Abstract
This study examined the bioproductivity of two poplar genotypes propagated by single-tree stem cuttings. The experiment compared variants using cuttings of different lengths (10–22 cm) and containers with volumes from 1 to 3 L. It was found that the best growth performance of seedlings in height according to the traditional container technology (70.6 ± 5.5–111.5 ± 5.0 cm) was observed in the intersectional hybrid of poplar ‘E.s.-38’ (genotype 1). The predominance of the genotype factor over the technology of rooting cuttings was established. The fast-growing genotype 1, E.s.-38, had higher productivity and plant height indices, suggesting it as a variety that can allow for the growth of standard planting material in containers in one season. For genotype 1, the length of cuttings was 10–14 cm when the container volume was increased to 3 L, which could increase the number of cuttings from one mother plant by 2–3 times. The revealed correlations between the height of the seedling and the diameter of the increment, as well as the analysis of the proportions of plant organs, showed that biological features of the rooting of stem cuttings depended on the genotype of poplars. The natural type G2 was characterized by the prevalence of root system growth over the growth of other organs; in the case of short cuttings, the proportion of leaves increased in plants to enhance photosynthesis and ensure rhizogenesis.
Keywords:
poplar; genotype; rhizogenesis; planting material; closed root system; biomass; pot-in-pot 1. Introduction
Forests play a critical role in climate regulation, covering 31% of the Earth’s land surface (4.06 billion hectares) and absorbing up to 2 billion tonnes of carbon dioxide annually. Additionally, wood provides up to 40% of renewable energy, reducing reliance on fossil fuels [1]. However, over the past 60 years, the global forest area has decreased by 81.7 million hectares, with a 60% reduction in forest area per capita [2]. Between 1990 and 2020 alone, 420 million hectares were lost to deforestation, with an annual loss of 10 million hectares between 2015 and 2020 [3]. Deforestation contributes to natural disasters and economic losses, with climate change potentially reducing global income by 19% by 2049 [4].
Climate change impacts, such as heatwaves, droughts, mild winters, floods, and reduced snow cover, negatively affect tree growth, forest health, and ecosystems [5,6,7,8]. These challenges necessitate innovative approaches to reforestation. Currently, three main methods are employed: natural regeneration, artificial regeneration, and a combined approach. Artificial regeneration, particularly through planting seedlings or sowing seeds, has gained prominence due to its efficiency [9]. Reforestation requires standardized planting material with good hereditary properties, making the optimization of seedling production in containers a critical task [10,11,12].
Poplar, a fast-growing and adaptive species that plays an important role in the context of global climate change [13] and is of great value for reforestation and bioenergy projects [14]. Modern clonal reproduction system (CRS) technologies significantly reduce the time required for forest crop formation, benefiting both forestry and the wood industry [15]. Optimizing parameters such as container volume and cuttings size can enhance seedling production efficiency and economic profitability [16]. Additionally, studying genotypic diversity in poplar opens opportunities for breeding programs to develop more resistant and productive varieties [17].
Populus species, members of the Salicaceae family, are among the fastest-growing trees in temperate and boreal zones. They are widely used for plantation cultivation, land restoration, and bioenergy production [18,19,20,21]. Poplar’s high biodiversity and adaptability to various environmental conditions make it a key species for agroforestry programs in Europe, North America, and the Russian Federation [22,23,24,25,26,27,28]. With 22 to 45 species and hybrids, poplar’s genetic diversity is a valuable resource for breeding and silvicultural production [29,30].
P. heterophylla and P. balsamifera subsp. trichocarpa (swamp poplars) are part of the Leucoides group. P. trichocarpa has a vast distribution, ranging from Alaska to California, while poplars (Populus spp.) are globally distributed, including in Europe, Asia, Africa, and South America [31,32]. In countries such as Canada, Sweden, Finland, and Norway over 90% of forest crops are planted using CRS seedlings [33]. High-tech containerized seedling production methods improve plant establishment and growth rates [34]. Key factors when choosing a container include volume, fertilizer [35], watering, material and shape, which affect the root structure. [36]. For example, tall pots suit species with taproots, while shallow, wide pots are better for fibrous root systems. Container volume must align with the growth rate of the species to avoid excessive root mass development at the expense of crown growth [37].
Studies have examined the issues of growing poplar plants in containers; for example, the influence of container depth and substrate heterogeneity on the growth of nodal seedlings of Populus balsamifera L. × Populus nigra L. (Populus sibirica) was established [38]. Studies in the field and in nurseries have shown that the root volume and survival rate of Pinus pinea L. seedlings increase with increasing container height and diameter [39]. The use of a larger-capacity container for planting Larix kaempferi was tested [40], and the variability indices of pine seedling morphological traits were determined depending on the container type [41].
The purpose of this study was to comprehensively analyse the effect of container volume and cuttings size on the growth parameters of poplar seedlings with closed root system grown on the basis of two different genotypes. The study involved a thorough evaluation of the interaction of these factors and their impact on key growth parameters such as vegetative development rate, root system formation and structure, and overall seedling survival rate. The results obtained will be of practical importance to foresters and nursery professionals, providing empirical evidence for optimizing growing processes and improving the efficiency of the production of quality planting material.
Thus, an in-depth study of this topic not only contributes to scientific progress in botany and forestry but also has practical applications that can have a positive impact on environmental programmes and the economics of the forestry sector.
2. Materials and Methods
2.1. Objects of Research and Methodology of Experimentation
The objects of the study were poplar genotypes differing in growth activity and root formation intensity in stem cuttings:
Genotype 1, designated as ‘E.s.-38’ (Elite Seedling-38), is an intersectional poplar hybrid created by crossing the species Populus deltoides Marsh, Populus balsamifera L., and the complex Populus alba L. + Populus tremula L. This hybrid was bred by Professor M. M. Veresin (Professor Breeder at Voronezh Forestry University) and demonstrates a high capacity for vegetative propagation by means of winter cuttings. It is characterised by intensive growth rates and increased winter hardiness, which is especially evident in the conditions of the forest-steppe zone, where its winter hardiness is superior to that of a number of Euro-American poplars.
Genotype 2 represents black poplar (Populus nigra L.), which is a native species for the forest-steppe zone of European Russia. This species is characterised by the formation of a spreading crown and predominantly grows on well-moistened floodplains. At maturity, plants of this species develop a powerful root system, including both superficial and deeply penetrating anchor roots [42].
Propagation of the genotypes was carried out by planting stem cuttings. Each cuttings contained at least four buds, and the average diameter at the top cut level was 1 cm. The cuttings were prepared from annual shoots in early spring on the floodplain areas of the Khopyorsky Reserve in the Voronezh Region. The cut whips were stored in a snowdrift in uncut form to prevent their desiccation.
The experiment plan included assessment of the factors influence, such as genotype, cuttings length, container volume, and their combinations, on the biometric parameters of the resulting poplar plants (planting material). Four cuttings, three container sizes, and two genotypes were factors in experiment. For the factor of cuttings length, 10 cm, 14 cm, 18 cm, and 22 cm stem cuttings were used. For the factor of container volume, 1 L (container height of 10 cm and diameter of 13 cm), 2 L (height of 19 cm and diameter of 12 cm), and 3 L (height of 23 cm and diameter of 13.5 cm) plastic containers were used. Two genotypes were used in the experiment.
The substrate used was a mixture of neutralised peat (Russia) and perlite in the ratio of 3:1. There were 40 plants in each experimental group in 3 repetitions.
In all variants of the experiment, 4 types of mineral fertilizers were successively added, and the application rate per 1 L of substrate was 5 g. In the spring, Fertika (JSC Fertika, Moscow, Russia), with an NPK content of 12:8:14, was used; a month later, Osmocote Pro was used (ICL, Amsterdam, Holland) (NPK—17:11:10 + 2 MgO); after 3 months, Fertika Universal-1 fertilizer (JSC Fertika, Moscow, Russia), with an NPK content of 4.8:20.8:31.3, was used; after 5 months, Autumn (JSC Fertika, Moscow, Russia; containing P2O5—5%, K2O—18%, CaO—8%, MgO—2.5%, S—12%) was used. All used fertilizers were added in the form of granules in the middle of the root zone, with a uniform distribution along the entire perimeter of the container.
Seedling height was measured using a measuring tape, and stem diameter was determined with a calliper at the base of the growth. In addition, the number of leaves per plant was counted, and their area was estimated using a portable laser leaf area meter CI-202 (CID Bio-Science, Camas, WA, USA).
At the end of the experiment, the wet and dry weights of leaves, shoots, and roots were measured with the Ohaus electronic laboratory scale (Parsippany, NJ, USA), with an accuracy of 0.01 g. To determine the dry weight of plant organs, they were dried in an RS422 Binder desiccator (Germany) to absolute dryness at 102 °C.
The biological productivity of plants was determined by the total dry mass of leaves, stems, and roots and using the productivity coefficient of the plant assimilation apparatus [43,44].
The site was prepared as follows: turf was removed, digging was carried out, levelling was done with a 2–5° slope, and then a 10–15 cm layer of coarse river sand was applied. The surface was covered with black spunbond of 60 g/m2 density with a UV stabilisation of 4%, which served to mulch the soil and prevent weed germination. The spunbond was secured with metal staples 40–50 cm in length. Irrigation was carried out by sprinkler irrigation using an oscillating sprinkler, and the irrigation regimen was controlled by a programmable electronic timer. Watering was carried out once a day in the early morning hours before sunrise for 60 min; under intense meteorological factors in the summer period, the frequency of watering was increased to two times in the morning and evening hours before and after sunset. For uniformity of watering, the sprinkler system was moved along the containers once a month.
