Influence of Container Volume and Cuttings Size on the Growth Parameters of Seedlings with a Closed Root System of Two Poplar Genotypes in the Voronezh Region
Abstract
1. Introduction
2. Materials and Methods
2.1. Objects of Research and Methodology of Experimentation
2.2. Statistical Analysis
3. Results
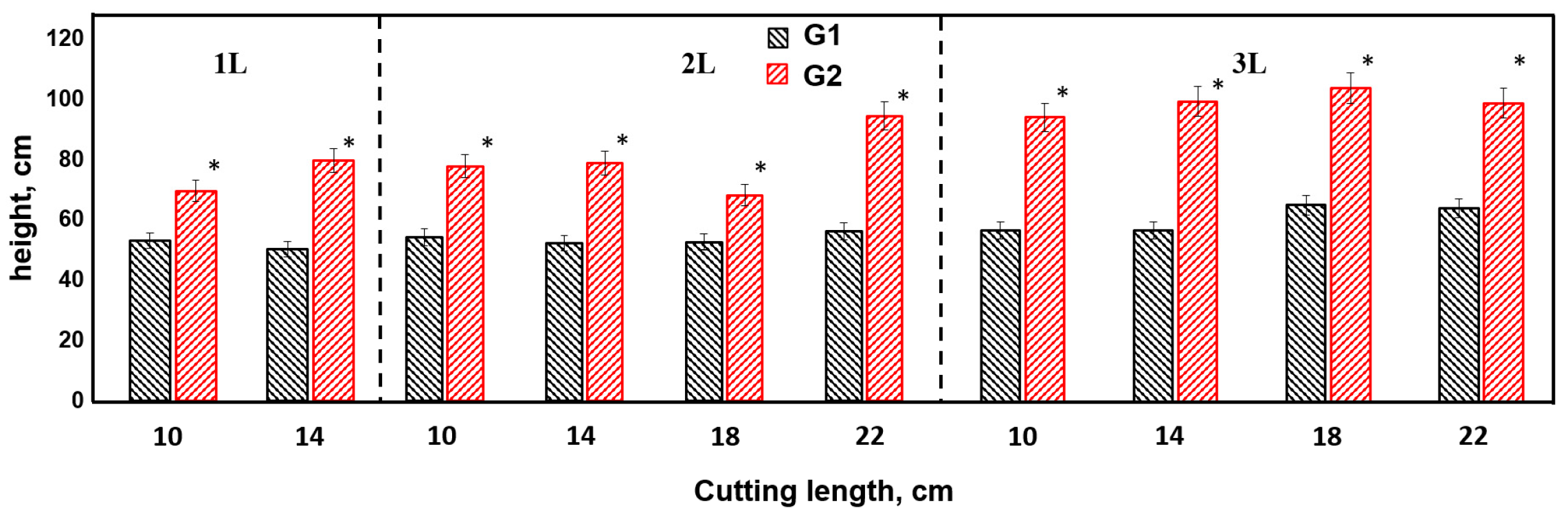
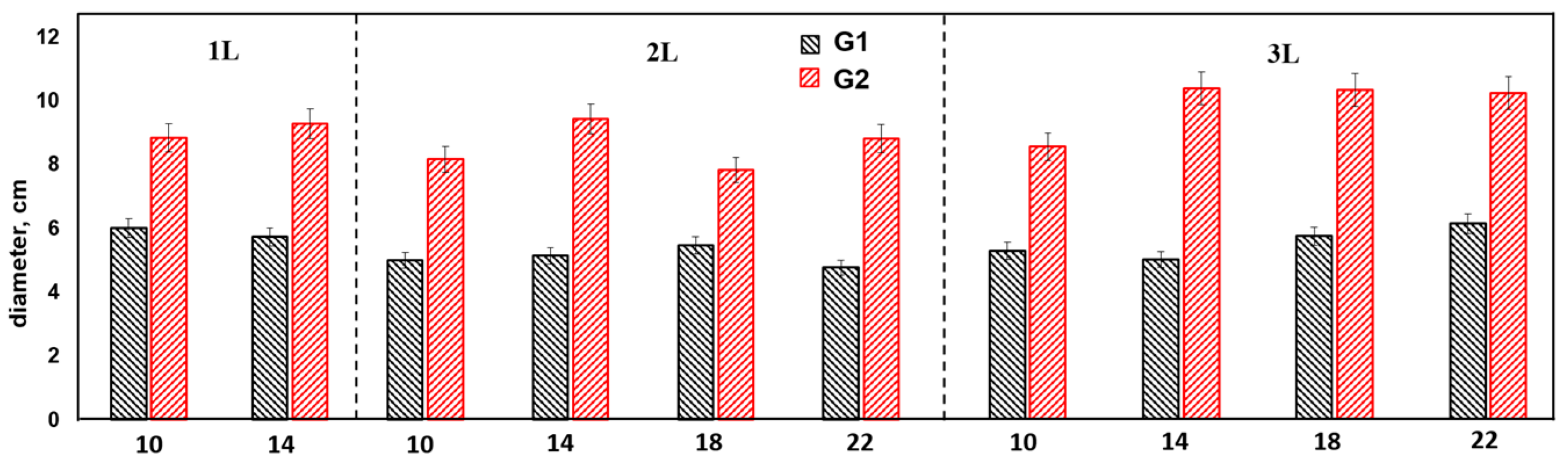
3.1. Effect of Cuttings Size, Container Volume, and Genotype on the Height of Annual Seedlings Obtained by Cuttings
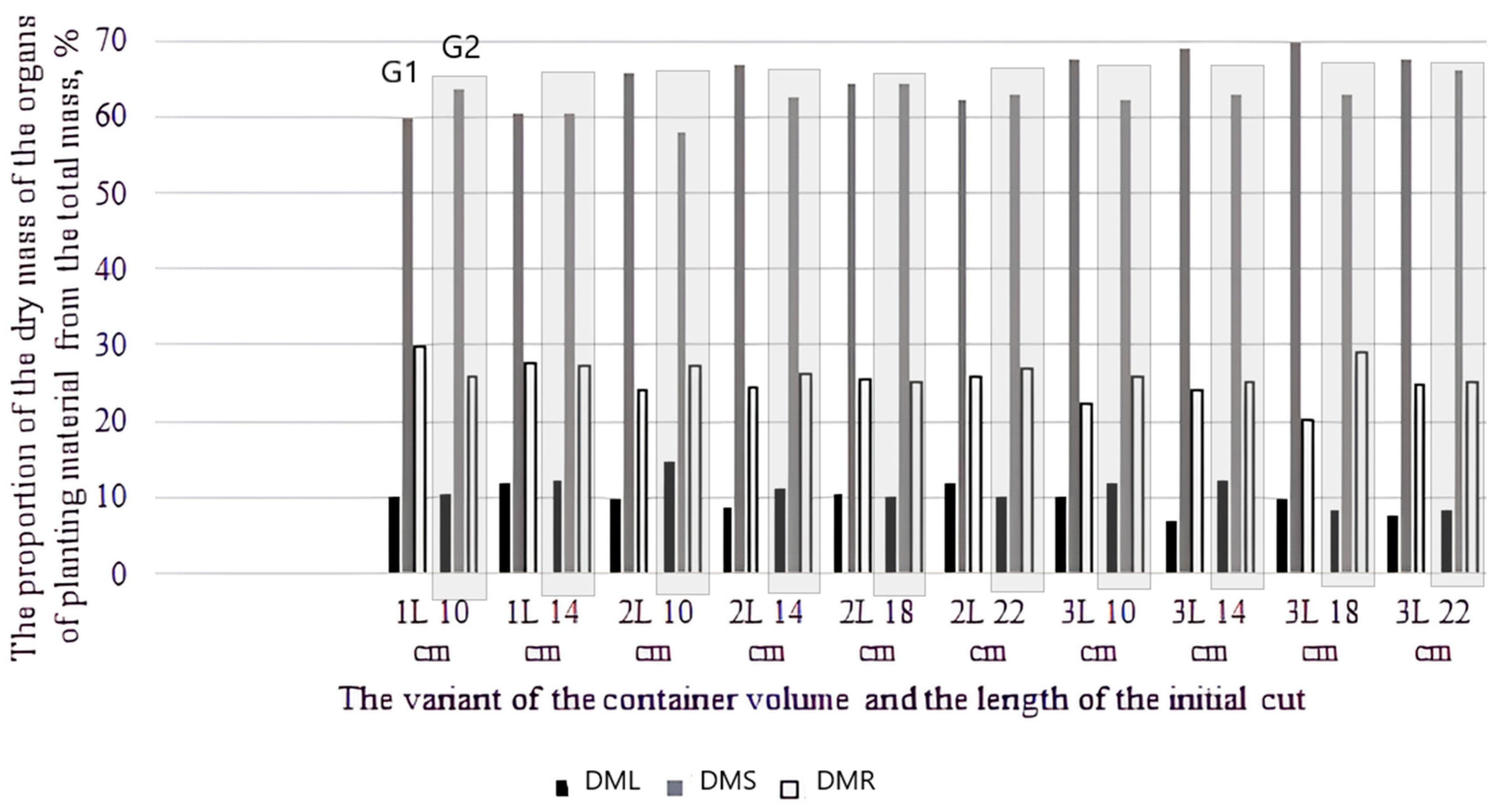
3.2. Effect of Cuttings Size, Container Volume, and Genotype on the Biological Productivity (Biomass) of the Annual Seedlings Obtained by Cuttings
