Abstract
The study of microorganisms associated with tropical plant species, particularly fungi, has garnered significant interest due to their potential applications in biological control and the synthesis of pharmacologically active compounds. This research aimed to identify and characterize the endophytic fungal communities associated with cocoa (Theobroma cacao L.) fruits across three municipalities in the Orellana province, located within the Ecuadorian Amazon. Fungi were isolated directly from cocoa fruits and analyzed through comprehensive cultural, morphological, and molecular analyses. The diversity of fungal taxa was evaluated using metrics of relative abundance and species richness. A total of 464 fungal isolates were obtained, representing 56 distinct morphotypes and 14 genera within the phylum Ascomycota. The most abundant genera included Penicillium sp. (27.8%), Epicoccum sp. (20.5%), Lasiodiplodia sp. (10.1%), Trichoderma sp. (9.91%), and Fusarium sp. (9.70%). Notably, in the municipality of La Joya de los Sachas, a higher number of endophytic fungi was observed, encompassing 14 genera. This study provides critical insights into the diversity and distribution of fungal communities associated with cocoa fruits in the northern Ecuadorian Amazon. These findings have important implications for the management of cocoa diseases and the discovery of novel bioactive compounds. Future investigations should explore the functional roles of these fungi, particularly their potential as biocontrol agents or sources of novel pharmaceuticals. Furthermore, examining the effects of environmental variables and agricultural practices on cocoa fruit mycobiota may contribute to a deeper understanding of the ecological dynamics within this system.
1. Introduction
Plants ubiquitously associate with diverse microorganisms [1], forming complex interactions that range from mutualism to antagonism [2]. Fungi play a crucial role in shaping the diversity and composition of terrestrial plant communities. Despite their ecological and functional significance, our understanding of these intricate plant–fungal interactions remains limited. Current estimates suggest that ecological relationships have been documented for less than 5% of the approximately 1.5 million extant fungal species [3]. This vast and largely unexplored fungal kingdom encompasses a wide functional diversity, with certain species acting as plant pathogens capable of infecting crops at any stage of development, while others enhance crop health and resilience [4]. These findings underscore the pressing need for further research to elucidate the complex interplay between plants and their associated fungal communities.
Endophytic fungi represent a highly diverse group of microorganisms that contribute significantly to plant health [5]. These fungi colonize the internal tissues of a wide range of plant hosts, including mosses, liverworts, ferns, conifers, and angiosperms, typically residing asymptomatically within healthy aerial tissues [6]. Endophytes exert beneficial effects on their hosts through various mechanisms, such as the production of phytohormones that stimulate plant growth and antimicrobial compounds that defend against pathogens [1]. Additionally, they compete with plant pathogens for resources and ecological niches [7], effectively limiting pathogen establishment and proliferation. While research on plant-associated microbes has predominantly focused on plant pathogens and mycorrhizal fungi, particularly in temperate environments and agricultural systems, increasing attention is being directed toward the potential of endophytic fungi in biological control and the production of pharmacologically active compounds [8].
The dominant fungal phylum in these associations is Ascomycota, followed by Basidiomycota and Mucoromycota, which are less frequently observed [9]. Fungi have been identified in association with approximately 300,000 plant species, colonizing diverse tissues such as leaves, flowers, petioles, roots, and fruits [10]. Coevolutionary processes between fungi and their plant hosts have often led to reduced fungal virulence or pathogenicity [11]. The nature of these interactions varies along a continuum from symbiotic to mutualistic to pathogenic [12,13], depending on environmental conditions and the host plant’s health status [8].
Several studies have investigated fungal communities associated with cocoa (Theobroma cacao L.). For instance, six fungal species have been identified: Lasiodiplodia pseudotheobromae, Arthrinium rasikravindrae, Diaporthe sp., Lasiodiplodia theobromae, and Colletotrichum sp., representing four families: Botryosphaeriaceae, Apiosporaceae, Diaporthaceae, and Glomerellaceae [7]. Additionally, other researchers have reported various fungi, including Trichoderma, Pestalotiopsis, Curvularia, Tolypocladium, Colletotrichum sp., Botryosphaeria sp., Xylaria sp., Clonostachys sp., Fusarium spp., Acremonium sp., and Phomopsis sp., isolated from T. cacao [4,14]. These fungi are environmentally acquired and contribute to plant defense through mechanisms such as induced systemic resistance, pathogen suppression, and inhibition via bioactive compound secretion [15].
In addition to endophytic fungi, pathogenic fungi and oomycetes significantly impact cocoa cultivation by causing diseases affecting flowers, foliage, and fruits. Key pathogens include Phytophthora spp., Moniliophthora roreri, Moniliophthora perniciosa, Ceratosystis cacaofunesta, and Oncobasidium theobromae. Among these, Phytophthora spp. and M. roreri are particularly notable, causing global cocoa production losses estimated between 20% and 60% [14]. These pathogens often exhibit resilience to adverse environmental conditions, such as high temperatures, excessive rainfall, and elevated relative humidity [16]. Climate change may exacerbate these challenges by influencing disease distribution and severity and potentially altering host resistance [17].
While previous studies have explored fungal communities associated with T. cacao in various regions, a significant gap remains in understanding endophytic fungal diversity specifically within cocoa fruits in the northern Ecuadorian Amazon. This knowledge gap is crucial, as endophytic fungi play vital roles in plant health, including disease resistance and nutrient acquisition. The unique environmental conditions of the northern Ecuadorian Amazon may harbor novel or understudied fungal species with significant biotechnological potential. This study, therefore, aims to comprehensively characterize the diversity of fungi associated with T. cacao fruits in this region using an integrative cultural, morphological, and molecular approach. This multifaceted methodology will provide a robust assessment of fungal diversity, advancing our understanding of the ecological roles of these fungi and their potential contributions to sustainable cocoa cultivation in the Amazon.