2.2. Statistical Analysis
Specialized software Statistica version 10.0 was used for statistical processing of the obtained data.
The reliability of the differences between the samples according to the main biometric parameters of plants (height of plants and diameter of plants) was assessed using the student’s t-test. In order to establish the influence of factors, such as genotype, container volume, and cuttings length, on the main biometric parameters of plants, one-way analysis of variance was used.
The multiway analysis of variance (ANOVA) was used to assess the significance of the influence of the considered combination factors on the studied plants’ parameters.
Correlation analysis was used to establish the relationships and patterns between the variables under study.
3. Results
3.1. Effect of Cuttings Size, Container Volume, and Genotype on the Height of Annual Seedlings Obtained by Cuttings
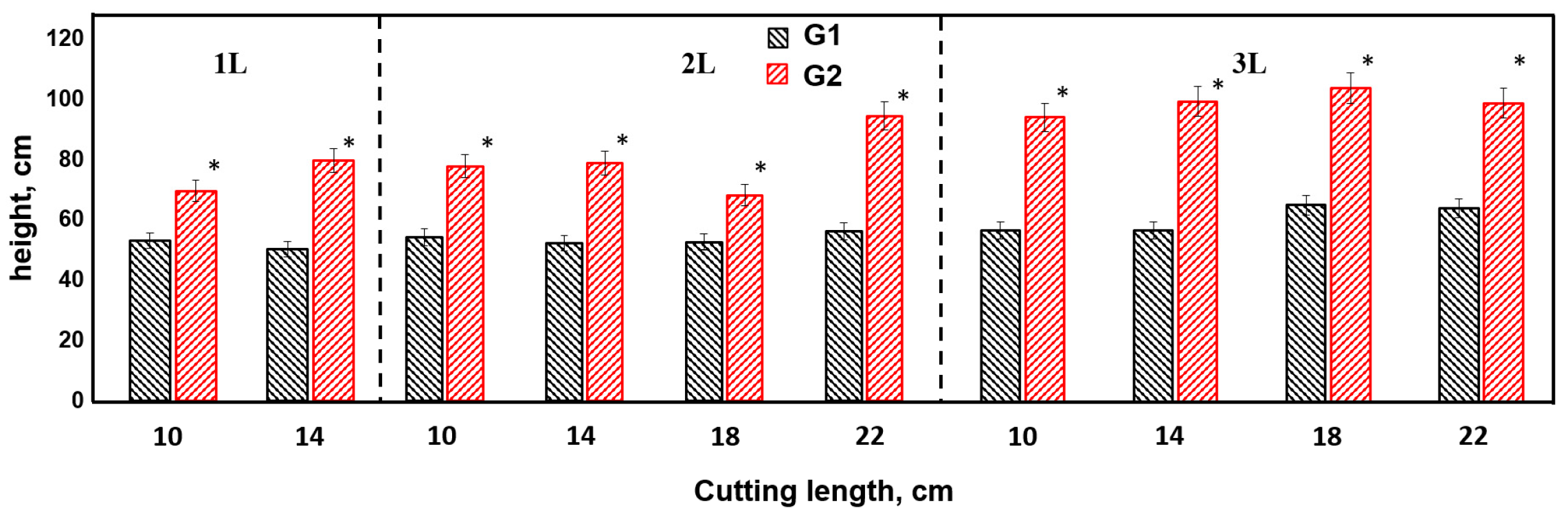
The results of the study showed that genotype 1 was significantly superior to genotype 2 in terms of height of annual seedling in all variants of the experiment, which was confirmed by statistically significant differences (p < 0.01) presented in Figure 1. This indicates a higher potential of genotype 1 with respect to growth during the first year of its development.
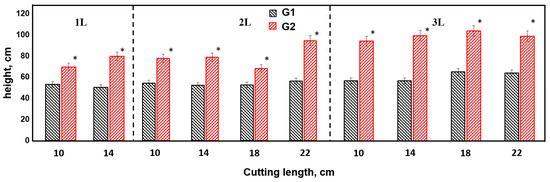
Figure 1.
Height of poplar plants of different genotypes.
Genotype 1 was characterized by the highest height growth. Among the tested variants of container volume and cuttings length, two main groups were distinguished by the intensity of growth processes in height. The group with the highest indices (111.9 ± 5.0–96.9 ± 5.9 cm) included experiment variants 3L22 cm, 3L18 cm, 3L14 cm, 3L10 cm, 2L22 cm, 2L18 cm, 2L14 cm, and 2L10 cm. The second group included variants with lower results for seedling height (89.4 ± 4.5–70.6 ± 5.5 cm), including 1L14 cm and 1L10 cm. Differences between groups were reliable at the level of p < 0.05.
By analysing the influence of the factor cuttings length on the height of annual seedlings of genotype 1 (E.s.-38), we observed that the greatest contribution of this factor was in the condition of using two-litre containers, where its influence was 59% (η2 = 0.59) of the total set of influencing factors. Consequently, this parameter should be taken into account in the harvest of cuttings, with a length of 18–22 cm being optimal. The lowest influence of this factor, equal to 26% (η2 = 0.26), was recorded for seedlings grown in three-litre containers. Such a decrease may be due to the fact that the effect of the factor cuttings length in this case was compensated for by the large volumes of root-covering coma and root system. Variants with one-litre pots occupied an intermediate position, with an influence of 38% (η2 = 0.38).
During the experiment with genotype 2, which was characterised by average growth and biological traits, differences in seedling height were also recorded in the different variants analysed, as illustrated in Figure 1. Although the differences in seedling height between the different experimental staging conditions were statistically significant, the severity of these differences was less marked compared to those for genotype 1.
This may indicate genotype 2’s greater tolerance to changing environmental conditions or its lower sensitivity to the factors considered, such as container volume and cuttings length. The lower degree of expression of growth differences in genotype 2 compared to genotype 1 may be related to its genetic characteristics affecting the morphological and physiological processes that determine plant growth.
For genotype 2, the highest seedling height values were observed in the variants using three-litre containers at cuttings lengths of 18 cm and 22 cm, where the average height was 68.1 ± 7.8 cm and 67.1 ± 6.3 cm, respectively. These results emphasise the importance of the optimal combination of container and cuttings size to achieving maximum plant growth.
The second group in terms of seedling height, with values of 61.1 ± 5.7 cm to 56.2 ± 3.2 cm, was formed by most of the variants studied, including 3L14 cm, 3L10 cm, 2L22 cm, 2L18 cm, 2L14 cm, 2L10 cm, and 1L10 cm. These results indicate that differences in container volume and cuttings length have a marked effect on growth, but the effect of these factors was less pronounced compared to that of the first group.
The lowest height increment was recorded in the variant with a root closure volume of 1 L and a cuttings length of 14 cm, where the seedling height was 53.7 ± 4.8 cm. This indicates limitations in the availability of resources for the root system in the limited space of the container, which restrains the growth of the plant.
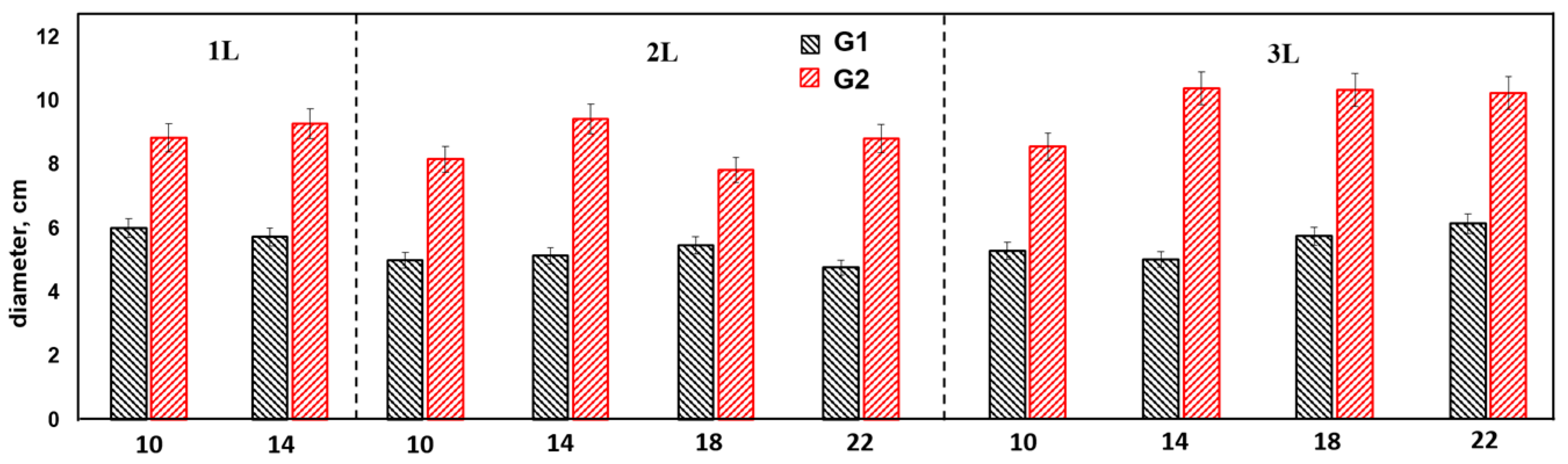
The average stem diameter values of the experiment variants are presented in Figure 2.
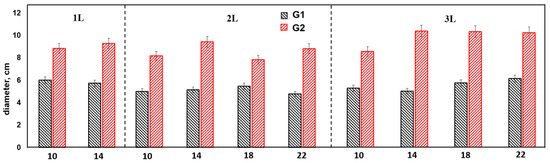
Figure 2.
