Optimizing Irrigation Rates and Antioxidant Foliar Spray Effects on Growth, Yield, and Fruit Quality of Manfalouty Pomegranate Trees
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Treatments and Experimental Design
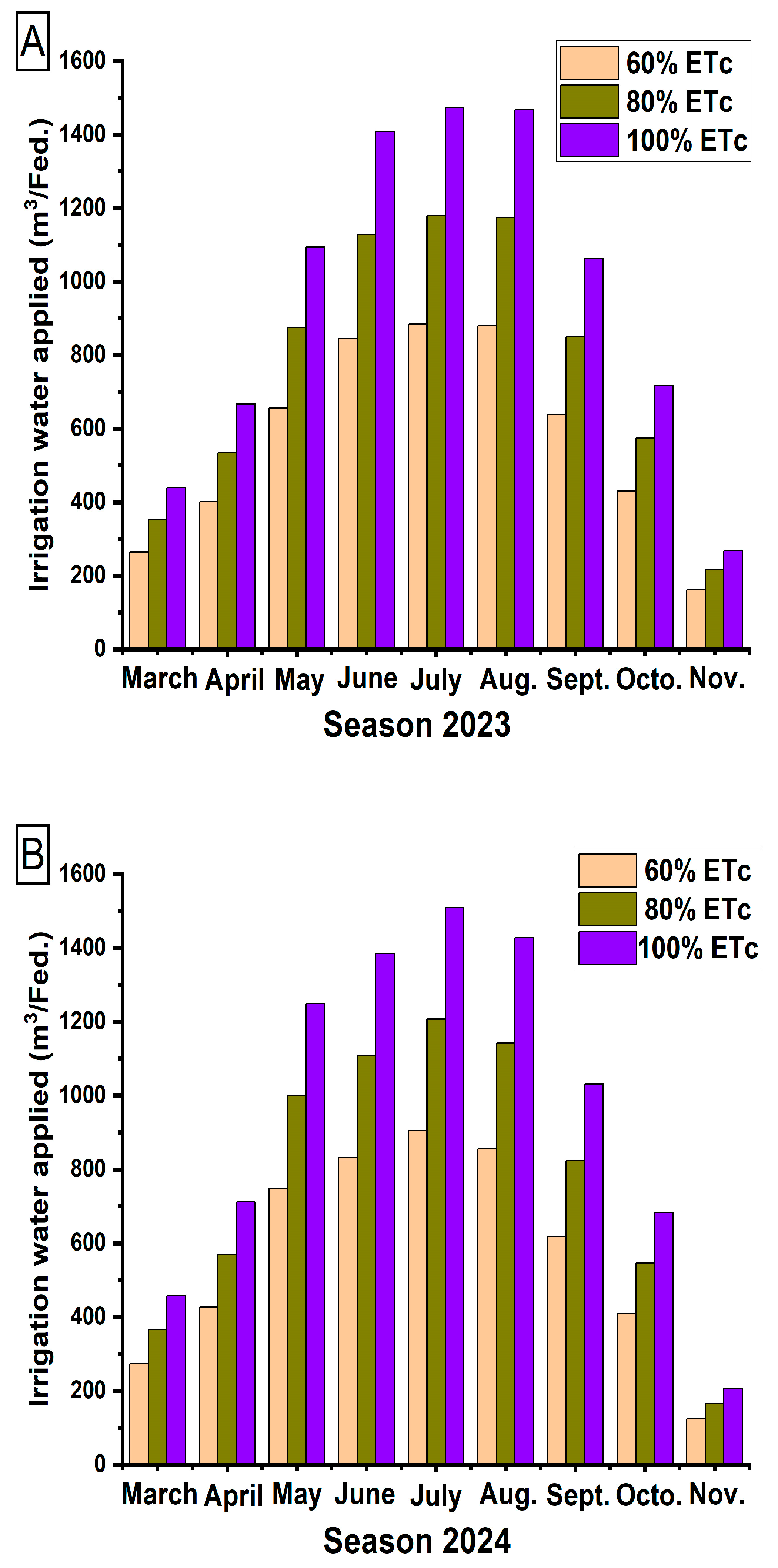
2.3. Irrigation Water Applied
2.4. Morphological and Physiological Assay of Tree Leaves
- Length of shoot (cm).
- Number of leaves/shoot.
- Area of leaf (cm2), was estimated by using the following formula as stated by Ahmed and Morsy [34]: Leaf area = 0.41 (Length of leaf × Width of leaf) + 1.83.
- The total chlorophyll (mg/g F.W.) of the leaves was extracted using methanol, the plant leaf was suspended in 10 mL of 95% methanol overnight in a falcon tube until the leaves loss their green color, discard the leaves tissue, and centrifuge at 6000× g for 5–10 min to eliminate any tissue residue; the supernatant then measured at 650 and 665 nm [9], and was computed using the equations of Lichtenthaler et al. [35].
- Total flavonoids (mg/g fresh weight) were measured in the leaves according to methods of Chang et al. [38] and Mahmoud et al. [9]. One mL of leaf extract was mixed with and one mL of aluminum chloride reagent and 0.5 mL potassium acetate buffer, incubated in room temperature for 15–20 min; then, the absorbance was measured at 496 nm using spectrophotometer; obtained data were calculated using standard curve of quercetin.
- Total phenols (mg/g fresh weight) were measured at 750 nm using Folin-Ciocalteu reagent [9,39]. The leaf extract was mixed with Folin-Ciocalteu diluted with 1:10 with distilled water and disodium carbonate (7.5%) and incubated in room for 30 min; then, the absorbance was measured at 750 nm using spectrophotometer and the data were calculated using gallic acid standard curve.
2.5. Yield Parameters
2.6. Fruit Quality
2.7. Statistical Analysis
3. Results
3.1. Irrigation Applied Water (L/Tree)
3.2. Impact of Antioxidant Spraying on Pomegranate Trees’ Vegetative Growth Under Three Irrigation Regimes
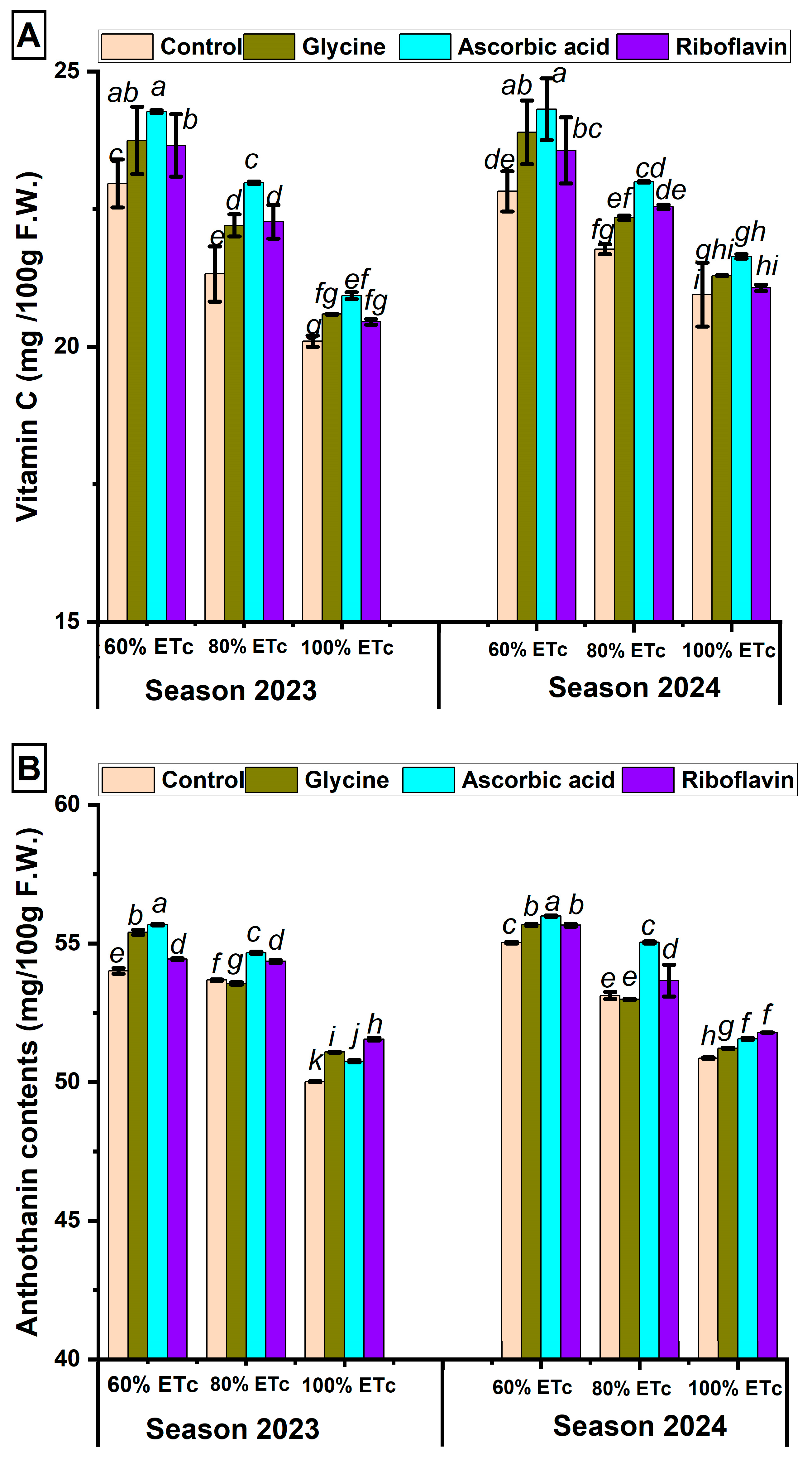
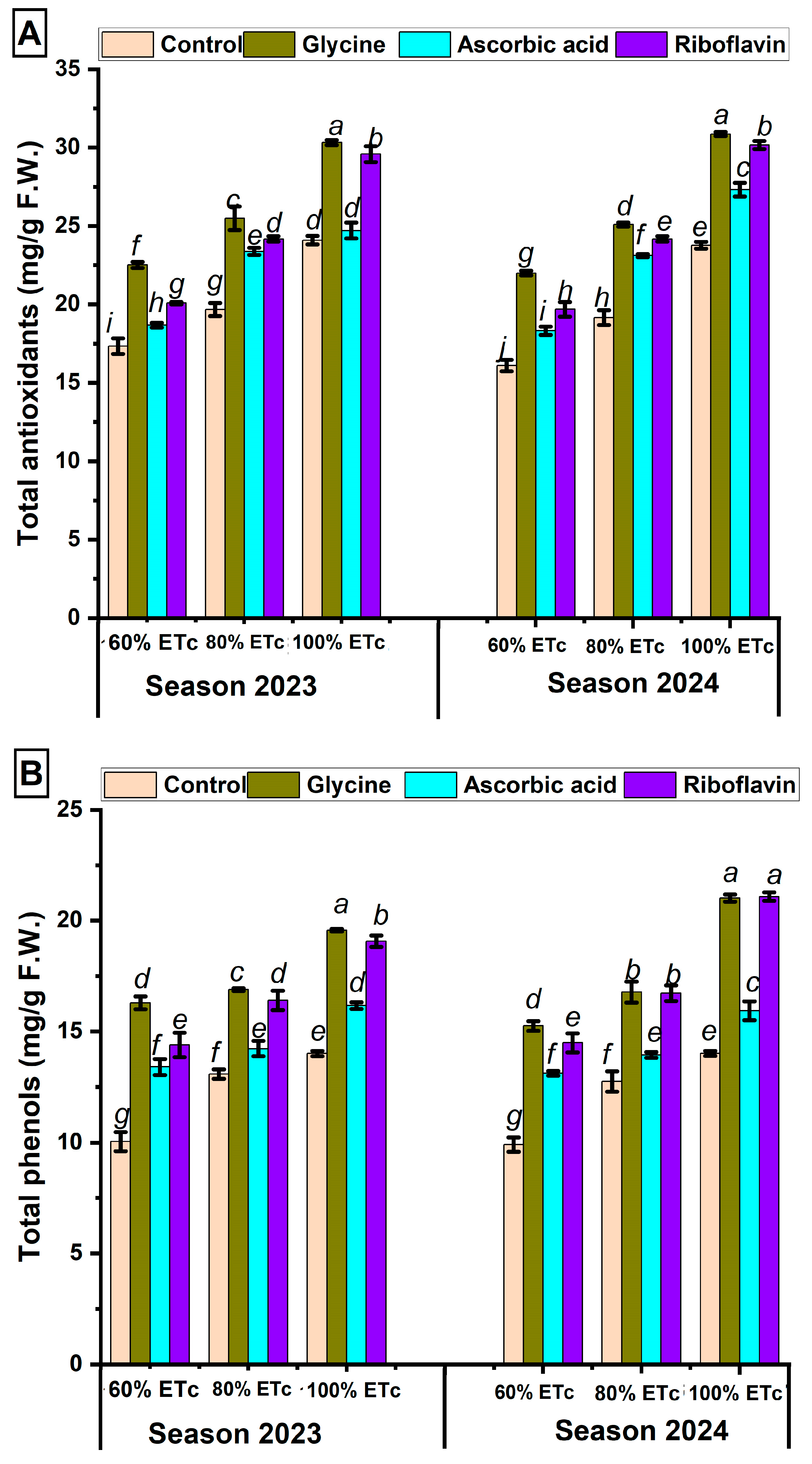
3.3. Impact of Antioxidant Spraying on Pomegranate Leaves’ Total Chlorophyll, Total Antioxidants, Total Phenols, and Total Flavonoids Under Three Irrigation Regimes
3.4. Impact of Antioxidant Spraying on Pomegranate Yield Parameters and Fruit Creaking Under Three Irrigation Regimes
3.5. Impact of Antioxidant Spraying on Pomegranate Fruit Quality Under Three Irrigation Regimes
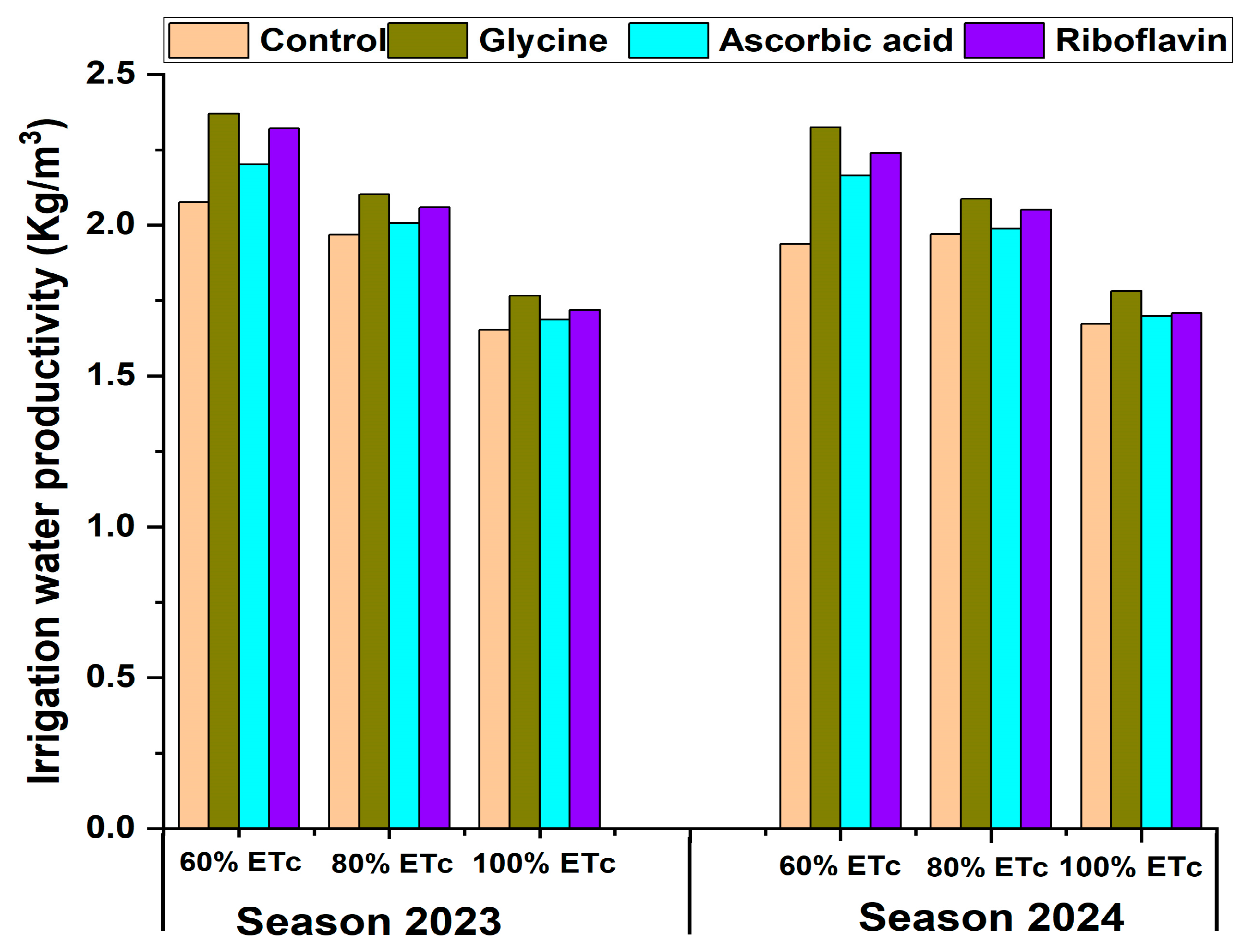
3.6. Irrigation Water Productivity (IWP kg/m3)
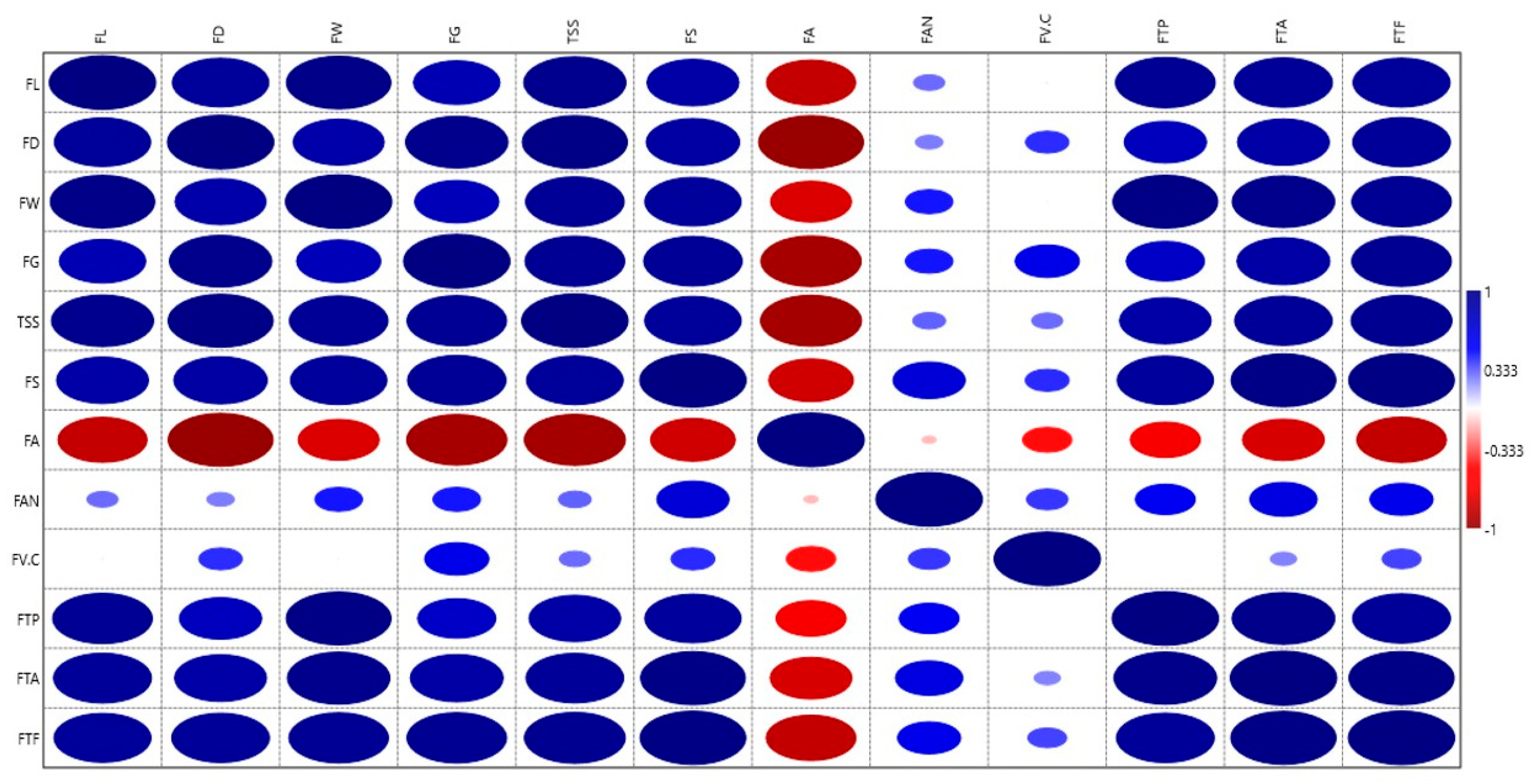
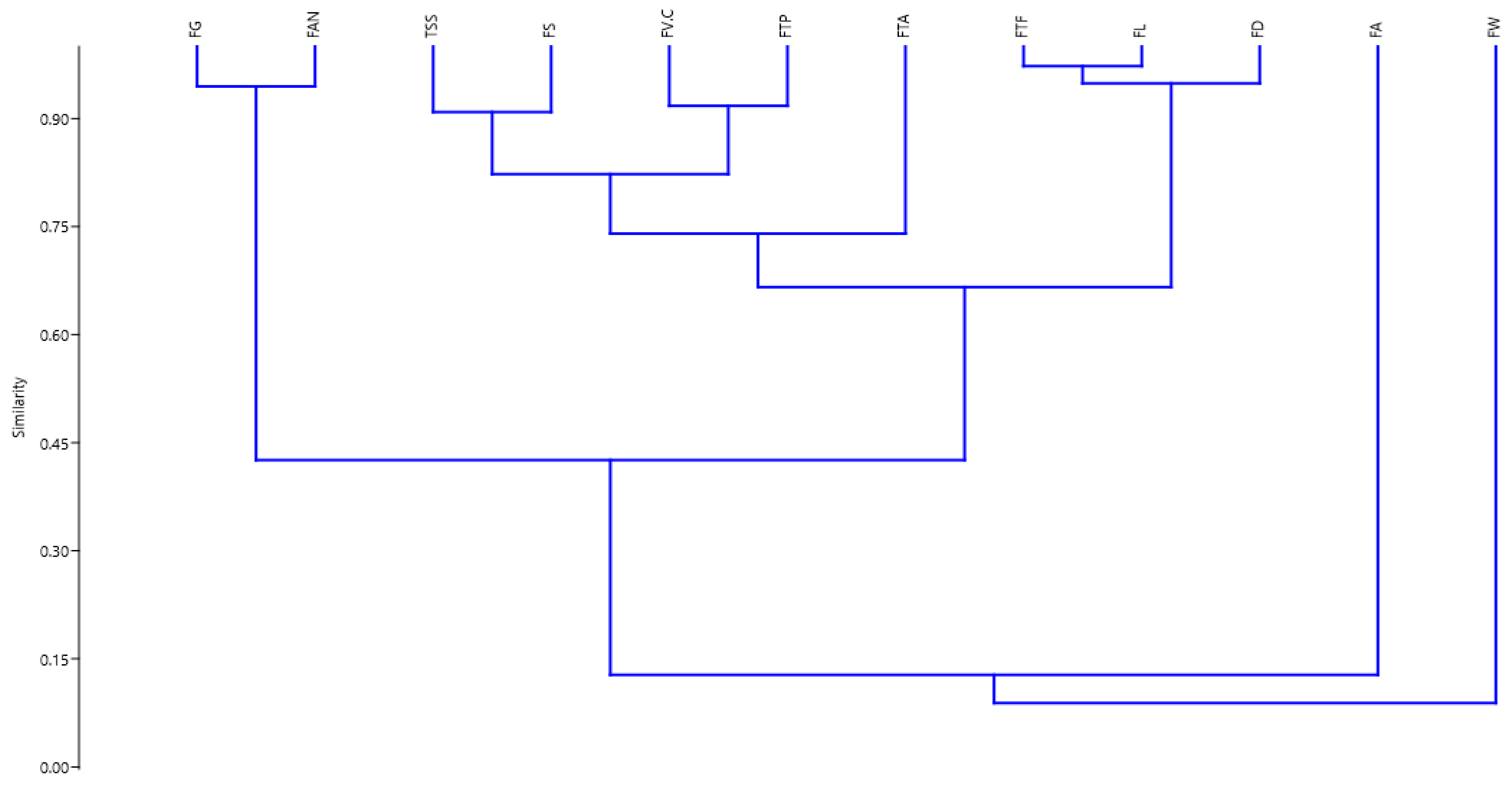
3.7. Statistical Correlations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alotaibi, M.; Alhajeri, N.S.; Al-Fadhli, F.M.; Elgabri, S.; Gabr, M.E. Impact of climate change on crop irrigation requirements in arid regions. Water Resour. Manag. 2023, 37, 1965–1984. [Google Scholar] [CrossRef]
- Mahmoud, G.A.E.; Hefzy, M.; Zahran, M.M.A.A. Synergistic Effects of Microbial Gibberellic Acid and Vitamins on Onion (Allium cepa L.). Yield, and Quality in Low-Fertility Soil. J. Soil. Sci. Plant Nutr. 2024, 24, 5342–5359. [Google Scholar] [CrossRef]
- Li, P.; He, Z.; Cai, J.; Zhang, J.; Belete, M.; Deng, J.; Wang, S. Identify the impacts of the Grand Ethiopian Renaissance Dam on watershed sediment and water yields dynamics. Sustainability 2022, 14, 7590. [Google Scholar] [CrossRef]
- Gabr, M.E. Land reclamation projects in the Egyptian Western Desert: Management of 1.5 million acres of groundwater irrigation. Water Int. 2023, 48, 240–258. [Google Scholar] [CrossRef]
- Tarantino, A.; Difonzo, G.; Lopriore, G.; Disciglio, G.; Paradiso, V.M.; Gambacorta, G.; Caponio, F. Bioactive compounds and quality evaluation of ‘Wonderful’pomegranate fruit and juice as affected by deficit irrigation. J. Sci. Food Agric. 2020, 100, 5539–5545. [Google Scholar]
