Abstract
Turmeric (Curcuma longa L. cv. Trang 1), a high-value cultivar known for its elevated curcuminoid and volatile oil content, holds significant potential in pharmaceutical and food applications. However, its commercial propagation is constrained by low rhizome productivity and the limitations of conventional vegetative propagation. This study aimed to improve the propagation efficiency of turmeric cv. Trang 1 by developing optimized protocols for explant sterilization, shoot proliferation, root induction, and acclimatization. Sprouted rhizome buds were sterilized and cultured on a Murashige and Skoog (MS) medium supplemented with various plant growth regulators, including cytokinins (benzyladenine [BA], thidiazuron [TDZ], and meta-topolin [mT]) and auxins (indole-3-butyric acid [IBA] and 1-naphthaleneacetic acid [NAA]). The shoot induction (4.60 ± 1.47 shoots per explant) and shoot height (2.34 ± 0.61 cm) were observed on the MS medium with 3.0 mg/L BA, while the TDZ, at 0.5 mg/L, also induced a high number of shoots (5.22 ± 0.64). When using single shoots derived from bud explants, mT at 1.5 mg/L significantly enhanced the shoot formation. For the root induction, 2.0 mg/L IBA yielded the highest number of roots (7.33 ± 1.49), while NAA was less effective. The plantlets acclimatized in a 1:1 soil and peat moss mixture showed the highest survival rate (86.67%). This improved protocol enables the efficient production of turmeric plantlets, supporting commercial deployment.
1. Introduction
Turmeric (Curcuma longa L.) is a perennial herb in the Zingiberaceae family, widely recognized for its uses in traditional medicine and culinary practices. The yellow–orange rhizomes are rich in bioactive compounds that contribute to its therapeutic properties. Among these, curcuminoids, particularly curcumin, have been extensively studied for their anti-tumor, anti-inflammatory, choleretic, wound-healing, and antioxidant activities [1,2]. This diverse biochemical profile has established turmeric as an important resource in both traditional remedies and modern pharmaceutical industries.
Despite broad cultivation, turmeric propagation remains largely restricted to asexual reproduction via rhizomes, as the species typically fails to produce viable seeds. This conventional method is inherently slow and inefficient, yielding only a limited number of progeny per rhizome. Furthermore, rhizome-based propagation carries a high risk of transmitting soil-borne pathogens, posing serious threats to plant health and productivity [3]. To overcome these limitations, plant tissue culture techniques, particularly micropropagation, have been developed as effective strategies for enhancing propagation efficiency. Micropropagation offers significant advantages, including the rapid production of large numbers of uniform, disease-free plants and shortened production cycles. This approach is especially valuable for high-value cultivars that are difficult to propagate conventionally. Indeed, various studies have reported successful in vitro regeneration in turmeric and related Zingiberaceae species, underscoring the potential of tissue culture for mass propagation [4,5,6].
Among turmeric cultivars, ‘Trang 1’ stands out for its superior phytochemical profile, including high curcuminoid and volatile oil content, promoted by the Department of Agriculture under the Ministry of Agriculture and Cooperatives, Thailand, making it a promising candidate for pharmaceutical and medicinal applications. However, despite its biochemical advantages, cv. Trang 1 exhibits relatively low rhizome productivity, which limits its potential for large-scale cultivation and commercialization.
A key determinant of micropropagation success is the strategic use of plant growth regulators (PGRs), particularly cytokinins and auxins. Cytokinins are crucial in promoting cell division and shoot initiation, often acting in concert with auxins, which primarily regulate root development and cell elongation. The interplay between these hormones influences various aspects of in vitro morphogenesis, and their effectiveness is context-dependent, varying by species, explant type, and physiological status [7]. Recent studies in both model and crop species have shown that cytokinin signaling involves a positive feedback loop that sustains shoot regeneration potential [8,9] while auxin biosynthesis and transport are tightly regulated to maintain developmental gradients essential for organogenesis [7]. Furthermore, specific cytokinin–auxin combinations have been found to significantly influence callus induction and shoot proliferation in various monocots, including maize and ginger [9,10]. Without such optimization, tissue cultures may exhibit poor response rates, abnormal growth, or low regeneration efficiency, undermining the reliability and scalability of the protocol. Therefore, systematic evaluation of PGR effects is a foundational step in developing a robust and reproducible micropropagation system, especially for economically significant cultivars like ‘Trang 1’.
This study aimed to improve the propagation efficiency of turmeric (C. longa L. cv. Trang 1) through the development of an optimized micropropagation protocol for explant sterilization, shoot proliferation, root induction, and post-culture acclimatization, ensuring successful transition of plantlets from in vitro to ex vitro conditions. By refining micropropagation techniques, the goal was to enable the rapid production of high-quality, disease-free plantlets, thereby supporting large-scale cultivation of this economically important cultivar.
2. Materials and Methods
2.1. Plant Materials
Rhizomes of C. longa L. cv. Trang 1 were sourced from the Trang Horticulture Research Center under the Horticulture Research Institute, Department of Agriculture, Thailand. Healthy rhizomes with visible buds were selected as explants.
2.2. Explant Sterilization
Sprouted rhizome buds (approximately 3–4 cm in length) were used as explants for initiation. The sprouted buds were first washed thoroughly with detergent and running tap water for 30 min. Surface sterilization was then carried out by immersing the buds in 70% ethanol for 1 min, followed by treatment with a 0.05% (w/v) mercuric chloride (HgCl2) solution for 15 min with constant agitation (120 rpm). After sterilization, the explants were rinsed three times with sterile distilled water (5 min per rinse) to remove any residual HgCl2. The sterilized buds were placed onto autoclaved an MS (Murashige and Skoog) [11] basal medium (pH 5.8) containing 30 g/L sucrose and solidified with 8 g/L agar.
