Abstract
Among the numerous diseases that affect papaya (Carica papaya L.) cultivation, anthracnose, caused by a complex of fungi from the genus Colletotrichum spp., stands out, primarily due to its damage to the commercial part of the papaya, the fruit, specifically the pulp. Although chemical control with synthetic molecules is the most commonly used method to combat anthracnose, it is not the most appropriate solution. The indiscriminate use of synthetic chemical products results in numerous harmful effects on the environment, the health of farmers, and the final consumers. Given these circumstances, the objective of this study was to analyze the efficacy of essential oils (EOs) from Citrus aurantium var. dulcis L., known as sweet orange, Citrus limon (L.), known as Sicilian lemon, and the major compound present in these oils, limonene, against the pathogens Colletotrichum okinawense, which cause anthracnose in papaya fruits. The percentage inhibition of mycelial growth was evaluated on the seventh day, with estimates of 50% and 90% inhibition, to compare the inhibitory effect among the fungal isolates. Chromatographic analysis revealed that sweet orange EO contains myrcene and limonene. Sicilian lemon essential oil includes myrcene, limonene, α- and β-pinene, and γ-terpinene. Both EOs and limonene exhibited activity against C. okinawense. The 50 µL/mL concentration was the most effective in inhibiting growth. The EOs and limonene showed similar IC50 values, with limonene at 48 µL/mL, Sicilian lemon EO at 51 µL/mL, and sweet orange EO at 57 µL/mL.
1. Introduction
Papaya (Carica papaya L.) is one of the most commercially important tropical fruits worldwide. According to the FAO (2023) [1], global papaya production reached approximately 13.8 million tons, with the prevalence concentrated in developing countries across Asia, Africa, and Latin America. India remained the world’s largest producer, accounting for more than 40% of total global output, followed by Indonesia, Nigeria, and Brazil. In the Americas, Brazil stood out as the leading producer, with over 1.1 million tons harvested in 2023, and it continues to be a major exporter of fresh papaya to Europe and North America. The cultivation of papaya presents significant socioeconomic potential for agriculture; however, its production and commercialization are impacted by abiotic and biotic factors that compromise fruit productivity and quality. When combined with suitable edaphoclimatic conditions, biotic factors promote the emergence of pathogens that hinder plant development and, consequently, affect fruit quality, leading to losses exceeding 15% during the commercialization phase [2]. A serious post-harvest disease in papaya is anthracnose, associated with fungi of the genus Colletotrichum spp.; this disease causes dark lesions on the fruit, making them visually unappealing and unsuitable for sale [3,4].
The management of post-harvest diseases in papaya, including anthracnose, a fungal disease associated with the complex of fungi from the genus Colletotrichum, is primarily based on chemical products such as fungicides [5,6]. However, concerns regarding human health risks, environmental damage, and the reduced efficacy of certain chemical products due to the emergence of resistant pathogen populations [7] and increasing consumer demand for environmentally friendly products have placed pressure on the production chain [8].
An alternative to synthetic chemical products in agriculture is using natural compounds that align with agroecological practices, such as essential oil-based products. These are less harmful to human health and the environment and represent a more economically viable option for farmers in the medium and long term [9]. The industrial production of citrus generates a large amount of waste, which contains high concentrations of EOs [10]. In the literature, some studies have reported that citrus essential oils exhibit various biological activities, including fungicidal activity [11]. Therefore, a potential use for citrus waste is extracting its essential oil to produce natural fungicides. Given this context, this study aimed to determine the antifungal activity of sweet orange and Sicilian lemon EOs and limonene, the major compound in these EOs, in the control of C. okinawense under in vitro conditions.
2. Materials and Methods
The experiment was conducted in the following laboratories: Phytochemistry and Catalysis Laboratory at the Federal Institute of Espírito Santo (IFES)—Alegre Campus; the Laboratory for Plant Breeding and Disease Resistance (LAMERP); the Agricultural and Environmental Biotechnology Laboratory (BIOTA); the Laboratory for Epidemiology and Diseases of Agricultural and Forest Plants (LEMP). These laboratories are part of the Center for Scientific and Technological Development in Phytosanitary Management of Pests and Diseases (NUDEMAFI) at the Center for Agricultural Sciences and Engineering of the Federal University of Espírito Santo (CCAE-UFES)—Alegre Campus.
The essential oils used in this study were obtained from commercial sources. The C. aurantium var. dulcis and C. limon EOs were acquired from Ferquima, batch 177, originating in Brazil. The limonene compound was purchased from Sigma-Aldrich, Shanghai, China.
2.1. Determination of the Chemical Composition of Essential Oils
The samples of sweet orange and Sicilian lemon essential oils were analyzed using gas chromatography with a flame ionization detector (GC-FID) on a Shimadzu GC-2010 Plus system, and gas chromatography coupled with mass spectrometry (GC-MS) on a Shimadzu QPMS-2010 system, following the methodology adapted by Dos Santos et al. [12].
