Residual Effect of Imidazolinone Herbicides on Emergence and Early Development of Forage Species in Rice-Livestock Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Origin and Sampling
2.2. Soil Characterization
2.3. Experimental Design of the Bioassays
- Emergence (%): determined at 21 DAS by counting the number of seedlings emerging per pot. Seedlings were considered to have emerged if they were visible above the soil surface with clearly unfolded cotyledons or first leaves. The value was expressed as a percentage relative to the total number of seeds sown. Although seed physiological quality was not assessed, the seed lots used showed germination and vigor levels above the minimum standards established by the National Seed Institute—Uruguay (INASE) [26].
- Plant height (cm): measured at 35 and 70 DAS. For grasses, height was determined from the soil surface to the tip of the last fully expanded true leaf. For legumes, it was measured from the base of the plant to the last visible node in the main stem.
- Shoot and root dry mass: at the end of the bioassay (70 DAS), plants were harvested. Shoots and roots systems were carefully separated, with excess soil gently removed. Both fractions were washed, dried, and then oven-dried at 60 °C until constant weight was reached. Results were expressed as grams of dry matter per plant (g·plant−1).
2.4. Statistical Analysis
3. Results and Discussion
3.1. Plant Response Variables
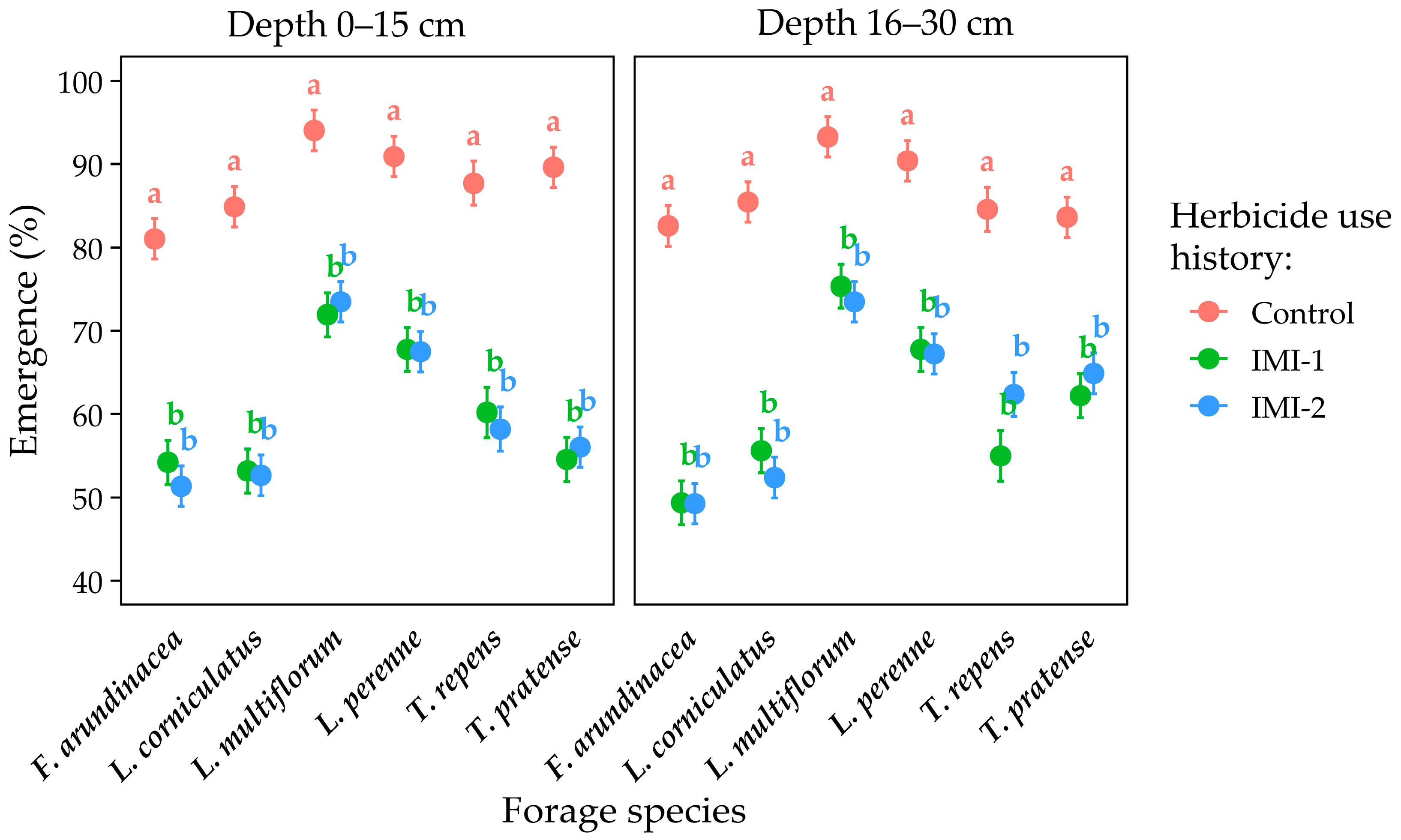
3.1.1. Emergence
3.1.2. Plant Height
3.1.3. Shoot and Root Dry Mass
3.2. Multivariate Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Deambrosi, E. Rice Production System in Uruguay and Its Sustainability. In Proceedings of the III International Conference of Temperate Rice, Punta del Este, Uruguay, 10–13 March 2003. [Google Scholar]
- Vilela, L.; Martha, G.B., Jr.; Marchão, R.L.; Guimarães, R., Jr.; Barioni, L.G.; Barcellos, A.d.O. Integração Lavoura-Pecuária. In Savanas: Desafios e Estratégias para o Equilíbrio Entre Sociedade, Agronegócio e Recursos Naturais; Faleiro, F.G., de Farias Neto, A.L., Eds.; Embrapa Cerrados: Fortaleza, Brazil, 2008; Volume 1, ISBN 978-85-7075-039-6. [Google Scholar]
- Lemaire, G.; Franzluebbers, A.; Carvalho, P.C.d.F.; Dedieu, B. Integrated Crop–Livestock Systems: Strategies to Achieve Synergy Between Agricultural Production and Environmental Quality. Agric. Ecosyst. Environ. 2014, 190, 4–8. [Google Scholar] [CrossRef]