2.3. Shoot Induction
To evaluate the effects of various plant growth regulators on shoot induction of turmeric cv. Trang 1, three sets of experiments were conducted using one-week-old in vitro shoots (for NAA with BA and TDZ with BA experiments) or single-shoot explants derived from initial rhizome cultures (mT experiments). All cultures were maintained at 25 ± 2 °C under a 16 h photoperiod (cool white fluorescent light, ~130 µmol photons m−2·s−1). For the NAA and BA, explants were cultured on an MS medium supplemented with a combination of BA (PhytoTech Labs, Lenexa, KS, USA) at 0, 1.0, 3.0, or 5.0 mg/L and NAA (Sisco Research Laboratory Pvt. Ltd., Mumbai, India) at 0 or 1.0 mg/L. For TDZ with or without BA, shoot explants were cultured on an MS medium containing TDZ (AK Scientific, Inc., Union City, CA, USA) at 0, 0.5, or 1.0 mg/L, either alone or combined with 3.0 mg/L BA. After four weeks, the percentage of shoot-inducing explants, average number of shoots per explant, and average shoot height (cm) were recorded. For mT, single shoots obtained from previous experiments were transferred to an MS medium supplemented with mT (PhytoTech Labs, Lenexa, KS, USA) at 0.0, 0.5, 1.0, 1.5, or 2.0 mg/L (without additional growth regulators). After eight weeks, the shoot induction rate, number of shoots per explant, and shoot length were evaluated.
2.4. Root Induction
For root induction, single regenerated shoots from the induction phase were transferred to an MS medium supplemented with auxin-type plant growth regulators. Two auxins were tested, IBA (PhytoTech Labs, Lenexa, KS, USA) or NAA, each at 0.0, 1.0, or 2.0 mg/L. After one week of culture on these rooting media, the percentage of shoots forming roots, the number of roots per shoot, and the average root length (cm) were recorded.
2.5. Acclimatization and Transplantation
Well-rooted plantlets were acclimatized under ambient conditions for 3–5 days before being removed from the culture flasks and gently washed under tap water to remove residual agar. The clean plantlets were transplanted into plastic pots (5 cm diameter) containing one of three sterilized potting substrates: (1) garden soil, (2) a 1:1 (v/v) mixture of garden soil and peat moss, or (3) a 1:1 (v/v) mixture of garden soil and coconut husk. Each substrate was thoroughly moistened before planting. To maintain high humidity, each pot was covered with a transparent plastic bag immediately after transplanting. The plantlets were acclimatized by gradually ventilating the bags; small holes were introduced in the plastic covers after 3–5 days, and the sizes of the openings were increased daily over a 2-week period. After 2 weeks, the plastic covers were removed completely, and the plantlets were grown under greenhouse conditions with regular watering for an additional three weeks. The survival rate (%) was recorded five weeks after transplantation for each substrate treatment (15 plantlets per treatment).
2.6. Data Analysis
Data analysis was performed using R Statistical Programming Version 4.3.3 via RStudio (version 2024.12.1 + 563) [12]. The Shapiro–Wilk test was applied to assess the normality, and the results indicated that the data were not normally distributed [13]. As the residuals were not normally distributed, the Kruskal–Wallis test was used to assess the differences among the treatments, followed by Fisher’s least significant difference (LSD) test with Holm’s method for p-value adjustment in post hoc comparisons [14,15].
3. Results
3.1. Effects of BA and NAA on Shoot Induction
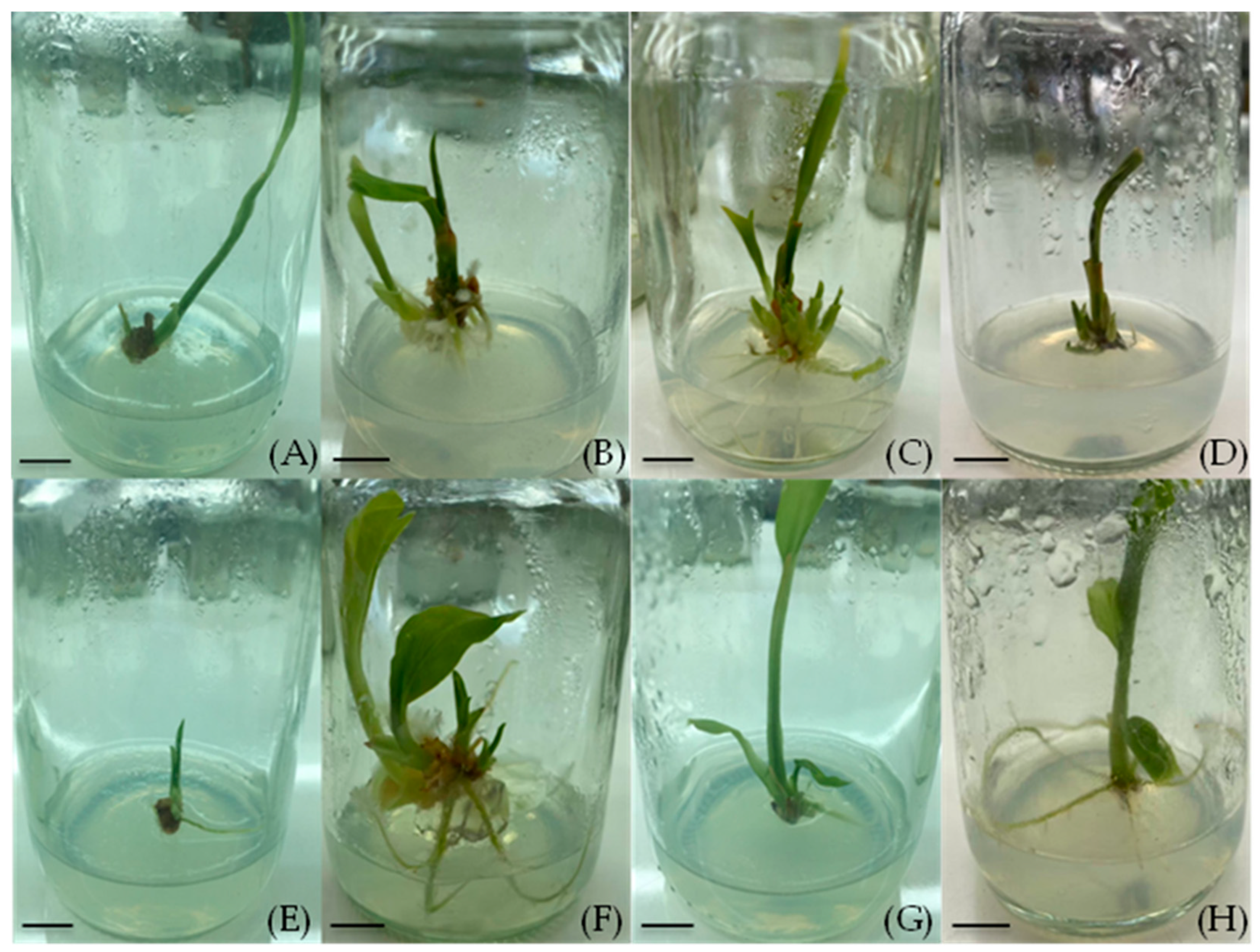