The analyses were performed using fused silica capillary columns (30 m × 0.25 mm) with an Rtx®-5MS stationary phase (film thickness of 0.25 μm). Nitrogen was used as the carrier gas for the GC-FID analysis, while helium was used as the carrier gas for the GC-MS analysis, with a flow rate of 3.0 mL/min.
The oven temperature was programmed in a temperature ramp, starting at 40 °C for the first 3 min, followed by an increase of 3 °C/min until reaching 240 °C, which was maintained for 5 min. The injector temperature was set at 250 °C, and the detector temperature was maintained at 280 °C, with a split ratio of 1:30.
The identification of components was carried out by comparing their mass spectra with those in the Willey7, NIST05, NIST05s, NIST12, and NIST62 spectral libraries, as well as by calculating the retention index (RI). For the RI calculation, a homologous series of n-alkanes (C7 to C40) was used, and the calculated values for each compound were compared with values from the literature [13]. Components present in the essential oils with a relative area greater than 1% were identified.
2.2. Selection of Emulsifier and Stability Testing of Emulsions
To determine the most suitable emulsifier for stabilizing the essential oil emulsions, emulsions with volumes of 1 mL were prepared at concentrations of 50 and 100 µL/mL for each essential oil and limonene. The emulsifiers tested were Tween 80 (ethoxylated sorbitan monooleate), Span 80 (sorbitan monooleate), and Span 85 (sorbitan trioleate), all sourced from Sigma-Aldrich. Each set of samples—comprising the essential oils and the isolated compound combined with different emulsifiers—was subjected to varying ultrasonication times (20, 30, 40, 50, and 60 min) using an ultrasonic cleaner from Cristófoli operating at 42 kHz.
The emulsions that remained stable, i.e., those that did not separate into phases during the ultrasonication process, proceeded to a heat stress test. In this test, the samples were heated in a water bath at 70 °C for 50 min.
The emulsions that maintained stability after heating were subjected to a centrifugation stress test, where they were centrifuged at 6000 rpm for 10 min. Following these tests, the emulsions with the highest stability were selected for biological assays.
The emulsions deemed stable after varying the concentrations of essential oils and emulsifiers, ultrasonication times, heating, and centrifugation consisted of 50 µL/mL (5% v/v) of each essential oil or the isolated compound and 2% v/v Tween 80.
2.3. Preparation of Stock Solution
To ensure homogeneous distribution of emulsions in PDA culture medium, a stock solution was prepared. This solution contained 5% (v/v) essential oils or limonene, 2% (v/v) Tween 80, and sterile distilled water, resulting in a stock solution with an oil concentration of 50 µL/mL (5% v/v). From this stock solution, antifungal tests were performed using serial dilutions (v/v) incorporated into the PDA culture medium.
2.4. Conducting Antifungal Activity
2.4.1. Isolation of Fungal Strains
The fungal isolates used in this study were Colletotrichum okinawense (LM 117), provided by Professor Marcos Paz Saraiva Câmara from the Department of Agronomy at the Federal Rural University of Pernambuco (UFRPE), were previously identified.
2.4.2. In Vitro Test for Mycelial Growth Inhibition Using Essential Oils and Limonene
An in vitro test was conducted to evaluate the effect of the essential oils and the isolated compound on the mycelial growth of the pathogen. For this test, 9 cm diameter Petri dishes were prepared, containing C. okinawense mycelium disks cultivated on Potato Dextrose Agar (PDA) medium for seven days prior to the experiment. The cultures were maintained in a bacteriological incubator at 28 °C ± 2 °C in darkness to standardize the developmental stage of the isolate.
To prevent bacterial growth, a solution of amoxicillin (100 mg/mL) was added to the PDA medium at a temperature of 50–55 °C. For the mycelial growth test, aliquots of the stock solution of emulsified essential oils and limonene were added to the molten PDA medium and poured into 5.5 cm diameter Petri dishes at the following concentrations: 1 µL/mL, 4 µL/mL, 8 µL/mL, 12 µL/mL, 16 µL/mL, 20 µL/mL, 40 µL/mL, and 50 µL/mL. Each concentration was prepared in four replicates.
As a positive control, the commercial fungicide Amistar Top SC (manufactured by Syngenta, Maharashtra, India) containing strobilurin and triazole as active ingredients was used. This fungicide, registered for controlling anthracnose in papaya, was tested at a concentration of 0.6 µL/mL, following the manufacturer’s recommendation (60 mL of product per 100 L of water). A negative control was prepared using a 2% (v/v) Tween 80 solution in distilled water. Each concentration was tested in four replicates.
After the culture medium with the test concentrations solidified, a 0.5 cm disk of fungal colony grown on PDA for seven days was placed at the center of each plate. The plates were sealed with plastic film and incubated in a bacteriological incubator at 28 °C ± 2 °C in darkness for seven days.