2. Materials and Methods
2.1. Samples Collection
A total of 59 healthy cocoa (Theobroma cacao L.) fruit samples, representing the traditional varieties CCN 51, Super Arbol, and Nacional, were collected for this study. These samples were sourced from cocoa producers in the northern region of the Ecuadorian Amazon. Collection sites were situated in the Orellana province, Ecuador, encompassing three distinct municipalities: Loreto (0°47′28″ S, 77°22′19″ W, 373 m a.s.l.), Francisco de Orellana (0°37′36″ S, 77°07′40″ W, 292 m a.s.l.), and La Joya de los Sachas (0°45′41″ S, 77°05′01″ W, 330 m a.s.l.) Table 1. These sites represent varying microclimates within the region. Loreto has an average temperature of 23.6 °C, with relative humidity ranging from 81% to 89% and an annual rainfall of 2516 mm. Francisco de Orellana experiences an average temperature of 26 °C, with relative humidity ranging from 86% to 89% and annual rainfall varying from 2800 mm to 4500 mm. La Joya de los Sachas is characterized by an average temperature of 25 °C, 90% relative humidity, and 4534 mm of annual precipitation. Healthy cocoa fruit samples were placed in sterile plastic bags, transported in coolers, and stored at 4–8 °C in the laboratory until processing.

Table 1.
Information on the sites sampled in each municipality and the cultivars used in this study.
2.2. Isolation of Fungi Associated with Healthy Cocoa Fruits
For the isolation of endophytic fungi, mature fruits were selected in the field. On these samples, basal areas of the fruit exposed to light were marked, from which five tissue fragments, approximately 25 mm2 each, were excised. The fragments were taken from the region between the exocarp and pericarp using a sterilized scalpel. Under sterile conditions within a laminar flow hood, the fragments were surface sterilized by sequential immersion in 70% ethanol for 30 s and 2% sodium hypochlorite for 2 min, followed by three rinses with sterile distilled water for 30 s each. The sterilized fragments were then placed on sterile paper towels for drying. Subsequently, the fragments were transferred to Petri dishes containing potato dextrose agar (PDA, Difco™) supplemented with chloramphenicol (25 μg/mL) and streptomycin (25 μg/mL). The plates were incubated at 26 °C in the dark for 12 days. Pure cultures were obtained by transferring a portion of the mycelium from the initial plates to fresh PDA plates. These plates were sealed with parafilm and incubated at 26 °C in the dark for an additional 12 days in an inverted position [14].
Monosporic cultures were prepared by transferring a small portion of mycelium from the pure cultures to test tubes containing 10 mL of sterile distilled water using a sterilized toothpick [18]. After vortexing for 10 s, the resulting suspension was spread onto synthetic nutrient-poor agar in Petri dishes. Excess water was removed, and the plates were sealed, labeled, and incubated laterally at 26 °C for 24 h. Germinated spores were identified using a stereomicroscope, and single spores were excised with a sterilized scalpel and transferred to fresh PDA plates. These plates were sealed, labeled, and incubated at 26 °C, with daily monitoring of the growth of isolates. All fungal isolates obtained from T. cacao fruits were preserved according to the method described by Castellani and stored at 4–8 °C [19].
2.3. Morphological and Cultural Characterization
Following the adhesive strip method [20], a drop of lactoglycerol was applied to a microscope slide, and a transparent adhesive tape was used to lift a portion of the fungal colony, taking care to avoid the formation of air bubbles. Microscopic and cultural analyses were conducted using a Leica DML optical microscope equipped with phase contrast and/or a Leica DMR epifluorescent light microscope equipped with a 50 W mercury lamp and a 450–490 nm excitation filter. Fungal identification was primarily based on macroscopic colony characteristics and the micromorphology of reproductive structures. Cultured colonies were characterized based on mycelial color (obverse and reverse) [21], margin shape, surface texture, elevation, edge characteristics, and mycelium color, using standard colors according to the Pantone Color Matching System. Trichoderma isolates were characterized by certain methods [22,23]. Fungal structures were observed and identified using fungi identification keys based on morphological characteristics, such as mycelium structure and spore morphology according to classical references [24,25]. For each fungus isolate, 10 random measurements were taken of the following structures: hyphae, conidia, conidiophores, conidiogenous cells, and pycnidia. Both morphological and cultural characteristics were used for fungal phenotypic characterization.
2.4. DNA Extraction, Amplification and Sequencing
Genomic DNA was extracted from monoconidial cultures using the Wizard® Genomic DNA Purification Kit. DNA was quantified on 1% agarose gels against a 50 ng lambda DNA molecular weight marker (Promega, Madison, WI, USA) and visualized using a Loccus Biotechnology Molecular Imaging Transilluminator. DNA quality and concentration were assessed using a Nanodrop® 2000c (Thermo Fisher Scientific, Waltham, MA, USA) spectrophotometer by measuring the 260/280 absorbance ratio. DNA was then diluted to a final concentration of 50 ng/µL. The internal transcribed spacer region of ribosomal DNA was amplified by PCR using 0.2 µM of ITS1/ITS4 primers [26], 1× Taq DNA Polymerase Master Mix (Promega, Madison, WI, USA), and approximately 50 ng of template DNA in a total reaction volume of 50 µL. PCR cycling conditions were as follows: initial denaturation at 95 °C for 5 min, followed by 35 cycles of 95 °C for 1 min, 56 °C for 45 s, and 72 °C for 90 s, with a final extension at 72 °C for 10 min, using a Thermo Fisher Scientific thermocycler (Thermo Fisher Scientific, Waltham, MA, USA). Amplification success was confirmed by electrophoresis on a 1% agarose gel. Amplicons were purified using the NZYGelpure kit and sent to Macrogen for Sanger sequencing. The resulting electropherograms were analyzed using 4Peaks software v 1.8, and sequences were analyzed using the BLAST algorithm at the National Center for Biotechnology Information (www.ncbi.nlm.nih.gov; accessed on 1 November 2024).