Sprouted rhizome buds of turmeric cv. Trang 1 were successfully sterilized and established on the MS medium (Figure 1). In the shoot induction experiment, sprouted rhizome buds were used, and the hormonal treatment significantly influenced the production of new shoots (Table 1). The MS medium supplemented with 3.0 mg/L BA without NAA achieved a 100% shoot induction rate, producing the highest number of shoots (4.60 ± 1.47 per explant) and the greatest average shoot height (2.34 ± 0.61 cm) after four weeks (Figure 2C). This response was significantly different from the hormone-free control, in which no new shoots were produced. The lower BA concentrations also induced shoot formation, but to a lesser extent: the 1.0 mg/L BA yielded about 1.00 ± 0.58 shoots per explant (Figure 2B), and the 5.0 mg/L BA yielded 1.50 ± 0.65 shoots per explant (Figure 2D). In contrast, the presence of the NAA in the medium had an inhibitory effect on the shoot bud induction. The MS medium containing 1.0 mg/L NAA (without BA) produced, on average, only 0.50 ± 0.29 shoots per explant. Even when the NAA (1.0 mg/L) was combined with the BA at 1.0–5.0 mg/L, the number of shoots per explant remained lower than in the corresponding BA-only treatments (Table 1). For example, the combination of 1.0 mg/L NAA + 3.0 mg/L BA produced 0.67 ± 0.67 shoots per explant on average, compared with 4.60 shoots with 3.0 mg/L BA alone. These results indicate that BA alone is effective at inducing multiple shoots from turmeric buds, whereas the addition of NAA tends to reduce shoot proliferation in this cultivar.

Figure 1.
Rhizomes (A) and sprouted rhizome buds (B) of turmeric cv. Trang 1 (scale bar = 1 cm).

Table 1.
MS media supplemented with different concentrations of NAA and BA for shoot induction for 4 weeks. Values are presented as means ± standard error (n ≥ 3). Means sharing the same letter(s) within a column do not differ significantly, based on Fisher’s Least Significant Difference (LSD) test with Holm’s correction for p-value adjustment at p ≤ 0.05. Asterisks denote statistically significant differences.

Figure 2.
Effects of NAA and BA on shoot induction in turmeric cv. Trang 1 after four weeks of culture: (A) MS medium without plant growth regulators (control), (B) MS + 1.0 mg/L BA, (C) MS + 3.0 mg/L BA, (D) MS + 5.0 mg/L BA, (E) MS + 1.0 mg/L NAA, (F) MS + 1.0 mg/L NAA + 1.0 mg/L BA, (G) MS + 1.0 mg/L NAA + 3.0 mg/L BA, and (H) MS + 1.0 mg/L NAA + 5.0 mg/L BA, (scale bar = 1 cm).
3.2. Effects of TDZ and BA on Shoot Induction
All cytokinin-containing treatments (BA and/or TDZ) induced high frequencies of shoot induction, whereas the hormone-free control had a very limited response. Specifically, every medium supplemented with BA and/or TDZ achieved 100% shoot induction, which was significantly higher than the control (only 37.5% of the explants produced shoots) (Table 2). The number of shoots per explant varied with the type and concentration of cytokinin. The highest shoot proliferation was obtained on the MS medium containing 0.5 mg/L TDZ (without BA), which produced 5.22 ± 0.64 shoots per explant on average. The MS with 1.0 mg/L TDZ (no BA) also supported a high induction rate (4.50 ± 0.53 shoots per explant). In the treatments that included 3.0 mg/L BA in combination with TDZ, the shoot numbers were slightly lower: 3.44 ± 0.47, 3.78 ± 0.70, and 4.56 ± 0.56 shoots per explant for 0, 0.5, and 1.0 mg/L TDZ, respectively. These values were not significantly different from the TDZ-only treatments, indicating that adding BA (3.0 mg/L) did not markedly enhance the shoot formation beyond what the TDZ alone achieved.

Table 2.
Shoot induction on MS media supplemented with different concentrations of BA and TDZ for 4 weeks. Values are presented as means ± standard error (n ≥ 3). Means sharing the same letter(s) within a column do not differ significantly, based on Fisher’s Least Significant Difference (LSD) test with Holm’s correction for p-value adjustment at p ≤ 0.05. Asterisks denote statistically significant differences.