- Turrini, F.; Boggia, R.; Donno, D.; Parodi, B.; Beccaro, G.; Baldassari, S.; Signorello, M.G.; Catena, S.; Alfei, S.; Zunin, P. From pomegranate marcs to a potential bioactive ingredient: A recycling proposal for pomegranate-squeezed marcs. Eur. Food Res. Technol. 2020, 246, 273–285. [Google Scholar]
- Asrey, R.; Kumar, K.; Sharma, R.R.; Meena, N.K. Fruit bagging and bag color affects physico-chemical, nutraceutical quality and consumer acceptability of pomegranate (Punica granatum L.) arils. J. Food Sci. Technol. 2020, 57, 1469–1476. [Google Scholar]
- Albergaria, E.T.; Oliveira, A.F.; Albuquerque, U.P. The effect of water deficit stress on the composition of phenolic compounds in medicinal plants. S. Afr. J. Bot. 2020, 131, 12–17. [Google Scholar] [CrossRef]
- Mahmoud, G.A.-E.; Sabra, M.A.; Mohamed, A.E.; Darwish, K.M.; Gaber, D.A. Optimization of Gibberellic Acid Production from Fusarium incarnatum and Its Effect on Zea mays Growth Promotion and Antioxidant Activity. J. Plant Growth Regul. 2025, 44, 3219–3235. [Google Scholar] [CrossRef]
- Hamed, H.A.; Mahmoud, G.A.-E.; Abeed, A.H.A. Unraveling growth and metabolic dynamics in drought-stressed spinach plants: Exploring the contribution of biological gibberellin. Sci. Hortic. 2025, 340, 113924. [Google Scholar] [CrossRef]
- Ilyas, M.; Nisar, M.; Khan, N.; Hazrat, A.; Khan, A.H.; Hayat, K.; Fahad, S.; Khan, A.; Ullah, A. Drought tolerance strategies in plants: A mechanistic approach. J. Plant Growth Regul. 2020, 40, 926–944. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Kumar, V.; Kohli, S.K.; Sidhu, G.P.; Bali, A.S.; Handa, N.; Kapoor, D.; Bhardwaj, R.; Zheng, B. Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules 2019, 9, 285. [Google Scholar] [CrossRef] [PubMed]
- Nazar, Z.; Akram, N.A.; Saleem, M.H.; Ashraf, M.; Ahmed, S.; Ali, S.; Alsahli, A.A.; Alyemeni, M.N. Glycinebetaine-induced alteration in gaseous exchange capacity and osmoprotective phenomena in safflower (Carthamus tinctorius L.). under water deficit conditions. Sustainability 2020, 12, 10649. [Google Scholar] [CrossRef]
- Shan, T.; Jin, P.; Zhang, Y.; Huang, Y.; Wang, X.; Zheng, Y. Exogenous glycine betaine treatment enhances chilling tolerance of peach fruit during cold storage. Postharvest Biol. Technol. 2016, 114, 104–110. [Google Scholar] [CrossRef]
- Mosa, W.F.; Salem, M.Z.; Al-Huqail, A.A.; Ali, H.M. Application of glycine, folic Acid, and moringa extract as bio-stimulants for enhancing the production of ‘Flame Seedless’ grape cultivar. Bioresources 2021, 16, 3391–3410. [Google Scholar] [CrossRef]
- Abd El-wahab, M.; HShakweer, N. Potential impacts of amino acids, putrescine and glycine betaine on productivity and fruit attributes of “Le-Conte” pear trees grown under water scarcity stress. Fayoum J. Agric. Res. Dev. 2024, 38, 15–35. [Google Scholar] [CrossRef]
- Almutairi, K.F.; Saleh, A.A.; Ali, M.M.; Paszt, L.S. 2021 Growth performance of Guava Trees after the Exogenous Application of Amino Acids Glutamic Acid, Arginine, and Glycine. Horticulturae 2022, 8, 1110. [Google Scholar] [CrossRef]
- Mhmood, R.A.; Alhayany, A.A.A. Effect of shading, glycine, and α-tocopherol spray on the growth of Yemeni pomegranate transplants. Euphrates J. Agric. Sci. 2024, 16, 474–483. [Google Scholar]
- Abo-Ogiala, A. Managing crop production of pomegranate cv. Wonderful via foliar application of ascorbic acid, proline and glycinbetaine under environmental stresses. Int. J. Environ. 2018, 7, 95–103. [Google Scholar]
- Xiao, M.; Li, Z.; Zhu, L.; Wang, J.; Zhang, B.; Zheng, F.; Zhao, B.; Zhang, H.; Wang, Y.; Zhang, Z. The Multiple Roles of Ascorbate in the Abiotic Stress Response of Plants: Antioxidant, Cofactor, and Regulator. Front. Plant Sci. 2021, 12, 598173. [Google Scholar] [CrossRef]