2.5. Fungal Diversity Analysis
The analyses of taxonomic composition were performed using the relative abundance matrix of genera identified, later grouped in Operational Taxonomic Units (OTUs), where each genus reflects one OTU. Plots with genera taxonomic composition were constructed using the R 4.4.1 program (R Development Core Team) with the ggplot library. The analysis of fungal diversity was calculated using the Hill series (effective number of genera) with the library iNEXT using the relative abundance of the taxa found in the samples. The Alpha diversity, which analyzes the diversity within each sample, was estimated by local (La Paz, Bajo Huino, La Belleza, San Carlos, San Jacinto, and Calumeña) and cocoa cultivar (Nacional, CCN51, and Super Arbol) obtaining Fungal Richness (q = 0), Shannon diversity (q = 1), and Simpson diversity (q = 2).
3. Results
3.1. Analysis of the Diversity of Fungi Present in Cocoa Fruits
A total of 464 fungal samples were isolated from T. cacao fruits. Most of the isolates were identified by their morphological and molecular characteristics, with the most abundant fungi of the genus being Penicillium sp. (27.8%), Epicoccum sp. (20.5%), Lasiodiplodia sp. (10.1%), Trichoderma sp. (9.91%), and Fusarium sp. (9.70%) and the least frequent being Nigrospora sp, Clonostachys sp., Diaporthe sp., Daldinia sp., Neopestalotiopsis sp., Alternaria sp., Purpureocillium sp., Coniochaeta sp., and Geotrichum (Table 2).

Table 2.
Frequency of fungi isolated from healthy Theobroma cacao fruit in the province of Orellana.
In the municipality of Loreto, 13 genera were found in the two localities, 7 in La Paz and 6 in Bajo Huino. In the two localities, four genera were found in common (Penicillium sp.: 33.33 and 36.61%, Epicoccum sp.: 22.22 and 27.78%, Lasiodiplodia sp.: 18.52 and 4.17%, and Fusarium sp.: 14.81 and 4.17%). In addition, in the two localities, the fungi were grouped in three (Diaporthe sp.: 1.86, Alternaria sp.: 5.56, and Purpureocillium sp.: 3.7%) and two (Trichoderma sp.: 18.05 and Nigrospora sp.: 9.72%) different genera, respectively. In the municipality of Francisco de Orellana, in the locality of La Belleza, in order of abundance, ten genera were determined: Penicillium sp. (25.53%), Lasiodiplodia sp. (21.28%), Trichoderma sp. (15.96%), Epicoccum sp. (15.95%), Diaporthe sp. (6.38%), Geotrichum sp. (4.26%), Clonostachys sp. (3.19%), Daldinia sp. (3.19%), Neopestalotiopsis sp. (2.13%), and Alternaria sp. (2.13%). In La Joya de los Sachas, 14 genera and 3 genera in common were identified in the three localities (Penicillium sp.: 38.14, 2.27, and 22.33%, Fusarium sp.: 4.12, 4.55, and 27.18%, and Clonotachys sp.: 4.12, 4.55, and 7.77%). In San Carlos and La Calumeña, three genera were found in common (Epicoccum sp.: 25.77 and 22.34, Lasiodiplodia sp.: 9.27 and 4.85%, Nigrospora sp.: 12.37 and 5.82%). In San Carlos and San Jacinto, two genera were identified in common: Coniochaeta sp. (2.6 and 9.09%) and Geotrichum sp. (2.07 and 2.27%) as well as in San Jacinto and La Calumeña, Diaporthe sp. (9.09 and 4.85%) and Daldinia sp. (6.28 and 4.85%). In addition, in San Carlos and San Jacinto, four more genera were identified (two per locality): in San Carlos, the genera Alternaria sp. (1.04%) and Purpureocillium sp. (2.6%), and in San Jacinto, Trichoderma sp. (40.91%) and Neopestalotiopsis sp. (20.14%). Finally, fungi not identified by genus were found in the locality La Belleza in Francisco de Orellana (4.26%) and in San Carlos in Joya de los Sachas (2.07%) (Figure 1).
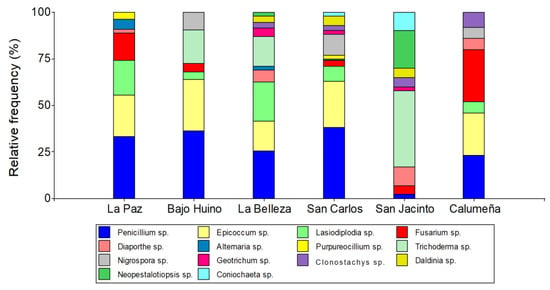
Figure 1.
Relative abundance at genus level of fungi isolated from T. cacao fruits in the province of Orellana.
The analysis of relative abundance for the group of fungal microorganisms identified in the fruits of T. cacao allowed them to be grouped in the fungal phylum Ascomycota. On the other hand, the analysis by class grouped the fungi into Sordariomycetes (39.85%), Dothidiomycetes (31.89%), Eurotiomycetes (27.8%), and Saccharomyces (0.43%). In addition, in the two localities (La Paz and Bajo Huino) of Loreto canton, in Francisco de Orellana (La Belleza), and in two localities (San Carlos and La Calumeña) in La Joya de los Sachas, three classes of fungi (Eurotiomycetes, Dothidiomycetes, and Sordariomycetes) were present; and Saccharomyces was identified in two localities (San Carlos and San Jacinto) of La Joya de los Sachas.
In the localities La Paz and Bajo Huino, the fungi were grouped in Eurotiomycetes (33.33 and 36.12%), Dothidiomycetes (46.3 and 31.94%), and Sordariomycetes (20.37 and 31.94%), respectively. In the canton of Francisco de Orellana, the most abundant class was Dothidiomycetes (39.09%), followed by Sordariomycetes (35.11%), and finally, Eurotiomycetes (25.53%). In the canton of La Joya de los Sachas, in San Carlos, San Jacinto, and La Calumeña, Eurotiomycetes (38.14, 2.27, and 22.33%) and Sordariomycetes (24.74, 95.46, and 50.49%), respectively, were identified. Also, the class Dothidiomycetes (36.09 and 27.18%) was identified only in San Carlos and La Calumeña; and Saccharomycetes (2.27 and 1.03%) in San Carlos and San Jacinto (Figure 2).
