic stress tolerance in grapevine rootstocks. These global multi-omics approaches (summarized in Table 5) provide a comprehensive framework for dissecting complex stress-response systems and accelerating the development of salt-resilient grapevine cultivars.

Table 5.
Summary of multiple genes in grapevine cultivars/rootstocks coping identified in grapevine cultivars and rootstocks coping with abiotic stresses, grouped by functional stress category.
5.5. Signaling Pathway in Grapevine Rootstock Under Salt Stress
Recent molecular advances demonstrate that salt tolerance in grapevine rootstocks is governed not by single genes but by complex transcriptional and signaling networks that coordinate ionic balance, redox control, and hormonal regulation. Central to these networks are transcription-factor (TF) families such as WRKY, NAC, MYB, and bHLH, which fine-tune ion homeostasis, reactive oxygen species (ROS) detoxification, and hormone-mediated stress signaling through multilayered regulatory cascades.
WRKY transcription factors act as key redox-sensitive regulators of salt-responsive genes. In Vitis vinifera and its hybrids, VvWRKY46, VvWRKY33, and VvWRKY54 activate the promoters of VvSOS1 and VvNHX1, enhancing Na+ extrusion and vacuolar sequestration under saline conditions [176]. Overexpression of VvWRKY46 in transgenic grape calli increases the K+/Na+ ratio and antioxidant enzyme activity, highlighting its dual role in ion transport and redox regulation. NAC transcription factors integrate osmotic stress responses with hormonal signaling pathways. VvNAC17 and VvNAC26 transcripts rapidly accumulate in both roots and leaves following salt exposure, activating genes involved in osmolyte biosynthesis and abscisic-acid (ABA)–dependent signaling [137]. NAC proteins also bind directly to the promoters of peroxidase and catalase genes, linking transcriptional regulation to antioxidant defense and ROS homeostasis. MYB transcription factors, such as VvMYB108A and VvMYB62, mediate hormonal cross-talk between ABA, ethylene, and the jasmonic-acid (JA) pathway. VvMYB108A enhances ethylene biosynthesis via upregulation of VvACS1 and works in coordination with NAC proteins to maintain leaf water potential and chlorophyll content during salt exposure [168]. Similarly, bHLH transcription factors—including VvbHLH104 and VvbHLH122—regulate iron homeostasis, root elongation, and tissue differentiation, reinforcing stress resilience and nutrient balance.
These TF families constitute an interactive regulatory network that strengthens salt tolerance through coordinated control of multiple processes. Co-expression analyses reveal that WRKY, NAC, and MYB modules frequently converge on ion-transport genes (SOS1, NHX, HKT, CLC), ROS-scavenging enzymes (SOD, APX, CAT), and osmoprotectant biosynthetic genes (P5CS, BADH). These interlinked pathways sustain balanced ion fluxes, maintain redox homeostasis, and modulate growth under salinity stress—hallmarks of tolerant rootstocks such as Ramsey and 1103 Paulsen. Figure 9 illustrates the integrated signaling framework controlling salt-stress responses in grapevine rootstocks. The model emphasizes transcriptional feedback loops among TFs, ion-transporters, antioxidant systems, and hormonal regulators that collectively enhance adaptation to saline environments.
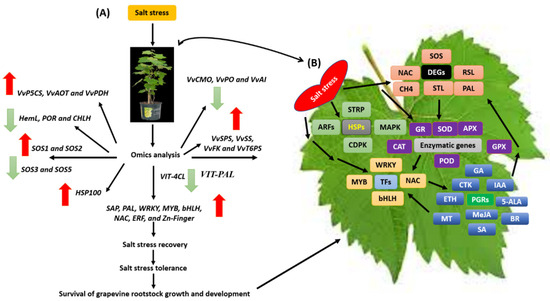
Figure 9.
A feasible model of grapevine rootstocks in response to salt stress. (A) The red arrow shows up-regulation and the green arrow shows down-regulation, and (B) represents the differentially expressed genes involved in grapevine defensive mechanism under salt stress tolerance. Abbreviations: ABA = abscisic acid; JA = jasmonic acid; SA = salicylic acid; ETH = ethylene; IAA = indole-3-acetic acid; BR = brassinosteroid; MT = melatonin; ROS = reactive oxygen species; APX = ascorbate peroxidase; POD = peroxidase; CAT = catalase; NHX = vacuolar Na+/H+ antiporter; SOS = Salt Overly Sensitive pathway; HKT = high-affinity K+ transporter; CLC = vacuolar Cl− channel; V-ATPase = vacuolar H+-ATPase; V-PPase = vacuolar H+-pyrophosphatase.
5.6. Integrated Omics Insights: From Molecules to Phenotypes
Advances in high-throughput sequencing and multi-omics platforms have greatly deepened our understanding of salt-stress responses in grapevine rootstocks. By integrating transcriptomic, proteomic, and metabolomic evidence, recent studies have moved beyond simple cataloging of differentially expressed genes to reveal coherent regulatory networks that connect molecular activity with physiological performance (Table 4 and Table 5) [162,166,168]. Transcriptomic analyses have consistently identified up-regulation of ion-transport and osmotic-balance genes—including VvSOS1, VvNHX1, VvHKT1, and vacuolar proton pumps (VvV-ATPase, VvV-PPase)—in tolerant genotypes such as 1103 Paulsen and Ramsey [164,169]. These genes coordinate Na+ exclusion and vacuolar sequestration, enabling the maintenance of cytosolic homeostasis under saline irrigation. Concomitant induction of antioxidant-related genes (SOD, CAT, APX, POD) and stress-responsive transcription factors (WRKY, NAC, MYB, bHLH) underscores the central role of redox signaling and transcriptional control in orchestrating defense responses (Table 5) [164,169].
Proteomic profiles reinforce these findings by highlighting differential abundance of enzymes associated with energy metabolism, osmolyte biosynthesis, and membrane stabilization [176,177]. Enhanced levels of ATP-synthase, peroxidases, and heat-shock proteins have been documented in tolerant rootstocks, suggesting active reinforcement of metabolic and protective pathways. Complementary metabolomic data reveal increased accumulation of compatible solutes such as proline, glycine betaine, and soluble sugars, which support osmotic adjustment and ROS detoxification [172]. The coordinated rise in these metabolites parallels transcriptional activation of biosynthetic genes such as VvP5CS (Δ1-pyrroline-5-carboxylate synthetase) and VvAOT, linking molecular regulation directly to phenotypic tolerance (Table 4) [171]. Cross-omics integration therefore provides a mechanistic bridge between molecular signatures and whole-plant responses. For instance, proline accumulation observed in tolerant rootstocks is not merely a metabolic marker but the functional outcome of synchronized transcriptional activation and enzyme accumulation revealed by transcriptomic-proteomic coupling [171,172]. Likewise, metabolite–gene correlation analyses demonstrate that increased flavonoid and phenolic synthesis aligns with up-regulation of PAL and CHS genes, reinforcing antioxidant capacity at the tissue level [177].
These convergent datasets collectively depict salt tolerance as a system-level property involving interconnected ion transport, redox modulation, hormonal signaling, and metabolic adjustment networks. The current challenge lies in refining this multi-omics framework into predictive models capable of guiding marker-assisted selection and molecular breeding of next-generation salt-resilient rootstocks [165].
5.7. Epigenomics and Single-Cell Omics in Salt Stress Research
Epigenomic studies have shown that chromatin modifications and non-coding RNAs are key regulators of salt-stress tolerance. DNA methylation and histone modifications act as molecular switches that enable plants to “remember” prior stress exposure, allowing rapid transcriptional reprogramming when stress recurs [173]. Salt-induced methylation patterns have been linked to the regulation of ion-transport genes, stress-responsive transcription factors, and hormone-signaling pathways in several crop species, and similar regulatory mechanisms are likely to operate in grapevine rootstocks. Beyond DNA-level control, small RNAs (miRNAs) and long non-coding RNAs (lncRNAs) modulate salt-responsive gene expression by targeting transcriptional regulators and ion-homeostasis genes. Combining such epigenetic signatures with transcriptomic datasets enhances understanding of heritable tolerance mechanisms that can be harnessed in breeding programs [178,179].
Single-cell omics technologies provide an additional dimension to stress-biology research. In contrast with bulk transcriptome analyses, single-cell RNA sequencing (scRNA-seq) resolves cell-type-specific transcriptional changes during salt stress [175]. This is especially relevant to grapevine, where root-cell heterogeneity determines ion exclusion, compartmentalization, and water-uptake efficiency. Studies in model plants indicate that salt stress activates distinct gene sets in epidermal and cortical cells, including aquaporins, ROS-scavenging enzymes, and regulators of the abscisic-acid (ABA) pathway. Applying these techniques to grapevine rootstocks can reveal how individual root and leaf cell types cooperate to maintain whole-plant salt tolerance [180]. The resulting high-resolution data will inform precise molecular interventions and facilitate the creation of salt-resilient cultivars.