On the seventh day, two diametrically opposite measurements of mycelial growth were taken using a digital caliper graduated in millimeters. The recorded values were used to calculate the percentage of mycelial growth inhibition for each concentration relative to the negative control.
The following formula proposed by Bastos [14] was used to calculate the percentage of mycelial growth inhibition (MGI):
where
: the percentage of mycelial growth inhibition;
: the diameter of mycelial growth in the control;
: the diameter of the mycelial growth in the treatments.
2.4.3. Mycelial Growth Inhibition Comparing the IC50 and IC90 of Treatments on C. okinawense
In vitro tests were conducted using the estimated inhibitory concentrations, IC50 and IC90, derived from scatter plots for each treatment (sweet orange essential oil, Sicilian lemon essential oil, and pure limonene), which inhibited the mycelial growth of C. okinawense by 50% and 90%, respectively.
The same methods for fungal isolate cultivation, inoculation, incubation, and evaluation used in the mycelial growth inhibition test with limonene on C. okinawense were adopted.
The treatments employed in this test included the estimated IC50 and IC90 concentrations of sweet orange oil, Sicilian lemon oil, and pure limonene. Positive (Amistar Top fungicide) and negative (PDA medium with Tween 80) controls were the same as those used in the mycelial growth inhibition test for C. okinawense. Each treatment was tested in four replicates.
2.4.4. Mode of Action of Essential Oils and Isolated Compound on C. okinawense
The fungistatic or fungicidal activity was evaluated by transferring PDA (Potato Dextrose Agar) medium disks containing fungal mycelia previously exposed to the most effective concentrations of the essential oils and pure limonene during the mycelial growth inhibition test. Disks of 0.5 cm were extracted from these colonies and transferred to new PDA culture media containing only an amoxicillin solution to inhibit bacterial growth. The same procedure was performed for the positive and negative control treatments. Subsequently, the presence or absence of mycelial growth was analyzed. Each treatment was tested in four replicates.
2.5. Statistical Analyses
The data on C. okinawense mycelial growth inhibition, relative to the different concentrations of essential oils, pure limonene, and fungicide, were subjected to analysis of variance (ANOVA) at a 5% probability level. Their means were grouped using Tukey’s test. Statistical analyses were performed using the R software, version 4.3.2.
3. Results and Discussion
3.1. Chemical Composition of Essential Oils
After the chemical analysis of sweet orange essential oil by gas chromatography with flame ionization detection (GC-FID) and gas chromatography–mass spectrometry (GC-MS), two compounds with relative areas exceeding 1.00% were identified. Limonene was the major compound, comprising 98.20% of the oil composition, followed by myrcene, with a relative area of 1.80%. The chromatographic profile of sweet orange essential oil is shown in Figure 1. Based on the chemical characterization of this essential oil, it can be concluded that the oil consists exclusively of hydrogenated monoterpenes.
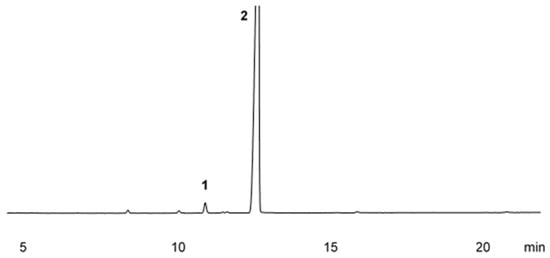
Figure 1.
Chromatographic profile of Citrus aurantium var. dulcis L. essential oil obtained by gas chromatography (GC-FID); Myrcene (1), Limonene (2).
Table 1 details each compound’s retention time, the calculated and tabulated retention indices (RIs), the names of the identified compounds, and their respective relative areas. Notably, the first retention peak occurred at 10.8 min, identifying myrcene, while the second peak occurred at 12.5 min, identifying limonene, the major compound in this oil, with a relative area of 98.20%.

Table 1.
Chemical characterization of Citrus aurantium var. dulcis L. essential oil based on the LTPRI index and gas chromatography–mass spectrometry (GC-MS).
According to Marriott, Shellie, and Cornwell [15], the primary composition of sweet orange essential oil includes the monoterpenes limonene (94%), α-pinene (0.54%), sabinene (0.74%), and myrcene (1.18%). These results differ from those obtained in the present study, identifying a higher concentration of limonene (98.20%) and myrcene (1.80%) in the sweet orange essential oil. However, such variations in the percentages of these two compounds between studies may be attributed to the genotype of the plant used for oil extraction, which varies by species. Furthermore, climatic parameters and agronomic factors, such as fertilization, altitude, the soil type, the harvesting season, the harvest date, and the distillation process, may also influence the composition and yield of the obtained oil, both qualitatively and quantitatively [16,17].
Five compounds with a relative area greater than 1% were identified in the analysis of Sicilian lemon essential oil. Limonene was the major compound, representing 72.35% of the oil’s composition, followed by β-pinene (14.79%), γ-terpinene (9.48%), α-pinene (1.85%), and myrcene (1.53%). The chromatographic profile of this essential oil can be observed in Figure 2.