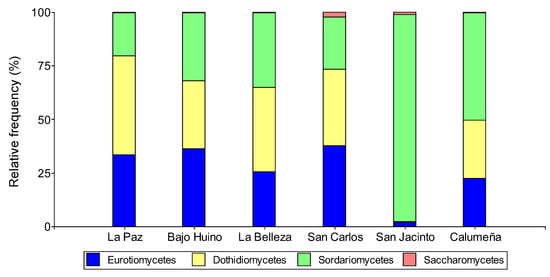
Figure 2.
Relative abundance at the class level of fungi from T. cacao fruits in the province of Orellana.
Our analysis at the order level allowed classifying the fungi in seven orders, whereby Eurotiales and Hypocreales (26.29 and 25.86%, respectively) were present in the six localities; Pleosporales, Xylariales, and Botryosphaeriales (20.46, 11.95, and 9.68%, respectively) were only found in five localities; and Diaporthales, Coniochaetales, and Saccharomycetales (3.70, 1.51, and 0.55%, respectively) were the lowest-frequency orders and were present in four and two localities, respectively.
In addition, the greatest number of orders were present in the municipalities of Francisco de Orellana and La Joya de los Sachas, six for each locality. On the other hand, in Loreto, in the two localities, the fungi were grouped into five orders. In La Paz and Bajo Huino, there was a higher presence of Eurotiales (36.11 and 33.33%), Pleosporales (27.68 and 27.78%), and Hypocreales (18.52 and 22.22%), respectively. Botryosphaeriales were present in greater numbers in La Paz (18.52%) with respect to Bajo Huiruno (4.17%). Xylariales was only present in Bajo Huiruno (9.72%) and Diaporthales in La Paz (1.85%). In Francisco de Orellana, the fungal microorganisms were grouped into six orders, with the most frequent being Eurotiales (25.53%), Botryosphaeriales (21.28%), Hypocreales (19.16%), Saccharomycetales (18.08%), Xylariales (9.57%), and Diaporthales (6.38%). In La Joya de los Sachas, the abundance of microorganisms by locality was very variable; in San Carlos, the orders identified were Eurotiales (38.15%), Pleosporales (26.8%), Xylariales (14.44%), Hypocreales (10.31%), Botryosphaeriales (9.27%), and Saccharomycetales (1.03%); in San Jacinto, the orders were Hypocreales (50%), Hylariales (34.95%), Diaporthales (9.1%), Coniochaetales (9.09%), and Saccharomycetales (2.27%); and in La Calumeña, Hypocreales (34.96%), Eurotiales (22.33%), Pleosporales (22.33%), Hylariales (10.68%), Botryosphaeriales (4.85%), and Diaporthales (4.85%) were present (Figure 3).
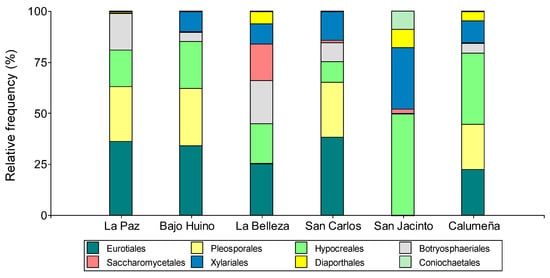
Figure 3.
Relative abundance at order level of fungi isolated from T. cacao fruits in Orellana province.
Relative abundance at the family level allowed us to group the fungal microorganisms into 15 families. In order of importance, we observed the presence of Aspergillaceae (26.28%), Didymellaceae (19.01%), Hypocreaceae (12.49%), Botryosphaeriaceae (9.68%), Nectriaceae (9.13%), Apiosporaceae (4.65%), Sporocadaceae (3.76%), Diaporthaceaceaceae (3.70%), Bionectriaceae (3.27%), Hypoxylaceae (2.48%), Plesporaceae (1.46%), Coniochaetaceae (1.52%), Xylariaceae (1.06%), Ophiocordycipitaceae (0.96%), and Dipodascaceae (0.55%).
In the Loreto municipality, in the two localities of La Paz and Bajo Huino, microorganisms were grouped into nine families, with the most abundant being Aspergillaceae (33.33 and 36.11%) and Didymellaceae (22.22 and 22.78%); in addition, two similar but less abundant families were identified in the two sites: Botryosphaeriaceae (21.28 and 4.17%) and Nectriaceae (14.81 and 4.17%). In La Paz, three families were identified (Plesporaceae 5.56%, Ophiocordycipitaceae 3.70%, and Diaporthaceaceae 1.86%) that were not present in Bajo Huiruno; and two that were present in Bajo Huiruno (Hypocreaceae 18.05% and Apiosporaceae 9.72%) but not in La Paz. In the canton Francisco de Orellana, in La Belleza, the microorganisms were grouped into ten families, four similar to those of the canton Loreto and six different: Aspergillaceae (25.53%), Didymellaceae (15. 95%) Botryosphaeriaceae (15.95%), Hypocreaceae (15.96%), Diaporthaceaceaceae (6.38%), Coniochaetaceae (4.26%), Biocectriaceae (3.19%), Hypoxylaceae (3.19%), Plesporaceae (2.13%), and Sporocadaceae (2.13%) (Figure 4).
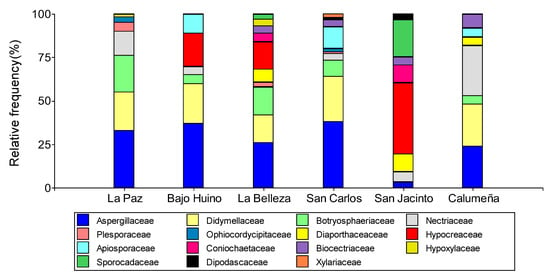
Figure 4.