4. Discussion
5. Conclusions
- Genotypic differences: The influence of genotype was found to be more significant than was rooting technology including the selection of cuttings length and container volume. This indicates the dominance of genetic factors over agronomic conditions in determining rooting success.
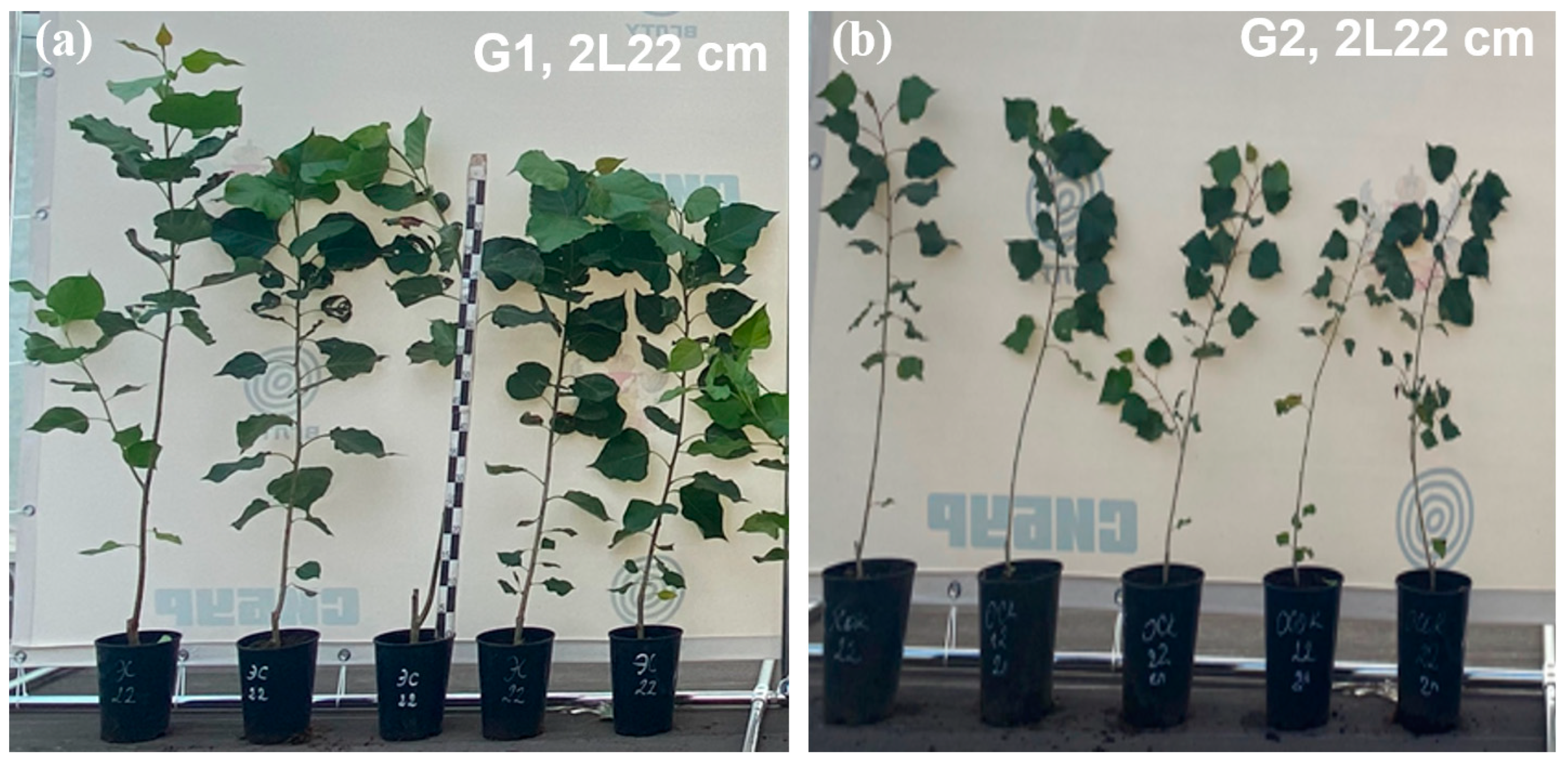
- Comparative productivity analysis: Genotype G1 outperformed the natural species G2 in terms of total biomass by 5.5 times, also showing superiority in height (1.74 times) and diameter (1.7 times). However, the rooting rate (RR) remained identical for both genotypes.