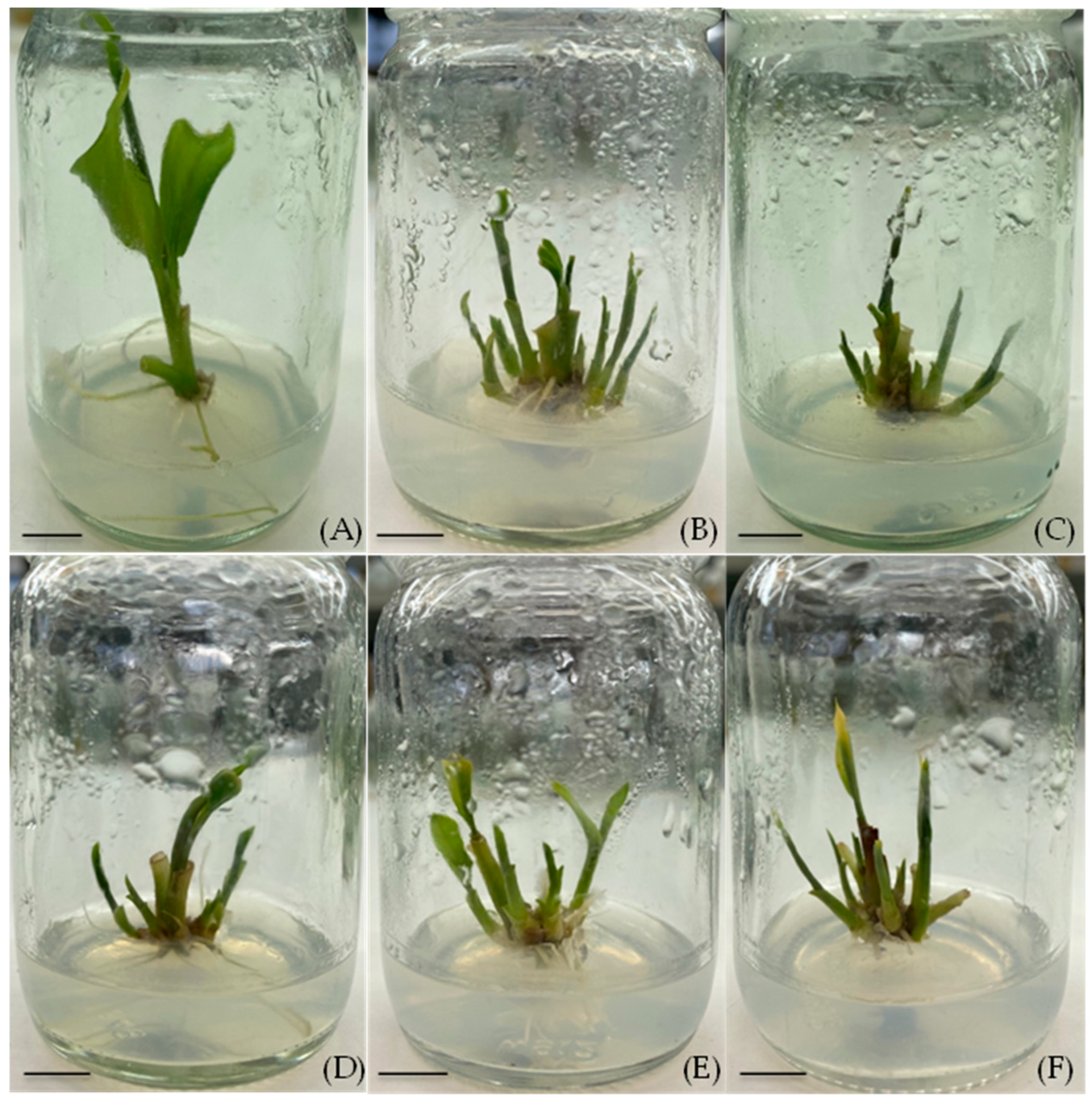
The presence of the TDZ had a notable effect on the shoot morphology (Figure 3). The hormone-free control produced the fewest and smallest shoots (only 1.00 ± 0.57 shoots of average lengths of 0.57 ± 0.37 cm). The TDZ alone tended to produce numerous but somewhat shorter shoots: at 0.5 mg/L TDZ, the shoots averaged 1.48 ± 0.23 cm in length, and at 1.0 mg/L TDZ, the shoots were 0.96 ± 0.13 cm tall on average. By contrast, the inclusion of the BA (3.0 mg/L) led to slightly taller shoots (around 1.21–1.53 cm) whether TDZ was present or not. For example, the BA alone (3.0 mg/L) yielded shoots 1.53 ± 0.17 cm long, and the BA + 0.5 mg/L TDZ produced shoots 1.52 ± 0.21 cm long. These shoots were longer than those on the TDZ-only medium. Overall, 0.5 mg/L TDZ emerged as the single most effective treatment for inducing a high number of shoots, though the BA-supported treatments produced slightly taller shoots.

Figure 3.
Shoot induction on media supplemented with different concentrations of BA and TDZ for 4 weeks: (A) MS medium without growth regulators (control), (B) TDZ 0.5 mg/L, (C) TDZ 1.0 mg/L, (D) BA 3.0 mg/L, (E) BA 3.0 mg/L + TDZ 0.5 mg/L, and (F) BA 3.0 mg/L + TDZ 1.0 mg/L, (scale bar = 1 cm).
3.3. Effect of mT on Shoot Induction
The mT proved to be highly effective in promoting shoot proliferation (Table 3 and Figure 4). All mT-supplemented treatments resulted in 100% shoot induction, whereas the control medium without growth regulators also had a high shoot induction (100%) but produced fewer shoots. The control (hormone-free) explants yielded 2.27 ± 0.38 shoots per explant on average. In contrast, even the lowest mT concentration (0.5 mg/L) produced significantly more shoots (4.20 ± 0.25). The number of shoots per explant increased with mT concentrations of up to 1.5 mg/L. The maximum induction was observed on the medium containing 1.5 mg/L mT, which produced 5.86 ± 0.67 shoots per explant. This was the highest shoot number recorded and was about 2.6 times the control. The medium with 2.0 mg/L mT also induced a high number of shoots (5.57 ± 0.61), not significantly different from the 1.5 mg/L mT treatment. Thus, the mT in the range of 0.5–2.0 mg/L greatly enhanced the shoot induction, with an optimum of around 1.5 mg/L for this cultivar.

Table 3.
Shoot induction on MS media supplemented with different concentrations of mT for 8 weeks. Values are presented as means ± standard error (n ≥ 7). Means sharing the same letter(s) within a column do not differ significantly, based on Fisher’s Least Significant Difference (LSD) test with Holm’s correction for p-value adjustment at p ≤ 0.05. Asterisks denote statistically significant differences.

Figure 4.
Shoot induction on solid media supplemented with different concentrations of mT for 4 weeks as follows: 0 (A), 0.5 (B), 1.0 (C), 1.5 (D), and 2.0 (E) mg/L, (scale bar = 1 cm).
Interestingly, the presence of the mT had an inverse effect on the shoot elongation. The longest shoots were observed on the control medium without cytokinin, where the shoots averaged 5.69 ± 0.79 cm in height. All mT-treated explants produced shorter shoots than the control. The shoot length decreased as the mT promoted the formation of more buds: for example, at the optimal 1.5 mg/L mT, the shoot length was 2.77 ± 0.24 cm on average, roughly half the height of the control shoots. Even at the lowest mT dose (0.5 mg/L), the shoot length was significantly reduced (3.05 ± 0.29 cm). Despite the reduction in the individual shoot height, the overall biomass (multiple shoots) increased due to the higher number of shoots.