- Nafady, N.A.; Bagy, M.M.K.; Abd-Alla, M.H.; Morsy, F.M.; Mahmoud, G.A.E. Improvement of medium components for high riboflavin production by Aspergillus terreus using response surface methodology. Rendiconti. Lincei 2015, 26, 335–344. [Google Scholar] [CrossRef]
- Cheng, S.; Liu, H.; Li, K.; Zheng, L.; Su, M.; Lin, X.; Huang, G.; Ren, Y. Riboflavin improves grain yield, 2-acetyl-1-pyrroline accumulation, and antioxidative properties of fragrant rice. J. Sci. Food Agric. 2024, 104, 1178–1189. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Cheng, S.; Ding, X.; Lin, X.; Deng, S.; Peng, L.; Tian, H. Exogenous Riboflavin Application at Different Growth Stages Regulates Photosynthetic Accumulation and Grain Yield in Fragrant Rice. Agriculture 2024, 14, 1979. [Google Scholar] [CrossRef]
- Aziz, A.; Akram, N.A.; Ashraf, M. Influence of natural and synthetic vitamin C (ascorbic acid). on primary and secondary metabolites and associated metabolism in quinoa (Chenopodium quinoa Willd.) plants under water deficit regimes. Plant Physiol. Biochem. 2018, 123, 192–203. [Google Scholar] [CrossRef]
- Huang, S.; Jin, S. Enhancing drought tolerance in horticultural plants through plant hormones: A strategic coping mechanism. Front. Plant Sci. 2025, 15, 1502438. [Google Scholar] [CrossRef]
- Kausar, A.; Zahra, N.; Zahra, H.; Hafeez, M.B.; Zafer, S.; Shahzadi, A.; Raza, A.; Djalovic, I.; Prasad, P.V. Alleviation of drought stress through foliar application of thiamine in two varieties of pea (Pisum sativum L.). Plant Signal. Behav. 2023, 18, 2186045. [Google Scholar] [CrossRef]
- El Refaey, A.; Mohamed, Y.I.; El-Shazly, S.M.; Abd El Salam, A.A. Effect of salicylic and ascorbic acids foliar application on Picual olive trees growth under water stress condition. Egypt. J. Soil. Sci. 2022, 62, 1–17. [Google Scholar] [CrossRef]
- Parvizi, H.; Sepaskhah, A.R.; Ahmadi, S.H. Effect of drip irrigation and fertilizer regimes on fruit yields and water productivity of a pomegranate (Punica granatum (L.). cv. Rabab). orchard. Agric. Water Manag. 2014, 146, 45–56. [Google Scholar] [CrossRef]
- Gómez-Bellot, M.J.; Garcia, C.J.; Parra, A.; Vallejo, F.; Ortuño, M.F. Influence of drought stress on increasing bioactive compounds of pomegranate (Punica granatum L.). juice. Exploratory study using LC–MS-based untargeted metabolomics approach. Eur. Food Res. Technol. 2023, 249, 2947–2956. [Google Scholar] [CrossRef]
- Klute, A. Methods of Soil Analysis. Part 1. Physical and Mineralogical Methods, 2nd ed.; American Society of Agronomy-Soil Science Society of America: Madison, WI, USA, 1986. [Google Scholar]
- Jackson, T.L. Soil Chemical Analysis; Prentice-Hall of India Private Limited: New Delhi, India, 1973. [Google Scholar]
- Smith, N. CROPWAT Model for ETo Calculation Using Penman-Monteith Method; FAO: Rome, Italy, 1991. [Google Scholar]
- Allen, R.G.; Pereira, S.L.; Raes, D.; Smith, M. Crop Evapotranspiration Guidelines for Computing Crop Water Requirements, Bulletin of the FAO Irrigation and Drainage; FAO: Rome, Italy, 1998. [Google Scholar]
- Ahmed, F.F.; Morsy, M.H. A new method for measuring leaf area in different fruit species. Minia J. Agric. Rec. Dev. 1999, 19, 97–105. [Google Scholar]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin, E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Ibrahim, A.B.M.; Mahmoud, G.A.-E.; Cordes, D.B.; Slawin, A.M.Z. Pb (II). and Hg (II). Thiosemicarbazones for Inhibiting the Broad-Spectrum Pathogen Cladosporium sphaerospermum ASU18 (MK387875) and Altering Its Antioxidant System. Appl. Organomet. Chem. 2022, 36, e6798. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis A.O.A.C, 17th ed.; A.O.A.C.: Washington, DC, USA, 2000. [Google Scholar]
- Ranganna, S. Manual of Analysis of Fruit and Vegetable Products; Tata Mc Graw Hill publisihing company Limeted: New Delhi, India, 1979; Volume 12, pp. 87–88. [Google Scholar]