- Effect of cuttings length: The strength of the effect of the factor cuttings length on height increment was 8–10% for G2 and 19–41% for G1. In terms of total biomass, this effect was 12–41% for G2 and 15–75% for G1, indicating variability in rooting across genotypes.
- Productivity grouping: Genotype G1 showed high productivity in 3L18 cm, 3L14 cm, 2L22 cm, whereas for G2, the 3L22 cm and 3L18 cm groups were highly productive. On the contrary, poor productivity was observed in groups with cuttings length of 10–14 cm for both genotypes.
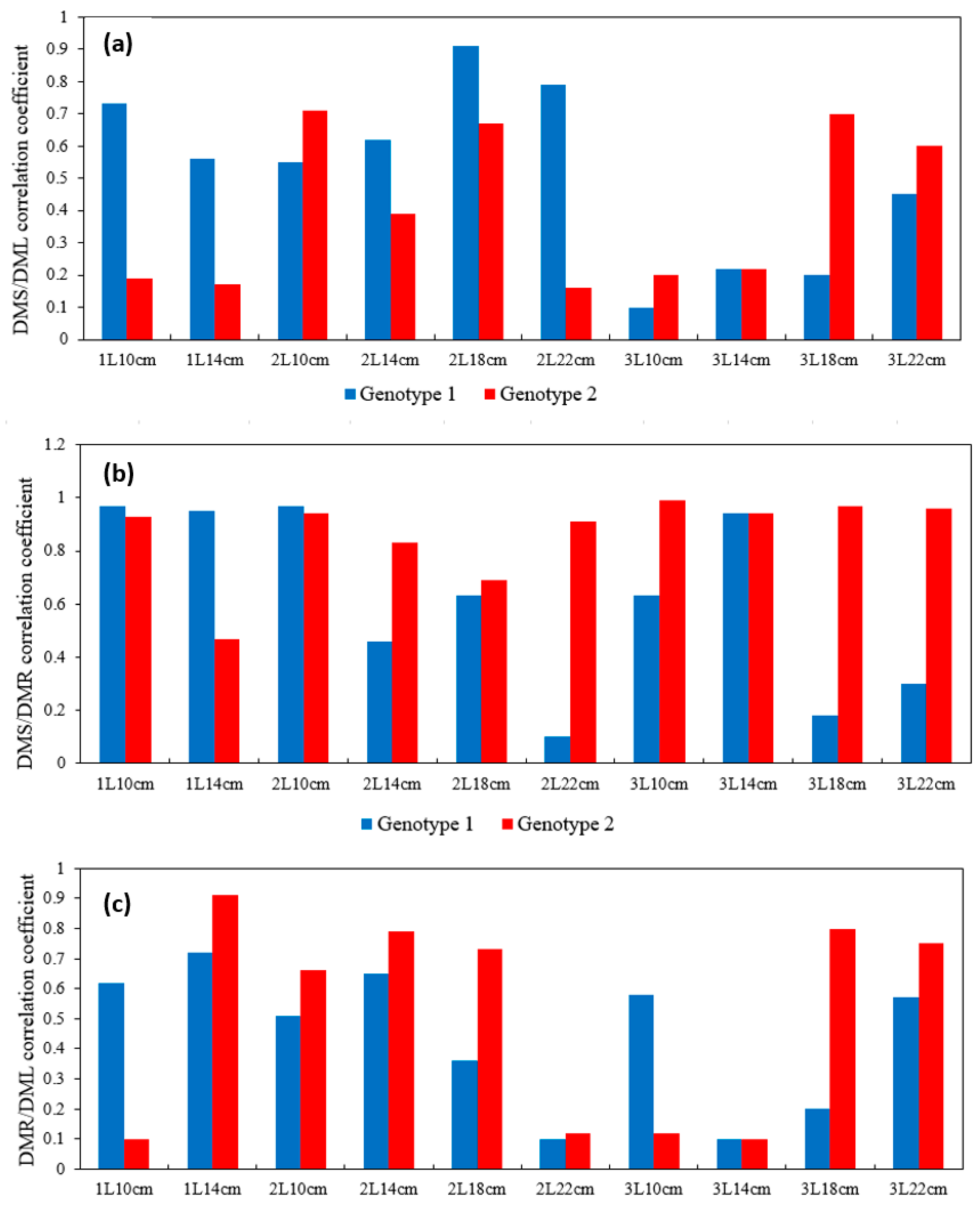
- Correlation and morphological features: Analysis of correlations between seedling height and growth diameter and dry weight distribution indicated biological rooting characteristics that differed between the two genotypes. The plants of the natural species G2 showed faster root system development, while the growth of the hybrid G1 was more proportional, especially when larger volume containers were used.
- Recommendations for propagation conditions: Consideration of biological traits and genetic characteristics is critical for optimising rooting conditions under greenhouse conditions. Genotype 1 (E.s.-38) showed high productivity, offering the potential for rapid production of standardised planting material.
- Recommendations for cuttings and container parameters: For genotype G1, cuttings of 10–14 cm in length with a container volume of 2 L or more are recommended for optimal results, including a height of more than 100 cm and a diameter of 8–10 mm. For genotypes with propagation difficulty, containers of at least 2 L are recommended, with cuttings 22 cm long.