- Kaspary, T.E.; Zarza, R. Efecto Residual de Los Herbicidas Utilizados En Arroz Clearfield Sobre La Implantación de Pastura En Sucesión. Rev. INIA Urug. 2022, 68, 23–26. [Google Scholar]
- DIEA (Dirección de Investigaciones Estadísticas Agropecuarias). Anuario Estadístico Agropecuario 2024, 27th ed.; Ministerio de Ganadería, Agricultura y Pesca (MGAP): Montevideo, Uruguay, 2024. [Google Scholar]
- Tan, S.; Evans, R.R.; Dahmer, M.L.; Singh, B.K.; Shaner, D.L. Imidazolinone-Tolerant Crops: History, Current Status and Future. Pest Manag. Sci. 2005, 61, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Shaner, D.; O’Connor, S. The Imidazolinone Herbicides; CRC Press: Boca Raton, FL, USA, 1991; ISBN 978-0-203-70999-3. [Google Scholar]
- Shaner, D.L. Herbicide Handbook; Weed Science Society of America: Lawrence, KS, USA, 2014; ISBN 978-0-615-98937-2. [Google Scholar]
- Gehrke, V.R.; Fipke, M.V.; de Avila, L.A.; Camargo, E.R. Understanding the Opportunities to Mitigate Carryover of Imidazolinone Herbicides in Lowland Rice. Agriculture 2021, 11, 299. [Google Scholar] [CrossRef]
- Ramezani, M.; Oliver, D.P.; Kookana, R.S.; Gill, G.; Preston, C. Abiotic Degradation (Photodegradation and Hydrolysis) of Imidazolinone Herbicides. J. Environ. Sci. Health Part B 2008, 43, 105–112. [Google Scholar] [CrossRef]
- Kraemer, A.F.; Marchesan, E.; Grohs, M.; de Avila, L.A.; Machado, S.L.d.O.; Zanella, R.; Massoni, P.F.S.; Sartori, G.M.S. Lixiviação do imazethapyr em solo de várzea sob dois sistemas de manejo. Cienc. Rural 2009, 39, 1660–1666. [Google Scholar] [CrossRef]
- Su, W.; Hao, H.; Ding, M.; Wu, R.; Xu, H.; Xue, F.; Shen, C.; Sun, L.; Lu, C. Adsorption and Degradation of Imazapic in Soils under Different Environmental Conditions. PLoS ONE 2019, 14, e0219462. [Google Scholar] [CrossRef]
- de Avila, L.A.; Marchesan, E.; Camargo, E.R.; Merotto, A., Jr.; Ulguim, A.d.R.; Noldin, J.A.; Andres, A.; Mariot, C.H.P.; Agostinetto, D.; Dornelles, S.H.B.; et al. Eighteen Years of ClearfieldTM Rice in Brazil: What Have We Learned? Weed Sci. 2021, 69, 585–597. [Google Scholar] [CrossRef]
- Bundt, A.C.; Avila, L.A.; Pivetta, A.; Agostinetto, D.; Dick, D.P.; Burauel, P. Imidazolinone Degradation in Soil in Response to Application History. Planta Daninha 2015, 33, 341–349. [Google Scholar] [CrossRef]