Epigenomic and single-cell omic approaches complement classical tools such as transcriptomics, proteomics, and metabolomics by introducing spatial and heritable dimensions to stress-response research. Integrating these technologies into grapevine studies will accelerate the identification of molecular markers and regulatory networks linked to salt tolerance, contributing to sustainable viticulture. Building on these molecular insights, CRISPR/Cas genome editing and marker-assisted selection (MAS) provide practical routes for translating omics discoveries into improved cultivars. Functional analyses have identified target genes—VvSOS1, VvNHX1, VvHKT1;1, and transcription factors such as VvWRKY46 and VvNAC17—whose manipulation enhances Na+ exclusion, vacuolar sequestration, and oxidative-stress mitigation. CRISPR/Cas9 allows precise modification of these loci without compromising agronomic performance, while MAS expedites the introgression of favorable alleles controlling ion transport and antioxidant activity. The convergence of epigenomic regulation, single-cell omics, and molecular breeding establishes a forward-looking framework for developing next-generation grapevine rootstocks adapted to saline environments and aligned with the goals of sustainable viticulture.
6. Regulation of Plant Growth Regulators in Grapevine Rootstock Under Salt Stress
Plant growth regulators (PGRs) play an influential role in the growth of grapevine rootstock and its responses to benign environmental conditions under stressful conditions. Regulation of PGR is well identified for their prevention mechanisms against salt stress function as a potential ROS scavenging (Figure 10), and PGR-related differentially expressed genes, proteins, transcription factors, and antioxidant enzymes (Figure 9B) [116,177,178]. PGR seems to be a critical signaling component that can enhance grapevine developmental stages when plants are subjected to salt stress. In addition, several scientific studies have documented how PGRs interact with other phytohormones under salt stress on grapevine rootstocks. The preliminary studies on the modulation of PGRs with the rest of the phytohormones are generally focused on auxin-Indole three acetic acid (IAA) [178,179], ethylene (ETH) [164], abscisic acid (ABA) [165], salicylic acid (SA) [176], methyl Jasmonate and Jasmonate (MeJA/JA) [167,168], melatonin (MT) [179], cytokinin (CTK) [180], 5-Aminolevulinic acid (5-ALA) [181] Strigolactones and among others [182,183]. Additionally, the published literature has focused on the correspondence between grapevine rootstocks as well as plant phytohormones involving interrelated corporation biological components such as stress responses, ROS production, gene expression, mechanical pathway, hormonal production, polyamines, nutritional status, seed metabolism, agronomic traits, plant morphological, biochemical and molecular responses under salt stress. However, under salt stress resilience, tolerant rootstocks (Salt Creek and Dogridge) [184] play pivotal roles in mitigating its diverse effects. For example, they help to improve grapevine plant growth and development, quantum yield, production, and tolerance mechanisms. The continuous performance of PGRs as well as their relative morpho-physiological investigations together with global omics techniques may provide an eco-friendly contributions to understand developmental deterioration of grapevine performance under salt stress. Most of the scientific studies have mentioned that, PGRs maintain various biological functions including baseline pathway, plant tolerance, ion signaling cascades, metabolic adjustments, production of free radicles, transmembrane proteins, housekeeping isozyme, hormone evolving enhancer protein kinases, phenotypical traits, eco-physiological aspects and genetically programmed developmental alteration in grapevine rootstocks under salt stress, thus, we did not discussed in-deep investigations about plant exogenous phytohormone regulations [2,169,170,183].
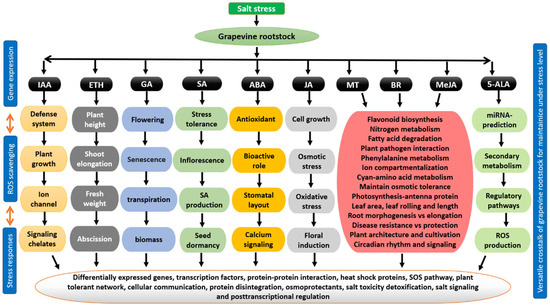
Figure 10.
A diagrammatic scheme illustrating the roles of various plant growth regulators with grapevine rootstock under salt stress. IAA: Indole acetic acid; ETH: Ethylene; GA: Gibberellic acid; SA: Salicylic acid; ABA: Abscisic acid; JA: Jasmonate; MT: Melatonin; BR: Brassinosteroids; MeJA: Methyl Jasmonate; and 5-ALA: 5-Aminolevulinic acid.
6.1. Abscisic Acid (ABA) Regulation
Abscisic acid (ABA) serves as a pivotal hormonal signal mediating grapevine adaptation to salt stress. Under salt exposure, elevated ABA levels in the roots and leaves trigger a wide range of physiological and molecular responses that collectively enhance plant resilience. The hormone primarily regulates stomatal closure through calcium-dependent signaling cascades, thereby minimizing transpirational water loss and maintaining cellular hydration. In grapevine rootstocks, ABA synthesis is rapidly induced via up-regulation of NCED (9-cis-epoxycarotenoid dioxygenase) genes, leading to increased accumulation of ABA in both vascular and guard cells [169].
Beyond its classical role in water conservation, ABA acts as a central integrator linking ionic stress with transcriptional regulation. It activates a network of ABA-responsive transcription factors, including AREB/ABF and bZIP, which regulate downstream genes involved in ion transport (VvSOS1, VvNHX1/2), osmolyte biosynthesis, and antioxidant defense (Figure 10). Cross-talk between ABA and calcium signaling promotes activation of anion channels such as SLAC1 and facilitates cytosolic Ca2+ oscillations that fine-tune ion homeostasis [169]. Studies in salt-tolerant rootstocks such as 1103 Paulsen and 140 R demonstrate that efficient ABA-mediated stomatal control and ROS detoxification are key determinants of sustained photosynthetic performance under saline irrigation. These findings, summarized in Table 5, highlight ABA-driven signaling as a major contributor to both short-term osmotic adjustment and long-term ionic homeostasis.
6.2. Indole-3-Acetic Acid (IAA) and Auxin Signaling
Auxin, predominantly in the form of indole-3-acetic acid (IAA), plays a critical role in modulating root system architecture and shoot development under salinity. Salt stress disrupts auxin transport and distribution, often leading to altered root elongation and branching patterns [183,184,185,186]. However, salt-tolerant grapevine rootstocks exhibit a more stable auxin gradient due to enhanced expression of auxin transporters (AUX1, PIN1, LAX) and signaling regulators (ARF, IAA genes). These molecular adjustments sustain root growth and lateral root formation, facilitating efficient water and nutrient uptake in saline soils (Figure 10).
IAA also interacts synergistically with ABA and ethylene to orchestrate stress-responsive growth modulation. Under moderate salinity, controlled auxin redistribution promotes selective inhibition of shoot elongation while supporting root proliferation, thereby optimizing the shoot-to-root ratio for osmotic balance. Proteomic and transcriptomic evidence indicates coordinated regulation of auxin transporters and expansin genes, allowing greater cell wall extensibility and improved ion uptake. Maintenance of an optimal ABA–IAA ratio is therefore crucial for sustaining root vigor and nutrient acquisition under salt stress [182,183,184].
6.3. Melatonin (MT) and Cross-Hormonal Synergy
Melatonin (MT), a multifunctional signaling molecule, has recently emerged as a vital regulator of salt stress tolerance in grapevines. Its antioxidant, hormonal, and transcriptional roles allow it to mitigate oxidative damage and modulate stress-responsive gene networks [168,186]. Exogenous or endogenous MT accumulation enhances the activity of ROS-scavenging enzymes (SOD, CAT, APX), thereby preserving redox balance (Figure 10). In grapevine tissues, MT interacts with ethylene and ABA pathways to coordinate stress adaptation—particularly through activation of VvMYB108A, which stimulates ethylene biosynthesis and enhances cellular protection mechanisms [168,185].
Additionally, melatonin regulates the expression of genes involved in ion transport and osmotic adjustment, including VvNHX1 and VvSOS1, reinforcing ionic stability under salt stress. Its signaling cross-talk with ABA and IAA fine-tunes physiological processes such as stomatal conductance, root elongation, and chlorophyll retention. Collectively, MT-mediated transcriptional activation complements ABA and IAA responses, integrating redox balance and hormonal control within the unified model presented in Figure 10. The mechanistic details summarized in Table 5 highlight MT as a promising molecular target for improving grapevine rootstock performance under saline environments.
7. Conclusions and Future Perspectives
Salinity continues to threaten grapevine productivity worldwide. This review integrates evidence across morphological, physiological, molecular, and omics levels to clarify how rootstocks mitigate salt stress. Tolerant genotypes maintain growth through ion exclusion, osmotic adjustment, and ROS detoxification, sustaining favorable Na+/K+ balance and protecting photosynthetic tissues. At the molecular scale, tolerance arises from coordinated activity of SOS, NHX, HKT, and CLC transporters, driven by WRKY, NAC, MYB, and bHLH transcription factors and modulated by hormonal signaling (ABA, JA, SA, IAA). Multi-omics analyses now connect these gene networks with metabolite and protein responses, revealing functional “evidence chains” linking molecular control to physiological performance. Emerging epigenomic and single-cell omics insights highlight additional regulatory layers, while CRISPR/Cas9 and marker-assisted selection provide practical tools for introducing salt-tolerance traits into elite rootstocks. Integrating physiology-based screening with molecular breeding will enable the creation of next-generation rootstocks resilient to salinity and drought. Strengthening this molecular understanding forms the foundation for sustainable viticulture under climate change, ensuring stable yield and fruit quality in salt-affected regions.