Average values of trunk diameter of annual poplar seedlings.
Comparative analysis of the average values of seedling growth diameter of the G1 and G2 seedlings revealed the superiority of G1 in all variants of initial cuttings length and container volume, which showed reliable differences, with the difference ranging from 1.28 (1L10 cm) to 1.6 (3L14 cm) times, depending on the experiment variant.
The strength of the influence of the categorical factor genotype is shown in Table 1.

Table 1.
The influence of the categorical factor genotype on the diameter and height of plants.
The maximum values were obtained for the following G1 variants: 3L22 cm, 3L18 cm, and 3L14 cm (12.8 ± 0.42–12.3 ± 0.24 mm). Average values were obtained for variants 3L10 cm, 2L22 cm, 2L18 cm, and 2L14 cm (11.8 ± 0.22–11.1 ± 0.27 mm mean), with the differences in diameter being significant (p < 0.05). The lowest mean values were recorded in variants 2L10 cm, 1L14 cm, and 1L10 cm (10.4 ± 0.44–8.7 ± 0.42). In general, the variability of the shoot diameter parameter for G1 was average, with a coefficient of variation of 13.2%.
For G2, the maximum values of diameter were observed at cuttings lengths of 18 and 22 cm in 3L, with average values in the range of 7.3 ± 41–7.9 ± 0.4 mm. The coefficient of variation of the parameter shoot diameter among the variants of genotype 2 was average, as was that of genotype 1, being at 15.2%. Genotype 2 showed a direct dependence of diameter growth on cuttings length, so in cuttings with a length of 10–14 cm, the average values of diameter were in the range of 6.2 ± 0.23 mm–6.7 ± 0.24, while in variants with a cuttings length of 18–22 cm, the average values exceeded 7.0 mm (p < 0.05).
Correlation analysis carried out for the pair of traits seedling height and diameter showed dependence on container volume. The correlation coefficient rs calculated on the basis of taking into account all variants for genotype 1 was 0.5, while for genotype 2, it was 0.55. The average strength of the correlation between height and diameter indicated the presence of morphogenetic specificities for genotypes with cuttings of material of different lengths and in different container variants. On the contrary, the overall considered correlation coefficient rs with the data for the two genotypes combined as a function of container volume was 0.88 for 1 L and 2 L and 0.9 for 3 L, showing a strong positive relationship.
While considering the correlation relationship depending on genotype and container type, we found differences for genotype. In genotype 2, the maximum correlation between the length of the initial cuttings and the diameter of the resulting growth was found for 3 L containers (rs = 0.62), 2 L container (rs = 0.47), and 1 L (rs = 0.26). Apparently, the limitation of root system volume had a negative effect on seedling growth. The opposite results were found for the fast-growing genotype 1—the correlation for 2 L (rs = 0.9) and 1 L containers (rs = 0.62) was stronger than that for the 3 L variant (rs = 0.2).
This trend for genotype 1 is probably related to the redirection of nutrients to the root system in developing the container root zone volume.
Thus, differences in the final plant height and diameter were revealed at the intensive growth phase (25 August). As shown in Figure 2 and Figure 3, changes in these conditions correlated directly with changes in plant growth parameters. This emphasises the importance of selecting the optimum parameters when growing seedlings in order to maximise plant growth and development, especially during critical phases of their life cycle when they enter the intensive growth stage. Thus, these results provide valuable data for further improvement of cultivation techniques under controlled environment conditions.
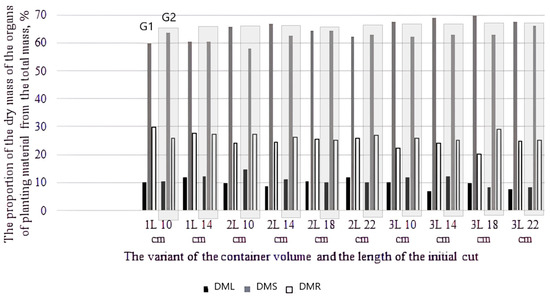
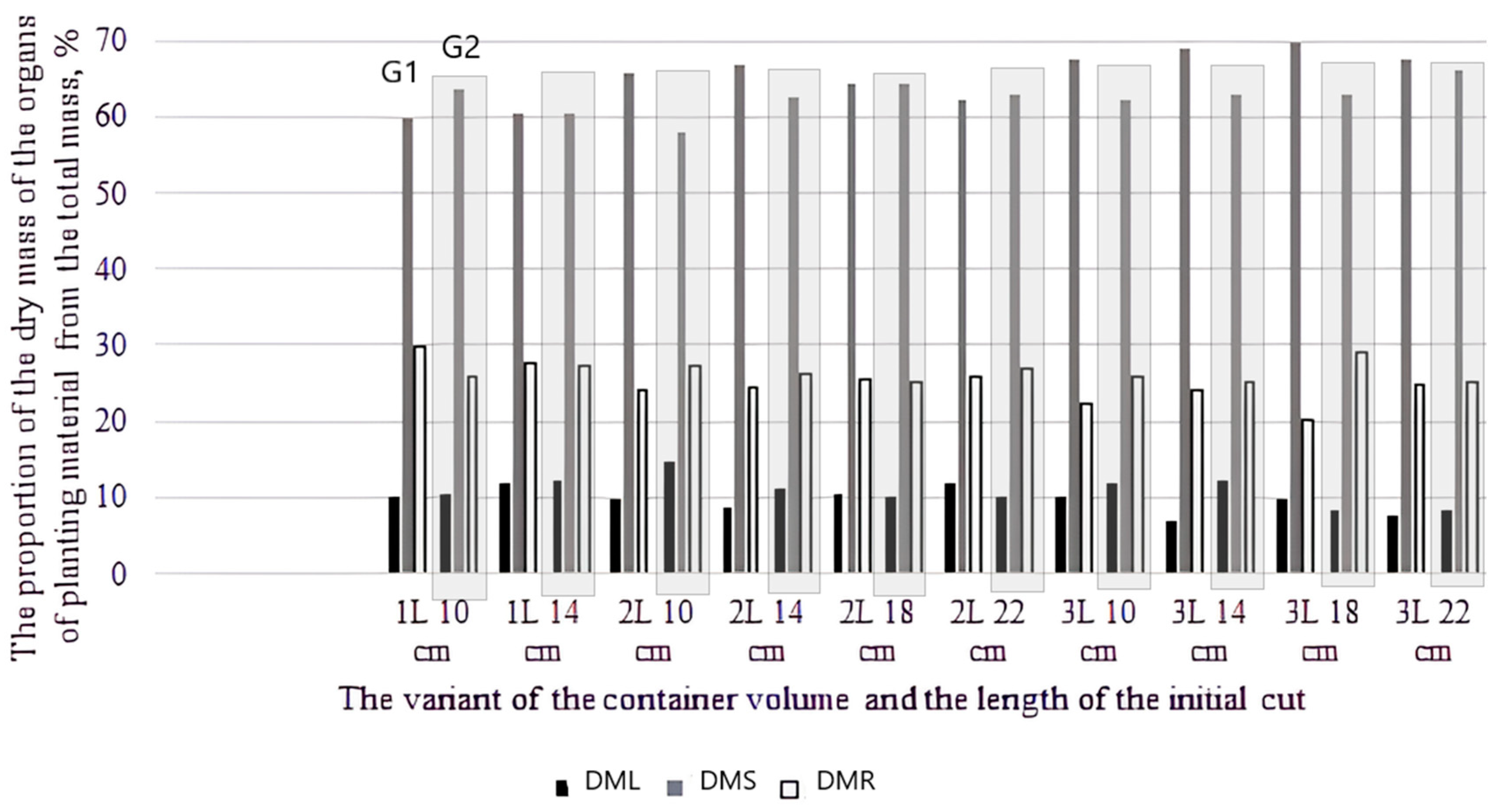
Figure 3.
Fractions of dry weights of individual organs of seedlings relative to the whole plant weight depending on genotype and experiment variant for genotype 1 and genotype 2 (DML—dry mass leaf; DMS—dry mass stem; DMR—dry mass root).
3.2. Effect of Cuttings Size, Container Volume, and Genotype on the Biological Productivity (Biomass) of the Annual Seedlings Obtained by Cuttings
The value of the formed biomass in a particular period of plant development is an important integral indicator of the efficiency of the productivity process [45]. The data on the dry mass of G1 plants are given in Table 2.

Table 2.
Estimation of dry biomass by fractions of underground and above-ground plant parts and the G1 ratio of whole plant mass to total assimilative surface area.
Analysis of the data in Table 2 allowed us to conclude that seedling productivity is not uniform and depends on the combination of container volume and initial cuttings height.
In general, fast-growing G1 is characterised by an increase in all plant organs with increasing length of the initial cuttings, and this dynamics can be traced for all container variants. The best results for the accumulation of total dry biomass were demonstrated by the G1 variants 3L22 cm, 3L18 cmm, and 3L14 cm. The second group included 3L10 cm, 2L22 cm, 2L18 cm, and 2L14 cm. The G1 variants 3L10 cm, 2L10 cm, 2L14 cm, and 2L10 cm were significantly behind the top group. This ranking clearly indicates the effect of container volume and cuttings length on overall plant productivity.
Slow-growing G2 was characterised by the increase of seedling organs to a greater extent with increasing length of the initial cuttings. The volume of the container provided an advantage only for the volume of the root system in the 3 L pot variant for long cuttings (18, 22 cm). The dry weight of annual seedlings (Table 3) with the ground method of exposing containers with plants was the highest in variants 3L22 cm and 3L18 cm but was significantly lower (p < 0.05) for plants in variants 3L14 cm, 3L10 cm, 2L22 cm, 2L18 cm, 2L14 cm, 2L10 cm, 1L14 cm, and 1L10 cm.