- Oliveira, M.L.; Marchesan, E.; Soares, C.F.; Farias, J.G.; Ulguim, A.d.R.; Fleck, A.G.; Coelho, L.L. Persistence of Imazapyr+imazapic in Irrigated Rice Area and Effect on Soybean Due to Soil Moisture and Phytoremediation in the Off-Season. Bragantia 2019, 78, 306–316. [Google Scholar] [CrossRef]
- Pinto, J.J.O.; Noldin, J.A.; Pinho, C.F.; Rossi, F.; Galon, L.; Almeida, G.F. Atividade residual de (imazethapyr+imazapic) para sorgo granífero (Sorghum bicolor) semeado em rotação com o arroz irrigado. Planta Daninha 2009, 27, 1015–1024. [Google Scholar] [CrossRef]
- Marchesan, E.; Santos, F.M.D.; Grohs, M.; Avila, L.A.D.; Machado, S.L.O.; Senseman, S.A.; Massoni, P.F.S.; Sartori, G.M.S. Carryover of Imazethapyr and Imazapic to Nontolerant Rice. Weed Technol. 2010, 24, 6–10. [Google Scholar] [CrossRef]
- Chiapinotto, D.M.; Avila, L.A.; Aranha, B.C.; Viana, V.E.; Benedetti, L.; Araújo, B.O.N.; Camargo, E.R. Potential Reduction of Non-Imidazolinone Rice Grain Yield by Imidazolinone Soil Residual Activity. Adv. Weed Sci. 2024, 42, e020240044. [Google Scholar] [CrossRef]
- Ulguim, A.R.; Carlos, F.S.; Zanon, A.J.; Ogoshi, C.; Bexaira, K.P.; Silva, P.R.F. Is Increasing Doses of Imazapyr + Imazapic Detrimental to the Main Crop Rotation Alternatives to Flooded Rice? Planta Daninha 2019, 37, e019217913. [Google Scholar] [CrossRef]
- Bundt, A.D.C.; Avila, L.A.; Agostinetto, D.; Nohatto, M.A.; Vargas, H.C. Carryover of Imazethapyr + Imazapic on Ryegrass and Non-Tolerant Rice as Affected by Thickness of Soil Profile. Planta Daninha 2015, 33, 357–364. [Google Scholar] [CrossRef]
- Saldain, N.; Bermudez, R.; Serrón, N.; Sosa, B. Efecto de la dosis de Kifix y del manejo del riego en la productividad inicial de la pradera subsiguiente. Ser. Act. Difus. 2014, 735, 11–13. [Google Scholar]
- Saldain, N.; López, A.; Sosa, B. Persistencia y actividad biológica del Kifix en suelos arroceros en el Este del Uruguay. Ser. Act. Difus. 2014, 735, 17–19. [Google Scholar]
- Saldain, N.; López, A.; Sosa, B. Efecto del Kifix asperjado en el arroz clearfield sobre el raigrás subsiguiente seguido por arroz sin resistencia (no clearfield) o sorgo forrajero en siembra directa. Ser. Act. Difus. 2014, 735, 14–16. [Google Scholar]
- Neal, J. Conducting a Bioassay for Herbicide Residues. N.C. Cooperative Extension, NC State University. Available online: https://content.ces.ncsu.edu/conducting-a-bioassay-for-herbicide-residues#section_heading_8801 (accessed on 1 June 2025).