Author Contributions
Conceptualization, A.M. and A.H.; methodology, A.M. and A.H.; software, S.L.; validation, A.M., S.L. and G.M.; formal analysis, A.H. and E.E.; investigation, J.F.; re-sources, G.M.; data curation, A.M. and A.H.; writing—original draft preparation, A.M. and A.H.; writing—review and editing, S.L. and C.Z.; visualization, E.E.; supervision, J.F. and C.Z.; project administration, J.F. and C.Z.; funding acquisition, C.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Special Fund for Science and Technology Plan of Jiangsu Province (International Science and Technology Cooperation/Hong Kong Macao Taiwan Science and Technology Cooperation Plan)—“the Belt and Road” innovation cooperation project (BZ2024050).
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
There is no conflict of interest whatsoever.
References
- Zhao, F.; Zheng, T.; Liu, Z.; Fu, W.; Fang, J. Transcriptomic analysis elaborates the resistance mechanism of grapevine rootstocks against salt stress. Plants 2022, 11, 1167. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, F.; Zheng, T.; Zhongjie, L.; Ji, X.; Zhang, Z.; Pervaiz, T.; Shangguan, L.; Fang, J. Whole-genome re-sequencing, diversity analysis, and stress-resistance analysis of 77 grape rootstock genotypes. Front. Plant Sci. 2023, 14, 1102695. [Google Scholar] [CrossRef]
- Zhu, J.-K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Hakeem, A.; Li, S.; Iqbal, S.; Elatafi, E.; Aziz, R.B.; Mauligen, E.; Liu, S.; Chen, X.; Zhang, R.; Shangguan, L. Effects of salt stress on growth, physio-biochemical traits, and tolerance mechanism of grapevine rootstocks. Euphytica 2025, 221, 122. [Google Scholar] [CrossRef]
- Sivritepe, N.; Sivritepe, H.Ö.; Çelik, H.; Katkat, A. Salinity Responses of Grafted Grapevines: Effects of Scion and Rootstock Genotypes. Not. Bot. Horti Agrobot. Cluj-Napoca 2010, 38, 193–201. [Google Scholar] [CrossRef]
- Upadhyay, A.K.; Sharma, J.; Satisha, J. Influence of rootstocks on salinity tolerance of Thompson Seedless grapevines. J. Appl. Hortic. 2013, 15, 173–177. [Google Scholar] [CrossRef]
- Elatafi, E.; Elhendawy, B.; Elshahat, A.; Iqbal, S.; Yauha, R.; Xuxian, X.; Wentao, L.; Feiyue, L.; Shaoxiao, F.; Hakeem, A. A Comprehensive Analysis of Cadmium Contamination in Viticulture: From Soil and Grape to Ecological Risks and Remediation. J. Soil Sci. Plant Nutr. 2025, 25, 1401–1431. [Google Scholar] [CrossRef]
- Gajjar, P.; Ismail, A.; Islam, T.; Darwish, A.; Abuslima, E.; Dawood, A.; El-Saady, A.; Tsolova, V.; El-kereamy, A.; Nick, P.; et al. Physiological Comparison of Two Salt-Excluder Hybrid Grapevine Rootstocks under Salinity Reveals Different Adaptation Qualities. Plants 2023, 12, 3247. [Google Scholar] [CrossRef]
- Jogaiah, S. Morphological, physio-biochemical and molecular response of grapevine rootstocks to moisture and salinity stress-A review. Progress. Hortic. 2015, 47, 179. [Google Scholar] [CrossRef]
- Elatafi, E.; Elhendawy, B.; Iqbal, S.; Ali, S.; Elshahat, A.; Hakeem, A.; Shaonan, L.; Ibrahim, A.; Shangguan, L.; Fang, J. Physicochemical, Metabolite, Osmolyte Synthesis, and Enzymatic Alterations of Grapevine Rootstocks in Response to Cadmium Stress. J. Soil Sci. Plant Nutr. 2025, 1–19. [Google Scholar] [CrossRef]
- Gambetta, G.A.; Manuck, C.; Drucker, S.; Shaghasi, T.; Fort, K.; Matthews, M.; Walker, M.A.; McElrone, A. The relationship between root hydraulics and scion vigour across Vitis rootstocks: What role do root aquaporins play? J. Exp. Bot. 2012, 63, 6445–6455. [Google Scholar] [CrossRef] [PubMed]
- Al-Taey, D.; Al-Ameer, A. Effect of Salinity on the Growth and Yield of Grapes: A review. IOP Conf. Ser. Earth Environ. Sci. 2023, 1262, 042038. [Google Scholar] [CrossRef]
- Owais, S.J. Morphological and physiological responses of six grape genotypes to NaCl salt stress. Pak. J. Biol. Sci. 2015, 18, 240–246. [Google Scholar] [CrossRef][Green Version]
- Lo’ay, A.; Abo El-Ezz, S. Performance of ‘Flame seedless’ grapevines grown on different rootstocks in response to soil salinity stress. Sci. Hortic. 2021, 275, 109704. [Google Scholar] [CrossRef]
- Maryum, Z.; Luqman, T.; Nadeem, S.; Khan, S.M.U.D.; Wang, B.; Ditta, A.; Khan, M.K.R. An overview of salinity stress, mechanism of salinity tolerance and strategies for its management in cotton. Front. Plant Sci. 2022, 13, 907937. [Google Scholar] [CrossRef]
- Zhang, X.; Walker, R.R.; Stevens, R.M.; Prior, L.D. Yield-salinity relationships of different grapevine (Vitis vinifera L.) scion-rootstock combinations. Aust. J. Grape Wine Res. 2002, 8, 150–156. [Google Scholar] [CrossRef]
- Henderson, S.W.; Baumann, U.; Blackmore, D.H.; Walker, A.R.; Walker, R.R.; Gilliham, M. Shoot chloride exclusion and salt tolerance in grapevine is associated with differential ion transporter expression in roots. BMC Plant Biol. 2014, 14, 273. [Google Scholar] [CrossRef]
- Peiro, R.; Jimenez, C.; Perpiñà, G.; Soler, J.X.; Gisbert, C. Evaluation of the genetic diversity and root architecture under osmotic stress of common grapevine rootstocks and clones. Sci. Hortic. 2020, 266, 109283. [Google Scholar] [CrossRef]
- Shani, U.; Waisel, Y.; Eshel, A.; Xue, S.; Ziv, G. Responses to salinity of grapevine plants with split root systems. New Phytol. 1993, 124, 695–701. [Google Scholar] [CrossRef]
- Prinsi, B.; Failla, O.; Scienza, A.; Espen, L. Root proteomic analysis of two grapevine rootstock genotypes showing different susceptibility to salt stress. Int. J. Mol. Sci. 2020, 21, 1076. [Google Scholar] [CrossRef] [PubMed]
- Jellouli, N.; Ben Jouira, H.; Skouri, H.; Ghorbel, A.; Mliki, A. Proteomic analysis of Tunisian grapevine cultivar Razegui under salt stress. J. Plant Physiol. 2008, 165, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Lupo, Y.; Schlisser, A.; Dong, S.; Rachmilevitch, S.; Fait, A.; Lazarovitch, N. Root system response to salt stress in grapevines (Vitis spp.): A link between root structure and salt exclusion. Plant Sci. 2022, 325, 111460. [Google Scholar] [CrossRef] [PubMed]
- Hayta, S.; Polat, R.; Selvi, S. Traditional uses of medicinal plants in Elazığ (Turkey). J. Ethnopharmacol. 2014, 154, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Ishtiaq, M.; Mahmood, A.; Maqbool, M. Indigenous knowledge of medicinal plants from Sudhanoti district (AJK), Pakistan. J. Ethnopharmacol. 2015, 168, 201–207. [Google Scholar] [CrossRef]
- Van Zelm, E.; Zhang, Y.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef]
- Tetik, F.; Civelek, S.; Cakilcioglu, U. Traditional uses of some medicinal plants in Malatya (Turkey). J. Ethnopharmacol. 2013, 146, 331–346. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, Y.; Lü, E.; Wang, C. Archaeobotanical evidence of plant utilization in the ancient Turpan of Xinjiang, China: A case study at the Shengjindian cemetery. Veg. Hist. Archaeobotany 2015, 24, 165–177. [Google Scholar] [CrossRef]
- Jiang, Y.; Timpe, A.; Lohrberg, F. Identifying urban agriculture as heritage: Traditional urban grape gardens in the ancient city of Xuanhua, China. J. Urban Hist. 2023, 49, 1199–1218. [Google Scholar] [CrossRef]
- Chen, T.; Wang, X.; Dai, J.; Li, W.; Jiang, H. Plant use in the Lop Nor region of southern Xinjiang, China: Archaeobotanical studies of the Yingpan cemetery (∼25–420 AD). Quat. Int. 2016, 426, 166–174. [Google Scholar] [CrossRef]