Relative abundance at the family level of fungal microorganisms isolated from T. cacao fruits in the province of Orellana.
In the municipality of La Joya de los Sachas, in San Carlos, San Jacinto, and La Calumeña, the microorganisms were grouped into ten, nine, and eight families, respectively. In the three localities, the similar families were Aspergillaceae (38.14, 2.27, and 22.34%), Bionectriaceae (4.12, 4.55, and 7.77%), and Nectriaceae (4.12, 4.55, and 27.18%). San Carlos and La Calumeña presented three identical families: Didymellaceae (25.77 and 22.34%), Botryosphaeriaceae (9.27 and 4.85%), and Apiosporaceae (12.37 and 5.82%); and between San Carlos and San Jacinto, one similar family was identified: Dipodascaceae (1.04 and 2.27%). In addition, two identical families were identified in San Jacinto and La Calumeña: Diaporthaceaceae (9.09 and 4.85%) and Hypoxylaceae (6.28 and 4.85%). Finally, three more families were identified in San Carlos (Plesporaceae: 1.04%, Xylariaceae: 2.07%, and Ophiocordycipitaceae: 2.06%) as in San Jacinto: Hypocreaceae 40.91%, Sporocadaceae 20.45%, and Coniochaetaceae 9.09% (Figure 4).
The highest number of fungal microorganisms was found in La Joya de los Sachas, with 14 genera, followed by Francisco de Orellana with 9 genera, and finally, Loreto with 9 genera. The analysis by locality shows that in La Paz and Bajo Huino, seven and six genera were present, respectively. In La Belleza, 10 genera of the 14 genera were found; and in San Carlos, San Jacinto, and La Calumeña, 11, 9, and 7 genera were present, respectively.
The average values recorded according to the Fungal Richness (q = 0) index in the cantons Loreto, Francisco de Orellana, and La Joya de los Sachas were 6.75, 10.00, and 9.66, respectively. In La Paz, the Fungal Richness index was 7.49 and Bajo Huino was 6.00. In La Belleza, in the canton Francisco de Orellana, the Fungal Richness index was 10.00. Finally, in the canton La Joya de los Sachas, the diversity of the San Carlos was 10.99, San Jacinto was 9.97, and La Calumeña was 8.00 (Figure 5A).
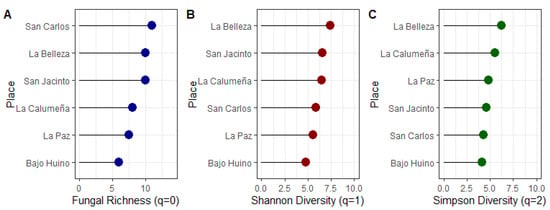
Figure 5.
Diversity indices of fungal microorganisms isolated from T. cacao fruits in the province of Orellana. (A) Fungal Richness index, (B) Shannon Diversity, and (C) Simpson Diversity.
The average values recorded according to the Shannon Diversity (q = 1) index in the cantons Loreto, Francisco de Orellana, and La Joya de los Sachas were 5.17, 7.44, and 6.28, respectively. In La Paz, the Shannon diversity index was 4.84 and Bajo Huino was 4.60. In the La Belleza municipality Francisco de Orellana, the Shannon diversity index was 7.44. Finally, in the canton La Joya de los Sachas, the diversity of the San Carlos was 5.87, San Jacinto was 6.54, and La Calumeña was 6.44 (Figure 6). According to the Shannon Diversity (q = 1) index, the sample population with the highest fungal diversity value was found in La Belleza, with 7.44 (Figure 5B).
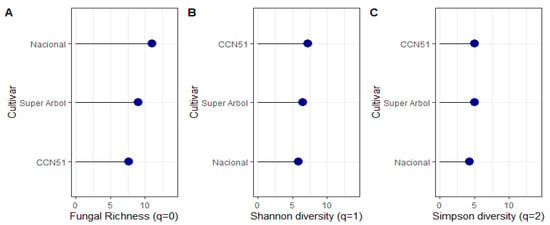
Figure 6.
Diversity indices of fungal microorganisms isolated from T. cacao fruits according to the different cultivars sampled. (A) Fungal Richness index, (B) Shannon Diversity, and (C) Simpson Diversity.
According to the results of the Simpson Diversity (q = 2) index, Bajo Huinohad showed a Simpson diversity of 6.20, with a higher probability of individuals of the same gender. In Loreto, Francisco de Orellana, and La Joya de los Sachas, the Simpson Diversity (q = 2) index was 4.48, 6.20, and 4.79, respectively (Figure 5C).
The Nacional cocoa cultivar displayed the highest fungal richness (q = 0), indicating a greater number of unique taxa. However, the CCN51 cultivar exhibited higher Shannon (q = 1) and Simpson (q = 2) diversity indices, suggesting a more even distribution of species within the fungal community. In contrast to the Nacional and CCN51 cultivars, the Super Arbol cultivar showed intermediate levels of diversity based on the Fungal Richness, Shannon, and Simpson indices (Figure 6).
3.2. Cultural, Morphological, and Molecular Characterization of Fungal Microorganisms
The relative abundance at the genus level of the fungi present in the fruits of T. cacao allowed the selection of the five most abundant genera in the three municipalities. Cultural (Table 3), morphological (Table 4), and molecular (Table 5) characterization was carried out for these genera. The cultural characterization of the fungi shows that the morphotypes presented circular, irregular, and filamentous mycelial growth; flat, curly, filamentous, and effuse elevations; smooth, rough, wavy, concentric, and crenulate surfaces; and colors varying from yellow, red, green, black, beige, to gray on the reverse (Table 3). Furthermore, morphological characterization of morphotypes was performed based on hyphal shape and conidial shape and size (Table 4).