- Need for further research: In order to optimise the propagation parameters for other poplar species and cultivars, further research is required to ensure the correct choice of container volume and cuttings length.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, Q.; Yang, H.; Liu, Y.; Liu, Z.; Xia, S.; Wu, Z.; Zhang, Y. Interdisciplinary perspectives on forest ecosystems and climate interplay: A Review. Environ. Rev. 2024, 33, 1–21. [Google Scholar] [CrossRef]
- Thom, D.; Rammer, W.; Seidl, R. The impact of future forest dynamics on climate: Interactive effects of changing vegetation and disturbance regimes. Ecol. Monogr. 2017, 87, 665–684. [Google Scholar] [CrossRef]
- The Food and Agriculture Organization. Global Forest Resources Assessment: Key Findings; FAO: Rome, Italy, 2020; ISBN 978-92-5-132581-0. [Google Scholar]
- Kotz, M.; Levermann, A.; Wenz, L. The economic commitment of climate change. Nature 2024, 628, 551–557. [Google Scholar] [CrossRef]
- Sicard, P.; Augustaitis, A.; Belyazid, S.; Calfapietra, C.; de Marco, A.; Fenn, M.; Bytnerowicz, A.; Grulke, N.; He, S.; Matyssek, R.; et al. Global topics and novel approaches in the study of air pollution, climate change and forest ecosystems. Environ. Pollut. 2016, 213, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Pollastrini, M.; Puletti, N.; Selvi, F.; Iacopetti, G.; Bussotti, F. Widespread crown defoliation after a drought and heat wave in the forests of tuscany (Central Italy) and their recovery—A case study from summer 2017. Front. For. Glob. Change 2019, 2, 74. [Google Scholar] [CrossRef]
- Pliura, A.; Jankauskiene, J.; Lygis, V.; Suchockas, V.; Bajerkevičiene, G.; Verbylaite, R. Response of juvenile progeny of seven forest tree species and their populations to simulated climate change-related stressors, heat, elevated humidity and drought. IForest 2018, 11, 374–388. [Google Scholar] [CrossRef]
- Gudynaitė-Franckevičienė, V.; Pliūra, A.; Suchockas, V. Ecogenetic plasticity and genetic variation in populus hybrids under the impact of simulated climate change related stressors. Balt. For. 2020, 26, 1–13. [Google Scholar] [CrossRef]
- Grossnickle, S.C.; Ivetić, V. Direct seeding in reforestation—A field performance review. Reforesta 2017, 4, 94–142. [Google Scholar] [CrossRef]
- Singh, A.; Husain, M.; Ali, S.R. Effect of container type and growing media on germination and seedling growth parameters at nursery stage in Allepo pine in Kashmir valley, India. Flora Fauna 2018, 24, 211. [Google Scholar] [CrossRef]
- Luoranen, J.; Rikala, R.; Smolander, H. Root egress and field performance of actively growing Betula pendula Container Seedlings. Scand. J. For. Res. 2003, 18, 133–144. [Google Scholar] [CrossRef]
- Simpson, D.G. Growing density and container volume affect nursery and field growth of interior spruce seedlings. Northern J. Appl. For. 1991, 8, 160–165. [Google Scholar] [CrossRef]
- Psistaki, K.; Tsantopoulos, G.; Paschalidou, A.K. An overview of the role of forests in climate change mitigation. Sustainability 2024, 16, 6089. [Google Scholar] [CrossRef]
- Lipka, O.N.; Korzukhin, M.D.; Zamolodchikov, D.G.; Dobrolyubov, N.Y.; Krylenko, S.V.; Bogdanovich, A.Y.; Semenov, S.M. The role of forests in the adaptation of natural systems to climate change. Forestry 2021, 5, 531–546. [Google Scholar] [CrossRef]
- Schwartz, A.; Starikov, I.V.; Kharlamov, V.S.; Golunov, R.Y.; Kobyakov, A.V.; Lukovtsev, F.Y.; Avtychnikov, A.V.; Tyuleneva, F.V.; Shmatkov, N.M.; Shtegolev, A.A.; et al. New urga look: Proposals in project strategies Development easy Complex. Sustain. For. 2020, 4, 2–25. [Google Scholar]
- Haralambides, H.E. Gigantism in container shipping, ports and global logistics: A time-lapse into the future. Marit. Econ. Logist. 2019, 21, 1–60. [Google Scholar] [CrossRef]
- Allier, A.; Moreau, L.; Charcosset, A.; Teyssèdre, S.; Lehermeier, C. Usefulness criterion and post-selection parental contributions in multi-parental crosses: Application to polygenic trait introgression. G3 Genes. Genomes Genet. 2019, 9, 1469–1479. [Google Scholar] [CrossRef]
- Giniyatullin, R.K.; Ivanov, R.S.; Tagirova, O.V.; Kulagin, A.Y. The content of photosynthetic pigments in the leaves of «healthy» and «weakened» balsam poplar trees (Populus balsamifera) growing under conditions of industrial pollution (Republic of Bashkortostan, Sterlitamak Industrial Center). Samara J. Sci. 2022, 11, 43–48. [Google Scholar] [CrossRef]
- Tsarev, A.P.; Tsareva, R.P.; Tsarev, V.A. Poplar testing and breeding in the Central Chernozem region of Russia. IOP Conf. Ser. Earth Environ. Sci. 2019, 392, 012010. [Google Scholar] [CrossRef]