3.4. Root Induction
Both the control (no auxin) and IBA-supplemented media induced high frequency of rooting in the excised turmeric shoots (Table 4). After one week on the rooting medium, 100% of the shoots formed roots in the control (0 auxin) as well as in all IBA treatments (1.0 and 2.0 mg/L IBA). In the absence of auxin, the shoots produced an average of 6.69 ± 0.57 roots per explant, indicating that some root initiation can occur spontaneously on an MS basal medium. The addition of 1.0 mg/L IBA resulted in fewer roots (4.40 ± 0.58) compared to the control, but these roots were significantly longer (4.13 ± 0.36 cm versus 3.01 ± 0.31 cm in the control). The highest number of roots was observed with 2.0 mg/L IBA, yielding 7.33 ± 1.49 roots per explant on average, which was slightly higher than the control (though not statistically different). The root length in the 2.0 mg/L IBA treatment (3.70 ± 0.46 cm) was intermediate between the control and the 1.0 mg/L IBA treatment.

Table 4.
Root induction on MS media supplemented with IBA and NAA for 1 week. Values are presented as means ± standard error (n ≥ 12). Means sharing the same letter(s) within a column do not differ significantly, based on Fisher’s Least Significant Difference (LSD) test with Holm’s correction for p-value adjustment at p ≤ 0.05. Asterisks denote statistically significant differences.
In sharp contrast, the media supplemented with NAA were much less effective at inducing roots. At 1.0 mg/L NAA, only 37.5% of the shoots produced any roots, and the few roots that did form were very short (0.09 ± 0.03 cm). Increasing the NAA to 2.0 mg/L further decreased the rooting frequency, to 28.6%, with an average of only 0.36 ± 0.17 roots per explant of a length of 0.11 ± 0.05 cm. These results demonstrate that IBA is a far more effective auxin than NAA for inducing adventitious roots in turmeric shoots. The IBA not only ensured a high rooting percentage (equal to the control) but also promoted the development of multiple relatively long roots. The NAA, on the other hand, severely inhibited the root initiation and growth at the concentrations tested.
3.5. Transplantation
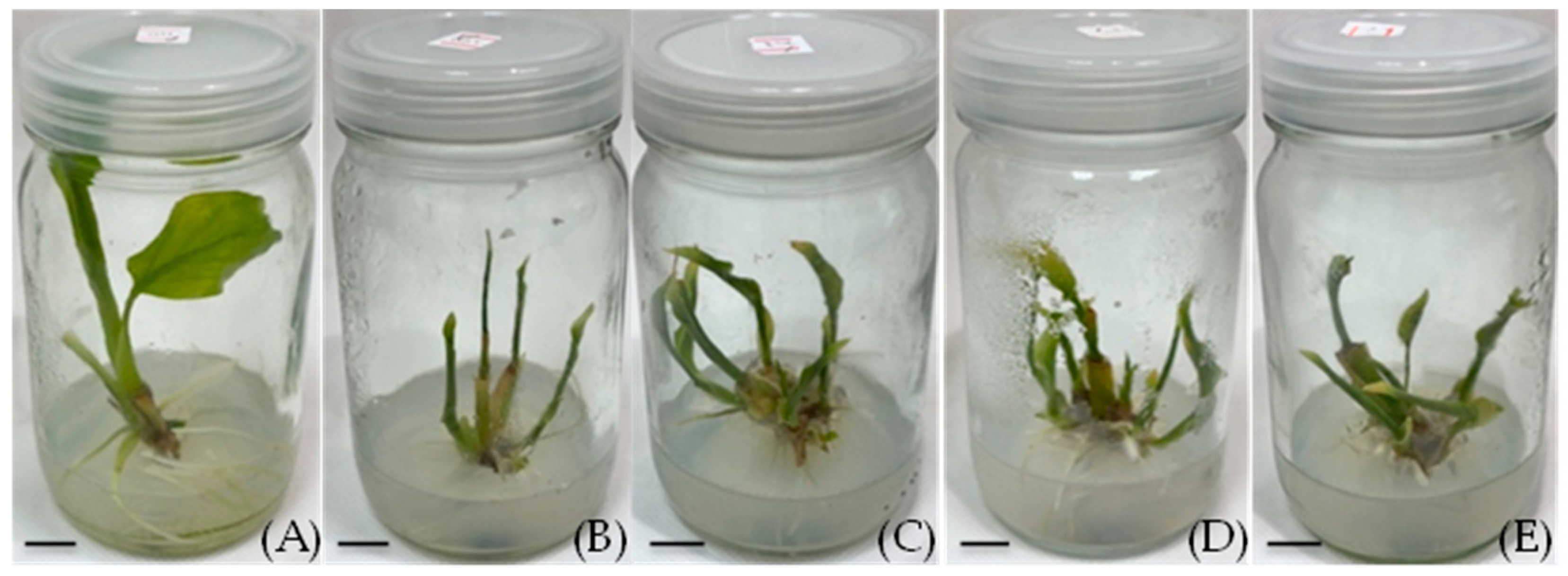
The type of potting medium significantly affected the survival of the transplanted plantlets after five weeks. The plantlets transferred to the sterile garden soil alone had a survival rate of only 26.67 ± 11.82%. In contrast, those transplanted to the 1:1 soil–peat moss mixture showed a dramatically higher survival rate of 86.67 ± 9.09%. These plantlets appeared healthy, with robust new growth (Figure 5G). The improvement in the survival with the peat moss was statistically significant compared to the soil alone. On the other hand, none of the plantlets survived in the soil + coconut husk (1:1) mixture (0% survival). The plantlets in the coconut husk mix quickly showed signs of stress, and the medium had problems with excessive moisture and fungal growth (Figure 5E). Overall, the soil + peat moss (1:1) substrate was the most effective for acclimatization, yielding a high survival rate and healthy plantlets.

Figure 5.
Acclimatization and transplantation: plantlets after washing off the agar medium (A), plantlets transplanted into various planting media (B), pots covered with plastic bags with holes punched and bags gradually opened (C,D), plantlets transplanted in soil mixed with coconut husk (E), plantlets transplanted in soil (F), and plantlets transplanted in soil mixed with peat moss (G) (scale bar = 1 cm).