Table 3.
Dry biomass by fractions of underground and aboveground plant parts for G2.
Analysis of the ratio of organ weights relative to the weight of the whole plant indicated differences between genotypes depending on cultivation options (container volume and cuttings length). Comparative diagrams are presented in Figure 3.
It is interesting to note that there were changes in relation to G2 leaf parameters for variants with minimal values of initial cuttings length when the 1 L and 2 L containers were compared: there was an increase in specific surface density of the leaf and the proportion relative to the mass of the whole plant, which demonstrated the activation of the photosynthetic processes.
For the 3 L pot variant, an increase in the proportion of stem and roots relative to the whole plant was observed with an overall increase in productivity. The variations found between the genotypes studied reflect the peculiarities of plant growth during the rooting process depending on the cuttings length and container volume. In genotype 2 (native species), obtained from short cuttings in a small container volume, the intensity of leaf growth increased, the proportion of stem mass relative to the whole plant decreased, and in containers of large volume, there was a recovery of organ mass ratios to the average for the genotype indicators in plants. For the fast-growing genotype, an increase in container volume led to an increase in the proportion of stem relative to the whole plant, along with an increase in total productivity, which lead to seedlings with a maximum stem height.
Comparison of the average values of plant dry weight between the two contrasting poplar genotypes showed significant biomass dominance of fast-growing G1 over hard-to-root G2. Thus, in the most productive variants, G1 3L22 cm and G2 3L22 cm, the excess was 3.75 times, while in the least productive variants, G1 1L10 cm and G2 1L10 cm, the difference was 2.8 times.
In general, by comparing the average height indices of G1 variants with the maximum productivity and similar samples of G2, we noted that G1 dominance was reliable (p < 0.05) (by 44–45.3%).
The assessment of the influence of a set of factors (pot volume, cutting length) on shoot diameter, leaf area, and the fresh and dry plant weight of the studied genotypes is presented in Table 4 and Table 5.

Table 4.
The influence power of the combination of factors container volume and cutting length on the genotype 1 plant parameters.

Table 5.
The influence power of the combination of factors container volume and cutting length on the genotype 2 plant parameters.
A significant effect of the combination of factors on the growth and accumulation of biomass of the slow-growing genotype 2 was revealed in the 2 L container variants. In the 2L14 cm and 2L18 cm variants, the effect of cultivation was observed relative to all the parameters studied. For the 3 L container variant, a similar effect was achieved with an initial cutting length of 14 cm. In the 3L18 cm and 3L22 cm variants, no reliable effect of reproduction conditions was revealed. For the 1 L containers, a reliable significant effect (a strength of influence from 42% to 57%) was recorded in the variant with a 14 cm cutting.
It can be noted that the variants with a significant influence of reproduction condition factors for the fast-growing genotype 1 had a similar direction to that from the analysis of the influence of reproduction conditions on the parameters of planting material for the slow-growing genotype 2.
For 2 L containers and 10–18 cm cuttings, a significant influence on the studied parameters was revealed. Among the variants of 1 L containers, the greatest influence of the combination of factors was noted for 14 cm cuttings. For 3-L containers, a reliable effect was established for cuttings 10 and 14 cm long.
The reliability of differences between the strength of influence for various studied parameters (reproduction conditions) for both studied genotypes is presented in Table 6 and Table 7. Differences between the strength of influence for pot volume and cutting length were significant, but not universally so; the highest reliability of differences was found between volumes of 2 L and 3 L and between cutting lengths of 14 and 22 cm (4.12 > 2.04).

Table 6.
Reliability of differences between the strength of influence for various studied parameters (genotype 1).

Table 7.
Reliability of the differences between the strength of influence for various studied parameters (genotype 2).
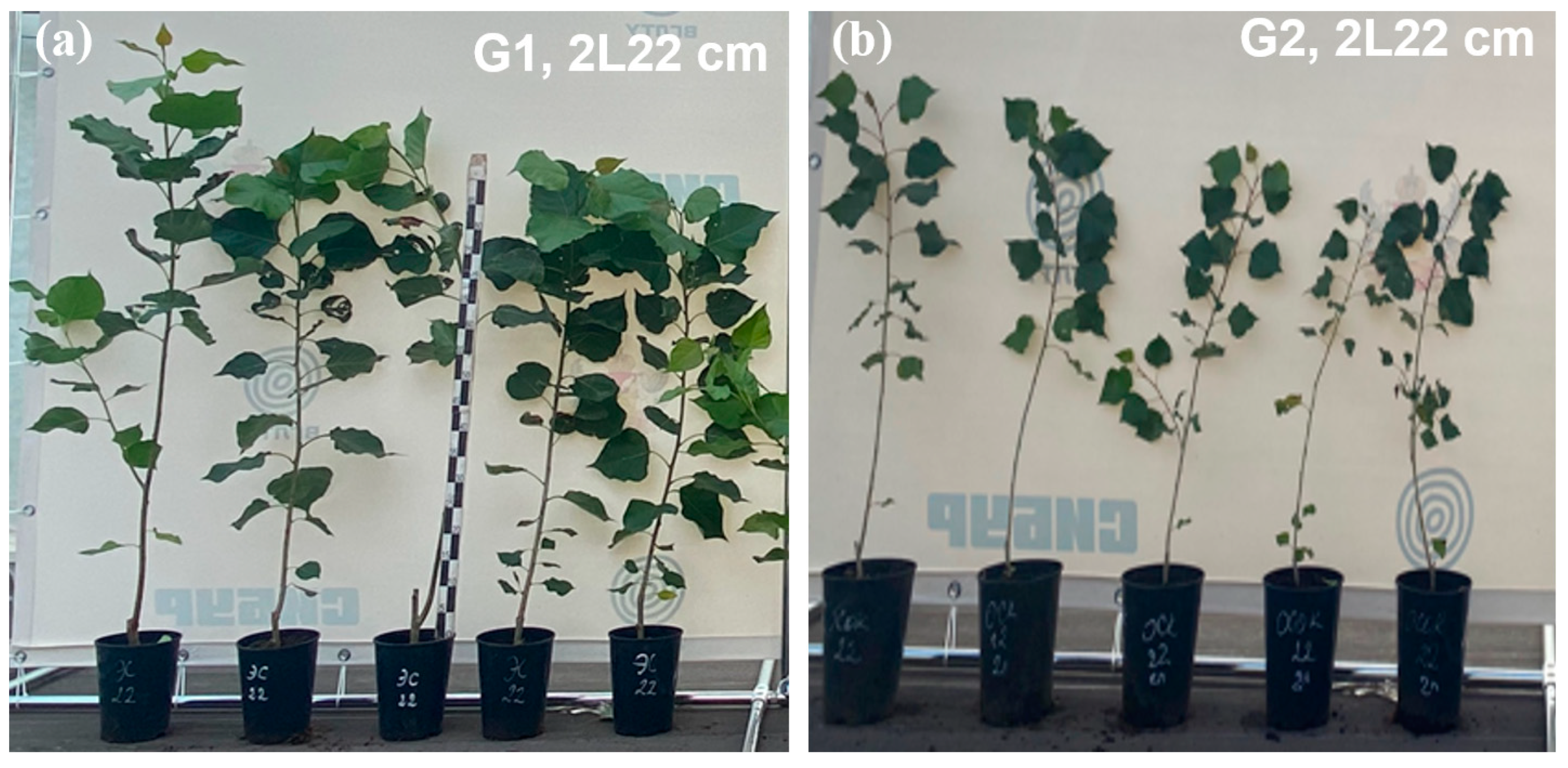
Figure 4 shows images of the main variants of the experiment, with plants showing normal development and standard quality.
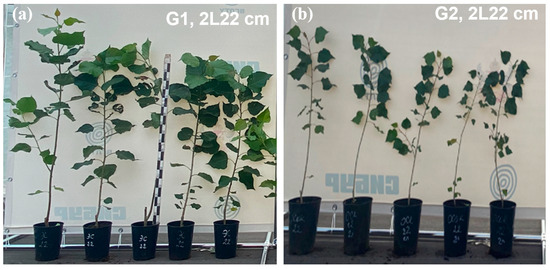
Figure 4.
Appearance of annual seedlings during the period of intensive poplar growth: (a) G1 2L22 cm and (b) G2 2L22 cm.
The groups of seedlings were determined according to the accumulation of crude and dry mass of the whole plant.
Three groups were identified: low productivity, medium productivity, and high productivity (Table 8).

Table 8.
Characterisation of the selected groups of seedlings of genotype 1 and 2 by biometric indices and productivity among the experimental variants.
The fast-growing genotype 1 low-productivity group included the following variants with a small cuttings length: 1L10 cm, 1L14 cm, 2L10 cm. The medium-productivity group included variants 2L14 cm, 2L18 cm, and 3L10 cm. The high-productivity group was represented by variants 3L14 cm, 3L18 cm, 3L22 cm, and 2L22 cm.
The G2 low-productivity group included the following variants with cuttings length of 10–14 cm: 1L10 cm, 1L14 cm, 2L10 cm, 2L14 cm, 3L10 cm, and 3L14 cm. The second cluster (intermediate group) included variants 2L18 and 2L22, while the third cluster was maximally distant from the first two (high-productivity group) and included variants 3L18 cm and 3L22 cm.