- Tsarev, A.; Tsareva, R.; Tsarev, V.; Miligula, E.; Lenchenkova, O. Introduced Poplar varieties and new hybrids for protective afforestation. IOP Conf. Ser. Earth Environ. Sci. 2020, 595, 012004. [Google Scholar] [CrossRef]
- Demidova, N.A.; Yaroslavtsev, S.V.; Durkina, T.M.; Fedotov, I.V.; Il’intsev, A.S. The growth course of Neva Poplar (Populus x Newesis Bogd.) and Californian Poplar (Populus Trichocarpa Torr. et Gray) in the European North of Russia. Bull. High. Educ. Institutions. Lesn. Zhurnal (For. J.) 2016, 3, 77–86. [Google Scholar] [CrossRef]
- Crowl, T.A.; Crist, T.O.; Parmenter, R.R.; Belovsky, G.; Lugo, A.E. The spread of invasive species and infectious disease as drivers of ecosystem change. Front. Ecol. Environ. 2008, 6, 238–246. [Google Scholar] [CrossRef]
- Reho, M.; Vilček, J.; Torma, S.; Koco, Š.; Lisnyak, A.; Klamár, R. Growing of the containerized seedlings of english oak (Quercus robur L.) to establish sustainable plantations in forest-steppe Ukraine. Forests 2022, 13, 1359. [Google Scholar] [CrossRef]
- Evlakov, P.M.; Tsarev, A.; Tsareva, R.P.; Rzhevsky, S.G.; Yu Zapletin, V. Evaluation of productivity at the leaf level of juvenile Poplars. IOP Conf. Ser. Earth Environ. Sci. 2019, 226, 012018. [Google Scholar] [CrossRef]
- Heimann, L.; Horst, I.; Perduns, R.; Dreesen, B.; Offermann, S.; Peterhansel, C. A Common histone modification code on c4 genes in maize and its conservation in Sorghum and Setaria italica. Plant. Physiol. 2013, 162, 456–469. [Google Scholar] [CrossRef]
- Boruszewski, P.; Laskowska, A.; Jankowska, A.; Klisz, M.; Mionskowski, M. Potential areas in poland for forestry plantation. Forests 2021, 12, 1360. [Google Scholar] [CrossRef]
- Tsaryov, A.; Tsaryova, R.; Tsaryov, V.; Tseplyaev, A.; Tregubov, O.; Laktionov, A.; Mizin, Y.; Miligula, Y.; Pokhvalenko, V. Rootability of poplar stem cuttings and height of 1-year-old seedlings using various cultivation methods. Astrakhan Bull. Environ. Educ. 2024, 1, 98–103. [Google Scholar] [CrossRef]
- Tsarev, A.P.; Tsareva, R.P.; Tsarev, V.A.; Miligula, E.N. The new poplar hybrids’ growth in the Central Black Earth region of Russia. IOP Conf. Ser. Earth Environ. Sci. 2019, 392, 012011. [Google Scholar] [CrossRef]
- Stanton, B.J.; Bourque, A.; Coleman, M.; Eisenbies, M.; Emerson, R.M.; Espinoza, J.; Gantz, C.; Himes, A.; Rodstrom, A.; Shuren, R.; et al. The practice and economics of hybrid poplar biomass production for biofuels and bioproducts in the pacific northwest. Bioenergy Res. 2021, 14, 543–560. [Google Scholar] [CrossRef]
- Choudhury, M.A.M.; Marcheggiani, E.; Despini, F.; Costanzini, S.; Rossi, P.; Galli, A.; Teggi, S. Urban tree species identification and carbon stock mapping for urban green planning and management. Forests 2020, 11, 1226. [Google Scholar] [CrossRef]
- Renninger, H.J.; Stewart, L.F.; Freeman, J.L.; Rousseau, R.J. Physiological functioning and productivity in eastern cottonwood and hybrid poplars on contrasting sites in the southeastern US. Bioenergy Res. 2022, 15, 1057–1070. [Google Scholar] [CrossRef]
- Smith, K.T. Whither compartmentalization of decay in trees? A commentary on: ‘Using the CODIT model to explain secondary metabolites of xylem in defence systems of temperate trees against decay Ffungi’. Ann. Bot. 2020, 125, iv–vi. [Google Scholar] [CrossRef] [PubMed]
- Perrette, G.; Delagrange, S.; Messier, C. Optimizing reduction pruning of trees under electrical lines: The influence of intensity and season of pruning on epicormic branch growth and wound compartmentalization. Arboric. Urban. For. 2020, 46, 432–449. [Google Scholar] [CrossRef]
- Sapronova, D.V.; Belyaev, A.I.; Semenyutina, A.V.; Khuzhakhmetova, A.S.; Sapronov, V.V. Improved technologies for growing seedlings of forest-forming species in the volgograd region. Sib. J. Life Sci. Agric. 2023, 15, 228–245. [Google Scholar] [CrossRef]
- Polyakov, V.; Abakumov, E.; Shamilishvily, G.; Chebykina, E.; Lavrishchev, A. Agrosoils in the city of St. Petersburg: Anthropogenic evolution and current state. In Advances in Understanding Soil Degradation; Springer: New York, NY, USA, 2022; pp. 775–796. [Google Scholar]
- Gallegos, J.; Álvaro, J.E.; Urrestarazu, M. Container Design Affects Shoot and Root Growth of Vegetable Plant. HortScience 2020, 55, 787–794. [Google Scholar] [CrossRef]
- Rahman, M.A.; Fleckenstein, C.; Dervishi, V.; Ludwig, F.; Pretzsch, H.; Rötzer, T.; Pauleit, S. How good are containerized trees for urban cooling? Urban. Urban. Green. 2023, 79, 127822. [Google Scholar] [CrossRef]