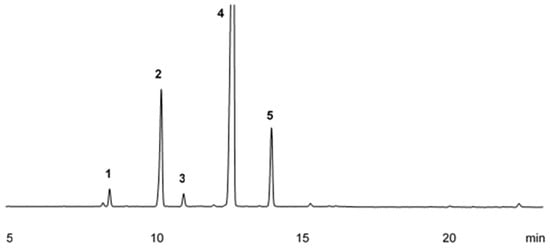
Figure 2.
Chromatographic profile of Citrus limon (L.) essential oil obtained by gas chromatography (GC-FID); α-Pinene (1), β-Pinene (2), Myrcene (3), Limonene (4), γ-Terpinene (5).
According to Noshad et al. [18], limonene was identified as the major compound in lemon essential oil. Similarly, Isvand, Karimaei, and Amini [19] also reported limonene as the predominant component in lemon oil.
The chemical characterization of Sicilian lemon essential oil is presented in Table 2, where it is possible to observe the retention time of each compound, the calculated and tabulated retention indices (RIs), the names of the identified compounds, and their respective relative areas. It can also be noted that, as in sweet orange essential oil, limonene appears as the major compound of this oil, with a relative area corresponding to 72.35% of the total composition. Furthermore, it can be confirmed that this essential oil consists exclusively of hydrogenated monoterpenes.

Table 2.
Chemical characterization of Citrus limon (L.) essential oil.
In the study conducted by Silva et al. [20] on the in vitro effect of plant compounds on C. gloeosporioides, it was also found that the major components of Sicilian lemon essential oil were limonene and citral. In addition to these compounds, other hydrogenated monoterpenes, oxygenated monoterpenes, hydrogenated sesquiterpenes, and oxygenated sesquiterpenes were identified. In other studies, Rozza et al. [21] and Ben Hsouna et al. [22], limonene and β-pinene were identified as the major constituents of Sicilian lemon essential oil.
In this study, it was possible to identify values related to the compounds present in Sicilian lemon essential oil, which corroborate the findings of Silva et al. [20], where limonene was shown to contribute the highest percentage to the composition of Sicilian lemon essential oil. It was further observed that limonene was the main contributor to their compositions in both essential oils, sweet orange and Sicilian lemon, which accounted for 98.20% (sweet orange) and 72.35% (Sicilian lemon), respectively. According to Jing et al. [23], sweet orange essential oils contain 83% to 97% limonene, while lemon essential oils contain 54% to 80%.
Fungicidal Activity of Sweet Orange and Sicilian Lemon Essential Oils on the Inhibition of Mycelial Growth in C. okinawense
For the tested concentrations of sweet orange essential oil, six groups of means for pathogen mycelial inhibition were formed (Table 3), as determined by Tukey’s test at a 5% probability level. The mean group “f” comprised the negative control, Tween 80, which showed no capacity to inhibit fungal growth. The mean group “ef”, represented by the concentration of 1.00 µL/mL of essential oil, inhibited an average of 15% of the mycelia and exhibited a standard deviation of 5% among the replicates. The mean group “de”, corresponding to the concentration of 4.00 µL/mL of essential oil, inhibited an average of 39% of the mycelia with a standard deviation of 6% among replicates. The mean group “cd”, represented by the concentration of 8.00 µL/mL of essential oil, inhibited an average of 52% of the mycelia and had a standard deviation of 11% among replicates. The concentrations of 12.00 µL/mL, 16.00 µL/mL, and 20.00 µL/mL did not differ significantly from each other and formed the mean group “bcd”, achieving an average inhibition of 66.6% of the mycelia. The group that demonstrated the highest mean mycelial inhibition included the positive control with the commercial product Amistar Top SC and the concentrations of 40.00 µL/mL and 50.00 µL/mL. All three treatments exhibited low standard deviations, indicating that the replicate data were close to the overall mean.

Table 3.
Inhibition of mycelial growth of C. okinawense with Citrus aurantium var. dulcis L. oil, Citrus limon (L.).
Applying different concentrations of Sicilian lemon essential oil, six groups of means were observed for the percentage of pathogen mycelial inhibition (Table 3). Again, the negative control (C(−)) showed no capacity to inhibit fungal growth and was classified in mean group “f” according to Tukey’s grouping test at a 5% probability level. The mean group “e”, represented by the concentration of 1.00 µL/mL of Sicilian lemon essential oil, responded similarly to the same concentration of sweet orange essential oil and inhibited an average of 15% of the fungal mycelia, with a standard deviation of 7% among replicates. The mean group “d”, represented by the concentration of 4.00 µL/mL of essential oil, inhibited an average of 53% of the mycelia with a standard deviation of 12% among replicates. The mean group “cd”, represented by the concentration of 8.00 µL/mL of Sicilian lemon essential oil, inhibited an average of 60% of the mycelia and exhibited a standard deviation of 8% among replicates. The concentrations of 12.00 µL/mL and 20.00 µL/mL did not differ significantly and formed the mean group “bc”, achieving an average inhibition of 68.5% of the mycelia. The concentration of 16.00 µL/mL and the positive control (C(+)), with doses of the commercial fungicide Amistar Top SC, did not exhibit statistically significant differences in their means, forming the group “b”, inhibiting approximately 73% of mycelial growth with a standard deviation of approximately 4.5%. Meanwhile, the group that demonstrated the highest means, “a”, for mycelial inhibition included the concentrations of 40.00 µL/mL and 50.00 µL/mL, showing greater inhibition capacity than the positive control (C(+)).