- Singh, K.K.; Chauhan, J. A review on vegetative propagation of grape (Vitis vinifera L.) through cutting. Glob. J. Bio-Sci. Biotechnol. 2020, 9, 50–55. [Google Scholar]
- Nikolaou, K.-E.; Chatzistathis, T.; Theocharis, S.; Argiriou, A.; Koundouras, S.; Zioziou, E. Effects of Salinity and Rootstock on Nutrient Element Concentrations and Physiology in Own-Rooted or Grafted to 1103 P and 101-14 Mgt Rootstocks of Merlot and Cabernet Franc Grapevine Cultivars under Climate Change. Sustainability 2021, 13, 2477. [Google Scholar] [CrossRef]
- Katnee, S.; Vijaya, D.; Rao, B.; Padma, M. Relative Salt Tolerance of Different Grape Rootstocks to NaCl. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 723–733. [Google Scholar] [CrossRef]
- Migicovsky, Z.; Sawler, J.; Gardner, K.M.; Aradhya, M.K.; Prins, B.H.; Schwaninger, H.R.; Bustamante, C.D.; Buckler, E.S.; Zhong, G.-Y.; Brown, P.J. Patterns of genomic and phenomic diversity in wine and table grapes. Hortic. Res. 2017, 4, 17035. [Google Scholar] [CrossRef]
- Parihar, S.; Sharma, D. A brief overview on Vitis vinifera. Sch Acad J Pharm 2021, 12, 231–239. [Google Scholar] [CrossRef]
- Ramos-Madrigal, J.; Runge, A.K.W.; Bouby, L.; Lacombe, T.; Samaniego Castruita, J.A.; Adam-Blondon, A.-F.; Figueiral, I.; Hallavant, C.; Martínez-Zapater, J.M.; Schaal, C. Palaeogenomic insights into the origins of French grapevine diversity. Nat. Plants 2019, 5, 595–603. [Google Scholar] [CrossRef]
- Fort, K.; Fraga, J.; Grossi, D.; Walker, M.A. Early Measures of Drought Tolerance in Four Grape Rootstocks. J. Am. Soc. Hortic. Sci. 2017, 142, 36–46. [Google Scholar] [CrossRef]
- Zhou-Tsang, A.; Wu, Y.; Henderson, S.; Walker, A.; Borneman, A.; Walker, R.; Gilliham, M. Grapevine salt tolerance. Aust. J. Grape Wine Res. 2021, 27, 149–168. [Google Scholar] [CrossRef]
- Satisha, J.; Ramteke, S.; Karibasappa, G. Physiological and biochemical characterisation of grape rootstocks. S. Afr. J. Enol. Vitic. 2007, 28, 163–168. [Google Scholar] [CrossRef]
- Arbabzadeh, F.; Dutt, G. Salt Tolerance of Grape Rootstocks Under Greenhouse Conditions. Am. J. Enol. Vitic. 1987, 38, 95–99. [Google Scholar] [CrossRef]
- Bacilieri, R.; Lacombe, T.; Le Cunff, L.; Di Vecchi-Staraz, M.; Laucou, V.; Genna, B.; Péros, J.-P.; This, P.; Boursiquot, J.-M. Genetic structure in cultivated grapevines is linked to geography and human selection. BMC Plant Biol. 2013, 13, 25. [Google Scholar] [CrossRef]
- Sharafan, M.; Malinowska, M.A.; Ekiert, H.; Kwaśniak, B.; Sikora, E.; Szopa, A. Vitis vinifera (Vine Grape) as a valuable cosmetic raw material. Pharmaceutics 2023, 15, 1372. [Google Scholar] [CrossRef] [PubMed]
- Galet, P.; Smith, J. Grape Varieties and Rootstock Varieties, English ed.; Oenoplurimédia: Chaintré, France, 1998. [Google Scholar]
- Nicol, J.; Stirling, G.; Rose, B.; May, P.; Van Heeswijck, R. Impact of nematodes on grapevine growth and productivity: Current knowledge and future directions, with special reference to Australian viticulture. Aust. J. Grape Wine Res. 1999, 5, 109–127. [Google Scholar] [CrossRef]
- Rahmani, H.; Rasoli, V.; Abdossi, V.; Ghanbari Jahromi, M. Ch1 (Vitis vinifera L.) rootstock control of scion response to water stress in some commercial grapevine cultivars. S. Afr. J. Enol. Vitic. 2023, 44, 1–8. [Google Scholar] [CrossRef]
- Galet, P. Cépages et vignobles de France: Tome 1, Les vignes américaines; Impr. P. Déhan: Montpellier, France, 1988. [Google Scholar]
- Khan, M.; Akram, M.; Waseem, R.; Qadri, R.; Al-Yahyai, R. Role of Grapevine Rootstocks in Mitigating Environmental Stresses: A review. Journal of Agricultural and Marine Sciences. J. Agric. Mar. Sci. 2020, 25, 7. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, K.; Yang, X.; Li, J.; Li, X. Comparative study of the key aromatic compounds of Cabernet Sauvignon wine from the Xinjiang region of China. J. Food Sci. Technol. 2021, 58, 2109–2120. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, Z.; Zhu, C.; Zhang, Z.; Shi, W.; Lu, Q.; Sun, J. Saline–Alkaline Stress Resistance of Cabernet Sauvignon Grapes Grafted on Different Rootstocks and Rootstock Combinations. Plants 2023, 12, 2881. [Google Scholar] [CrossRef]
- Wang, W.-n.; Min, Z.; Wu, J.-r.; Liu, B.-c.; Xu, X.-l.; Fang, Y.; Ju, Y. Physiological and transcriptomic analysis of Cabernet Sauvginon (Vitis vinifera L.) reveals the alleviating effect of exogenous strigolactones on the response of grapevine to drought stress. Plant Physiol. Biochem. 2021, 167, 400–409. [Google Scholar] [CrossRef]
- George, I.; Pascovici, D.; Mirzaei, M.; Haynes, P. Quantitative proteomic analysis of cabernet sauvignon grape cells exposed to thermal stresses reveals alterations in sugar and phenylpropanoid metabolism. Proteomics 2015, 15, 3048–3060. [Google Scholar] [CrossRef]
- Han, X.; Yao, F.; Xue, T.-t.; Wang, Z.; Wang, Y.; Cao, X.; Hui, M.; Wu, D.; Li, Y.-h.; Wang, H.; et al. Sprayed biodegradable liquid film improved the freezing tolerance of cv. Cabernet Sauvignon by up-regulating soluble protein and carbohydrate levels and alleviating oxidative damage. Front. Plant Sci. 2022, 13, 1021483. [Google Scholar] [CrossRef]
- Li, Y.; Du, Y.P.; Fu, Y.D.; Zhai, H. Physiological Responses of Waterlogging on Different Rootstock Combinations of Cabernet Sauvignon Grape. Acta Hortic. Sin. 2013, 40, 2105. [Google Scholar]
- Dami, I.; Zhang, Y. Variations of freezing tolerance and sugar concentrations of grape buds in response to foliar application of abscisic acid. Front. Plant Sci. 2023, 14, 1084590. [Google Scholar] [CrossRef]
- Lu, X.; Ma, L.; Zhang, C.; Yan, H.; Bao, J.; Gong, M.; Wang, W.; Li, S.; Ma, S.; Chen, B. Grapevine (Vitis vinifera) responses to salt stress and alkali stress: Transcriptional and metabolic profiling. BMC Plant Biol. 2022, 22, 528. [Google Scholar] [CrossRef]
- Han, X.; Li, Y.-H.; Yao, M.-H.; Yao, F.; Wang, Z.; Wang, H.; Li, H. Transcriptomics Reveals the Effect of Short-Term Freezing on the Signal Transduction and Metabolism of Grapevine. Int. J. Mol. Sci. 2023, 24, 3884. [Google Scholar] [CrossRef] [PubMed]
- Verslype, N.; Nascimento, A.; Musser, R.; de Souza Caldas, R.M.; Martins, L.; Leão, P. Drought tolerance classification of grapevine rootstock by machine learning for the São Francisco Valley. Smart Agric. Technol. 2023, 4, 100192. [Google Scholar] [CrossRef]
- Gao, Z.; Li, J.; Zhu, H.; Sun, L.; Du, Y.; Zhai, H. Using differential thermal analysis to analyze cold hardiness in the roots of grape varieties. Sci. Hortic. 2014, 174, 155–163. [Google Scholar] [CrossRef]
- Jogaiah, S.; Ramteke, S.; Sharma, J.; Upadhyay, A.K. Moisture and Salinity Stress Induced Changes in Biochemical Constituents and Water Relations of Different Grape Rootstock Cultivars. Int. J. Agron. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Scott, D.H. Breeding Salt Tolerant Grapevine Rootstocks. Master’s Thesis, University of California, Davis, CA, USA, 2022. [Google Scholar]
- Lo’ay, A.; Hamza, D. The potential of vine rootstocks impacts on ‘Flame Seedless’ bunches behavior under cold storage and antioxidant enzyme activity performance. Sci. Hortic. 2020, 260, 108844. [Google Scholar] [CrossRef]
- Miller, D.; Howell, G.; Striegler, R. Cane and Bud Hardiness of Selected Grapevine Rootstocks. Am. J. Enol. Vitic. 1988, 39, 55–59. [Google Scholar] [CrossRef]