- Thom, D.; Keeton, W.S. Disturbance-Based Silviculture for Habitat Diversification: Effects on Forest Structure, Dynamics, and Carbon Storage. For. Ecol. Manag. 2020, 469, 118132. [Google Scholar] [CrossRef]
- Dominguez-Lerena, S.; Herrero Sierra, N.; Carrasco Manzano, I.; Ocaña Bueno, L.; Peñuelas Rubira, J.L.; Mexal, J.G. container characteristics influence Pinus pinea seedling development in the nursery and field. For. Ecol. Manag. 2006, 221, 63–71. [Google Scholar] [CrossRef]
- Harayama, H.; Tobita, H.; Kitao, M.; Kon, H.; Ishizuka, W.; Kuromaru, M.; Kita, K. Enhanced summer planting survival of japanese larch container-grown seedlings. Forests 2021, 12, 1115. [Google Scholar] [CrossRef]
- Jokanović, D.; Devetaković, J.; Nikolić Jokanović, V.; Živanović, K.; Mijatović, L. Variability of anatomical and morphological traits of Pinus nigra and Pinus sylvestris seedlings affected by different container type. Wood Res. 2024, 69, 37–49. [Google Scholar] [CrossRef]
- Tseplyaev, A.; Popova, A.; Paltseva, A. Thuja occidentalis “Smaragd” root system growth modeling, grown using above ground Pot-in-Pot. BIO Web Conf. 2024, 145, 01009. [Google Scholar] [CrossRef]
- Sandhya, S.; Mehta, S.; Pandey, S.; Husen, A. Adventitious root formation in cuttings as influenced by genotypes, leaf area, and types of cuttings. In Environmental, Physiological and Chemical Controls of Adventitious Rooting in Cuttings; Elsevier: Amsterdam, The Netherlands, 2022; pp. 381–395. [Google Scholar]
- Choudhary, B.S.; T., R.K.; Dewangan, S.K. Review Study for Performance Analysis of an Evaporative (Pot in Pot) Cooling System. E3S Web Conf. 2023, 430, 01253. [Google Scholar] [CrossRef]
- Casati, M.; Kichey, T.; Decocq, G. Monographs on Invasive Plants in Europe N°7: Rhododendron Ponticum L. Bot. Lett. 2022, 169, 213–236. [Google Scholar] [CrossRef]
- Hafez, M.; Rashad, M.; Popov, A.I. The Biological Correction of Agro-Photosynthesis of Soil Plant Productivity. J. Plant Nutr. 2020, 43, 2929–2980. [Google Scholar] [CrossRef]
- Eliasson, L. Dependence of Root Growth on Photosynthesis in Populus tremula. Physiol. Plant 1968, 21, 806–810. [Google Scholar] [CrossRef]
- Okoro, O.O.; Grace, J. The physiology of rooting Populus cuttings. Physiol. Plant 1976, 36, 133–138. [Google Scholar] [CrossRef]
- Gudynaitė-Franckevičienė, V.; Pliūra, A. The impact of different environmental conditions during vegetative propagation on growth, survival, and biochemical characteristics in Populus hybrids in clonal field trial. Forests 2021, 12, 892. [Google Scholar] [CrossRef]
- Vigl, F.; Rewald, B. Size Matters?—The diverging influence of cutting length on growth and allometry of two salicaceae clones. Biomass Bioenergy 2014, 60, 130–136. [Google Scholar] [CrossRef]
- Park, B.B.; Han, S.H.; Hernandez, J.O.; An, J.Y.; Nyam-Osor, B.; Jung, M.H.; Lee, P.S.-H.; Lee, S.I. The use of deep container and heterogeneous substrate as potentially effective nursery practice to produce good quality nodal seedlings of Populus sibirica tausch. Forests 2021, 12, 418. [Google Scholar] [CrossRef]
- Puri, S.; Thompson, F.B. Relationship of Water to Adventitious Rooting in Stem Cuttings of Populus Species. Agrofor. Syst. 2003, 58, 1–9. [Google Scholar] [CrossRef]
- McIvor, I.R.; Sloan, S.; Pigem, L.R. Genetic and Environmental Influences on Root Development in Cuttings of Selected Salix and Populus Clones—A Greenhouse Experiment. Plant Soil. 2014, 377, 25–42. [Google Scholar] [CrossRef]
- Meyer, M.; Morgenstern, K.; Heilig, D.; Heil, B.; Kovács, G.; Leibing, C.; Krabel, D. Biomass Allocation and Root Characteristics of Early-Stage Poplars (Populus spp.) for Assessing Their Water-Deficit Response During SRC Establishment. Bioenergy Res. 2021, 14, 385–398. [Google Scholar] [CrossRef]
- Montagnoli, A.; Dumroese, R.K.; Negri, G.; Scippa, G.S.; Chiatante, D.; Terzaghi, M. Asymmetrical Copper Root Pruning May Improve Root Traits for Reforesting Steep and/or Windy Sites. New For. 2022, 53, 1093–1112. [Google Scholar] [CrossRef]
- Konatowska, M.; Rutkowski, P.; Budka, A.; Goliński, P.; Szentner, K.; Mleczek, M. The Interactions between Habitat, Sex, Biomass and Leaf Traits of Different Willow (Salix) Genotypes. Int. J. Environ. Res. 2021, 15, 395–412. [Google Scholar] [CrossRef]
- Boytsov, A.K.; Zhigunov, A.V. Ten-Year Breeding Tests for Growing Clones of Hybrid Aspen and Other Hybrid Poplars in the Conditions of the North-West of Russia. Proc. St. Petersburg For. Res. Inst. 2023, 3, 38–52. [Google Scholar] [CrossRef]
- Douglas, G.B.; McIvor, I.R.; Lloyd-West, C.M. Early Root Development of Field-Grown Poplar: Effects of Planting Material and Genotype. NZ J. For. Sci. 2016, 46, 1. [Google Scholar] [CrossRef]