- Klein, R.; Bernards, M.; Shea, P. A Quick Test For Herbicide Carry-Over in the Soil; University Nebraska–Lincoln Extension Publications: Lincoln, NE, USA, 2008. [Google Scholar]
- Instituto Nacional de Semillas. Available online: https://www.inase.uy/Certificacion/EstandaresProduccion.aspx (accessed on 1 June 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025. [Google Scholar]
- Wixson, M.B.; Shaw, D.R. Effects of Soil-Applied AC 263,222 on Crops Rotated with Soybean (Glycine max). Weed Technol. 1992, 6, 276–279. [Google Scholar] [CrossRef]
- Alister, C.; Kogan, M. Efficacy of Imidazolinone Herbicides Applied to Imidazolinone-Resistant Maize and Their Carryover Effect on Rotational Crops. Crop Prot. 2005, 24, 375–379. [Google Scholar] [CrossRef]
- Guimarães, S.; Galon, L.; Lima, A.; Bastiani, M.O.; Belarmino, J.G.; Burg, G.M.; Zadoná, R.R.; Coneço, G.; Silva, A.F. Especies Tolerantes Aos Herbicidas Do Grupos Das Imidazoinonas Con Potencial de Uso Em Fitorremediação. In Proceedings of the XXVIII Congresso Brasileiro da Ciencia das Plantas Daninhas na era da Biotecnologia, Campo Grande, MS, Brasil, 3–6 September 2012. [Google Scholar]
- Saldain, N.; Bermudez, R.; Serrón, N.; Sosa, B. Efecto del Kifix (Imazapir + Imazapic) Asperjado En El Arroz Clearfield Sobre Las Plantas Forrajeras Subsiguientes En El Este del Uruguay. Ser. Act. Difus. 2012, 686, 32–37. [Google Scholar]
- Zaccaro, M.L.M.; Byrd, J.D., Jr.; Russell, D.P. Tolerance of Several Legumes to Residual Imazapyr Applied Under Greenhouse Conditions. Weed Technol. 2018, 32, 66–71. [Google Scholar] [CrossRef]
- Bundt, A.D.C.; de Avila, L.A.; Pinto, J.J.d.O.; dos Santos, T.T.; Agostinetto, D.; Martins, K. Transporte ascendente da mistura formulada de imazethapyr e imazapic em resposta à profundidade do lençol freático. Cienc. Rural 2013, 43, 1597–1604. [Google Scholar] [CrossRef]
- Shaw, D.R.; Watkins, R.M.; Garris, S.B., Jr.; Cole, A.W. Forage Species Tolerande to Imazapyr and Imazapic; MAFES Mississippi Agricultural and Forestry Experiment Station: Mississippi State, MS, USA, 2001. [Google Scholar]
- Shinn, S.L.; Thill, D.C. Tolerance of Several Perennial Grasses to Imazapic. Weed Technol. 2004, 18, 60–65. [Google Scholar] [CrossRef]
- Chu, L.; Gao, Y.; Chen, L.; McCullough, P.E.; Jespersen, D.; Sapkota, S.; Bagavathiannan, M.; Yu, J. Impact of Environmental Factors on Seed Germination and Seedling Emergence of White Clover (Trifolium repens L.). Agronomy 2022, 12, 190. [Google Scholar] [CrossRef]