Comparative analysis of the tree diagram data showed that cuttings length had a considerable influence on the overall productivity of seedlings in both genotypes in the selected groups. It is possible to increase the productivity by increasing the volume of root zone by increasing the container volume. The association distance of contrasting clusters in the two genotypes differed by almost an order of magnitude (0.5 in the main clusters in G2 versus 5 in G1), indicating different levels of biomass accumulation.
The parameters of the selected groups showed that for the fast-growing genotype 1, it is possible to increase the growth activity of seedlings from short cuttings of 10 and 14 cm by increasing the container volume to 2 L and 3 L.
For the slow-growing genotype 2, the growth activity of seedlings obtained from short cuttings (10, 14 cm) was low and did not increase with increasing container volume. Maximum productivity for genotype 2 could only be achieved using 18 and 22 cm cuttings, and increasing the container volume from 2 L to 3 L led to a 1.5-fold increase in plant dry weight (from 4.4 to 6.4 g).
Thus, in the case of genotype 2, seedling growth can be reliably increased by increasing the root ball to three litres and using a cuttings of at least 18 cm. This was confirmed by determining the strength of the influence of the factor cuttings length on plant height, which was maximum in the variants with genotype 2 in the three-litre containers (η2 = 0.24). In 1 L and 2 L containers, the influence of this factor did not exceed 10%. Probably, in the case of black poplar G2, the influence of genotype had a decisive impact on the growth processes taking place. The positive influence of phenotypic features manifested as the increase in the root zone, which improved conditions for the growth of the above-ground and underground parts of the plant.
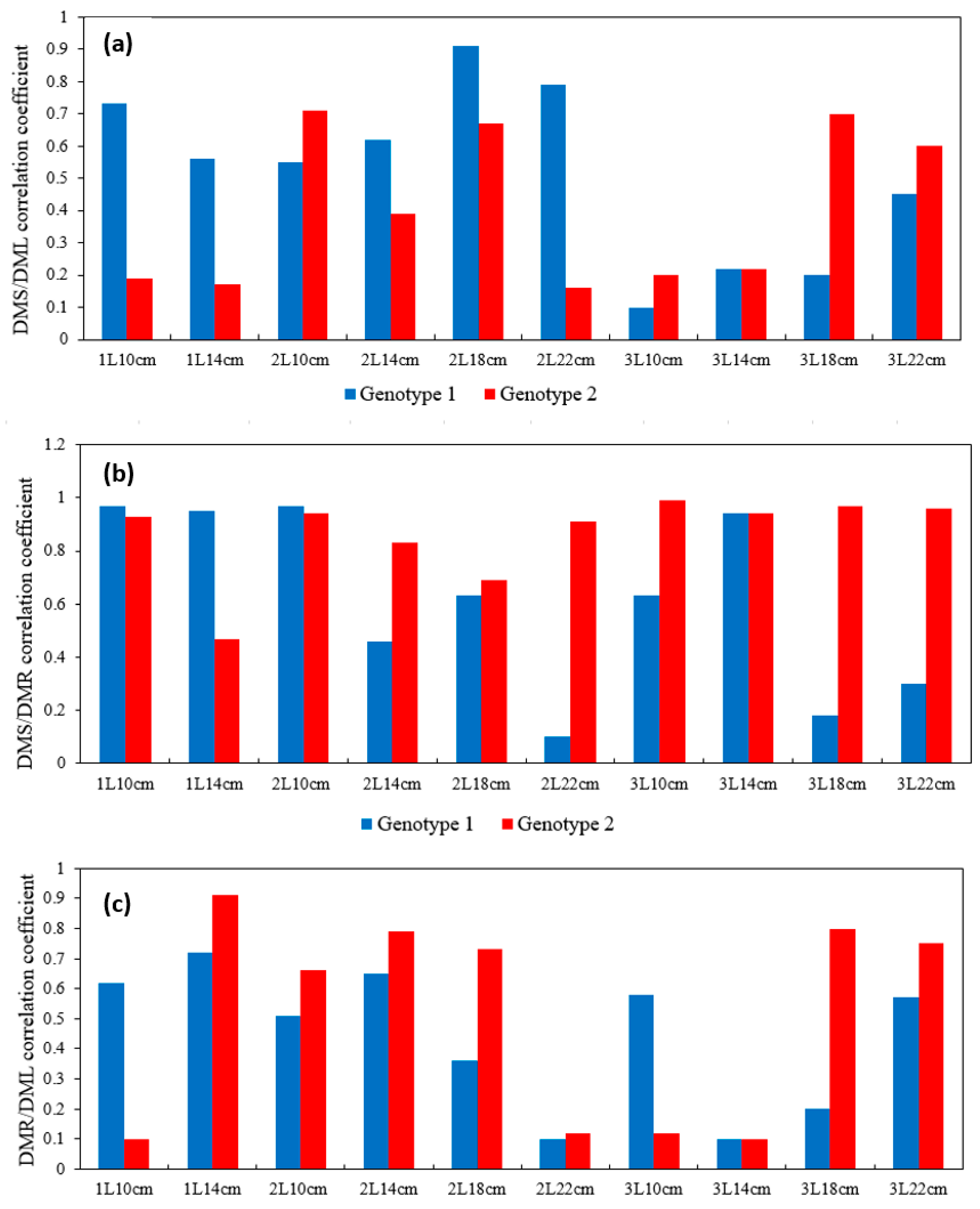
Taking into account the detected variability of the rooting and growth of cuttings depending on the genotype and cuttings variants, we conducted a correlation analysis of the relationship between seedling organ biomass formation and the parameters of root, stem, and leaf dry weight. The characteristics of growth for the genotypes in the studied variants of the experiment were revealed (Figure 5).
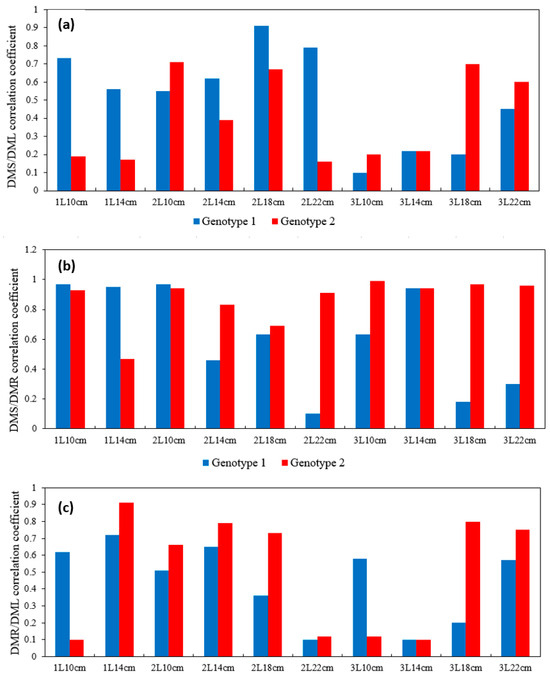
Figure 5.
Diagram of the correlation coefficient depending on poplar genotype and experimental variant (a) for the DMS/DML pair, (b) DMS/DMR pair, and (c) DMR/DML pair. (DML—dry mass leaf; DMS—dry mass stem; DMR—dry mass root).
The obtained data indicated a high degree of positive correlation between the dry biomass of roots and stems, and for the difficult-to-root genotype 2, this was observed for all experimental variants (the exception was variant 1L14 cm with a reduced correlation). For genotype 1, rs varied from a minimum of 0.1 to a maximum of 0.97. The strongest correlation was observed in variants with a small container volume and short cuttings length (1L10 cm, 1L14 cm, 2L10 cm) and in cases of a large container volume and a short length of initial cuttings (3L10 cm, 3L14 cm).
Thus, for the hard-to-root genotype, the f value of the root system depends on the size of the initial stem cuttings.
Interesting results were obtained when we evaluated the relationship between leaf and root and between leaf and stem dry biomass. For hard-to-root G2, in general, the relationship between leaf and stem biomasses was low, with the exceptions being the variants of the high-yielding group (3L18 cm, 3L22 cm), as well as some of the medium-yielding groups. At the same time, the correlation between root and leaf biomasses for these variants was significantly high (rs from 0.73 to 0.91). Thus, in the hard-to-root genotype, the growth of the root system depends on the biomass of leaves and, consequently, on their photosynthetic activity. The absence of a strong correlation between cuttings size (with their equal thickness) and leaf size showed that the stock of carbohydrates contained in the initial cuttings was redistributed to the root, and the root formation process prevailed over stem and leaf growth at the first stages of rooting.
For the fast-growing genotype 1, the correlation between leaf and stem biomasses was stronger than that for genotype 1, with rs exceeding 0.45 in 7 out of 11 variants of the experiment. The strongest relationship was shown for high-yielding variants in the medium-volume container (2L18 cm, 2L22 cm). The correlation between root and leaf biomasses was weaker lower in comparison with genotype 2. The strongest relationship was found for the low- and medium-productivity (intermediate) variants 1L10 cm, 1L14 cm, 2L10 cm, 2L14 cm (rs from 0.51 to 0.72), as well for the two high-productivity variants. In the fast-growing G1 poplar, organ growth was more proportional, and the dependence of stem and leaf biomass is higher. Thus, during rooting, the leaf biomass rapidly increases simultaneously with the growth of the root system.
Regarding the values of the raw leaf-specific surface density and dry leaf-specific surface density (SSD), no regular variation among the variants could be distinguished for either genotype. The mean values of the specific surface density of raw and dry leaf weight for G1 were 0.024 ± 0.0004 and 0.0057 ± 0.000142, respectively. The mean values of the specific surface density of the crude and dry leaf weight for G2 were 0.025 ± 0.0005 and 0.007 ± 0.0002, respectively.