- Ryynänen, L.; Aronen, T. Genome Fidelity during Short- and Long-Term Tissue Culture and Differentially Cryostored Meristems of Silver Birch (Betula pendula). Plant Cell Tissue Organ. Cult. 2005, 83, 21–32. [Google Scholar] [CrossRef]
| Container Volume | Handle Length | G1 | G2 | ||
|---|---|---|---|---|---|
| Plant Height | Plant Diameter | Plant Height | Plant Diameter | ||
| 1 | 10 | 0.005855 | 0.005855 | 0.017272 | 0.125750 |
| 1 | 14 | 0.074592 | 0.074592 | 0.155515 | 0.289592 |
| 2 | 10 | 0.278529 | 0.278529 | 0.247487 | 0.215629 |
| 2 | 14 | 0.358922 | 0.358922 | 0.325162 | 0.395622 |
| 2 | 18 | 0.452787 | 0.452787 | 0.618914 | 0.589637 |
| 2 | 22 | 0.554790 | 0.554790 | 0.635565 | 0.652345 |
| 3 | 10 | 0.589626 | 0.589626 | 0.716373 | 0.745633 |
| 3 | 14 | 0.725634 | 0.725634 | 0.536536 | 0.518962 |
| 3 | 18 | 0.745639 | 0.745639 | 0.785397 | 0.777615 |
| 3 | 22 | 0.805239 | 0.805239 | 0.623138 | 0.626384 |
| Experimental Option | Leaf Mass Dry, M, g ± mM | Specific Surface Density of Dry Leaves g ± mM | Stem Dry Mass g ± mM | Root Dry Mass g ± mM | Weight of Whole Plant g ± mM | Productivity Coefficient of the Assimilation Apparatus (g/m2) |
|---|---|---|---|---|---|---|
| G1, E.s.-38, 1 L | ||||||
| 10 cm | 1.0 ± 0.16 | 2.08 ± 0.08 | 5.8 ± 0.54 | 2.9 ± 0.27 | 9.7 ± 0.92 | 20.2 |
| 14 cm | 1.3 ± 0.08 | 2.44 ± 0.14 | 6.5 ± 0.79 | 3 ± 0.7 | 10.8 ± 1.1 | 20.3 |
| G1, E.s.-38, 2 L | ||||||
| 10 cm | 1.1 ± 0.17 | 2.32 ± 0.09 | 7.3 ± 1.19 | 2.7 ± 0.58 | 11.1 ± 1.2 | 23.6 |
| 14 cm | 1.2 ± 0.12 | 2.4 ± 0.14 | 9 ± 0.51 | 3.3 ± 0.41 | 13.5 ± 1.2 | 25 |
| 18 cm | 1.4 ± 0.17 | 2.4 ± 0.11 | 9.6 ± 1.45 | 3.5 ± 0.46 | 14.5 ± 1.3 | 25 |
| 22 cm | 2.0 ± 0.15 | 2.6 ± 0.05 | 10.5 ± 0.8 | 4.4 ± 0.36 | 16.9 ± 1.5 | 21 |
| G1, E.s.-38, 3 L | ||||||
| 10 cm | 1.3 ± 0.14 | 2.32 ± 0.05 | 8.5 ± 0.54 | 2.8 ± 0.25 | 12.6 ± 1.5 | 22.5 |
| 14 cm | 1.4 ± 0.2 | 2.48 ± 0.05 | 13.8 ± 1.69 | 4.8 ± 0.77 | 20.0 ± 1.8 | 35.7 |
| 18 cm | 2.1 ± 0.18 | 2.58 ± 0.09 | 14.7 ± 0.61 | 4.3 ± 0.6 | 21.1 ± 1.9 | 26 |
| 22 cm | 1.8 ± 0.19 | 2.56 ± 0.13 | 15.9 ± 1.82 | 5.9 ± 1.14 | 23.6 ± 1.98 | 33.7 |
| Experimental Option | Leaf Mass Dry, M, g ± mM | Specific Surface Density of Dry Leaves g ± mM | Stem Dry Mass g ± mM | Root Dry Mass g ± mM | Weight of Whole Plant g ± mM | Productivity Coefficient of the Assimilation Apparatus (g/m2) |
|---|---|---|---|---|---|---|
| G2, 1 L | ||||||
| 10 cm | 0.37 ± 0.02 | 0.78 ± 0.03 | 2.2 ± 0.21 | 0.9 ± 0.13 | 3.5 ± 0.32 | 7.4 |
| 14 cm | 0.44 ± 0.04 | 0.83 ± 0.04 | 2.2 ± 0.13 | 1.0 ± 0.14 | 3.64 ± 0.35 | 6.8 |
| G2, 2 L | ||||||
| 10 cm | 0.53 ± 0.07 | 0.77 ± 0.05 | 2.1 ± 0.3 | 1 ± 0.3 | 3.63 ± 0.3 | 5.3 |
| 14 cm | 0.43 ± 0.07 | 0.71 ± 0.06 | 2.4 ± 0.28 | 1 ± 0.13 | 3.8 ± 0.4 | 6.3 |
| 18 cm | 0.45 ± 0.06 | 0.77 ± 0.02 | 2.8 ± 0.38 | 1.1 ± 0.22 | 4.3 ± 0.4 | 7.4 |
| 22 cm | 0.46 ± 0.06 | 0.72 ± 0.08 | 2.8 ± 0.26 | 1.2 ± 0.2 | 4.4 ± 0.4 | 6.9 |
| G2, 3 L | ||||||
| 10 cm | 0.46 ± 0.07 | 0.62 ± 0.07 | 2.4 ± 0.42 | 1.0 ± 0.39 | 3.9 ± 0.4 | 5.2 |
| 14 cm | 0.48 ± 0.04 | 0.66 ± 0.03 | 2.5 ± 0.4 | 1.0 ± 0.2 | 3.4 ± 0.4 | 5.4 |
| 18 cm | 0.51 ± 0.07 | 0.73 ± 0.03 | 3.9 ± 0.55 | 1.8 ± 0.48 | 6.2 ± 0.5 | 8.9 |
| 22 cm | 0.53 ± 0.04 | 0.74 ± 0.07 | 4.2 ± 0.2 | 1.6 ± 0.16 | 6.3 ± 0.5 | 8.9 |
| Probabilities for Poster Criteria Error: Intergroup MS = 0.61629, cc = 45.000 | |||||
|---|---|---|---|---|---|
| Pot Volume | Stem Length | Diameter | Area | Wet Weight | Dry Weight |
| 1 | 10 | 0.018503 | 0.011526 | 0.156259 | 0.002229 |
| 1 | 14 | 0.683412 | 0.852393 | 0.329205 | 0.326940 |
| 2 | 10 | 0.529061 | 0.761087 | 0.937806 | 0.516362 |
| 2 | 14 | 0.051967 | 0.866085 | 0.918322 | 0.630463 |
| 2 | 18 | 0.795126 | 0.558827 | 0.365238 | 0.245495 |
| 2 | 22 | 0.000025 | 0.000144 | 0.000026 | 0.002397 |
| 3 | 10 | 0.310299 | 0.128877 | 0.182473 | 0.829074 |
| 3 | 14 | 0.004127 | 0.518215 | 0.412465 | 0.579407 |
| 3 | 18 | 0.004808 | 0.000951 | 0.000178 | 0.001520 |
| 3 | 22 | 0.006494 | 0.018377 | 0.007283 | 0.029225 |
| Probabilities for Poster Criteria Error: Intergroup MS = 0.61629, cc = 45.000 | |||||
|---|---|---|---|---|---|
| Pot Volume | Stem Length | Diameter | Area | Wet Weight | Dry Weight |
| 1 | 10 | 0.008275 | 0.007272 | 0.028244 | 0.040502 |
| 1 | 14 | 0.575592 | 0.652915 | 0.487722 | 0.423510 |
| 2 | 10 | 0.718649 | 0.047487 | 0.146815 | 0.055874 |
| 2 | 14 | 0.295463 | 0.202025 | 0.483404 | 0.470582 |
| 2 | 18 | 0.448057 | 0.805141 | 0.661465 | 0.833698 |
| 2 | 22 | 0.660695 | 0.003648 | 0.035057 | 0.040165 |
| 3 | 10 | 0.779258 | 0.016373 | 0.102808 | 0.289789 |