- Kirch, W. (Ed.) Pearson’s Correlation Coefficient. In Encyclopedia of Public Health; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, D.; Ryan, P. PAST—PAlaeontological Statistics; Paleontological Museum, University of Oslo: Oslo, Norway, 2001; Available online: https://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 22 June 2001).
- Adiba, A.; Khachtib, Y.; Boutagayout, A.; Hamdani, A.; Kouighat, M.; Haddioui, A.; Hssaini, L.; Razouk, R. Bioactive compounds and quality attributes of pomegranate fruit as affected by continuous deficit irrigation. Vegetos 2024, 38, 1392–1405. [Google Scholar] [CrossRef]
- Martínez, J.J.; Melgarejo, P.; Hernández, F. Irrigation regime effects on pomegranate (Punica granatum L.) fruit quality. Agric. Water Mgmt. 2009, 96, 1633–1639. [Google Scholar]
- Lurie, S.; Friedman, H.; Weksler, A. Effects of irrigation and harvesting time on pomegranate fruit quality. Postharv. Biol. Technol. 2012, 65, 162–168. [Google Scholar]
- Jin, Y.; Wang, C.K.; Sang, Y. Contribution of stem water storage to daily transpiration of three temperate trees in northeastern China. Chin. J. Plant Ecol. 2011, 35, 1310–1317. [Google Scholar] [CrossRef]
- Volschenk, T. Effect of water deficits on pomegranate tree performance and fruit quality–A review. Agric. Water Manag. 2021, 246, 106499. [Google Scholar] [CrossRef]
- Gaber, S.H.; Ahmed, A.A.; Abdel Rahman, M.A.E.; Hefzy, M. Enhance of water productivity, yield and fruit quality of pomegranate trees on newly reclaimed soils. Arch. Agric. Sci. J. 2025, 8, 42–63. [Google Scholar] [CrossRef]
- Khattab, M.M.; Shaban, A.E.; El-Shrief, A.H.; Mohamed, A.S.E. Growth and productivity of pomegranate trees under different irrigation Levels I: Vegetative growth and fruiting. JHSOP 2011, 3, 194–198. [Google Scholar]
- Zaghloul, A.E.; Moursi, E.A. Effect of irrigation scheduling under some biostimulants foliar application for Navel orange trees on some water relations, productivity, fruit quality and storability in the North Nile Delta region. Alex. Sci. Exch. J. 2017, 38, 671–686. [Google Scholar] [CrossRef]
- Jamshidi, S.; Zand-Pars, S.; Kamgar-Haghighi, A.A.; Shahsavar, A.R.; Niyogi, D. Evapotranspiration, crop coefficients, and physiological responses of citrus trees in semi-arid climatic conditions. Agric. Water Manag. 2020, 227, 105838. [Google Scholar] [CrossRef]
- Mansour, A.H.A.; Darwesh, R.K.; Hefzy, M. Effect of irrigation regime and spraying salicylic acid on characteristics and quality of (Banzahir). lime fruits (Citrus aurantifolia B.) at harvest, marketing and some water relations. Environ. Biodivers. Soil. Secur. 2020, 4, 313–331. [Google Scholar]
- Leal, L.d.S.G.; Marinho, L.B.; Macário de, G.; Pereira dos, M.É.; Clemente, V.D.; Aires, E.S.; Figueiredo Neto, A. Irrigation Management of Punica granatum in the Sub-Middle São Francisco River Valley: Morphophysiological Aspects. Com. Sci. 2024, 15, e4023. [Google Scholar] [CrossRef]
- Hassan, I.F.; Ajaj, R.; Abd El-Khalek, A.F.; Alam-Eldein, S.M.; Gaballah, M.S.; Athar, H.u.R.; Hatterman-Valenti, H.M. Effects of Deficit Irrigation on Growth, Yield, and Quality of Pomegranate (Punica granatum). Grown in Semi-Arid Conditions. Horticulturae 2025, 11, 101. [Google Scholar] [CrossRef]
- Tarantino, A.; Frabboni, L.; Disciglio, G. Water-Yield Relationship and Vegetative Growth of Wonderful Young Pomegranate Trees under Deficit Irrigation Conditions in Southeastern Italy. Horticulturae 2021, 7, 79. [Google Scholar] [CrossRef]
- Gómez-Bellot, J.; Parra, A.; Nortes, P.; Alarcón, J.J.; Ortuño, M.F. Searching for a deficit irrigation strategy to save water and improve fruit quality without compromising pomegranate production. Sci. Hortic. 2024, 324, 112631. [Google Scholar] [CrossRef]