Differences between genotypes were reliable according to Fisher’s criterion (p < 0.01 and p < 0.001, respectively). However, no differences were found among the selected groups in terms of productivity. Thus, there was no change in leaf thickness depending on the rooting and growth characteristics of the variants.
However, changes were found, as mentioned above, in the proportion of leaf mass relative to the mass of the whole plant (Figure 4). The most pronounced regularity was revealed for hard-rooting G2: the greater the productivity, the smaller the proportion of leaves relative to the weight of the whole plant (in variants of the high-yielding groups, the proportion of leaves was 8.2–9.0%, while in the low-yielding groups, it was 10.6–14.6%). Thus, it can be assumed that for variants of cuttings with lengths of 10 and 14 cm, the carbohydrate reserve is insufficient for root formation, and there is an increase in photosynthetic activity due to an increase in leaf mass.
4. Discussion
The first thing to note is the differences in growth parameters of the studied genotypes. Despite relatively identical growing medium conditions, one of the genotypes showed a higher growth rate and a developed root system. This supports the hypothesis that genetic factors play a predominant role in determining the growth rate and rhizogenesis compared to the effects of environmental or agronomic measures [46].
Containerised seedling cultivation has its own specific characteristics, such as space limitations for the root system and the need to maintain optimal microconditions. Genotypes more adapted to such conditions will exhibit better adaptive performance, which is related to their natural tendency to utilise the resource more efficiently. These findings are consistent with other work [47], where enhanced root formation was observed in certain genotypes of Willow under container cultivation.
Comparative analysis of the obtained seedlings of two contrasting poplar genotypes in terms of growth intensity and rhizogenesis showed a significant contribution of genotype when planting material were grown in containers, which is consistent with the results of studies on the evaluation of container cultivation of poplar and willow genotypes. Poplar is characterised by the persistence of genotype influence on growth features not only in the first year of rooting but also in subsequent years, as well as the selection of genotypes and genomic groups with the highest rooting ability based on growth parameters [48,49]. In Salix and Populus clones, significant differences in the initiation of root formation, total root length, and dry mass of plants have been established, which will affect the growth characteristics of seedlings both in the container and in the open ground [50]. Significant differences in the development of root biomass within 2 years after planting have also been found [51].
Recent studies have shown differences in rooting and growth of contrasting poplar genotypes at the morphological, physiological, and genetic levels. In experiments studying the effect of light on the rooting of P. tremula stem cuttings, it was shown that in darkness, root growth slows down within 24 h, and on the second to third day, it stops completely. After removal of leaves or steam destruction of tissues on the stem cuttings under the leaves, it stops within 24 h [52]. In the case of using root cuttings, it was found that there is no effect of light on their germination, but light inhibits the rooting of root cuttings.
Meanwhile, stored carbohydrates are consumed during rooting of stem cuttings, but this does not determine the biological rooting potential of genotypes. For example, in a rooting study of Populus tremula L. × Populus tremuloides Michx, there was no evidence to support the view that failure to root was due to inadequate carbohydrate reserves. In leafless hardwood cuttings, carbohydrate content was initially very high (14–19%) but declined rapidly (to 5–10%) as roots, callus, or shoots developed [47]. One of the differences between easily rooted P. × euramericana and hard-to-root P. tremula is the apparent downward transfer of assimilates in P. × euramericana cuttings [53].
Analyses of plant dry mass and the distribution of dry mass between plant parts are similar to those of other poplars. Fast-growing poplars have a greater root-mass-per-unit leaf area and a higher rate of root elongation after transplantation compared to very slow-growing clones, indicating a better water balance and drought prevention, at least at the beginning of growth [54].
Thus, both the process of photosynthesis and carbohydrate supply from leaves and downward transfer of assimilants in cuttings are important for the rooting of stem cuttings. This explains the results we obtained on productivity in the selected groups and the revealed differences in the strength of correlations between the masses of plant organs.
The type and volume of the container have a significant influence on the development of the root system of plants. Our study has shown that due to genotypic differences and peculiarities of the source material, it is necessary to carefully select appropriate containers. The use of maximum volume containers is not always justified from an economic point of view, as it may lead to increased cultivation costs and difficulties in transporting the plants [55]. It is expected that seedling characteristics generated according to cultivation methods will remain influential in the early stages of field cultivation [56]. In addition, the length of the initial cuttings is an important factor influencing the development of the root system.
In studies conducted on Populus clone Max 4 and Salix clone Inger, it was found that when 20 and 40 cm cuttings were used, longer cuttings showed an increase in biomass but had different effects on shoot growth and root system distribution in both clones. It was found that longer cuttings of Populus cl. Max contribute to greater aboveground biomass, mainly at the expense of leaves, compared to 20 cm long cuttings. Meanwhile, 40 cm cuttings of Salix cl. Inger develop longer but thinner roots. Thus, long cuttings may be more suitable for forming clonal plantations of Salix cl. Inger on sites with limited water and nutrient resources. One of the differences between easily rooted P. × euramericana and hard-to-root P. tremula was the apparent downward transfer of assimilates in P. × euramericana cuttings [57] Despite the difficulties associated with planting and the higher cost of long cuttings, their use may be justified by increased survival and aboveground productivity for some clones.
In the future, it is necessary to determine the optimal length of cuttings and to take into account not only different environmental conditions but also the specifics of poplar species and clones [58]. The possibility of regulating the biological features of seedlings opens up prospects for improving their adaptation to open-ground conditions. Moisture-deficient cuttings show a longer rooting process and form fewer roots, whereas pre-soaking favours rooting, especially in drier soils. The initial water potential of cuttings decreases with time but stabilises with the formation of a root system [54].
Soil density is an important limiting factor in the development of root systems. Studies show that poplar and willow clones show significant differences in root initiation, root length, and root dry mass. However, for both genera, more fine roots have been found to form in soils with low bulk density compared to higher density soils. Increasing soil density significantly inhibits the development of root systems in both Salix and Populus clones [56].
Based on the results of the study on the influence of container depth and substrate heterogeneity on the growth of nodal seedlings of Populus sibirica, an effective methodology for growing high-quality planting material is proposed. The use of deeper containers and heterogeneous substrate favours the formation of root systems adapted to difficult conditions, including arid and semi-arid environments [59]. Heterogeneous soil structure in combination with deep containers favours increased carbon supply to the root system and hence intensification of its growth [57]. The depth of the container and the depth of planting of the cuttings influence the spatial organisation of the root systems of seedlings, and the use of chemical pruning with copper allows for the formation of planting material with asymmetric root systems [51].
Our data are consistent with previous studies on the topic under study. In studies conducted by Boytsov A.I. and co-authors [59], similar results were obtained for several poplar genotypes, which supports our conclusion regarding the significant role of genotype in the formation of growth and root characteristics. This suggests that genotypic specificity plays a key role in the adaptive abilities of plants when cultivation methods are altered. The identification of genotypes that can develop most efficiently under container growing conditions has significant practical implications for silviculture and agroecological programmes. Such genotypes have the potential to improve the efficiency of production processes and can contribute to the success of programmes to restore disturbed ecosystems [51].
The presented study emphasises the importance of selecting suitable containers for seedling production and of taking into account the genotypic differences and specific requirements of the source material. It was found that the use of maximum volume containers is not always appropriate for economic and logistical reasons. The choice of cuttings length was also identified as a critically important factor affecting the development of both the aboveground part and the root system, with differences in responses observed between the Populus and Salix clones.
In addition, soil density has a significant effect on the formation of root systems, where light soils favour the development of fine roots, while a higher density limits their growth. These results emphasise the need for containers with optimal depth and the use of heterogeneous substrates to improve the quality of planting material. Also, the proposed methods for regulating the biological characteristics of cuttings, such as pre-soaking and chemical pruning, can enhance plant adaptation to outdoor conditions, especially in arid and semi-arid environments. In further studies, it is necessary to test the indices of functional activity of the leaf apparatus at different stages of growth, including intensity of photosynthesis, transpiration, stomatal conductance, water use efficiency, and the anatomical structure of the leaf, to gain a deeper understanding of the growth and development of poplar plants with a closed root system.
Thus, an integrated approach, including the selection of containers, cuttings length, and substrate parameters, can optimise the seedling growth processes, opening up new opportunities to improve seedling productivity and persistence under different environmental conditions. This study provides valuable recommendations for improving practices in silviculture and crop production.
5. Conclusions
A study on the propagation of two poplar genotypes differing in rhizogenesis and productivity characteristics by stem cuttings revealed the following key aspects:
- Genotypic differences: The influence of genotype was found to be more significant than was rooting technology including the selection of cuttings length and container volume. This indicates the dominance of genetic factors over agronomic conditions in determining rooting success.
- Comparative productivity analysis: Genotype G1 outperformed the natural species G2 in terms of total biomass by 5.5 times, also showing superiority in height (1.74 times) and diameter (1.7 times). However, the rooting rate (RR) remained identical for both genotypes.
- Effect of cuttings length: The strength of the effect of the factor cuttings length on height increment was 8–10% for G2 and 19–41% for G1. In terms of total biomass, this effect was 12–41% for G2 and 15–75% for G1, indicating variability in rooting across genotypes.
- Productivity grouping: Genotype G1 showed high productivity in 3L18 cm, 3L14 cm, 2L22 cm, whereas for G2, the 3L22 cm and 3L18 cm groups were highly productive. On the contrary, poor productivity was observed in groups with cuttings length of 10–14 cm for both genotypes.
- Correlation and morphological features: Analysis of correlations between seedling height and growth diameter and dry weight distribution indicated biological rooting characteristics that differed between the two genotypes. The plants of the natural species G2 showed faster root system development, while the growth of the hybrid G1 was more proportional, especially when larger volume containers were used.