- Ruperti, B.; Botton, A.; Populin, F.; Eccher, G.; Brilli, M.; Quaggiotti, S.; Trevisan, S.; Cainelli, N.; Guarracino, P.; Schievano, E.; et al. Flooding Responses on Grapevine: A Physiological, Transcriptional, and Metabolic Perspective. Front. Plant Sci. 2019, 10, 339. [Google Scholar] [CrossRef]
- Wang, X.; Yan, L.; Wang, B.; Qian, Y.; Wang, Z.; Wu, W. Comparative Proteomic Analysis of Grapevine Rootstock in Response to Waterlogging Stress. Front. Plant Sci. 2021, 12, 749184. [Google Scholar] [CrossRef]
- Shi, W.; He, W.; Zhang, Z.; Sun, J.; Zhu, C.; Liu, Z.; Xu, Y.; Zhao, B. Study on the Resistance of ‘Cabernet Sauvignon’ Grapevine with Different Rootstocks to Colomerus vitis. Sustainability 2022, 14, 15193. [Google Scholar] [CrossRef]
- Barakat, A.; Hussein, B.; Abd-Elfattah, N.; Soliman, M. Evaluation of the two rootstocks (SO4 and Freedom) for the salt stress in vitro conditions. Plant Arch. 2019, 19, 500–507. [Google Scholar]
- Verma, Y.; Upadhyay, A.K.; Taynath, B.; Raut, S.; Yadav, S.; Ghadge, S. Role of Dogridge Rootstock for Salinity Tolerance in Thompson Seedless Grapevine: Nutrient Distribution, Physiological and Biochemical Functions. Agric. Mech. Asia 2023, 54, 13965–13986. [Google Scholar]
- Baghdady, M.; Abdrabboh, G.; Abdel-Aziz, H. In vitro responses of some grape rootstocks to salt stress. In Proceedings of the 3rd International Conference on Biotechnology Applications in Agriculture (ICBAA), Benha University, Moshtohor and Sharm El-Sheikh, Egypt, 5–9 April 2016. [Google Scholar]
- Zhu, X.; Li, X.; Jiu, S.; Zhang, K.; Wang, C.; Fang, J. Analysis of the regulation networks in grapevine reveals response to waterlogging stress and candidate gene-marker selection for damage severity. R. Soc. Open Sci. 2018, 5, 172253. [Google Scholar] [CrossRef] [PubMed]
- Sultan, M. Effect of rootstocks on growth, yield and fruit quality of Red Globe grape. Ann. Agric. Sci. Moshtohor 2016, 54, 339–344. [Google Scholar]
- Ramajayam, D.; Singh, S. In Vitro Screening of Grape Root-Stocks for NaCl Tolerance; LAP LAMBERT Academic Publishing: Saarbrücken, Germany, 2018. [Google Scholar]
- Ramajayam, D. Mycorrhization alleviates salt stress in grape rootstocks during in vitro acclimatization. Indian J. Hortic. 2013, 70, 26–32. [Google Scholar]
- Cakir, B.; Özmen, C.; Kibar, U.; Mutaf, F.; Büyük, P.; Bakır, M.; Ergul, A. Salt stress induces endoplasmic reticulum stress-responsive genes in a grapevine rootstock. PLoS ONE 2020, 15, e0236424. [Google Scholar] [CrossRef]
- Berdeja Aramayo, M.; Hilbert, G.; Dai, Z.; Blank, M.; Stoll, M.; Delrot, S. Effects of drought stress and rootstock genotype on grape berry quality. In Proceedings of the 7th International Symposium on Irrigation of Horticultural Crops, Geisenheim, Germany, 16–20 July 2012; p. 1038. [Google Scholar] [CrossRef]
- Kidman, C.; Olarte Mantilla, S.M.; Dry, P.; McCarthy, M.; Collins, C. Effect of Water Stress on the Reproductive Performance of Shiraz (Vitis vinifera L.) Grafted to Rootstocks. Am. J. Enol. Vitic. 2014, 65, 96–108. [Google Scholar] [CrossRef]
- Kang, S.-B.; Lee, I.-B.; Park, J.; Lim, T. Effect of Waterlogging Conditions on the Growth, Root Activities and Nutrient Content of ‘Campbell Early’ Grapevine. Wonye Kwahak Kisulchi = Korean J. Hortic. Sci. Technol. 2010, 28, 172–179. [Google Scholar]
- Rashedy, A. Growth Evaluation of Some Grape Cultivars and Rootstocks Under Saline Stress Conditions. Ph.D. Thesis, Cairo University, Giza, Egypt, 2011. [Google Scholar] [CrossRef]
- Küskü, D.; Soylemezoglu, G. Responses of Potential Rootstock Hybrids (Vinifera × American) to Drought and Salt Stress. Erwerbs-Obstbau 2023, 65, 2143–2152. [Google Scholar] [CrossRef]
- Suarez, D.; Celis, N.; Anderson, R.; Sandhu, D. Grape Rootstock Response to Salinity, Water and Combined Salinity and Water Stresses. Agronomy 2019, 9, 321. [Google Scholar] [CrossRef]
- Han, Y.; Li, X. Current progress in research focused on salt tolerance in Vitis vinifera L. Front. Plant Sci. 2024, 15, 1353436. [Google Scholar]
- Daldoul, S.; Gargouri, M.; Weinert, C.; Jarrar, A.; Egert, B.; Mliki, A.; Nick, P. A Tunisian Wild Grape Leads to Metabolic Fingerprints of Salt Tolerance. Plant Physiol. 2023, 193, 371–388. [Google Scholar] [CrossRef]
- Ojeda, M.; Pire, R. Effect of salinity on two grafted or ungrafted grape rootstocks. Rev. Fitotec. Mex. 2011, 34, 43–52. [Google Scholar]
- Pire, R.; Pereira, A.; Diez, J.; Fereres, E. Drought tolerance assessment of a Venzuelan grape rootstock and possible conditioning mechanisms. Agrociencia 2007, 41, 435–446. [Google Scholar]
- Fahim, S.; Ghanbari, A.; Naji, A.; Shokohian, A.; Maleki, H.; Gohari, G.; Hano, C. Multivariate Discrimination of Some Grapevine Cultivars under Drought Stress in Iran. Horticulturae 2022, 8, 871. [Google Scholar] [CrossRef]
- Stevens, R.M.; Walker, R. Response of grapevines to irrigation-induced saline–sodic soil conditions. Anim. Prod. Sci. 2002, 42, 323–331. [Google Scholar] [CrossRef]
- Pommer, C.V.; Ribeiro, I.J.A.; Jr, M.J.; Hernandes, J.; Martins, F.P.; Gallo, P. IAC JULIANA—Table grape cultivar. Crop. Breed. Appl. Biotechnol. 2002, 2, 323–324. [Google Scholar] [CrossRef]
- da Silva, E.; Santos, H.; Ometto, J.; Maniçoba da Rosa Ferraz Jardim, A.; Silva, T.; Hermínio, P.; Simões, A.; Souza, E.; Ferreira-Silva, S. Salt-excluder rootstock improves physio-biochemical responses of grafted grapevine plants subjected to salinity stress. Curr. Plant Biol. 2024, 37, 100316. [Google Scholar] [CrossRef]
- Daldoul, S.; Hanzouli, F.; Hamdi, Z.; Chenenaoui, S.; Wetzel, T.; Nick, P.; Mliki, A.; Gargouri, M. The root transcriptome dynamics reveals new valuable insights in the salt-resilience mechanism of wild grapevine (Vitis vinifera subsp. sylvestris). Front. Plant Sci. 2022, 13, 1077710. [Google Scholar] [CrossRef]
- Guan, L.; Muhammad Salman, H.; Khan, N.; Nasim, M.; Jiu, S.; Fiaz, M.; Zhu, X.; Zhang, K.; Fang, J. Transcriptome Sequence Analysis Elaborates a Complex Defensive Mechanism of Grapevine (Vitis vinifera L.) in Response to Salt Stress. Int. J. Mol. Sci. 2018, 19, 4019. [Google Scholar] [CrossRef] [PubMed]
- Bari, L.R.; Ghanbari, A.; Darvishzadeh, R.; Giglou, M.T.; Baneh, H.D. Discernment of grape rootstocks base on their response to salt stress using selected characteristics in combination with chemometric tools. Food Chem. 2021, 365, 130408. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hui, M.; Shi, X.-Q.; Wu, D.; Wang, Y.; Han, X.; Cao, X.; Yao, F.; Li, H.; Wang, H. Characteristics of the Seed Germination and Seedlings of Six Grape Varieties (V. vinifera). Plants 2022, 11, 479. [Google Scholar] [CrossRef] [PubMed]
- Cuena Lombraña, A.; Lallai, A.; Belhadj, F.; Gharbi, B.; Bacchetta, G. Carignan Grape Cultivar Salt Tolerance during the Germination Phase across the Mediterranean Basin. Seeds 2022, 1, 136–145. [Google Scholar] [CrossRef]
- Nasim, M.; Zheng, T.; Naseri, E.; Leng, X.; Zhang, Z.; Jia, H.; Fang, J. Molecular Evaluation of Vitality and Survival Rate of Dormant Kyoho Grape Seedlings: A Step toward Molecular Farming. Plants 2019, 8, 577. [Google Scholar] [CrossRef]
- İlhan, M.; EkbİÇ, H. Determination of Tolerance to Drought Stress of Two American Grapevine Rootstocks by PEG Application. Akad. Ziraat Derg. 2023, 153–162. [Google Scholar] [CrossRef]