| 3 | 14 | 0.718649 | 0.336169 | 0.733309 | 0.899434 |
| 3 | 18 | 0.177615 | 0.039725 | 0.073395 | 0.108325 |
| 3 | 22 | 0.026384 | 0.013768 | 0.074460 | 0.064087 |
| Pot Volume/Cutting Length | The Power of Influence | t0.05 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1/10 | 1/14 | 1/18 | 1/22 | 2/10 | 2/14 | 2/18 | 2/22 | 3/10 | 3/14 | 3/18 | 3/22 | ||
| 1/10 | – | 0.21 | 0.12 | 0.44 | 0.88 | 0.95 | 1.11 | 1.02 | 2.01 | 2.15 | 2.54 | 4.2 | 2.04 |
| 1/14 | 0.21 | – | 0.08 | 0.33 | 0.74 | 1.14 | 2.01 | 2.22 | 2.54 | 3.14 | 3.22 | 2.98 | 2.04 |
| 2/10 | 0.88 | 0.74 | 0.98 | 1.95 | – | 0.4 | 1.12 | 0.95 | 1.54 | 1.89 | 2.01 | 2.04 | 2.04 |
| 2/14 | 0.95 | 1.14 | 1.24 | 2.11 | 0.4 | – | 0.46 | 0.61 | 0.88 | 0.74 | 2.0 | 2.03 | 2.04 |
| 2/18 | 1.11 | 2.01 | 1.54 | 2.34 | 1.12 | 0.56 | – | 1.45 | 2.15 | 2.25 | 2.87 | 3.01 | 2.04 |
| 2/22 | 1.02 | 2.22 | 1.88 | 2.16 | 0.95 | 0.61 | 1.45 | – | 0.23 | 0.51 | 0.96 | 1.45 | 2.04 |
| 3/10 | 2.01 | 2.54 | 1.51 | 3.18 | 1.54 | 0.88 | 2.15 | 0.23 | – | 1.54 | 2.13 | 2.28 | 2.04 |
| 3/14 | 2.15 | 3.14 | 2.17 | 3.33 | 1.89 | 0.74 | 2.25 | 0.51 | 1.54 | – | 1.22 | 1.56 | 2.04 |
| 3/18 | 3.54 | 3.22 | 3.01 | 4.12 | 2.01 | 2.0 | 2.87 | 0.96 | 2.13 | 1.22 | – | 0.02 | 2.04 |
| 3/22 | 4.2 | 2.98 | 3.52 | 4.54 | 2.04 | 2.03 | 3.01 | 1.45 | 2.28 | 1.56 | 0.02 | – | 2.04 |
| Pot Volume/Cutting Length | The Power of Influence | t0.05 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1/10 | 1/14 | 1/18 | 1/22 | 2/10 | 2/14 | 2/18 | 2/22 | 3/10 | 3/14 | 3/18 | 3/22 | ||
| 1/10 | – | 0.86 | 0.95 | 1.05 | 2.05 | 2.11 | 2.10 | 3.65 | 3.84 | 3.96 | 3.44 | 3.88 | 2.04 |
| 1/14 | 0.86 | – | 0.47 | 0.44 | 0.87 | 1.12 | 1.23 | 2.14 | 2.22 | 2.35 | 3.11 | 3.56 | 2.04 |
| 2/10 | 2.05 | 0.87 | 0.08 | 0.12 | – | 0.02 | 0.08 | 0.22 | 0.65 | 0.14 | 2.14 | 2.63 | 2.04 |
| 2/14 | 2.11 | 1.12 | 1.01 | 0.10 | 0.02 | – | 0.15 | 0.74 | 2.22 | 2.88 | 3.54 | 4.12 | 2.04 |
| 2/18 | 2.10 | 1.23 | 1.15 | 0.34 | 0.08 | 0.15 | – | 0.01 | 0.08 | 0.15 | 0.85 | 2.14 | 2.04 |
| 2/22 | 3.65 | 2.14 | 0.98 | 0.52 | 0.22 | 0.74 | 0.01 | – | 0.88 | 0.25 | 0.17 | 0.65 | 2.04 |
| 3/10 | 3.84 | 2.22 | 0.88 | 2.32 | 0.65 | 2.22 | 0.08 | 0.88 | – | 0.07 | 0.14 | 0.25 | 2.04 |
| 3/14 | 3.96 | 2.35 | 1.85 | 2.15 | 0.14 | 2.88 | 0.15 | 0.25 | 0.07 | – | 0.12 | 0.05 | 2.04 |
| 3/18 | 3.44 | 3.11 | 2.09 | 2.06 | 2.14 | 3.54 | 0.85 | 0.17 | 0.14 | 0.12 | – | 0.09 | 2.04 |
| 3/22 | 3.88 | 3.56 | 2.08 | 2.38 | 2.63 | 4.12 | 2.14 | 0.65 | 0.25 | 0.05 | 0.09 | – | 2.04 |
| Selected Groups by Productivity | G1 | G2 | ||
|---|---|---|---|---|
| Total Dry Mass, g | Cultivation Options | Total Dry Mass, g | Cultivation Options | |
| High-productivity group | 21.5 ± 0.97 | 3L22 cm; 3L18 cm; 3L14 cm; 2L22 cm. | 6.4 ± 0.19 | 3L22 cm; 3L18 cm. |
| Medium-productivity group | 14.4 ± 0.49 | 3L10 cm; 2L14 cm; 2L18 cm. | 4.4 ± 0.05 | 2L22 cm; 2L18 cm. |
| Low-productivity group | 10.5 ± 0.42 | 2L10 cm; 1L10 cm; 1L14 cm. | 3.6 ± 0.08 | 3L14 cm; 3L10 cm; 2L14 cm; 2L10 cm; 1L14 cm; 1L10 cm. |
| Average productivity of all variants of the experiment | 17.9 ± 2.6 | 4.5 ± 0.38 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evlakov, P.; Tseplyaev, A.; Popova, A.; Zapletin, V.; Ryzhkova, V.; Repnikova, L.; Zhuzhukin, K. Influence of Container Volume and Cuttings Size on the Growth Parameters of Seedlings with a Closed Root System of Two Poplar Genotypes in the Voronezh Region. Int. J. Plant Biol. 2025, 16, 49. https://doi.org/10.3390/ijpb16020049
Evlakov P, Tseplyaev A, Popova A, Zapletin V, Ryzhkova V, Repnikova L, Zhuzhukin K. Influence of Container Volume and Cuttings Size on the Growth Parameters of Seedlings with a Closed Root System of Two Poplar Genotypes in the Voronezh Region. International Journal of Plant Biology. 2025; 16(2):49. https://doi.org/10.3390/ijpb16020049
Chicago/Turabian StyleEvlakov, Peter, Alexey Tseplyaev, Anna Popova, Vladimir Zapletin, Vladlena Ryzhkova, Lyudmila Repnikova, and Konstantin Zhuzhukin. 2025. "Influence of Container Volume and Cuttings Size on the Growth Parameters of Seedlings with a Closed Root System of Two Poplar Genotypes in the Voronezh Region" International Journal of Plant Biology 16, no. 2: 49. https://doi.org/10.3390/ijpb16020049
APA StyleEvlakov, P., Tseplyaev, A., Popova, A., Zapletin, V., Ryzhkova, V., Repnikova, L., & Zhuzhukin, K. (2025). Influence of Container Volume and Cuttings Size on the Growth Parameters of Seedlings with a Closed Root System of Two Poplar Genotypes in the Voronezh Region. International Journal of Plant Biology, 16(2), 49. https://doi.org/10.3390/ijpb16020049