- Recommendations for propagation conditions: Consideration of biological traits and genetic characteristics is critical for optimising rooting conditions under greenhouse conditions. Genotype 1 (E.s.-38) showed high productivity, offering the potential for rapid production of standardised planting material.
- Recommendations for cuttings and container parameters: For genotype G1, cuttings of 10–14 cm in length with a container volume of 2 L or more are recommended for optimal results, including a height of more than 100 cm and a diameter of 8–10 mm. For genotypes with propagation difficulty, containers of at least 2 L are recommended, with cuttings 22 cm long.
- Need for further research: In order to optimise the propagation parameters for other poplar species and cultivars, further research is required to ensure the correct choice of container volume and cuttings length.
Thus, a comprehensive study of factors affecting poplar rooting allows for optimising stem cuttings techniques, improving the production characteristics of planting material depending on the genetic background and agronomic environment, opening up new opportunities for improving the quality and productivity of planting material, and stimulating sustainable development of forestry and related industries. This not only improves the economic efficiency of production but also supports efforts to conserve biodiversity and ecosystem services provided by forest ecosystems.
Author Contributions
Conceptualization, A.T., A.P. and K.Z.; Data curation, A.P., V.Z. and L.R.; Formal analysis, P.E., A.P. and L.R.; Funding acquisition, P.E.; Investigation, A.P.; Methodology, A.T., V.Z., V.R., L.R. and K.Z.; Project administration, P.E.; Resources, A.P.; Software, P.E. and A.T.; Visualization, V.Z. and V.R.; Writing—original draft, P.E., A.T., V.Z., V.R., L.R. and K.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The work was carried out within the framework of the state assignment of the Ministry of Science and Higher Education of the Russian Federation No. 1023013000020-6.4.1.2 “Selection of economically valuable and climate-resistant tree crops characterised by high biological productivity and sequestration potential, taking into account regional soil and climatic features for the implementation of climate projects” (FZUR-2023-0002).
Data Availability Statement
Dataset available on request from the authors.
Acknowledgments
We would like to thank Voronezh State University of Forestry and Technologies named after G.F. Morozov for the opportunity to conduct this research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Feng, Q.; Yang, H.; Liu, Y.; Liu, Z.; Xia, S.; Wu, Z.; Zhang, Y. Interdisciplinary perspectives on forest ecosystems and climate interplay: A Review. Environ. Rev. 2024, 33, 1–21. [Google Scholar] [CrossRef]
- Thom, D.; Rammer, W.; Seidl, R. The impact of future forest dynamics on climate: Interactive effects of changing vegetation and disturbance regimes. Ecol. Monogr. 2017, 87, 665–684. [Google Scholar] [CrossRef]
- The Food and Agriculture Organization. Global Forest Resources Assessment: Key Findings; FAO: Rome, Italy, 2020; ISBN 978-92-5-132581-0. [Google Scholar]
- Kotz, M.; Levermann, A.; Wenz, L. The economic commitment of climate change. Nature 2024, 628, 551–557. [Google Scholar] [CrossRef]
- Sicard, P.; Augustaitis, A.; Belyazid, S.; Calfapietra, C.; de Marco, A.; Fenn, M.; Bytnerowicz, A.; Grulke, N.; He, S.; Matyssek, R.; et al. Global topics and novel approaches in the study of air pollution, climate change and forest ecosystems. Environ. Pollut. 2016, 213, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Pollastrini, M.; Puletti, N.; Selvi, F.; Iacopetti, G.; Bussotti, F. Widespread crown defoliation after a drought and heat wave in the forests of tuscany (Central Italy) and their recovery—A case study from summer 2017. Front. For. Glob. Change 2019, 2, 74. [Google Scholar] [CrossRef]
- Pliura, A.; Jankauskiene, J.; Lygis, V.; Suchockas, V.; Bajerkevičiene, G.; Verbylaite, R. Response of juvenile progeny of seven forest tree species and their populations to simulated climate change-related stressors, heat, elevated humidity and drought. IForest 2018, 11, 374–388. [Google Scholar] [CrossRef]
- Gudynaitė-Franckevičienė, V.; Pliūra, A.; Suchockas, V. Ecogenetic plasticity and genetic variation in populus hybrids under the impact of simulated climate change related stressors. Balt. For. 2020, 26, 1–13. [Google Scholar] [CrossRef]
- Grossnickle, S.C.; Ivetić, V. Direct seeding in reforestation—A field performance review. Reforesta 2017, 4, 94–142. [Google Scholar] [CrossRef]
- Singh, A.; Husain, M.; Ali, S.R. Effect of container type and growing media on germination and seedling growth parameters at nursery stage in Allepo pine in Kashmir valley, India. Flora Fauna 2018, 24, 211. [Google Scholar] [CrossRef]
- Luoranen, J.; Rikala, R.; Smolander, H. Root egress and field performance of actively growing Betula pendula Container Seedlings. Scand. J. For. Res. 2003, 18, 133–144. [Google Scholar] [CrossRef]
- Simpson, D.G. Growing density and container volume affect nursery and field growth of interior spruce seedlings. Northern J. Appl. For. 1991, 8, 160–165. [Google Scholar] [CrossRef]
- Psistaki, K.; Tsantopoulos, G.; Paschalidou, A.K. An overview of the role of forests in climate change mitigation. Sustainability 2024, 16, 6089. [Google Scholar] [CrossRef]
- Lipka, O.N.; Korzukhin, M.D.; Zamolodchikov, D.G.; Dobrolyubov, N.Y.; Krylenko, S.V.; Bogdanovich, A.Y.; Semenov, S.M. The role of forests in the adaptation of natural systems to climate change. Forestry 2021, 5, 531–546. [Google Scholar] [CrossRef]
- Schwartz, A.; Starikov, I.V.; Kharlamov, V.S.; Golunov, R.Y.; Kobyakov, A.V.; Lukovtsev, F.Y.; Avtychnikov, A.V.; Tyuleneva, F.V.; Shmatkov, N.M.; Shtegolev, A.A.; et al. New urga look: Proposals in project strategies Development easy Complex. Sustain. For. 2020, 4, 2–25. [Google Scholar]
- Haralambides, H.E. Gigantism in container shipping, ports and global logistics: A time-lapse into the future. Marit. Econ. Logist. 2019, 21, 1–60. [Google Scholar] [CrossRef]
- Allier, A.; Moreau, L.; Charcosset, A.; Teyssèdre, S.; Lehermeier, C. Usefulness criterion and post-selection parental contributions in multi-parental crosses: Application to polygenic trait introgression. G3 Genes. Genomes Genet. 2019, 9, 1469–1479. [Google Scholar] [CrossRef]
- Giniyatullin, R.K.; Ivanov, R.S.; Tagirova, O.V.; Kulagin, A.Y. The content of photosynthetic pigments in the leaves of «healthy» and «weakened» balsam poplar trees (Populus balsamifera) growing under conditions of industrial pollution (Republic of Bashkortostan, Sterlitamak Industrial Center). Samara J. Sci. 2022, 11, 43–48. [Google Scholar] [CrossRef]
- Tsarev, A.P.; Tsareva, R.P.; Tsarev, V.A. Poplar testing and breeding in the Central Chernozem region of Russia. IOP Conf. Ser. Earth Environ. Sci. 2019, 392, 012010. [Google Scholar] [CrossRef]
- Tsarev, A.; Tsareva, R.; Tsarev, V.; Miligula, E.; Lenchenkova, O. Introduced Poplar varieties and new hybrids for protective afforestation. IOP Conf. Ser. Earth Environ. Sci. 2020, 595, 012004. [Google Scholar] [CrossRef]
- Demidova, N.A.; Yaroslavtsev, S.V.; Durkina, T.M.; Fedotov, I.V.; Il’intsev, A.S. The growth course of Neva Poplar (Populus x Newesis Bogd.) and Californian Poplar (Populus Trichocarpa Torr. et Gray) in the European North of Russia. Bull. High. Educ. Institutions. Lesn. Zhurnal (For. J.) 2016, 3, 77–86. [Google Scholar] [CrossRef]
- Crowl, T.A.; Crist, T.O.; Parmenter, R.R.; Belovsky, G.; Lugo, A.E. The spread of invasive species and infectious disease as drivers of ecosystem change. Front. Ecol. Environ. 2008, 6, 238–246. [Google Scholar] [CrossRef]
- Reho, M.; Vilček, J.; Torma, S.; Koco, Š.; Lisnyak, A.; Klamár, R. Growing of the containerized seedlings of english oak (Quercus robur L.) to establish sustainable plantations in forest-steppe Ukraine. Forests 2022, 13, 1359. [Google Scholar] [CrossRef]
- Evlakov, P.M.; Tsarev, A.; Tsareva, R.P.; Rzhevsky, S.G.; Yu Zapletin, V. Evaluation of productivity at the leaf level of juvenile Poplars. IOP Conf. Ser. Earth Environ. Sci. 2019, 226, 012018. [Google Scholar] [CrossRef]