- Pinto, J.J.O.; Noldin, J.A.; Rosenthal, M.D.; Pinho, C.F.; Rossi, F.; Machado, A.; Piveta, L.; Galon, L. Atividade residual de (imazethapyr + imazapic) sobre azevém anual (Lolium multiflorum), semeado em sucessão ao arroz irrigado, sistema clearfield®. Planta Daninha 2009, 27, 609–619. [Google Scholar] [CrossRef]
- Galon, L.; Lima, A.M.; Guimarães, S.; Belarmino, J.G.; Burg, G.M.; Concenço, G.; Bastiani, M.O.; Beutler, A.N.; Zandona, R.R.; Radünz, A.L. Potential of Plant Species for Bioremediation of Soils Applied with Imidazolinone Herbicides. Planta Daninha 2014, 32, 719–726. [Google Scholar] [CrossRef]
- Santos, L.O.; Pinto, J.J.O.; Piveta, L.B.; Noldin, J.A.; Galon, L.; Concenço, G. Carryover Effect of Imidazolinone Herbicides for Crops Following Rice. Am. J. Plant Sci. 2014, 5, 1049–1058. [Google Scholar] [CrossRef]
- Tranel, P.J.; Wright, T.R. Resistance of Weeds to ALS-Inhibiting Herbicides: What Have We Learned? Weed Sci. 2002, 50, 700–712. [Google Scholar] [CrossRef]
- Avila, L.A.; Marchezan, M.; François, T.; Cezimbra, D.M.; Souto, K.M.; Refatti, J.P. Injury Caused by the Formulated Mixture of the Herbicide Imazethapyr and Imazapic in Raygrass as Affected by Soil Moisture. Planta Daninha 2010, 28, 1041–1046. [Google Scholar] [CrossRef]
- Giménez, J.P.; Istilart, C.M.; Giménez, D.O.; Yanniccari, M. Evaluación de la residualidad de imidazolinonas sobre la implantación de trigo, avena y alfafa/ryegrass luego del cultivo de girasol imi-tolerante. Ser. Inf. Tec. 2014, 5, 124–128. [Google Scholar]
- Souto, K.M.; de Avila, L.A.; Cassol, G.V.; Machado, S.L.d.O.; Marchesan, E. Phytoremediation of Lowland Soil Contaminated with a Formulated Mixture of Imazethapyr and Imazapic1. Rev. Ciênc. Agron. 2015, 46, 185–192. [Google Scholar] [CrossRef]
- Souto, K.M.; Jacques, R.J.S.; Zanella, R.; Machado, S.L.d.O.; Balbinot, A.; Avila, L.A. de Phytostimulation of Lowland Soil Contaminated with Imidazolinone Herbicides. Int. J. Phytoremediation 2020, 22, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, A.V.; Souza, J.R.P.; Shaner, D. Persistence and Carryover Effect of Imazapic and Imazapyr in Brazilian Cropping Systems. Weed Technol. 2005, 19, 986–991. [Google Scholar] [CrossRef]
- Gianelli, V.R.; Bedmar, F.; Costa, J.L. Persistence and Sorption of Imazapyr in Three Argentinean Soils. Environ. Toxicol. Chem. 2014, 33, 29–34. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, W. Correlation of Imazapyr Adsorption and Desorption with Soil Properties. Soil Sci. 1999, 164, 411. [Google Scholar] [CrossRef]






| Sampling Area | Year(s) | Herbicide Use History | Product (Formulation) | Dose (g a.i. ha−1) | Number of Applications | Time Since Last Application |
|---|---|---|---|---|---|---|