The different concentrations of the major compound, limonene, led to the formation of five groups of means for the percentage of inhibition of pathogen mycelial growth. This highlights the existence of distinct inhibitory responses (Table 3). According to Tukey’s grouping test at a 5% probability level, the negative control (C(−)) did not differ statistically from the treatment with a concentration of 1.00 µL/mL, both of which were classified as treatments that promoted the lowest mycelial inhibition averages.
The mean group “e”, represented by the concentration of 4.00 µL/mL of limonene essential oil, achieved an average pathogen inhibition of 46%, with a standard deviation of 2% among replicates. The treatment with a concentration of 8.00 µL/mL was assigned to the “cde” mean group and inhibited 58% of the fungal structures. The mean group “de”, represented by the concentration of 12.00 µL/mL of essential oil, inhibited an average of 66% of the mycelia with a standard deviation of 2% among replicates.
The group with the highest mycelial inhibition averages was composed of the following five treatments: the positive control (C(+)) with doses of the commercial product Amistar Top SC, and the concentrations of 16.00 µL/mL, 20.00 µL/mL, 40.00 µL/mL, and 50.00 µL/mL. These five treatments did not differ significantly, as determined by Tukey’s test at a 5% probability level.
Although the concentrations of 40.00 µL/mL and 50.00 µL/mL in the sweet orange essential oil treatment were statistically grouped with the positive control (C(+)), it was observed that these two concentrations were well defined and provided an average inhibition of 89% of mycelial growth, as shown in Figure 3.
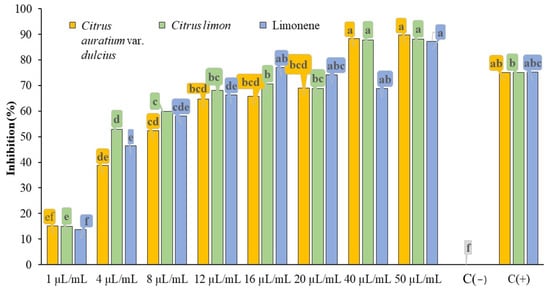
Figure 3.
Inhibition of mycelial growth of C. okinawense with Citrus aurantium var. dulcis L. oil, Citrus limon (L.) oil, and the compound limonene in an in vitro test.
In the treatments containing concentrations of 40.00 µL/mL and 50.00 µL/mL of Sicilian lemon essential oil, these concentrations stood out from the others (Figure 3), exhibiting an inhibitory capacity of approximately 88% of mycelial growth, with an average standard deviation of 0.5%. Despite all of these treatments presenting the same pathogen inhibition response, it was further observed that the treatment with a concentration of 50.00 µL/mL of limonene was well established and defined within the group with the highest inhibition averages (Figure 3), achieving an average of 87% mycelial inhibition, with a standard deviation of 4% among replicates.
Means followed by the same letter do not differ significantly, as determined by Tukey’s test (p < 0.05).
Studies on sweet orange essential oil have demonstrated its effectiveness in controlling plant fungal diseases. For instance, Velázquez-Nuñez et al. [24] identified the antifungal properties of essential oil obtained from the peel of sweet orange (Citrus sinensis var. Valencia) against the pathogen Aspergillus flavus. The main constituents identified were limonene, β-pinene, β-myrcene, α-pinene, and citral. Research by Gomes et al. [25] using essential oil extracted from orange peel (Citrus sinensis) at a concentration of 2000 μg/mL observed inhibitory effects on the growth of Fusarium oxysporum and Alternaria alternata, achieving inhibitory growth rates of 60% and 51%, respectively. These findings suggest that essential oils from oranges can effectively control phytopathogenic fungi responsible for plant diseases.
Limonene exhibits notable antifungal activity [26,27]. In vitro assays evaluating 18 essential oils against major phytopathogenic fungi affecting fruits revealed that 3 mL/L of limonene was sufficient to achieve complete (100%) mycelial inhibition of the primary pathogens [28].