- Heimann, L.; Horst, I.; Perduns, R.; Dreesen, B.; Offermann, S.; Peterhansel, C. A Common histone modification code on c4 genes in maize and its conservation in Sorghum and Setaria italica. Plant. Physiol. 2013, 162, 456–469. [Google Scholar] [CrossRef]
- Boruszewski, P.; Laskowska, A.; Jankowska, A.; Klisz, M.; Mionskowski, M. Potential areas in poland for forestry plantation. Forests 2021, 12, 1360. [Google Scholar] [CrossRef]
- Tsaryov, A.; Tsaryova, R.; Tsaryov, V.; Tseplyaev, A.; Tregubov, O.; Laktionov, A.; Mizin, Y.; Miligula, Y.; Pokhvalenko, V. Rootability of poplar stem cuttings and height of 1-year-old seedlings using various cultivation methods. Astrakhan Bull. Environ. Educ. 2024, 1, 98–103. [Google Scholar] [CrossRef]
- Tsarev, A.P.; Tsareva, R.P.; Tsarev, V.A.; Miligula, E.N. The new poplar hybrids’ growth in the Central Black Earth region of Russia. IOP Conf. Ser. Earth Environ. Sci. 2019, 392, 012011. [Google Scholar] [CrossRef]
- Stanton, B.J.; Bourque, A.; Coleman, M.; Eisenbies, M.; Emerson, R.M.; Espinoza, J.; Gantz, C.; Himes, A.; Rodstrom, A.; Shuren, R.; et al. The practice and economics of hybrid poplar biomass production for biofuels and bioproducts in the pacific northwest. Bioenergy Res. 2021, 14, 543–560. [Google Scholar] [CrossRef]
- Choudhury, M.A.M.; Marcheggiani, E.; Despini, F.; Costanzini, S.; Rossi, P.; Galli, A.; Teggi, S. Urban tree species identification and carbon stock mapping for urban green planning and management. Forests 2020, 11, 1226. [Google Scholar] [CrossRef]
- Renninger, H.J.; Stewart, L.F.; Freeman, J.L.; Rousseau, R.J. Physiological functioning and productivity in eastern cottonwood and hybrid poplars on contrasting sites in the southeastern US. Bioenergy Res. 2022, 15, 1057–1070. [Google Scholar] [CrossRef]
- Smith, K.T. Whither compartmentalization of decay in trees? A commentary on: ‘Using the CODIT model to explain secondary metabolites of xylem in defence systems of temperate trees against decay Ffungi’. Ann. Bot. 2020, 125, iv–vi. [Google Scholar] [CrossRef] [PubMed]
- Perrette, G.; Delagrange, S.; Messier, C. Optimizing reduction pruning of trees under electrical lines: The influence of intensity and season of pruning on epicormic branch growth and wound compartmentalization. Arboric. Urban. For. 2020, 46, 432–449. [Google Scholar] [CrossRef]
- Sapronova, D.V.; Belyaev, A.I.; Semenyutina, A.V.; Khuzhakhmetova, A.S.; Sapronov, V.V. Improved technologies for growing seedlings of forest-forming species in the volgograd region. Sib. J. Life Sci. Agric. 2023, 15, 228–245. [Google Scholar] [CrossRef]
- Polyakov, V.; Abakumov, E.; Shamilishvily, G.; Chebykina, E.; Lavrishchev, A. Agrosoils in the city of St. Petersburg: Anthropogenic evolution and current state. In Advances in Understanding Soil Degradation; Springer: New York, NY, USA, 2022; pp. 775–796. [Google Scholar]
- Gallegos, J.; Álvaro, J.E.; Urrestarazu, M. Container Design Affects Shoot and Root Growth of Vegetable Plant. HortScience 2020, 55, 787–794. [Google Scholar] [CrossRef]
- Rahman, M.A.; Fleckenstein, C.; Dervishi, V.; Ludwig, F.; Pretzsch, H.; Rötzer, T.; Pauleit, S. How good are containerized trees for urban cooling? Urban. Urban. Green. 2023, 79, 127822. [Google Scholar] [CrossRef]
- Thom, D.; Keeton, W.S. Disturbance-Based Silviculture for Habitat Diversification: Effects on Forest Structure, Dynamics, and Carbon Storage. For. Ecol. Manag. 2020, 469, 118132. [Google Scholar] [CrossRef]
- Dominguez-Lerena, S.; Herrero Sierra, N.; Carrasco Manzano, I.; Ocaña Bueno, L.; Peñuelas Rubira, J.L.; Mexal, J.G. container characteristics influence Pinus pinea seedling development in the nursery and field. For. Ecol. Manag. 2006, 221, 63–71. [Google Scholar] [CrossRef]
- Harayama, H.; Tobita, H.; Kitao, M.; Kon, H.; Ishizuka, W.; Kuromaru, M.; Kita, K. Enhanced summer planting survival of japanese larch container-grown seedlings. Forests 2021, 12, 1115. [Google Scholar] [CrossRef]
- Jokanović, D.; Devetaković, J.; Nikolić Jokanović, V.; Živanović, K.; Mijatović, L. Variability of anatomical and morphological traits of Pinus nigra and Pinus sylvestris seedlings affected by different container type. Wood Res. 2024, 69, 37–49. [Google Scholar] [CrossRef]
- Tseplyaev, A.; Popova, A.; Paltseva, A. Thuja occidentalis “Smaragd” root system growth modeling, grown using above ground Pot-in-Pot. BIO Web Conf. 2024, 145, 01009. [Google Scholar] [CrossRef]
- Sandhya, S.; Mehta, S.; Pandey, S.; Husen, A. Adventitious root formation in cuttings as influenced by genotypes, leaf area, and types of cuttings. In Environmental, Physiological and Chemical Controls of Adventitious Rooting in Cuttings; Elsevier: Amsterdam, The Netherlands, 2022; pp. 381–395. [Google Scholar]
- Choudhary, B.S.; T., R.K.; Dewangan, S.K. Review Study for Performance Analysis of an Evaporative (Pot in Pot) Cooling System. E3S Web Conf. 2023, 430, 01253. [Google Scholar] [CrossRef]
- Casati, M.; Kichey, T.; Decocq, G. Monographs on Invasive Plants in Europe N°7: Rhododendron Ponticum L. Bot. Lett. 2022, 169, 213–236. [Google Scholar] [CrossRef]
- Hafez, M.; Rashad, M.; Popov, A.I. The Biological Correction of Agro-Photosynthesis of Soil Plant Productivity. J. Plant Nutr. 2020, 43, 2929–2980. [Google Scholar] [CrossRef]
- Eliasson, L. Dependence of Root Growth on Photosynthesis in Populus tremula. Physiol. Plant 1968, 21, 806–810. [Google Scholar] [CrossRef]
- Okoro, O.O.; Grace, J. The physiology of rooting Populus cuttings. Physiol. Plant 1976, 36, 133–138. [Google Scholar] [CrossRef]
- Gudynaitė-Franckevičienė, V.; Pliūra, A. The impact of different environmental conditions during vegetative propagation on growth, survival, and biochemical characteristics in Populus hybrids in clonal field trial. Forests 2021, 12, 892. [Google Scholar] [CrossRef]
- Vigl, F.; Rewald, B. Size Matters?—The diverging influence of cutting length on growth and allometry of two salicaceae clones. Biomass Bioenergy 2014, 60, 130–136. [Google Scholar] [CrossRef]
- Park, B.B.; Han, S.H.; Hernandez, J.O.; An, J.Y.; Nyam-Osor, B.; Jung, M.H.; Lee, P.S.-H.; Lee, S.I. The use of deep container and heterogeneous substrate as potentially effective nursery practice to produce good quality nodal seedlings of Populus sibirica tausch. Forests 2021, 12, 418. [Google Scholar] [CrossRef]
- Puri, S.; Thompson, F.B. Relationship of Water to Adventitious Rooting in Stem Cuttings of Populus Species. Agrofor. Syst. 2003, 58, 1–9. [Google Scholar] [CrossRef]
- McIvor, I.R.; Sloan, S.; Pigem, L.R. Genetic and Environmental Influences on Root Development in Cuttings of Selected Salix and Populus Clones—A Greenhouse Experiment. Plant Soil. 2014, 377, 25–42. [Google Scholar] [CrossRef]
- Meyer, M.; Morgenstern, K.; Heilig, D.; Heil, B.; Kovács, G.; Leibing, C.; Krabel, D. Biomass Allocation and Root Characteristics of Early-Stage Poplars (Populus spp.) for Assessing Their Water-Deficit Response During SRC Establishment. Bioenergy Res. 2021, 14, 385–398. [Google Scholar] [CrossRef]
- Montagnoli, A.; Dumroese, R.K.; Negri, G.; Scippa, G.S.; Chiatante, D.; Terzaghi, M. Asymmetrical Copper Root Pruning May Improve Root Traits for Reforesting Steep and/or Windy Sites. New For. 2022, 53, 1093–1112. [Google Scholar] [CrossRef]
- Konatowska, M.; Rutkowski, P.; Budka, A.; Goliński, P.; Szentner, K.; Mleczek, M. The Interactions between Habitat, Sex, Biomass and Leaf Traits of Different Willow (Salix) Genotypes. Int. J. Environ. Res. 2021, 15, 395–412. [Google Scholar] [CrossRef]
- Boytsov, A.K.; Zhigunov, A.V. Ten-Year Breeding Tests for Growing Clones of Hybrid Aspen and Other Hybrid Poplars in the Conditions of the North-West of Russia. Proc. St. Petersburg For. Res. Inst. 2023, 3, 38–52. [Google Scholar] [CrossRef]
- Douglas, G.B.; McIvor, I.R.; Lloyd-West, C.M. Early Root Development of Field-Grown Poplar: Effects of Planting Material and Genotype. NZ J. For. Sci. 2016, 46, 1. [Google Scholar] [CrossRef]
- Ryynänen, L.; Aronen, T. Genome Fidelity during Short- and Long-Term Tissue Culture and Differentially Cryostored Meristems of Silver Birch (Betula pendula). Plant Cell Tissue Organ. Cult. 2005, 83, 21–32. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).