- Cancado, G.; Ribeiro, A.; Piñeros, M.; Miyata, L.; Alvarenga, Â.; Villa, F.; Pasqual, M.; Purgatto, E. Evaluation of aluminium tolerance in grapevine rootstocks. VITIS 2009, 48, 167–173. [Google Scholar]
- Jogaiah, S.; Porika, H. Scion preconditioning and cytokinin treatment improved graft compatibility in Red Globe grape grafted on Dogridge rootstock. Indian J. Hortic. 2023, 80, 165–170. [Google Scholar] [CrossRef]
- Titova, L.; Magomadov, A.; Avdeenko, I.; Grigoriev, A.; Palaeva, D. Analysis of the development of grafted grape seedlings on a nursery garden of different graft-rootstock combinations. IOP Conf. Ser. Earth Environ. Sci. 2021, 723, 022105. [Google Scholar] [CrossRef]
- Titova, L.; Magomadov, A.; Avdeenko, I.; Grigoriev, A. Development of grafted grape seedlings depending on the length and variety of rootstock. IOP Conf. Ser. Earth Environ. Sci. 2021, 723, 022085. [Google Scholar] [CrossRef]
- Dardeniz, A.; Müftüoğlu, N.; Altay, H. Determination of salt tolerance of some American grape rootstocks. Bangladesh J. Bot. 2006, 35, 143–150. [Google Scholar]
- Sabir, A.; Yazar, K.; Doğan, O.; Şit, M.; Kara, Z. Effects of Colchicine Treatments on Some Grape Rootstock and Grape Varieties at Cotyledon Stage. Selcuk J. Agric. Food Sci. 2018, 32, 424–429. [Google Scholar] [CrossRef]
- Sabir, A. Influences of Self- and Cross-pollinations on Berry Set, Seed Characteristics and Germination Progress of Grape (Vitis vinifera cv. Italia). Int. J. Agric. Biol. 2011, 13, 591–594. [Google Scholar]
- Kucukbasmaci, A.; Sabir, A. Long-term impact of deficit irrigation on the physiology and growth of grapevine cv. ‘Prima’ grafted on various rootstocks. Acta Sci. Pol. Hortorum Cultus 2019, 18, 57–70. [Google Scholar] [CrossRef]
- Dardeniz, A.; Gökbayrak, Z.; Müftüoğlu, N.; Turkmen, C.; Beşer, K. Cane Quality Determination of 5 BB and 140 Ru Grape Rootstocks. Eur. J. Hortic. Sci. 2008, 73, 254–258. [Google Scholar] [CrossRef]
- Somkuwar, R.; Bondage, D.; M, S.; S, N.; Sharma, A. Yield, raisin recovery and biochemical characters of fresh and dried grapes (raisin). Indian J. Agric. Sci. 2013, 83, 924–927. [Google Scholar]
- Sucu, S.; Yağci, A.; yıldırım, K. Changes in Morphological, Physiological Traits and Enzyme Activity of Grafted and Ungrafted Grapevine Rootstocks Under Drought Stress. Erwerbs-Obstbau 2018, 60, 1–10. [Google Scholar] [CrossRef]
- Lu, J.; Ren, Z.; Cousins, P. Field Evaluation of Grape Rootstock Response to Natural Infection by Pierce’s Disease. Available online: https://static.cdfa.ca.gov/PiercesDisease/proceedings/2004/2004_45-47.pdf (accessed on 5 July 2025).
- Miras-Avalos, J.; Intrigliolo, D. Grape Composition under Abiotic Constrains: Water Stress and Salinity. Front. Plant Sci. 2017, 8, 851. [Google Scholar] [CrossRef]
- yıldırım, K.; Yağci, A.; Sucu, S.; Tunç, S. Responses of grapevine rootstocks to drought through altered root system architecture and root transcriptomic regulations. Plant Physiol. Biochem. 2018, 127, 256–268. [Google Scholar] [CrossRef]
- Elaidy, A.; Khalf, A.; Abo-Ogiala, A. Effect of different grape rootstocks on the growth, yield and quality of Superior grape under salt stress. Middle East J. 2019, 8, 167–175. [Google Scholar]
- Corso, M.; Bonghi, C. Grapevine rootstock effects on abiotic stress tolerance. Plant Sci. Today 2014, 1, 108–113. [Google Scholar] [CrossRef]
- Flexas, J.; Barón, M.; Bota, J.; Ducruet, J.-M.; Gallé, A.; Galmés, J.; Jiménez, M.; Pou, A.; Ribas-Carbó, M.; Sajnani, C.; et al. Photosynthesis limitations during water stress acclimation and recovery in the drought-adapted Vitis hybrid Richter-110 (V. berlandieri×V. rupestris). J. Exp. Bot. 2009, 60, 2361–2377. [Google Scholar] [CrossRef] [PubMed]
- Ezzahouani, A.; Williams, L. The Influence of Rootstock on Leaf Water Potential, Yield, and Berry Composition of Ruby Seedless Grapevines. Am. J. Enol. Vitic. 1995, 46, 559–563. [Google Scholar] [CrossRef]
- Rashedy, A. Response of two grape rootstocks to some salt tolerance treatments under saline water conditions. J. Hortic. Sci. Ornam. Plants 2010, 2079–2158, 93–106. [Google Scholar]
- Buesa, I.; Pérez-Pérez, J.G.; Visconti, F.; Strah, R.; Intrigliolo, D.S.; Bonet, L.; Gruden, K.; Pompe-Novak, M.; de Paz, J.M. Physiological and Transcriptional Responses to Saline Irrigation of Young ‘Tempranillo’ Vines Grafted Onto Different Rootstocks. Front Plant Sci 2022, 13, 866053. [Google Scholar] [CrossRef]
- Bettiga, L.J. Wine Grape Varieties in California; UCANR Publications: Davis, CA, USA, 2003; Volume 3419. [Google Scholar]
- Ferreira, J.H.S. Effect of Rootstock on the Incidence of Dying Arm of Chenin blanc vines. S. Afr. J. Enol. Vitic. 1985, 6, 23–24. [Google Scholar] [CrossRef][Green Version]
- Reynolds, A.G.; Wardle, D.A.J.H. Rootstocks impact vine performance and fruit composition of grapes in British Columbia. HortTechnology 2001, 11, 419–427. [Google Scholar] [CrossRef]
- Rahemi, A. Breeding of grapevine rootstocks for adaptability to the environmental stresses. In Proceedings of the Third National Conference of Grape and Raisin Conducted by Research Institute of Grape and Raisin (RIGR), Malayer, Iran, 27–29 September 2016; Malayer University: Malayer, Iran, 2016; pp. 27–29. (In Persian) [Google Scholar]
- Lin, Y.; Liu, S.; Fang, X.; Ren, Y.; You, Z.; Xia, J.; Hakeem, A.; Yang, Y.; Wang, L.; Fang, J. The physiology of drought stress in two grapevine cultivars: Photosynthesis, antioxidant system, and osmotic regulation responses. Physiol. Plant. 2023, 175, e14005. [Google Scholar] [CrossRef]
- Bota, B.J.; Flexas, J.; Medrano, H. Genetic variability of photosynthesis and water use in Balearic grapevine cultivars. Ann. Appl. Biol. 2001, 138, 353–361. [Google Scholar] [CrossRef]
- Pool, B.; Lerch, S.; Howard, G.; Johnson, T.; Weimann, D. Rootstocks for planting or replanting New York vineyards, Finger lakes vineyard notes. Cornell Univ. 2005, 1–14. [Google Scholar]
- Bica, D.; Gay, G.; Morando, A.; Soave, E. Effects of rootstock and Vitis vinifera genotype on photosynthetic parameters. In Proceedings of the V International Symposium on Grapevine Physiology, Jerusalem, Israel, 25–30 May 1997; Volume 526, pp. 373–380. [Google Scholar]
- Regina, M.; Carbonneau, A. Gas exchanges in Vitis vinifera under water stress regime. I. Characterization of the variety behavior. Pesqui. Agropecu. Bras. 1996, 31, 869–876. [Google Scholar]
- Amir, J.; Eshghi, S.; Tafazoli, E.; Kholdebarin, B.; Abbaspour, N. Ameliorative effects of Salicylic acid on mineral concentrations in roots and leaves of two grapevine (Vitis vinifera L.) cultivars under salt stress. Vitis—J. Grapevine Res. 2014, 53, 181–188. [Google Scholar]
- Zhang, Z.; Liu, H.; Sun, J.; Yu, S.; He, W.; Li, T.; Baolong, Z. Nontarget Metabolomics of Grape Seed Metabolites Produced by Various Scion–Rootstock Combinations. J. Am. Soc. Hortic. Sci. 2020, 145, 1–10. [Google Scholar] [CrossRef]
- Dubrovina, A.; Kiselev, K.; Khristenko, V.; Aleynova, O. VaCPK20, a calcium-dependent protein kinase gene of wild grapevine Vitis amurensis Rupr., mediates cold and drought stress tolerance. J. Plant Physiol. 2015, 185, 1–12. [Google Scholar] [CrossRef]
- Lupo, Y.; Prashanth, K.; Lazarovitch, N.; Fait, A.; Rachmilevitch, S. Importance of Leaf Age in Grapevines Under Salt Stress. bioRxiv 2023, 2023.04. 02, 535264. [Google Scholar] [CrossRef]