Table 3.
Cultural characterization of fungi isolated from Theobroma cacao fruits in the province of Orellana.

Table 4.
Morphological characterization of fungi isolated from Theobroma cacao fruits in the province of Orellana.

Table 5.
Most abundant fungal genera present in T. cacao fruits, identified by morphology and morphological and molecular characterization in Orellana Province.
Finally, the isolates were identified by ITS sequences in order to determine species richness and abundance (Table 5). Amplification of the PCR products of the ITS regions of the rDNA of the fungal isolates generated fragments of 300 to 600 bp with primers ITS1 and ITS4. Such size variations were initial evidence that they corresponded to different genera. Analysis with BLASTN confirmed this observation by obtaining first hits with percentage identity values > 99% for the genera presented in Table 5. The results of the BLASTN comparisons corresponded to a local alignment leading to putative identifications.
4. Discussion
The analysis of fungal diversity in cocoa fruits revealed a rich mycobiome comprising 14 genera. The most prevalent taxa included Penicillium sp. (27.8%), Epicoccum sp. (20.5%), Lasiodiplodia sp. (10.1%), Trichoderma sp. (9.91%), and Fusarium sp. (9.70%). Additional genera, including Nigrospora sp., Clonostachys sp., Diaporthe sp., Daldinia sp., Neopestalotiopsis sp., Alternaria sp., Purpureocillium sp., Coniochaeta sp., and Geotrichum sp., were identified at lower frequencies. Notably, several of these genera, such as Epicoccum, Lasiodiplodia, Clonostachys, Fusarium, and Trichoderma, are known for producing metabolites with plant-protective properties [27]. The presence of these potentially beneficial fungi within the cocoa fruit mycobiome suggests a complex ecological interplay between the cacao plant and its associated fungi. This interplay may contribute to natural defense mechanisms against phytopathogens, warranting further investigation into the specific roles and interactions of these fungi within the cocoa ecosystem.
Representing a significant proportion of the fungal community, Epicoccum spp. is recognized for its antagonistic activity against a broad spectrum of plant pathogens [28]. Studies have documented their inhibitory effects on Pythium ultimum, Aphanomyces cochlioides, and Rhizoctonia solani, as well as antibiotic activity against Escherichia coli and multidrug-resistant strains of Bacillus subtilis and Staphylococcus aureus [28]. Their presence in cocoa fruits may contribute to intrinsic defense mechanisms. Research should focus on elucidating the specific bioactive metabolites produced by Epicoccum spp. and their roles in the cocoa fruit environment.
Frequently identified as endophytes, Lasiodiplodia spp. have demonstrated biocontrol efficacy against two major cocoa pathogens, Moniliophthora roreri (frosty pod rot) and Moniliophthora perniciosa (witches’ broom disease) [29]. These findings highlight their potential for inclusion in integrated disease management programs. Future research should explore the optimization of formulations and application methods to maximize their utility as biocontrol agents.
Widely recognized for their antifungal properties, Trichoderma spp. employ diverse mechanisms, including antibiosis, antagonism, mycoparasitism, and induced systemic resistance [22]. For example, Trichoderma asperellum has been shown to effectively suppress Anthracnose dieback in cocoa [4]. Research should prioritize strain selection, delivery system development, and ecological impact assessments to enhance the application of Trichoderma spp. in sustainable cocoa cultivation.
Both Clonostachys and Fusarium spp. exhibit biocontrol activity against Phytophthora palmivora (black pod rot) and M. roreri. While some Fusarium species are pathogenic, others possess beneficial properties, underscoring the complexity of fungal interactions within the cocoa ecosystem. Further studies should delineate the specific roles of these genera in disease suppression and ecosystem balance [30].
The diverse fungal community inhabiting cocoa fruits, particularly the presence of genera known for their plant-protective properties, offers promising avenues for developing sustainable disease management strategies. Further research focusing on the specific mechanisms of action, interactions, and potential applications of these beneficial fungi will be crucial for harnessing the full potential of the cocoa fruit mycobiome for enhanced disease resistance and sustainable cocoa production.
This study identified fungi belonging to four classes within the Ascomycota phylum: Sordariomycetes (39.85%), Dothidiomycetes (31.89%), Eurotiomycetes (27.8%), and Saccharomycetes (0.43%). These findings align with reports from studies conducted in diverse regions, including China, India, Germany, and Brazil, where similar class distributions have been observed [9]. Eight fungal orders were identified: Eurotiales (26.29%), Hypocreales (25.86%), Pleosporales (20.46%), Xylariales (11.95%), Botryosphaeriales (9.68%), Diaporthales (3.70%), Coniochaetales (1.51%), and Saccharomycetales (0.55%). These orders, consistent with global reports, demonstrate the ecological and geographical factors influencing fungal diversity [26].
The diversity of the endophytic fungal community associated with healthy cocoa fruits underscores the complex interplay between cacao plants and their mycobiomes. This dynamic relationship has significant implications for developing environmentally friendly and economically viable disease management strategies.
This study employed a culture-dependent approach to investigate fungal diversity and community composition within cacao fruits from various locations and cultivars in the Ecuadorian Northern Amazon. Ascomycota dominated the fungal communities across all sites, consistent with previous research [31]. Fungal diversity, measured by the number of genera, varied among locations and cultivars. The highest diversity (11 genera) was observed in San Carlos, where the Nacional cocoa cultivar is grown. This traditional cultivar has served as a source for cocoa improvement programs [32,33]. The high diversity observed here may be related to plant characteristics, as previous studies have shown that domestication and breeding can reduce microbial diversity in crops such as agave, barley, and apple [34,35].