Studies have demonstrated that limonene compromises the integrity of fungal cell membranes by increasing their fluidity and permeability, thereby disrupting cellular homeostasis and enhancing fungal susceptibility to its antifungal activity [29,30]. These findings are consistent with the results of this study, in which essential oils and the isolated compound demonstrated efficacy in controlling the development of the tested fungal. Studies by Bicas and Pastore [31] investigated the inhibitory action of limonene on the development of certain microorganisms isolated from various inoculum sources, including five fungal species. They observed that limonene was ineffective in inhibiting all fungal isolates’ growth. The inhibitory concentrations capable of inhibiting 50% (IC50) and 90% (IC90) of mycelial growth were determined to be 6.54 µL/mL and 50.88 µL/mL, respectively, for sweet orange essential oil (Figure 4). Using citrus essential oils from industrial residues exemplifies an approach aligned with circular economy (CE) principles. Instead of being discarded, citrus processing by-products, such as peels and seeds, are valorized through essential oil extraction, promoting resource efficiency and reducing environmental impacts [11]. In this study, the antifungal activity of sweet orange and Sicilian lemon essential oils against Colletotrichum okinawense demonstrates the potential of waste-derived products to replace synthetic fungicides, contributing to more sustainable agricultural practices. Adding value to agro-industrial residues supports waste minimization and strengthens agroecological management systems, in line with CE goals [11].
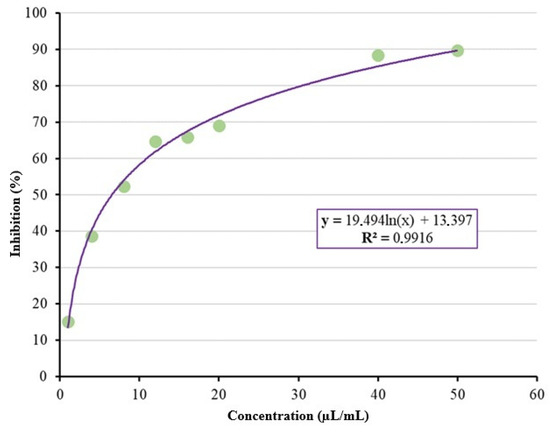
Figure 4.
Estimation of IC50 and IC90 for Citrus aurantium var. dulcis L. oil.
The inhibitory concentrations capable of inhibiting 50% (IC50) and 90% (IC90) of mycelial growth were 5.50 µL/mL and 45.05 µL/mL of Sicilian lemon EO, respectively, as shown in Figure 5.
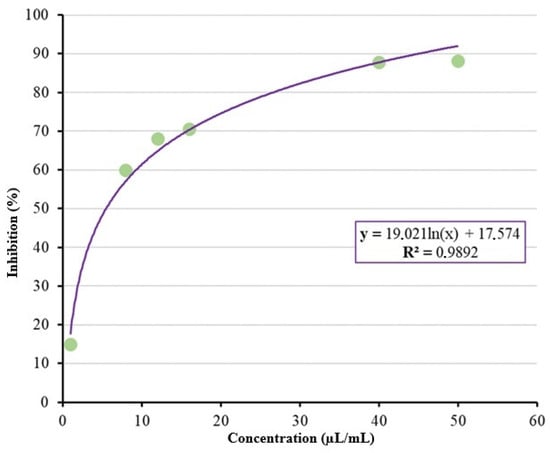
Figure 5.
Estimation of IC50 and IC90 for Citrus limon (L.) essential oil.
The inhibitory concentrations capable of inhibiting 50% (IC50) and 90% (IC90) of mycelial growth were 5.68 µL/mL and 47.61 µL/mL of the limonene compound, respectively, as shown in Figure 6.
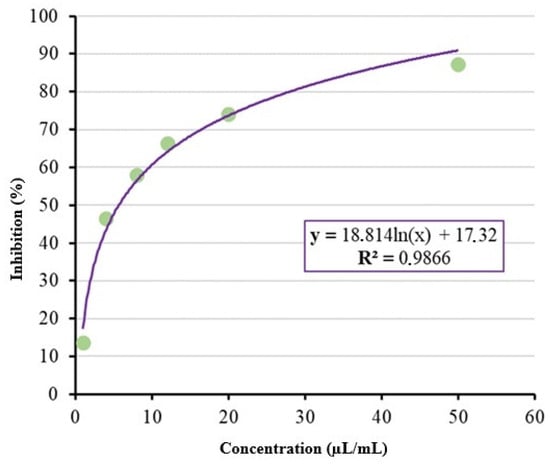
Figure 6.
Estimation of IC50 and IC90 for the limonene compound.
4. Conclusions
The 50 µL/mL concentration for sweet orange essential oil, Sicilian lemon essential oil, and pure limonene presented the highest mean inhibition for the fungal isolate C. okinawense. EO from Sicilian lemon achieved the best result from this tree treatment. This result is likely related to more chemical compounds (α-pinene, β-pinene, and γ-terpinene) in Sicilian lemon essential oil than sweet orange essential oil and the limonene compound. This study highlights the potential use of these essential oils—sweet orange and Sicilian lemon—for managing anthracnose in papaya fruits caused by C. okinawense. Therefore, citrus essential oils can be considered a viable alternative to synthetic chemical products for controlling anthracnose, both in pre-harvest and post-harvest stages of papaya production.