4. Discussion
In this experiment, sprouted rhizome buds were shown to be suitable explants for initiating turmeric cultures, leading to healthy shoots. Firstly, shoot induction was achieved on an MS medium with 3.0 mg/L BA, which is consistent with findings from previous studies on turmeric and related species. BA is a well-established cytokinin known to stimulate cell division and promote the outgrowth of axillary buds. For instance, Kambaska and Santilata (2009) [5] reported that a medium containing 6.0 mg/L BA effectively induced prolific shoot formation in Aframomum corrorima, a member of the Zingiberaceae family. Similarly, Raihana et al. [16] found that BA was capable of inducing a high number of shoots in C. mangga. In some cases, the combination of BA with a low level of auxin (such as NAA) has been used to enhance simultaneous shoot initiation. For example, Jala (2012) [17] reported that an MS medium supplemented with 2.0–3.0 mg/L BA plus 1.0 mg/L NAA yielded the highest number of shoots in C. longa, a protocol similar to that used by Salvi et al. [4] for turmeric shoot culture. However, our results indicate that adding NAA did not improve the shoot proliferation in cv. Trang 1. In fact, the media containing NAA produced fewer shoots than BA alone. This observation aligns with the findings of Rahman et al. [18], who demonstrated that BA alone (2.0 mg/L) was sufficient to induce shoot formation in turmeric without the need for auxin. It is possible that exogenous auxins in the proliferation stage can disrupt the cytokinin/auxin balance or promote callus formation at the expense of shoot buds in some genotypes, thus explaining the inhibitory effect of NAA in our study.
TDZ, a phenylurea-derived plant growth regulator with strong cytokinin-like activity, has been reported to be very effective for shoot induction in many plant species. In the present study, TDZ at 0.5 mg/L (without BA) produced the highest number of shoots per explant, although all TDZ and/or BA treatments resulted in 100% shoot induction. These results are in line with reports from other species, where TDZ outperformed BA in inducing multiple shoots. Yorgancılar and Erişen (2011) [19] found that TDZ induced a greater number of shoots in Astragalus schizopterus compared to BA. Likewise, in Hedychium coronarium, TDZ was more effective than BA for shoot organogenesis [20]. The superiority of TDZ in promoting bud break and adventitious shoot formation is well-documented. TDZ is known to induce endogenous cytokinin responses and can release axillary buds from apical dominance. However, a common observation is that TDZ-induced shoots may be smaller or less physiologically developed than those induced by BA. In our study, we noted that while the TDZ alone gave the highest shoot numbers, the shoots were somewhat shorter, and the combination of BA with TDZ did not significantly increase the shoot numbers beyond the TDZ alone. In some species, combining TDZ with BA can have a synergistic effect on shoot induction in faba beans [21], but in turmeric cv. Trang 1, this combination offered no clear advantage. It appears that a low concentration of TDZ can effectively initiate multiple shoots, but subsequent transfer to a BA-containing medium (or hormone-free medium) may be beneficial for the elongation and development of those shoots, as BA generally supports better shoot growth and morphogenesis [19,20].
mT, an aromatic cytokinin, proved highly effective in our experiments, significantly improving both shoot proliferation and rooting in vitro compared to the control (no PGR). This finding is in agreement with other studies that have identified mT as a potent alternative to BA in tissue cultures. Gentile et al. [22] reported that mT efficiently promoted shoot regeneration in Prunus rootstocks and noted an associated improvement in root induction. Werbrouck et al. [23] similarly showed that mT stimulated better overall development (shoots and roots) than BA in several ornamental species, resulting in more robust and adaptable plantlets. The superior performance of mT over BA has been documented in a range of plants: for example, Behera et al. [24] achieved high-frequency shoot organogenesis in Hedychium coronarium using 3.0 mg/L mT, and Waman et al. [25] found mT to be the most effective cytokinin for shoot induction in C. mangga. The optimal concentration of mT can vary by species: for example, Elayaraja et al. [26] reported 6.21 μM mT to be the best for regenerating sesame (Sesamum indicum), whereas Wojtania (2010) [27] found that 0.5–1.0 mg/L mT maximized geranium shoot induction. In our case, 1.5 mg/L mT was optimal for the turmeric cv. Trang 1. A notable effect of the mT was the reduced shoot elongation accompanying the increased shoot number. Nonetheless, the use of mT in micropropagation is often advantageous because it tends to produce shoots that root more easily and show less tissue vitrification or hyperhydricity compared to BA-derived shoots [23,28]. Collectively, our results and previous studies highlight mT as a superior cytokinin for turmeric tissue cultures, particularly for obtaining a high proliferation rate coupled with strong potential for root development.
Our rooting experiments confirmed that IBA is more effective than NAA for inducing roots in turmeric shoots. The shoots readily formed roots on the control MS medium or with the added IBA, whereas the NAA drastically inhibited the rooting. The promotive effect of IBA on adventitious root formation is well-documented across many plant species. Tien et al. [29] reported that in stem cuttings of Solanum procumbens, IBA treatment produced more roots than either NAA or indole-3-acetic acid (IAA). Al-Saqri and Alderson (1996) [30] similarly found IBA to significantly enhance rooting in Rosa centifolia cuttings, yielding both a higher number of roots and greater root lengths compared to other auxins. Within Zingiberaceae, our findings are supported by earlier studies: Jualang et al. [31] observed that IBA was an efficient root-inducing agent for Etlingera coccinea, and Haque and Ghosh (2018) [32] reported that in Kaempferia angustifolia, IBA treatment resulted in superior rooting success than NAA, consistent with the findings of Tien et al. [29], who reported that IBA was superior to both NAA and IAA for root induction. Similarly, Al-Saqri and Alderson (1996) [30] observed that IBA significantly promoted root formation in R. centifolia, resulting in greater root numbers and lengths compared to other treatments. In turmeric micropropagations, IBA is frequently the auxin of choice for rooting because of its stability and effectiveness in inducing vigorous roots. NAA, on the other hand, can cause basal callusing and often results in fewer, weaker roots [32]. In our study, 2.0 mg/L IBA gave the highest root count (though statistically similar to the no-auxin control) and 1.0 mg/L IBA produced the longest roots. This suggests that a moderate IBA concentration can promote quality root development (longer roots) while a higher concentration will boost root number without reducing rooting frequency. The ability of turmeric shoots to root even on auxin-free media (as we saw in the control) may indicate the presence of sufficient endogenous auxins or the carry-over effect of cytokinin treatments. Nonetheless, the application of the IBA ensured uniform and earlier rooting, which is beneficial for synchronizing plantlet development prior to transfer ex vitro.