- Fisher, A. The Impact of Glycine Betaine Applications on Drought Response in Wild Blueberries. Master’s Thesis, The Graduate School of Natural and Applied Science of Universtiy of Maine, Orono, ME, USA, 2022; 40p. [Google Scholar]
- Yang, Z.; Yu, J.; Merewitz, E.; Huang, B. Differential effects of abscisic acid and glycine betaine on physiological responses to drought and salinity stress for two perennial grass species. J. Am. Soc. Hort. Sci. 2012, 137, 96–106. [Google Scholar] [CrossRef]
- Küçükyumuk, C.; Küçükyumuk, Z.; İmrak, B.; Çömlekçioğlu, S. The Applications of Different Glycine Betaine Doses on Young Pear Trees Under Drought Stress Conditions. Horticulturae 2024, 10, 1217. [Google Scholar] [CrossRef]
- Kheder, A.M.A.; Abo-Elmagd, A.M. Improving vegetative growth and productivity of navel orange (Citrus sinensis L.). trees under salt affected soil using glycinebetaine and potassium silicate. J. Plant Prod. 2021, 12, 279–286. [Google Scholar] [CrossRef]
- Hassan, I.F.; Ajaj, R.; Gaballah, M.S.; Ogbaga, C.C.; Kalaji, H.M.; Hatterman-Valenti, H.M.; Alam-Eldein, S.M. Foliar Application of Nano-Silicon Improves the Physiological and Biochemical Characteristics of ‘Kalamata’ Olive Subjected to Deficit Irrigation in a Semi-Arid Climate. Plants 2022, 11, 1561. [Google Scholar] [CrossRef] [PubMed]
- Haleem, A.Y.; Ghieth, W.M.; Hegazy, A.A.H. Performance of Some Pomegranate Cultivars under Different Irrigation levels in North Sinai. Middle East. J. Agric. Res. 2021, 9, 1021–1031. [Google Scholar]
- Nasrabadi, M.; Ramezanian, A.; Eshghi, S.; Sarkhosh, A. Chilling and heat requirement of pomegranate (Punica granatum L.). trees grown under sustained deficit irrigation. Sci. Hortic. 2020, 263, 109117. [Google Scholar] [CrossRef]
- Mahmoud, G.A.-E.; Gaber, S.H. Nanotechnology Applications in Postharvest Disease Management. In Recent Advances in Postharvest Technologies; Benkeblia, N., Ed.; Springer: Cham, Switzerland, 2024; Volume 2, pp. 225–245. [Google Scholar]
- Mahmoud, G.A.-E.; Rashed, N.M.; El-Ganainy, S.M.; Salem, S.H. Unveiling the Neem (Azadirachta indica). Effects on Biofilm Formation of Food-Borne Bacteria and the Potential Mechanism Using a Molecular Docking Approach. Plants 2024, 13, 2669. [Google Scholar] [CrossRef]
- Zahir, A.; Abbasi, B.H.; Adil, M.; Anjum, S.; Zia, M. Synergistic effects of drought stress and photoperiods on phenology and secondary metabolism of Silybum marianum. Appl. Biochem. Biotech. 2014, 174, 693–707. [Google Scholar]
- Król, A.; Amarowicz, R.; Weidner, S. Changes in the composition of phenolic compounds and antioxidant properties of grapevine roots and leaves (Vitis vinifera L.) under continuous of long-term drought stress. Acta Physiologiae. Plantarum. 2014, 36, 1491–1499. [Google Scholar]
- Farooq, M.; Hussain, M.; Wahid, A.; Siddique, K.H.M. Drought Stress in Plants: An Overview. Plant Responses to Drought Stress: From Morphological to Molecular Features; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–33. [Google Scholar]
- Osman, H.S. Enhancing antioxidant–yield relationship of pea plant under drought at different growth stages by exogenously applied glycine betaine and proline. Ann. Agric. Sci. 2015, 60, 389–402. [Google Scholar] [CrossRef]
- Jabeen, Z.; Mahmood, B.; Rehman, S.; Butt, A.M.; Hussain, N. Biodegradable glycine betaine encapsulated chitosan nanoparticles induce the expression of antioxidant enzyme genes to improve drought tolerance in maize. S. Afr. J. Bot. 2024, 171, 571–582. [Google Scholar] [CrossRef]
- Shafiq, S.; Akram, N.A.; Ashraf, M.; García-Caparrós, P.; Ali, O.M.; Latef, A.A.H.A. Influence of Glycine Betaine (Natural and Synthetic). on Growth, Metabolism and Yield Production of Drought-Stressed Maize (Zea mays L.) Plants. Plants 2021, 10, 2540. [Google Scholar] [CrossRef]