Author Contributions
Conceptualization, C.R.d.O.M.; methodology, M.F.C.S.; software and validation, A.N.V.; formal analysis, investigation, and resources, M.P.S.C.; data curation and writing—original draft preparation, L.M.; writing—review and editing, visualization, and supervision, C.d.S.B.; project administration, L.A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- FAO. Major Tropical Fruits Market Review—Preliminary Results 2023; Food and Agriculture Organization of the United Nations: Rome, Italy, 2023; Available online: https://openknowledge.fao.org/handle/20.500.14283/cb9621en (accessed on 30 April 2025).
- Machado, R.M.A.; Dias, V.M.; de Souza, C.L.M.; da Silva, L.B.; Freire, M.D.G.M. Avaliação de óleos essenciais sobre o crescimento in vitro do fungo Colletotrichum gloeosporioides. Biol. Saude 2013, 3, 64–75. [Google Scholar]
- Tan, G.H.; Ali, A.; Siddiqui, Y. Major fungal postharvest diseases of papaya: Current and prospective diagnosis methods. Crop Prot. 2023, 174, 106399. [Google Scholar] [CrossRef]
- Zakaria, L. Diversity of Colletotrichum species associated with anthracnose disease in tropical fruit crops—A review. Agriculture 2021, 11, 297. [Google Scholar] [CrossRef]
- Bautista-Banõs, S.; Sivakumar, D.; Bello-Pérez, A.; Villanueva-Arce, R.; Hernández-López, M. A review of the management alternatives for controlling fungi on papaya fruit during the postharvest supply chain. Crop Prot. 2013, 49, 8–20. [Google Scholar] [CrossRef]
- Santos, L.A.D.L.; Silva, H.S.A.; Pinheiro, L.R.B.; Rocha, L.S.; Bragança, C.A.D.; Silva, H.S.A. Biocontrole da antracnose em frutos de mamoeiro por bactérias epifíticas formadoras de biofilme. Summa Phytopathol. 2021, 47, 45–53. [Google Scholar] [CrossRef]
- Casemiro, J.C.L.; Bacchi, L.M.A.; Reis, H.F.; Gavassoni, W.L. Chitosan associated with plant extracts in the post-harvest con-trol of anthracnose in papaya ‘formosa’. Summa Phytopathol. 2019, 45, 64–69. [Google Scholar] [CrossRef]
- Fornazier, M.J.; Martins, D.S.; Ventura, J.A.; Zanuncio Júnior, J.S.; Costa, H. Agrotóxicos e contaminação de ali-mentos. Incaper Rev. Vitoria 2017, 8, 17–31. [Google Scholar]
- Dantas, A.M.M.; Nascimento, S.R.D.C.; Cruz, B.L.S.D.; Silva, F.H.A.D.; Ambrósio, M.M.D.Q.; Senhor, R.F. Alternative control of post-harvest diseases in Tainung 1 papaya1. Pesqui. Agropecu. Trop. 2018, 48, 29–35. [Google Scholar] [CrossRef]
- Zema, D.A.; Calabrò, P.S.; Folino, A.; Tamburino, V.I.; Zappia, G.; Zimbone, S.M. Valorisation of citrus processing waste: A review. Waste Manag. 2018, 80, 252–273. [Google Scholar] [CrossRef]
- BRAH, A.S.; Armah, F.A.; Obuah, C.; Akwetey, S.A.; Adokoh, C.K. Toxicity and therapeutic applications of citrus essential oils (CEOs): A review. Int. J. Food Prop. 2023, 26, 301–326. [Google Scholar] [CrossRef]
- Dos Santos, A.T.B.; Junior, J.S.Z.; Parreira, L.A.; De Abreu, K.M.P.; De Oliveira Bernardes, C.; De Carvalho, J.R.; Menini, L. Chemical identification and insecticidal effect of Tephrosia vogelii essential oil against Cerosipha forbesi in strawberry crop. Crop Prot. 2021, 139, 26. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Bastos, C.N. Efeito do Óleo de Piper aduncum Sobre Crinipellis perniciosae e Outros Fungos Fitopatogênicos. Fitopatol. Bras. 1997, 22, 441–443. [Google Scholar]
- Marriott, P.J.; Shellie, R.; Cornwell, C. Gas Chromatographic Technologies for the Analysis of Essential Oils. J. Chromatogr. A 2001, 936, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Kerrola, K.; Galambosi, B.; Kallio, H. Volatile Components and Odor Intensity of Four Phenotypes of Hyssop (Hyssopus officinalis L.). J. Agric. Food Chem. 1994, 42, 776–781. [Google Scholar] [CrossRef]