- Minarovicová, L.; Karovicová, J.; Kohajdová, Z.; Kuchtová, V. The Chemical Composition of Grape Fibre. Potravin. Slovak J. Food Sci 2015, 9, 53–57. [Google Scholar]
- Aloy, K.G.; Lemos, P.L.P.K.; Jacobs, S.A.; Schumacher, R.L.; Jacobs, B.; Potter, G.H.; Costa, V.B. Climatological variables and their influence on the quality of Vitis vinifera grapes in Dom Pedrito, region of Campanha, Brazil. Rev. DELOS 2023, 16, 3753–3770. [Google Scholar] [CrossRef]
- Monteiro, E.; Gonçalves, B.; Cortez, I.; Castro, I. The role of biostimulants as alleviators of biotic and abiotic stresses in grapevine: A review. Plants 2022, 11, 396. [Google Scholar] [CrossRef]
- Oliveira, M.; Fernandes-Silva, A. Vineyard and Olive Orchard Management to Maintain Yield and Quality Under Abiotic Stress Conditions. In Modern Fruit Industry; IntechOpen: London, UK, 2019. [Google Scholar]
- Razzaq, A.; Zafar, M.M.; Ali, A.; Hafeez, A.; Sharif, F.; Guan, X.; Deng, X.; Pengtao, L.; Shi, Y.; Haroon, M. The pivotal role of major chromosomes of sub-genomes A and D in fiber quality traits of cotton. Front. Genet. 2022, 12, 642595. [Google Scholar] [CrossRef]
- Razzaq, A.; Zafar, M.M.; Li, P.; Qun, G.; Deng, X.; Ali, A.; Hafeez, A.; Irfan, M.; Liu, A.; Ren, M. Transformation and overexpression of primary cell wall synthesis-related zinc finger gene Gh_A07G1537 to improve fiber length in cotton. Front. Plant Sci. 2021, 12, 777794. [Google Scholar] [CrossRef]
- Bilal, H.; Shakeel, A.; Chattha, W.S.; Tehseen, M.; Azhar, S.A.; Rizwan, M. Genetic variability studies of seed cotton yield and fibre quality in upland cotton (Gossypium hirsutum L.) grown under salinity stress. Pak. J. Bot 2022, 54, 1995–1999. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, C.; Yan, D.; Chen, W.; Luo, L. Estimation of characteristic parameters of grape clusters based on point cloud data. Front. Plant Sci. 2022, 13, 885167. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Huang, B. Mechanism of Salinity Tolerance in Plants: Physiological, Biochemical, and Molecular Characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Pardo, J.; Batelli, G.; Oosten, M.; Bressan, R.; Li, X. The Salt Overly Sensitive (SOS) Pathway: Established and Emerging Roles. Mol. Plant 2013, 6, 275–286. [Google Scholar] [CrossRef]
- Raza, A.; Tabassum, J.; Fakhar, A.; Sharif, R.; Chen, H.; Zhang, C.; Ju, L.; Fotopoulos, V.; Siddique, K.; Singh, R.; et al. Smart reprograming of plants against salinity stress using modern biotechnological tools. Crit. Rev. Biotechnol. 2022, 43, 1035–1062. [Google Scholar] [CrossRef]
- Wei, L.; Du, Y.; Xiang, J.; Zheng, T.; Cheng, J.; Wu, J. Integrated mRNA and miRNA transcriptome analysis of grape in responses to salt stress. Front. Plant Sci. 2023, 14, 1173857. [Google Scholar] [CrossRef]
- Catacchio, C.; Alagna, F.; Perniola, R.; Bergamini, C.; Rotunno, S.; Calabrese, F.; Crupi, P.; Antonacci, D.; Ventura, M.; Cardone, M. Transcriptomic and genomic structural variation analyses on grape cultivars reveal new insights into the genotype-dependent responses to water stress. Sci. Rep. 2019, 9, 2809. [Google Scholar] [CrossRef]
- Zhong, H.; Zhongjie, L.; Zhang, F.; Zhou, X.; Sun, X.; Li, Y.; Liu, W.; Xiao, H.; Wang, N.; Lu, H.; et al. Metabolomic and transcriptomic analyses reveal the effects of self- and hetero-grafting on anthocyanin biosynthesis in grapevine. Hortic. Res. 2022, 9, 103. [Google Scholar] [CrossRef]
- Aswathi, K.; Ul-Allah, S.; Puthur, J.T.; Siddique, K.H.; Frei, M.; Farooq, M. The Plant Mind: Unraveling Abiotic Stress Priming, Memory, and Adaptation. Physiol. Plant. 2025, 177, e70372. [Google Scholar] [CrossRef]
- Zrig, A.; Mohamed, H.B.; Tounekti, T.; Khemira, H.; Serrano, M.; Valero, D.; Vadel, A. Effect of rootstock on salinity tolerance of sweet almond (cv. Mazzetto). S. Afr. J. Bot. 2016, 102, 50–59. [Google Scholar] [CrossRef]
- Upreti, K.K.; Murti, G. Response of grape rootstocks to salinity: Changes in root growth, polyamines and abscisic acid. Biol. Plant. 2010, 54, 730–734. [Google Scholar] [CrossRef]
- Wei, P.; Che, B.; Shen, L.; Cui, Y.; Wu, S.; Cheng, C.; Liu, F.; Li, M.-W.; Yu, B.; Lam, H.-M. Identification and functional characterization of the chloride channel gene, GsCLC-c2 from wild soybean. BMC Plant Biol. 2019, 19, 121. [Google Scholar] [CrossRef] [PubMed]
- Mohammadkhani, N.; Heidari, R.; Abbaspour, N. Effects of salinity on antioxidant system in four grape (Vitis vinifera L.) genotypes. Vitis 2013, 52, 105–110. [Google Scholar]
- Raziq, A.; Wang, Y.; Mohi Ud Din, A.; Sun, J.; Shu, S.; Guo, S. A comprehensive evaluation of salt tolerance in tomato (Var. ailsa craig): Responses of physiological and transcriptional changes in RBOH’s and ABA biosynthesis and signalling genes. Int. J. Mol. Sci. 2022, 23, 1603. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ma, H.; Chen, T.; Pen, J.; Yu, S.; Zhao, X. Morphological and physiological responses of cotton (Gossypium hirsutum L.) plants to salinity. PLoS ONE 2014, 9, e112807. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Unraveling salt stress signaling in plants. J. Integr. Plant Biol. 2018, 60, 796–804. [Google Scholar] [CrossRef]
- Zhu, J.-K. Genetic analysis of plant salt tolerance using Arabidopsis. Plant Physiol. 2000, 124, 941–948. [Google Scholar] [CrossRef]
- Qiu, Q.-S.; Guo, Y.; Dietrich, M.A.; Schumaker, K.S.; Zhu, J.-K. Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc. Natl. Acad. Sci. USA 2002, 99, 8436–8441. [Google Scholar] [CrossRef]
- Muhammad Salman, H.; Jogaiah, S.; Pervaiz, T.; Zhao, Y.; Khan, N.; Fang, J. Physiological and Transcriptional Variations Inducing Complex Adaptive Mechanisms in Grapevine by Salt Stress. Environ. Exp. Bot. 2019, 162, 455–467. [Google Scholar] [CrossRef]
- Jin, Z.; Lv, X.; Sun, Y.; Fan, Z.; Xiang, G.; Yao, Y. Comprehensive discovery of salt-responsive alternative splicing events based on Iso-Seq and RNA-seq in grapevine roots. Environ. Exp. Bot. 2021, 192, 104645. [Google Scholar] [CrossRef]
- Lu, X.; Ma, L.; Zhang, C.; Yan, H.; Bao, J.; Gong, M.; Wang, W.; Li, S.; Ma, S.; Chen, B. Transcriptional and metabolic profiles revealed the difference in grapevine (Vitis vinifera) plants responses to neutral salt stress and alkali salt stress. BMC Plant Bio. 2022. preprint. [Google Scholar] [CrossRef]
- Upadhyay, A.; Gaonkar, T.; Upadhyay, A.K.; Jogaiah, S.; Shinde, M.; Kadoo, N.; Gupta, V. Global transcriptome analysis of grapevine (Vitis vinifera L.) leaves under salt stress reveals differential response at early and late stages of stress in table grape cv. Thompson Seedless. Plant Physiol. Biochem. 2018, 129, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Ayadi, M.; Martins, V.; Ben Ayed, R.; Jbir-Koubaa, R.; Feki, M.; Mzid, R.; Gerós, H.; Sami, A.; Hanana, M. Genome Wide Identification, Molecular Characterization, and Gene Expression Analyses of Grapevine NHX Antiporters Suggest Their Involvement in Growth, Ripening, Seed Dormancy, and Stress Response. Biochem. Genet. 2020, 58, 102–128. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Jiao, B.; Liu, Z.; Wang, X.; Wang, J.; Zhang, J.; Wang, Q.; Xu, Y.; Wang, Q.C. Crosstalk between grapevine leafroll-associate virus-3 (GLRaV-3) and NaCl-induced salt stress in in vitro cultures of the red grape ‘Cabernet Sauvignon’. Plant Cell Tissue Organ Cult. (PCTOC) 2021, 144, 1–12. [Google Scholar] [CrossRef]