- Darwesh, R.K. Water productivity for Egyptian clover as affected by different irrigation regimes and cultivation methods in the North Middle Nile Delta region. Environ. Biodivers. Soil. Secur. 2018, 2, 193–203. [Google Scholar] [CrossRef]
- Abdelfattah, I.M.; Attia, E.; El-Banna, G.M. Irrigation scheduling and its impacts on freesia-water productivity, vegetative and flowering parameters under greenhouse cultivation. Environ. Biodivers. Soil. Secur. 2020, 4, 59–71. [Google Scholar] [CrossRef]


| Physical Properties | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Particle size distribution (%) | Texture Class | Moisture content (Volumetric %) | OM (%) | CaCO3 (%) | Bulk density (g/cm3) | ||||
| Sand | Silt | clay | S.P. | F.C. | W.P. | ||||
| 90.20 | 6.10 | 3.70 | Sandy | 23.7 | 10.6 | 4.7 | 0.43 | 32.15 | 1.61 |
| Chemical Properties | |||||||||
| pH (1-1) | EC (dS/ m) (1:1) | Available phosphorus (mg/L) | TN (%) | Soluble cations (mg/L) | Soluble anions (mg/L) | ||||
| Ca++ | Mg++ | Na+ | K+ | Co3−−+HCo3− | Cl− | ||||
| 8.12 | 0.6 | 7.85 | 0.03 | 2.48 | 1.58 | 0.68 | 0.85 | 2.57 | 1.81 |
| Year | Month | Temperature (T max °C) | Temperature (T min °C) | Relative Humidity (RH %) | Wind Speed (km/h) | Sunshine (h/day) | ETo (mm/day) |
|---|---|---|---|---|---|---|---|
| 2023 | March | 27.1 | 12.1 | 34.3 | 9.9 | 9.9 | 5.74 |
| April | 31.5 | 15.1 | 25.5 | 14.2 | 10.3 | 7.49 | |
| May | 34.6 | 19.2 | 26.4 | 13.5 | 11.4 | 8.35 | |
| June | 38.3 | 24.2 | 28.6 | 16.7 | 12.3 | 9.90 | |
| July | 39.7 | 24.5 | 30.9 | 14.8 | 12.2 | 10.02 | |
| August | 38.7 | 24 | 37.7 | 15.7 | 11.9 | 9.98 | |
| September | 37.4 | 22.7 | 34.4 | 14.5 | 10.8 | 8.06 | |
| October | 31.9 | 18.7 | 47.5 | 13.9 | 10.0 | 6.04 | |
| November | 28.9 | 14.8 | 50 | 10.4 | 9.4 | 5.17 | |
| 2024 | March | 26.6 | 11.1 | 36.5 | 10.9 | 9.9 | 5.97 |
| April | 32.1 | 16.6 | 32.6 | 13.7 | 10.3 | 7.97 | |
| May | 36.1 | 20.5 | 24.1 | 14.6 | 11.4 | 9.49 | |
| June | 40.9 | 24.8 | 25.1 | 13.8 | 12.3 | 9.5 | |
| July | 40.3 | 26.1 | 26.8 | 11.6 | 12.2 | 10.6 | |
| August | 39.9 | 26 | 28.5 | 15.1 | 11.9 | 9.7 | |
| September | 36.7 | 23.5 | 38.8 | 16.7 | 10.8 | 8.1 | |
| October | 31.4 | 17.4 | 44.8 | 15.9 | 10.0 | 6.0 | |
| November | 25.3 | 12.2 | 56.3 | 15.4 | 9.4 | 4.0 |
| Season 2023 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Irrigation
(ETc) | 60% ETc | 80% ETc | 100% ETc | 60% ETc | 80% ETc | 100% ETc | 60% ETc | 80% ETc | 100% ETc |
| Spraying | Shoot length (cm) | Number leaves/shoot | Leaf area (cm2) | ||||||
| Control | 51.77 ± 0.23 i | 55.42 ± 0.42 g | 59.99 ± 0.01 cd | 61.20 ± 0.20 k | 67.74 ± 0.04 i | 71.77 ± 0.38 d | 5.22 ± 0.02 e | 5.62 ± 0.03 d | 6.15 ± 0.25 c |
| Glycine | 57.58 ± 0.50 f | 60.55 ± 1.00 c | 63.67 ± 0.10 a | 70.21 ± 0.21 f | 71.87 ± 0.10 d | 77.70 ± 0.20 a | 5.63 ± 0.03 d | 6.15 ± 0.10 c | 6.91 ± 0.01 a |
| Ascorbic acid | 52.88 ± 0.10 h | 56.98 ± 0.02 f | 59.21 ± 0.21 e | 63.53 ± 0.23 j | 69.53 ± 0.03 g | 73.19 ± 0.19 c | 5.36 ± 0.20 e | 5.97 ± 0.00 c | 6.72 ± 0.12 b |
| Riboflavin | 55.48 ± 0.48 g | 59.58 ± 0.30 de | 62.56 ± 0.50 b | 68.48 ± 0.40 h | 70.90 ± 0.10 e | 76.67 ± 0.22 b | 5.55 ± 0.05 d | 5.99 ± 0.00 c | 6.68 ± 0.20 b |
| Season 2024 | |||||||||
| Control | 45.65 ± 0.05 i | 53.44 ± 0.44 g | 59.33 ± 0.57 c d | 53.23 ± 0.23 j | 65.32 ± 0.32 h | 71.69 ± 0.33 d | 4.46 ± 0.10 g | 5.11 ± 0.21 e | 5.67 ± 0.10 c |
| Glycine | 55.56 ± 0.40 f | 58.54 ± 0.46 d | 64.70 ± 0.30 a | 68.54 ± 0.46 f | 70.54 ± 0.54 e | 79.13 ± 0.13 a | 4.75 ± 0.15 f | 5.51 ± 0.09 c d | 6.17 ± 0.00 a |
| Ascorbic acid | 50.88 ± 0.12 h | 54.98 ± 1.00 f | 60.21 ± 0.21 c | 61.03 ± 0.03 i | 66.77 ± 0.22 g | 73.97 ± 0.03 c | 4.56 ± 0.11 f g | 5.35 ± 0.10 d | 6.11 ± 0.01 ab |
| Riboflavin | 53.45 ± 0.45 g | 56.87 ± 1.64 e | 62.27 ± 0.83 b | 66.88 ± 0.12 g | 70.12 ± 0.12 e | 78.60 ± 0.10 b | 4.61 ± 0.06 f g | 5.43 ± 0.17 d | 5.92 ± 0.02 b |