| Rio Branco | 2021, 2022, 2023 | Control | - | - | - | - |
| IMI-1 | imazapyr + imazapic | 52.5 + 17.5 | 2 | ≈17 months | ||
| IMI-2 | ≈5 months | |||||
| Treinta y Tres | 2021, 2022, 2023 | Control | - | - | - | - |
| IMI-1 | imazapyr + imazapic | 52.5 + 17.5 | 2 | ≈17 months | ||
| IMI-2 | ≈5 months | |||||
| UEPL 1 | 2022 | Control | - | - | - | - |
| IMI-2 | imazapyr + imazapic | 52.5 + 17.5 | 1 | ≈5 months | ||
| Tacuarembó | 2023 | Control | - | - | - | - |
| IMI-1 | imazapyr + imazapic | 52.5 + 17.5 | 2 | ≈17 months | ||
| IMI-2 | ≈5 months |
| Sampling Area | Herbicide Use History | pH (H2O) | Organic Carbon (%) | Texture | ||
|---|---|---|---|---|---|---|
| 0–15 cm 2 | 16–30 cm | 0–15 cm | 16–30 cm | |||
| Rio Branco | Control | 5.4 | 6.4 | 1.15 | 0.64 | Loam |
| IMI-1 | 5.2 | 6.2 | 0.85 | 0.55 | ||
| IMI-2 | 5.4 | 6.2 | 0.79 | 0.59 | ||
| Treinta y Tres | Control | 5.3 | 5.9 | 1.80 | 0.92 | Silty clay loam |
| IMI-1 | 6.4 | 7.6 | 1.67 | 0.77 | ||
| IMI-2 | 5.3 | 6.5 | 1.44 | 0.79 | ||
| UEPL 1 | Control | 5.4 | 6.5 | 1.35 | 0.75 | Silty clay loam |
| IMI-1 | 6.2 | 7.7 | 0.81 | 0.52 | ||
| Tacuarembó | Control | 4.8 | 5.4 | 1.09 | 0.49 | Sandy loam |
| IMI-1 | 5.4 | 6.6 | 1.30 | 0.64 | ||
| IMI-2 | 5.2 | 6.5 | 0.89 | 0.81 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez-De-Barbieri, V.; González-Barrios, P.; Rovira, P.; Marchesi, C.; Cuadro, R.; Zarza, R.; Kaspary, T.E. Residual Effect of Imidazolinone Herbicides on Emergence and Early Development of Forage Species in Rice-Livestock Systems. Int. J. Plant Biol. 2025, 16, 110. https://doi.org/10.3390/ijpb16030110
Rodriguez-De-Barbieri V, González-Barrios P, Rovira P, Marchesi C, Cuadro R, Zarza R, Kaspary TE. Residual Effect of Imidazolinone Herbicides on Emergence and Early Development of Forage Species in Rice-Livestock Systems. International Journal of Plant Biology. 2025; 16(3):110. https://doi.org/10.3390/ijpb16030110
Chicago/Turabian StyleRodriguez-De-Barbieri, Valentina, Pablo González-Barrios, Pablo Rovira, Claudia Marchesi, Robin Cuadro, Rodrigo Zarza, and Tiago Edu Kaspary. 2025. "Residual Effect of Imidazolinone Herbicides on Emergence and Early Development of Forage Species in Rice-Livestock Systems" International Journal of Plant Biology 16, no. 3: 110. https://doi.org/10.3390/ijpb16030110
APA StyleRodriguez-De-Barbieri, V., González-Barrios, P., Rovira, P., Marchesi, C., Cuadro, R., Zarza, R., & Kaspary, T. E. (2025). Residual Effect of Imidazolinone Herbicides on Emergence and Early Development of Forage Species in Rice-Livestock Systems. International Journal of Plant Biology, 16(3), 110. https://doi.org/10.3390/ijpb16030110