Plant species, and even different genotypes within a species, can exert a strong influence on their associated microbial communities. The phylogenetic distance between plant species has also been shown to correlate with variations in microbial assemblages [34]. The locality of La Belleza, where the improved CCN51 cultivar is cultivated, also exhibited high fungal diversity (10 genera). CCN51 is one of the most widely cultivated and productive cocoa cultivars in Ecuador [36]. San Jacinto, home to the Super Árbol cultivar (a Trinitario x Nacional hybrid) [33], harbored significant fungal diversity with nine genera. This hybrid is currently the most widespread in the northern Amazon region of Ecuador [33]. The lowest diversity (6sixgenera) was found in Bajo Huino, where the CCN51 cultivar is also grown.
The observed differences in fungal diversity may be related to crop management practices. San Carlos and La Belleza are managed agroecologically with minimal pesticide application, while other locations utilize more conventional practices. Previous studies have linked frequent pesticide use to reduced microbial diversity in cocoa. This suggests that agroecological practices may contribute to higher fungal diversity in cocoa fruits [37].
A comprehensive understanding of the mechanisms employed by beneficial fungi to suppress pathogens is essential for advancing sustainable disease management in cocoa cultivation. This includes investigating the production of antifungal metabolites, competition for resources, mycoparasitism, and the induction of systemic resistance in the cacao plant. Identifying and characterizing the bioactive compounds involved will provide a foundation for developing targeted biocontrol strategies. The cocoa fruit mycobiome represents a complex ecological community where interactions between different fungal species can significantly influence their collective impact on plant health. Research is needed to elucidate synergistic or antagonistic relationships between beneficial fungi and potential pathogens, as well as the factors governing community composition and stability.
Environmental variables, such as temperature, humidity, and soil conditions, play a pivotal role in shaping the composition and activity of the cocoa fruit mycobiome. Understanding how these factors influence the prevalence and efficacy of beneficial fungi is crucial for optimizing biocontrol strategies. Practical methods for applying beneficial fungi in cocoa cultivation must also be developed to ensure the successful translation of laboratory findings into field applications. This includes exploring different formulation techniques, delivery systems, and application schedules to maximize the effectiveness of biocontrol agents under diverse cultivation conditions.
Beyond disease suppression, the potential impact of beneficial endophytic fungi on cocoa bean quality and flavor should be considered. Research should investigate whether these fungi affect the chemical composition of cocoa beans and the characteristics of the resulting chocolate products, ensuring that biocontrol strategies align with quality standards. By addressing these critical research areas, a comprehensive understanding of the role of beneficial fungi in cocoa fruit health can be achieved. This knowledge will enable the development of sustainable disease management strategies that leverage the cocoa fruit mycobiome, providing an environmentally friendly and economically viable approach to cocoa production while contributing to the long-term sustainability of the industry.
5. Conclusions
Investigations into fungal communities represent a burgeoning field of research, providing insights into the diverse microorganisms inhabiting plants. This study characterized 464 fungal isolates from healthy cocoa fruits, classifying them into 56 morphotypes and 14 genera, all within the Ascomycota phylum. The most prevalent genera were Penicillium (27.8%), Epicoccum (20.5%), Lasiodiplodia (10.1%), Trichoderma (9.91%), and Fusarium (9.70%), highlighting the rich fungal diversity associated with cocoa. This diverse assemblage of endophytic fungi suggests a complex interplay between the cocoa plant and its associated mycobiome, warranting further investigation into their ecological roles and potential impact on cocoa health and disease.
This study revealed a diverse endophytic fungal community associated with cocoa fruits, emphasizing the importance of understanding the intricate interplay between these fungi and their host plant. The identified genera, including Penicillium, Epicoccum, Lasiodiplodia, Trichoderma, and Fusarium, represent a complex ecosystem with potential implications for cocoa health and disease management. The dominance of these genera suggests their adaptation to the cocoa fruit environment and raises questions about their specific roles and interactions.
The observed fungal diversity also reflects broader ecological patterns. The dominance of Ascomycota, particularly Sordariomycetes, Dothidiomycetes, and Eurotiomycetes, aligns with findings from similar studies conducted in diverse geographical regions. This suggests a conserved pattern of fungal community structure associated with cocoa across different environments. Understanding these global patterns can inform broader biocontrol strategies and contribute to a more holistic understanding of cocoa-fungal interactions.
Further research is crucial to fully explore the potential of these beneficial fungi for developing sustainable disease management strategies in cocoa cultivation. This includes investigating the specific mechanisms of action, optimizing application methods, and evaluating the long-term efficacy and environmental impact of biocontrol strategies. Reducing the reliance on synthetic fungicides is a key goal as it promotes environmentally friendly agricultural practices and minimizes potential risks to human health and the environment. By harnessing the natural protective properties of beneficial fungi, we can move toward more sustainable and resilient cocoa cultivation practices.
Author Contributions
Conceptualization and draft preparation, P.I.A.-R.; methodology, L.A.H.-S., S.E.S.-C., A.F.T.A.F.e.F. and D.A.R.-R.; supervision and editing, P.I.A.-R., L.A.H.-S., M.A.G.-C. and E.P.S.-C.; analysis and editing, P.I.A.-R., A.F.T.A.F.e.F., M.A.G.-C. and L.A.H.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Research Project “IDIPIM-002—Implementación de una Plataforma Informática de Alertas Tempranas Fitosanitarias Agrícolas en los cantones La Joya de los Sachas, Loreto y Francisco de Orellana” funded by the Escuela Superior Politécnica de Chimborazo through the DDI-ESPOCH.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon request to the corresponding author.