| Season 2023 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Irrigation
(ETc) | 60% ETc | 80% ETc | 100% ETc | 60% ETc | 80% ETc | 100% ETc | 60% ETc | 80% ETc | 100% ETc |
| Spraying | Total antioxidants | Total phenols | Total flavonoids | ||||||
| Control | 104.61 ± 0.50 d | 88.51 ± 0.10 g | 77.32 ± 0.28 j | 89.78 ± 0.16 e | 71.17 ± 0.82 h | 67.79 ± 1.32 j | 44.13 ± 0.23 d e | 43.52 ± 0.46 e | 35.76 ± 0.91 i |
| Glycine | 112.67 ± 0.60 a | 105.14 ± 0.93 d | 88.32 ± 0.28 g | 96.72 ± 0.14 a | 92.91 ± 0.02 d | 79.28 ± 0.63 g | 49.10 ± 0.55 a | 44.34 ± 0.16 d | 41.86 ± 0.17 f |
| Ascorbic acid | 110.16 ± 0.27 c | 92.24 ± 0.05 f | 84.46 ± 0.44 i | 94.13 ± 0.03 c | 85.07 ± 0.98 f | 68.13 ± 0.81 j | 46.46 ± 0.41 c | 44.19 ± 0.27 d | 37.94 ± 0.05 h |
| Riboflavin | 111.21 ± 0.37 b | 96.69 ± 0.53 e | 87.38 ± 0.33 h | 95.10 ± 0.10 b | 92.32 ± 1.06 d | 69.61 ± 0.85 i | 47.24 ± 0.21 b | 44.36 ± 0.05 d | 38.78 ± 0.14 g |
| Season 2024 | |||||||||
| Control | 103.89 ± 0.78 d | 87.68 ± 0.78 g h | 76.82 ± 0.75 j | 89.81 ± 0.10 f | 70.23 ± 0.12 i | 66.33 ± 0.11 l | 44.40 ± 0.10 d | 42.86 ± 0.31 e | 34.87 ± 0.52 i |
| Glycine | 113.29 ± 0.62 a | 103.93 ± 0.81 d | 87.82 ± 0.75 g | 96.91 ± 0.05 a | 92.71 ± 0.22 d | 78.11 ± 0.67 h | 49.56 ± 0.23 a | 44.17 ± 0.29 d | 41.33 ± 0.34 f |
| Ascorbic acid | 110.49 ± 0.50 c | 92.11 ± 0.12 f | 83.96 ± 0.93 i | 94.22 ± 0.07 c | 83.75 ± 0.48 g | 67.37 ± 0.06 k | 46.59 ± 0.53 c | 44.13 ± 0.15 d | 37.57 ± 0.50 h |
| Riboflavin | 111.71 ± 0.08 b | 95.92 ± 0.56 e | 86.88 ± 0.82 h | 95.18 ± 0.08 b | 90.82 ± 0.75 e | 68.51 ± 0.46 j | 47.31 ± 0.27 b | 44.23 ± 0.20 d | 38.41 ± 0.36 g |
| Season 2023 | ||||||
|---|---|---|---|---|---|---|
| Irrigation (ETc) | 60% ETc | 80% ETc | 100% ETc | 60% ETc | 80% ETc | 100% ETc |
| Spraying | Yield (Ton/fed.) | Fruit Creaking (%) | ||||
| Control | 10.71 ± 0.07 h | 13.54 ± 0.36 e | 14.21 ± 0.27 cd | 6.98 ± 0.02 f | 8.03 ± 0.03 d | 10.34 ± 0.04 a |
| Glycine | 12.23 ± 0.63 f | 14.46 ± 0.21 bc | 15.18 ± 0.15 a | 6.02 ± 0.02 i | 7.55 ± 0.15 e | 9.87 ± 0.07 c |
| Ascorbic acid | 11.36 ± 0.11 g | 13.80 ± 0.27 de | 14.51 ± 0.14 bc | 6.22 ± 0.02 h | 7.55 ± 0.15 e | 10.07 ± 0.07 b |
| Riboflavin | 11.97 ± 0.10 f | 14.16 ± 0.06 cd | 14.78 ± 0.17 ab | 6.65 ± 0.05 g | 7.52 ± 0.03 e | 9.99 ± 0.01 b |
| Season 2024 | ||||||
| Control | 10.07 ± 0.07 i | 13.64 ± 0.02 e | 14.48 ± 0.10 cd | 6.95 ± 0.05 f | 8.00 ± 0.50 d | 11.08 ± 0.37 a |
| Glycine | 12.08 ± 0.21 f | 14.46 ± 0.08 cd | 15.43 ± 0.06 a | 6.02 ± 0.02 h | 7.34 ± 0.04 ef | 9.91 ± 0.32 c |
| Ascorbic acid | 11.25 ± 0.13 h | 13.77 ± 0.10 e | 14.72 ± 0.21 bc | 6.32 ± 0.02 gh | 7.41 ± 0.01 e | 10.39 ± 0.06 b |
| Riboflavin | 11.64 ± 0.22 g | 14.21 ± 0.19 d | 14.79 ± 0.21 b | 6.54 ± 0.04 g | 7.48 ± 0.08 e | 10.52 ± 0.10 b |
| Season 2023 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Irrigation (ETc) | 60% ETc | 80% ETc | 100% ETc | 60% ETc | 80% ETc | 100% ETc | 60% ETc | 80% ETc | 100% ETc | 60% ETc | 80% ETc | 100% ETc |
| Spraying | Fruit Length (cm2) | Fruit Diameter (cm2) | Fruit Weight (g) | Arils Weight (%) | ||||||||
| Control | 7.76 ± 0.05 g | 8.28 ± 0.03 d | 8.54 ± 0.04 c | 8.78 ± 0.06 g | 9.08 ± 0.09 ef | 9.46 ± 0.06 cd | 336.69 ± 1.00 k | 395.98 ± 4.35 g | 420.00 ± 2.00 d | 50.11 ± 0.11 i | 53.97 ± 1.00 ef | 55.02 ± 0.33 bc d |
| Glycine | 8.15 ± 0.05 de | 8.54 ± 0.01 c | 8.77 ± 0.07 a | 9.15 ± 0.15 e | 9.63 ± 0.06 b | 9.79 ± 0.01 a | 368.00 ± 1.00 h | 419.04 ± 1.00 d | 455.00 ± 1.00 a | 53.74 ± 0.52 fg | 55.00 ± 0.30 cd | 56.46 ± 0.46 a |
| Ascorbic acid | 8.01 ± 0.01 f | 8.48 ± 0.02 c | 8.58 ± 0.18 bc | 8.96 ± 0.02 f | 9.39 ± 0.02 d | 9.52 ± 0.02 bc | 345.00 ± 1.00 j | 402.75 ± 1.75 f | 428.31 ± 0.30 c | 52.99 ± 0.00 gh | 54.53 ± 1.03 de f | 55.99 ± 0.00 ab |