- Borsato, A.V.; Doni-Filho, L.; Côcco, L.C.; Paglia, E.C. Yield and Chemical Composition of Essential Oil of the Chamomile [Chamomilla recutita (L.) Raeuchert] Extracted for Steam Distillation. Semin. Cienc. Agrar. 2008, 29, 129–136. [Google Scholar] [CrossRef]
- Noshad, M.; Alizadeh Behbahani, B.; Jooyandeh, H.; Rahmati-Joneidabad, M.; Hemmati Kaykha, M.E.; Ghodsi Sheikhjan, M. Utilization of Plantago major Seed Mucilage Containing Citrus limon Essential Oil as an Edible Coating to Improve Shelf-Life of Buffalo Meat Under Refrigeration Conditions. Food Sci. Nutr. 2021, 9, 1625–1639. [Google Scholar] [CrossRef]
- Isvand, A.; Karimaei, S.; Amini, M. Assessment of Chitosan Coating Enriched with Citrus limon Essential Oil on the Quality Characteristics and Shelf Life of Beef Meat During Cold Storage. Int. J. Food Microbiol. 2024, 423, 110825. [Google Scholar] [CrossRef]
- Silva, A.C.D.; Sales, N.D.L.P.; Araújo, A.V.D.; Caldeira Júnior, C.F. Efeito in vitro de Compostos de Plantas Sobre o Fungo Colletotrichum gloeosporioides Penz: Isolado do Maracujazeiro. Cienc. Agrotecnologia 2009, 33, 1853–1860. [Google Scholar] [CrossRef]
- Rozza, A.L.; Moraes, T.M.; Kushima, H.; Tanimoto, A.; Marques, M.O.M.; Bauab, T.M.; Hiruma-Lima, C.A.; Pellizon, C.H. Gastroprotective Mechanisms of Citrus limon (Rutaceae) Essential Oil and Its Majority Compounds Limonene and β-Pinene: Involvement of Heat-Shock Protein-70, Vasoactive Intestinal Peptide, Glutathione, Sulfhydryl Compounds, Nitric Oxide and Prostaglandin E2. Chem. Biol. Interact. 2011, 189, 82–89. [Google Scholar] [CrossRef]
- Ben Hsouna, A.; Ben Halima, N.; Smaoui, S.; Hamdi, N. Citrus limon Essential Oil: Chemical Composition, Antioxidant and Antimicrobial Activities with Its Preservative Effect Against Listeria monocytogenes Inoculated in Minced Beef Meat. Lipids Health Dis. 2017, 16, 146. [Google Scholar] [CrossRef]
- Jing, L.; Lei, Z.; Li, L.; Xie, R.; Xi, W.; Guan, Y.; Zhou, Z. Antifungal Activity of Citrus Essential Oils. J. Agric. Food Chem. 2014, 62, 3011–3033. [Google Scholar] [CrossRef] [PubMed]
- Velázquez-Nuñez, M.J.; Avila-Sosa, R.; Palou, E.; López-Malo, A. Antifungal Activity of Orange (Citrus sinensis var. Valencia) Peel Essential Oil Applied by Direct Addition or Vapor Contact. Food Control 2013, 31, 1–4. [Google Scholar] [CrossRef]
- Gomes, M.S.; Cardoso, M.G.; Andrade, J.; Miranda, C.A.S.F.; Machado, S.F.; Passos, L.O. Citrus sinensis: Caracterização Química e Atividade Biológica Frente a Dois Fungos Fitopatogênicos. In Proceedings of the 34th Annual Meeting of the Brazilian Chemical Society, São Paulo, Brazil, 4 July 2011. [Google Scholar]
- Sharma, N.; Tripathi, A. Effects of Citrus sinensis (L.) Osbeck Epicarp Essential Oil on Growth and Morphogenesis of Aspergillus niger (L.) Van Tieghem. Microbiol. Res. 2008, 163, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.A. Natural Fungicides Obtained from Plants. In Fungicides for Plant and Animal Diseases; IntechOpen: London, UK, 2012. [Google Scholar]
- Combrinck, S.; Regnier, T.; Kamatou, G.P.P. In Vitro Activity of Eighteen Essential Oils and Some Major Components Against Common Postharvest Fungal Pathogens of Fruit. Ind. Crops Prod. 2011, 33, 344–349. [Google Scholar] [CrossRef]
- Onken, J.; Berger, R.G. Effects of R-(+)-Limonene on Submerged Cultures of the Terpene Transforming Basidiomycete Pleurotus sapidus. J. Biotechnol. 1999, 69, 163–168. [Google Scholar] [CrossRef]
- Kumar, A.; Kudachikar, V.B. Antifungal Properties of Essential Oils Against Anthracnose Disease: A Critical Appraisal. J. Plant Dis. Prot. 2018, 125, 133–144. [Google Scholar] [CrossRef]
- Bicas, J.L.; Pastore, G.M. Isolation and Screening of d-Limonene-Resistant Microorganisms. Braz. J. Microbiol. 2007, 38, 563–567. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).