- Vila, H.; Filippini, M.; Venier, M.; Di Filippo, M.; Hugalde, I. A dynamic model for sodium intoxication unravels salt tolerance in grapevine (Vitis vinifera L.) rootstocks. Rev. Fac. Cienc. Agrar. 2020, 52, 88–101. [Google Scholar]
- Jellouli, N.; Ben Jouira, H.; Daldoul, S.; Chenenaoui, S.; Ghorbel, A.; Bensalem-Fnayou, A. Proteomic and Transcriptomic Analysis of Grapevine PR10 Expression During Salt Stress and Functional Characterization in Yeast. Plant Mol. Biol. Rep. 2010, 28, 1–8. [Google Scholar] [CrossRef]
- Li, P.; Tan, X.; Liu, R.; Rahman, F.; Jianfu, J.; Sun, L.; Fan, X.; Liu, J.; Liu, C.; Zhang, Y. QTL detection and candidate gene analysis of grape white rot resistance by interspecific grape (V. vinifera L. x V. davidii Foex.) crossing. Hortic. Res. 2023, 10, uhad063. [Google Scholar] [CrossRef]
- Karaagac, E.; Vargas, A.M.; de Andrés, M.; Carreño, I.; Ibáñez, J.; Carreño, J.; Martínez-Zapater, J.; Cabezas, J.A. Marker assisted selection for seedlessness in table grape breeding. Tree Genet. Genomes 2012, 8, 1003–1015. [Google Scholar] [CrossRef]
- Debreceni, D.; Lencsés, A.; Szőke, A.; Veres, A.; Hoffmann, S.; Kozma, P.; Kovacs, L.; Heszky, L.; Kiss, E. Marker-assisted selection for two dominant powdery mildew resistance genes introgressed into a hybrid grape population. Sci. Hortic. 2010, 126, 448–453. [Google Scholar] [CrossRef]
- Das, P.; Lahiri Majumder, A. Transcriptome analysis of grapevine under salinity and identification of key genes responsible for salt tolerance. Funct. Integr. Genom. 2019, 19, 61–73. [Google Scholar] [CrossRef]
- Lima, M.; Machado, A.; Gubler, W. Metabolomic Study of Chardonnay Grapevines Double Stressed with Esca-Associated Fungi and Drought. Phytopathology 2017, 107, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Sun, J.; Zhao, S.; Lu, Q.; Pan, L.; Zhao, B.; Yu, S. Effects of different rootstocks on Phenolics in skin of “cabernet sauvignon” and widely targeted metabolome and transcriptome analysis. Hortic. Res. 2022, 9, uhac053. [Google Scholar] [CrossRef] [PubMed]
- Karimi, R.; Ebrahimi, M.; Amerian, M. Abscisic acid mitigates NaCl toxicity in grapevine by influencing phytochemical compounds and mineral nutrients in leaves. Sci. Hortic. 2021, 288, 110336. [Google Scholar] [CrossRef]
- Rooy, S.S.; Salekdeh, G.H.; Ghabooli, M.; Gholami, M.; Mohsenifard, E.; Karimi, R. Effect of gradual and shock chilling stress on abscisic acid, soluble sugars and antioxidant enzymes changes in ‘Sultana’ grapevine. Iran. J. Plant Physiol. 2017, 7, 2211–2224. [Google Scholar]
- Karimi, R.; Merati, M.; Shayganfar, A. Phytochemical characterization of five commercial Vitis vinifera cultivars in response to salinity. Acta Physiol. Plant. 2023, 45, 108. [Google Scholar] [CrossRef]
- Liang, X.; Li, J.; Yang, Y.; Jiang, C.; Guo, Y. Designing salt stress-resilient crops: Current progress and future challenges. J. Integr. Plant Biol. 2024, 66, 303–329. [Google Scholar] [CrossRef]
- Shi, L.; Cui, X.; Shen, Y. The roles of histone methylation in the regulation of abiotic stress responses in plants. Plant Stress 2024, 11, 100303. [Google Scholar] [CrossRef]
- Vodiasova, E.; Sinchenko, A.; Khvatkov, P.; Dolgov, S. Genome-Wide Identification, Characterisation, and Evolution of the Transcription Factor WRKY in Grapevine (Vitis vinifera): New View and Update. Int. J. Mol. Sci. 2024, 25, 6241. [Google Scholar] [CrossRef]
- Giribaldi, M.; Gény, L.; Delrot, S.; Schubert, A. Proteomic analysis of the effects of ABA treatments on ripening Vitis vinifera berries. J. Exp. Bot. 2010, 61, 2447–2458. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, D.; Zhang, Y.; Li, G.; Sun, D.; Zhou, B.; Li, J. Insights into the Epigenetic Basis of Plant Salt Tolerance. Int. J. Mol. Sci. 2024, 25, 11698. [Google Scholar] [CrossRef]
- Abdulraheem, M.I.; Xiong, Y.; Moshood, A.Y.; Cadenas-Pliego, G.; Zhang, H.; Hu, J. Mechanisms of plant epigenetic regulation in response to plant stress: Recent discoveries and implications. Plants 2024, 13, 163. [Google Scholar] [CrossRef]
- Liu, Q.; Kang, J.; Du, L.; Liu, Z.; Liang, H.; Wang, K.; He, H.; Zhang, X.; Wang, Q.; Hong, Y. Single-cell multiome reveals root hair-specific responses to salt stress. New Phytol. 2025, 246, 2634–2651. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Liu, Y.; Hassan, M.M.; Abraham, P.E.; Merlet, J.; Townsend, A.; Jacobson, D.; Buell, C.R.; Tuskan, G.A.; Yang, X. Advances in the application of single-cell transcriptomics in plant systems and synthetic biology. BioDesign Res. 2024, 6, 0029. [Google Scholar] [CrossRef] [PubMed]
- Corso, M.; Vannozzi, A.; Ziliotto, F.; Zouine, M.; Maza, E.; Nicolato, T.; Vitulo, N.; Meggio, F.; Valle, G.; Bouzayen, M.; et al. Grapevine Rootstocks Differentially Affect the Rate of Ripening and Modulate Auxin-Related Genes in Cabernet Sauvignon Berries. Front. Plant Sci. 2016, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, C.; Davies, C. Hormonal Control of Grape Berry Development and Ripening. Biochem. Grape Berry 2012, 1, 194–217. [Google Scholar]
- Cakir, B.; Kiliçkaya, O.; Olcay, A. Genome-wide analysis of Aux/IAA genes in Vitis vinifera: Cloning and expression profiling of a grape Aux/IAA gene in response to phytohormone and abiotic stresses. Acta Physiol. Plant 2013, 35, 365–377. [Google Scholar] [CrossRef]
- Böttcher, C.; Burbidge, C.; Boss, P.; Davies, C. Interactions between ethylene and auxin are crucial to the control of grape (Vitis vinifera L.) berry ripening. BMC Plant Biol. 2013, 13, 222. [Google Scholar] [CrossRef]
- Karimi, R.; Gavili-Kilaneh, K.; Khadivi, A. Methyl jasmonate promotes salinity adaptation responses in two grapevine (Vitis vinifera L.) cultivars differing in salt tolerance. Food Chem. 2021, 375, 131667. [Google Scholar] [CrossRef]
- Ismail, A.; Riemann, M.; Nick, P. The jasmonate pathway mediates salt tolerance in grapevines. J. Exp. Bot. 2012, 63, 2127–2139. [Google Scholar] [CrossRef]
- Xu, L.; Xiang, G.; Sun, Q.; Ni, Y.; Jin, Z.; Gao, S.; Yao, Y. Melatonin enhances salt tolerance by promoting MYB108A-mediated ethylene biosynthesis in grapevines. Hortic. Res. 2019, 6, 114. [Google Scholar] [CrossRef]
- Montanaro, G.; Briglia, N.; Lopez, L.; Amato, D.; Panara, F.; Petrozza, A.; Cellini, F.; Nuzzo, V. A synthetic cytokinin primes photosynthetic and growth response in grapevine under ion-independent salinity stress. J. Plant Interact. 2022, 17, 789–800. [Google Scholar] [CrossRef]
- Yang, Y.; Xia, J.; Fang, X.; Jia, H.; Wang, X.; Lin, Y.; Liu, S.; Ge, M.; Pu, Y.; Fang, J.; et al. Drought stress in ‘Shine Muscat’ grapevine: Consequences and a novel mitigation strategy–5-aminolevulinic acid. Front. Plant Sci. 2023, 14, 1129114. [Google Scholar] [CrossRef]
- Cetin, E.; Canbay, H.; Daler, S. The roles of strigolactones: Mineral compounds, indole-3 acetic acid and GA3 content in grapevine on drought stress. J. Plant Stress Physiol. 2022, 8, 1–7. [Google Scholar] [CrossRef]
- Jogaiah, S.; Prakash, G.S.; Murti, G.S.R.; Upreti, K.K. Water stress and rootstocks influences on hormonal status of budded grapevine. Eur. J. Hortic. Sci. 2007, 72, 202–205. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).