The choice of substrate greatly influenced the success of the acclimatization for the in vitro-derived plantlets. We found that mixing the peat moss with the soil improved the plantlet survival and growth dramatically. Peat moss is known to enhance substrate moisture retention and aeration while also providing some level of sterility and disease suppression. Our results are in line with Alqadasi et al. [33], who noted improved regeneration and growth of ginger plantlets when cultured in a soil–peat medium of appropriate pH. Faridah et al. [34] achieved efficient regeneration of Zingiber zerumbet and reported that incorporating organic components like peat into the potting mix supported better plantlet establishment. Yunus et al. [35] likewise observed a 93.3% survival rate for Etlingera elatior plantlets in a mixture of soil, peat moss, and sand, compared with only 24.4% survival in soil alone, highlighting the importance of a balanced medium. In our study, the plantlets in the plain soil likely faced higher evaporative stress, resulting in over 70% mortality. The soil + coconut husk mixture retained too much water and became quickly infested with fungi, completely killing the plantlets. The severe fungal contamination observed in that treatment underscores the need for good drainage and perhaps antifungal measures during acclimatization. By contrast, the soil + peat moss mix maintained a stable moisture level and good aeration, allowing the delicate turmeric plantlets to harden off gradually and develop new roots and shoots. These findings emphasize that optimizing post-culture growing conditions is as critical as the in vitro steps to ensuring the high survival and vigor of micropropagated plants.
5. Conclusions
We have developed a suitable micropropagation protocol for C. longa cv. Trang 1. For shoot induction, an MS medium containing 3.0 mg/L BA was the most effective, yielding a 100% induction rate and producing the highest number of shoots per explant as well as the tallest shoots. An MS medium with 0.5 mg/L TDZ also induced a high number of shoots, although TDZ tended to produce shorter shoots compared to BA. The use of mT significantly enhanced shoot proliferation relative to the control, with an optimal concentration of 1.5 mg/L mT yielding about 5–6 shoots per explant. For dedicated root induction, an MS medium supplemented with 2.0 mg/L IBA produced the greatest number of roots, while 1.0 mg/L IBA produced the longest roots; both concentrations were far superior to NAA in inducing healthy roots. Upon transfer to soil, the choice of potting substrate was critical. A 1:1 mixture of soil and peat moss provided the highest survival (86.67%) and supported the vigorous growth of the plantlets, whereas soil alone resulted in moderate survival and a soil–coconut husk mix was unsuitable. This micropropagation system will facilitate the mass propagation of this valuable cultivar, overcoming the limitations of traditional rhizome propagation and providing disease-free planting materials for growers and researchers.
Author Contributions
Conceptualization, A.B. and N.W.; data curation, N.W.; formal analysis, A.B., P.C., N.S., E.K. and N.W.; investigation, A.B. and N.W.; methodology, A.B., E.K. and N.W.; visualization, A.B., P.C., S.V. and N.W.; writing—original draft, A.B., S.V., N.S. and N.W.; writing—review and editing, S.V. and N.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by Faculty of Science, Kasetsart University, through the Graduate Fellowship Program.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We thank the International SciKU Branding (ISB), Faculty of Science, Kasetsart University, for their support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, S.; Yuan, W.; Deng, G.; Wang, P.; Yang, P.; Aggarwal, B.B. Chemical composition and product quality control of turmeric (Curcuma longa L.). Pharm. Crop. 2011, 5, 28–54. [Google Scholar] [CrossRef]
- Razavi, B.M.; Ghasemzadeh Rahbardar, M.; Hosseinzadeh, H. A review of therapeutic potentials of turmeric (Curcuma longa) and its active constituent, curcumin, on inflammatory disorders and pain, and their related patents. Phytother. Res. 2021, 35, 6489–6513. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, P.; Monisha, S.; Selvaraj, K.V.; Chitra, M.; Prabha, T.; Santhakumar, M.; Bharathi, A.; Velayutham, A. Nutritional value, phytochemistry, pharmacological and in vitro regeneration of turmeric (Curcuma longa L.): An updated review. Ann. Phytomed. 2022, 11, 236–246. [Google Scholar] [CrossRef]
- Salvi, N.D.; George, L.; Eapen, S. Micropropagation and field evaluation of micropropagated plants of turmeric. Plant Cell Tissue Organ Cult. 2002, 68, 143–151. [Google Scholar] [CrossRef]
- Kambaska, K.B.; Santilata, S. Effect of plant growth regulators on micropropagation of ginger (Zingiber officinale Rosc.) cvs. Suprava and Suruchi. J. Agric. Technol. 2009, 5, 271–280. [Google Scholar]
- Hagos, R.; Gebremdhin, H. Effects of cytokinin types and their concentration on in vitro shoot induction and multiplication of korarima (Aframomum corrorima). Int. J. Genet. Mol. Biol. 2015, 7, 8–14. [Google Scholar]
- Di, D.W.; Zhang, C.; Luo, P.; An, C.W.; Guo, G.Q. The biosynthesis of auxin: How many paths truly lead to IAA? Plant Growth Regul. 2016, 78, 275–285. [Google Scholar] [CrossRef]
- Lee, K.; Yoon, H.; Park, O.S.; Seo, P.J. A positive feedback loop of cytokinin signaling ensures efficient de novo shoot regeneration in Arabidopsis. New Phytol. 2025, 246, 18–27. [Google Scholar] [CrossRef]
- Astutik, M.; Suhartanto, B.; Umami, N.; Suseno, N.; Haq, M.S. Auxin and cytokinin effect on in vitro callus induction of maize (Zea mays L.) Srikandi Putih. In Proceedings of the 6th International Seminar of Animal Nutrition and Feed Science (ISANFS 2021), Yogyakarta, Indonesia, 7–8 July 2021; Atlantis Press: Dordrecht, The Netherlands, 2022; pp. 1–5. [Google Scholar]
- Chakraborty, A.; Santra, I.; Haque, S.M.; Ghosh, B. In vitro conservation of commercial and threatened members of Zingiberaceae: An Indian scenario. Biodivers. Conserv. 2023, 32, 2155–2195. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- R Core Team R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 21 April 2025).