| Riboflavin | 8.04 ± 0.04 ef | 8.51 ± 0.02 c | 8.68 ± 0.08 ab | 9.15 ± 0.05 e | 9.45 ± 0.00 cd | 9.60 ± 0.10 b | 361.69 ± 1.00 i | 409.50 ± 1.50 e | 438.00 ± 2.00 b | 52.19 ± 0.19 h | 54.89 ± 1.00 cd e | 55.58 ± 0.40 ab c |
| Season 2024 | ||||||||||||
| Control | 7.59 ± 0.05 h | 8.19 ± 0.07 e | 8.56 ± 0.02 c | 8.91 ± 0.08 h | 9.22 ± 0.04 e | 9.47 ± 0.04 cd | 333.31 ± 0.30 j | 387.83 ± 3.53 g | 420.40 ± 1.44 d | 50.02 ± 0.02 g | 53.43 ± 0.43 de | 56.56 ± 0.42 b |
| Glycine | 8.06 ± 0.06 f | 8.55 ± 0.05 c | 8.88 ± 0.02 a | 9.11 ± 0.04 f | 9.53 ± 0.06 bc | 9.80 ± 0.10 a | 351.69 ± 1.00 h | 418.67 ± 1.61 d | 463.27 ± 2.61 a | 53.88 ± 1.00 cd | 54.98 ± 1.00 c | 58.06 ± 0.94 a |
| Ascorbic acid | 7.87 ± 0.02 g | 8.34 ± 0.02 d | 8.61 ± 0.01 c | 8.88 ± 0.02 h | 9.40 ± 0.01 d | 9.61 ± 0.01 b | 338.31 ± 1.30 i | 392.09 ± 0.31 f | 430.17 ± 1.76 c | 52.55 ± 0.40 ef | 54.20 ± 0.20 cd | 57.55 ± 0.89 ab |
| Riboflavin | 8.03 ± 0.03 f | 8.42 ± 0.02 d | 8.76 ± 0.10 b | 9.01 ± 0.01 g | 9.43 ± 0.01 d | 9.61 ± 0.01 b | 350.07 ± 1.10 h | 396.75 ± 3.75 e | 455.27 ± 2.61 b | 52.02 ± 0.98 f | 54.28 ± 0.28 cd | 57.37 ± 0.63 ab |
| Season 2023 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Irrigation (ETc) | 60% ETc | 80% ETc | 100% ETc | 60% ETc | 80% ETc | 100% ETc | 60% ETc | 80% ETc | 100% ETc |
| Spraying | TSS (%) | Total Sugar (%) | TA (%) | ||||||
| Control | 16.22 ± 0.22 c | 15.36 ± 0.06 e | 14.32 ± 0.02 h | 13.00 ± 0.20 b | 12.40 ± 0.10 e | 12.12 ± 0.12 f | 1.13 ± 0.05 | 1.10 ± 0.08 | 1.08 ± 0.11 |
| Glycine | 16.93 ± 0.05 a | 15.78 ± 0.08 d | 14.83 ± 0.07 f | 13.44 ± 0.04 a | 12.87 ± 0.03 bc | 12.23 ± 0.03 ef | 1.08 ± 0.07 | 1.05 ± 0.04 | 1.02 ± 0.02 |
| Ascorbic acid | 16.66 ± 0.06 b | 15.34 ± 0.04 e | 14.54 ± 0.12 gh | 13.02 ± 0.02 b | 12.55 ± 0.05 d | 12.19 ± 0.09 f | 1.10 ± 0.07 | 1.09 ± 0.09 | 1.06 ± 0.00 |
| Riboflavin | 16.79 ± 0.03 ab | 15.50 ± 0.5 e | 14.76 ± 0.06 fg | 12.98 ± 0.02 b | 12.76 ± 0.10 c | 12.21 ± 0.01 f | 1.13 ± 0.11 | 1.09 ± 0.00 | 1.07 ± 0.03 |
| Season 2024 | |||||||||
| Control | 16.67 ± 0.03 c | 15.56 ± 0.04 f | 14.78 ± 0.02 j | 14.00 ± 0.30 c | 13.43 ± 0.03 e | 12.34 ± 0.04 g | 1.13 ± 0.09 | 1.10 ± 0.05 | 1.09 ± 0.08 |
| Glycine | 16.99 ± 0.08 a | 15.78 ± 0.02 d | 15.34 ± 0.06 g | 14.36 ± 0.06 a | 13.69 ± 0.09 d | 12.65 ± 0.05 f | 1.08 ± 0.08 | 1.03 ± 0.02 | 1.02 ± 0.03 |
| Ascorbic acid | 16.82 ± 0.03 b | 15.66 ± 0.04 e | 14.98 ± 0.02 i | 14.03 ± 0.03 bc | 13.56 ± 0.04 de | 12.54 ± 0.04 f | 1.10 ± 0.07 | 1.07 ± 0.01 | 1.06 ± 0.05 |
| Riboflavin | 16.88 ± 0.02 b | 15.69 ± 0.01 e | 15.07 ± 0.11 h | 14.21 ± 0.01 ab | 13.65 ± 0.05 d | 12.61 ± 0.01 f | 1.11 ± 0.10 | 1.08 ± 0.07 | 1.07 ± 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaber, S.H.; Mansour, A.H.A.; Mahmoud, G.A.-E.; Hefzy, M. Optimizing Irrigation Rates and Antioxidant Foliar Spray Effects on Growth, Yield, and Fruit Quality of Manfalouty Pomegranate Trees. Int. J. Plant Biol. 2025, 16, 123. https://doi.org/10.3390/ijpb16040123
Gaber SH, Mansour AHA, Mahmoud GA-E, Hefzy M. Optimizing Irrigation Rates and Antioxidant Foliar Spray Effects on Growth, Yield, and Fruit Quality of Manfalouty Pomegranate Trees. International Journal of Plant Biology. 2025; 16(4):123. https://doi.org/10.3390/ijpb16040123
Chicago/Turabian StyleGaber, Shimaa Hosny, Ahmed H. A. Mansour, Ghada Abd-Elmonsef Mahmoud, and Mohamed Hefzy. 2025. "Optimizing Irrigation Rates and Antioxidant Foliar Spray Effects on Growth, Yield, and Fruit Quality of Manfalouty Pomegranate Trees" International Journal of Plant Biology 16, no. 4: 123. https://doi.org/10.3390/ijpb16040123
APA StyleGaber, S. H., Mansour, A. H. A., Mahmoud, G. A.-E., & Hefzy, M. (2025). Optimizing Irrigation Rates and Antioxidant Foliar Spray Effects on Growth, Yield, and Fruit Quality of Manfalouty Pomegranate Trees. International Journal of Plant Biology, 16(4), 123. https://doi.org/10.3390/ijpb16040123