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Kruskal, W.H.; Wallis, W.A. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Holm, S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 1979, 6, 65–70. [Google Scholar]
- Raihana, R.; Faridah, Q.Z.; Julia, A.A.; Abdelmageed, A.H.A.; Kadir, M. In vitro culture of Curcuma mangga from rhizome bud. J. Med. Plant Res. 2011, 5, 6418–6422. [Google Scholar] [CrossRef]
- Jala, A. Effects of NAA, BA and sucrose on shoot induction and rapid micropropagation by trimming shoot of Curcuma longa L. Thammasat Int. J. Sci. Technol. 2012, 17, 54–60. [Google Scholar]
- Rahman, M.; Amin, M.; Jahan, H.; Ahmed, R. In vitro regeneration of plantlets of Curcuma longa Linn.: A valuable spice plant in Bangladesh. Asian J. Plant Sci. 2004, 3, 306–309. [Google Scholar] [CrossRef]
- Yorgancılar, M.; Erişen, S. The effect of thidiazuron (TDZ) on shoot regeneration of Astragalus schizopterus. J. Anim. Plant Sci. 2011, 21, 519–524. [Google Scholar]
- Verma, M.; Bansal, Y.K. Effect of a potent cytokinin thidiazuron (TDZ) on in vitro regeneration of Hedychium coronarium J. Koenig, a valuable medicinal plant. Int. J. Rec. Biotechnol. 2014, 2, 38–44. [Google Scholar]
- Khalafalla, M.M.; Hattori, K. A combination of thidiazuron and benzyladenine promotes multiple shoot production from cotyledonary node explants of faba bean (Vicia faba L.). Plant Growth Regul. 1999, 27, 145–148. [Google Scholar] [CrossRef]
- Gentile, A.; Jaquez Gutiérrez, M.; Martinez, J.; Frattarelli, A.; Nota, P.; Caboni, E. Effect of meta-topolin on micropropagation and adventitious shoot regeneration in Prunus rootstocks. Plant Cell Tissue Organ Cult. 2014, 118, 373–381. [Google Scholar] [CrossRef]
- Werbrouck, S.P.; Strnad, M.; Van Onckelen, H.A.; Debergh, P.C. Meta-topolin, an alternative to benzyladenine in tissue culture? Physiol. Plant. 1996, 98, 291–297. [Google Scholar] [CrossRef]
- Behera, S.; Barik, D.P.; Naik, S.K. In vitro propagation and genetic fidelity assessment of Hedychium coronarium J. Koenig regenerated from axenic cotyledonary nodes on meta-topolin-supplemented medium. Ind. Crops Prod. 2019, 134, 206–215. [Google Scholar] [CrossRef]
- Waman, A.A.; Bohra, P.; Karthika Devi, R.; Pixy, J. In vitro multiplication protocol for Curcuma mangga: Studies on carbon, cytokinin source and explant size. J. Hortic. Sci. 2021, 16, 69–76. [Google Scholar] [CrossRef]
- Elayaraja, D.; Subramanyam, K.; Vasudevan, V.; Sathish, S.; Kasthurirengan, S.; Ganapathi, A.; Manickavasagam, M. Meta-topolin (mT) enhances the in vitro regeneration frequency of Sesamum indicum L. Biocatal. Agric. Biotechnol. 2019, 21, 101320. [Google Scholar]
- Wojtania, A. Effect of meta-topolin on in vitro propagation of Pelargonium × hortorum and Pelargonium × hederaefolium cultivars. Acta Soc. Bot. Pol. 2010, 79, 101–106. [Google Scholar] [CrossRef]
- Bairu, M.W.; Stirk, W.A.; Doležal, K.; Van Staden, J. Optimizing the micropropagation protocol for the endangered Aloe polyphylla: Can meta-topolin and its derivatives serve as replacements for benzyladenine and zeatin? Plant Cell Tissue Organ. Cult. 2007, 90, 15–23. [Google Scholar] [CrossRef]
- Tien, L.X.; Chac, L.X.; Oanh, L.T.; Ly, P.T.; Sau, H.V.; Hung, N.H.; Thanh, V.D.; Doudkin, R.; Thinh, B.D. Effect of auxins (IAA, IBA and NAA) on clonal propagation of Solanum procumbens stem cuttings. Plant Cell Biotechnol. Mol. Biol. 2020, 21, 113–120. [Google Scholar]
- Al-Saqri, F.; Alderson, P. Effects of IBA, cutting type and rooting media on the rooting of Rosa centifolia cuttings. J. Hortic. Sci. 1996, 71, 729–737. [Google Scholar] [CrossRef]
- Jualang, A.G.; Nurul Humairah, T.A.; Devina, D.; Hartinie, M. In vitro shoot regeneration from rhizome bud of native ginger in Borneo, Etlingera coccinea. J. Trop. Plant Physiol. 2015, 7, 36–46. [Google Scholar]
- Haque, S.M.; Ghosh, B. Micropropagation of Kaempferia angustifolia Roscoe—An aromatic, essential oil yielding, underutilized medicinal plant of Zingiberaceae. J. Crop Sci. Biotechnol. 2018, 21, 147–153. [Google Scholar] [CrossRef]
- Alqadasi, A.S.; Al-Madhagi, I.; Al-Kershy, A.; Al-Samaei, M. Effect of cytokinin type and pH level on regeneration of ginger in vitro. Int. J. Hortic. Sci. Technol. 2022, 9, 293–302. [Google Scholar]
- Faridah, Q.Z.; Abdelmageed, A.; Julia, A.A.; Hafizah, R.N. Efficient in vitro regeneration of Zingiber zerumbet Smith (a valuable medicinal plant) from rhizome bud explants. Afr. J. Biotechnol. 2011, 10, 9303–9308. [Google Scholar] [CrossRef]
- Yunus, M.F.; Abd Aziz, M.; Kadir, M.A.; Rashid, A.A. In vitro propagation of Etlingera elatior (Jack) (torch ginger). Sci. Hortic. 2012, 135, 145–150. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).