Acknowledgments
The authors thank to Escuela Superior Politécnica de Chimborazo because this study is a part of the Project “IDIPIM-002—Implementación de una Plataforma Informática de Alertas Tempranas Fitosanitarias Agrícolas en los cantones La Joya de los Sachas, Loreto y Francisco de Orellana”.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Alves-Júnior, M.; De Sousa, F.O.; Silva, T.F.; Albino, U.B.; Garcia, M.G.; Moreira, S.M.C.D.O.; Vieira, M.R.D.S. Functional and Morphological Analysis of Isolates of Phylloplane and Rhizoplane Endophytic Bacteria Interacting in Different Cocoa Production Systems in the Amazon. Curr. Res. Microb. Sci. 2021, 2, 100039. [Google Scholar] [CrossRef]
- Gilbert, G.S. Evolutionary Ecology of Plant Diseases in Natural Ecosystems. Annu. Rev. Phytopathol. 2002, 40, 13–43. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.E.; Mejía, L.C.; Kyllo, D.; Rojas, E.I.; Maynard, Z.; Robbins, N.; Herre, E.A. Fungal Endophytes Limit Pathogen Damage in a Tropical Tree. Proc. Natl. Acad. Sci. USA 2003, 100, 15649–15654. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Ospina, J.; Molina-Hernández, J.B.; Chaves-López, C.; Romanazzi, G.; Paparella, A. The Role of Fungi in the Cocoa Production Chain and the Challenge of Climate Change. J. Fungi 2021, 7, 202. [Google Scholar] [CrossRef]
- Rubini, M.R.; Silva-Ribeiro, R.T.; Pomella, A.W.V.; Maki, C.S.; Araújo, W.L.; Dos Santos, D.R.; Azevedo, J.L. Diversity of Endophytic Fungal Community of Cacao (Theobroma cacao L.) and Biological Control of Crinipellis perniciosa, Causal Agent of Witches’ Broom Disease. Int. J. Biol. Sci. 2005, 1, 24–33. [Google Scholar] [CrossRef]
- Berbee, M.L. The Phylogeny of Plant and Animal Pathogens in the Ascomycota. Physiol. Mol. Plant Pathol. 2001, 59, 165–187. [Google Scholar] [CrossRef]
- Chaithra, M.; Vanitha, S.; Ramanathan, A.; Jegadeeshwari, V.; Rajesh, V.; Hegde, V.; Apshara, E. Morphological and Molecular Characterization of Endophytic Fungi Associated with Cocoa (Theobroma cacao L.) in India. CJAST 2020, 38, 1–8. [Google Scholar] [CrossRef]
- Carlsson-Granér, U.; Thrall, P.H. The Spatial Distribution of Plant Populations, Disease Dynamics and Evolution of Resistance. Oikos 2002, 97, 97–110. [Google Scholar] [CrossRef]
- Arnold, A.E. Understanding the Diversity of Foliar Endophytic Fungi: Progress, Challenges, and Frontiers. Fungal Biol. Rev. 2007, 21, 51–66. [Google Scholar] [CrossRef]
- Yu, J.; Wu, Y.; He, Z.; Li, M.; Zhu, K.; Gao, B. Diversity and Antifungal Activity of Endophytic Fungi Associated with Camellia oleifera. Mycobiology 2018, 46, 85–91. [Google Scholar] [CrossRef]
- Sieber, T.N. Endophytic Fungi in Forest Trees: Are They Mutualists? Fungal Biol. Rev. 2007, 21, 75–89. [Google Scholar] [CrossRef]
- Usuki, F.; Narisawa, K. A Mutualistic Symbiosis between a Dark Septate Endophytic Fungus, Heteroconium chaetospira, and a Nonmycorrhizal Plant, Chinese Cabbage. Mycologia 2007, 99, 175–184. [Google Scholar] [CrossRef]
- Tellenbach, C.; Grünig, C.R.; Sieber, T.N. Negative Effects on Survival and Performance of Norway Spruce Seedlings Colonized by Dark Septate Root Endophytes Are Primarily Isolate-Dependent. Environ. Microbiol. 2011, 13, 2508–2517. [Google Scholar] [CrossRef]
- Hanada, R.E.; Pomella, A.W.V.; Costa, H.S.; Bezerra, J.L.; Loguercio, L.L.; Pereira, J.O. Endophytic Fungal Diversity in Theobroma cacao (Cacao) and T. grandiflorum (Cupuaçu) Trees and Their Potential for Growth Promotion and Biocontrol of Black-Pod Disease. Fungal Biol. 2010, 114, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Fontana, D.C.; De Paula, S.; Torres, A.G.; De Souza, V.H.M.; Pascholati, S.F.; Schmidt, D.; Dourado Neto, D. Endophytic Fungi: Biological Control and Induced Resistance to Phytopathogens and Abiotic Stresses. Pathogens 2021, 10, 570. [Google Scholar] [CrossRef] [PubMed]
- Marelli, J.-P.; Guest, D.I.; Bailey, B.A.; Evans, H.C.; Brown, J.K.; Junaid, M.; Barreto, R.W.; Lisboa, D.O.; Puig, A.S. Chocolate Under Threat from Old and New Cacao Diseases. Phytopathology 2019, 109, 1331–1343. [Google Scholar] [CrossRef] [PubMed]
- Schroth, G.; Läderach, P.; Martinez-Valle, A.I.; Bunn, C.; Jassogne, L. Vulnerability to Climate Change of Cocoa in West Africa: Patterns, Opportunities and Limits to Adaptation. Sci. Total Environ. 2016, 556, 231–241. [Google Scholar] [CrossRef]
- Valle-Ramírez, S.B.; Torres-Gutiérrez, R.; Caicedo-Quinche, W.O.; Abril-Saltos, R.V.; Sucoshañay-Villalba, D.J. Aislamiento y caracterización de Metarhizium spp. de cultivos de caña de azúcar y su patogenicidad contra Mahanarva andigena (Hemiptera: Cercopidae). CTA 2022, 23, e2361. [Google Scholar] [CrossRef]
- Castellani, A. Further Researches on the Long Viability and Growth of Many Pathogenic Fungi and Some Bacteria in Sterile Distilled Water. Mycopathol. Mycol. Appl. 1963, 20, 1–6. [Google Scholar] [CrossRef]
- Urzí, C.; De Leo, F. Sampling with Adhesive Tape Strips: An Easy and Rapid Method to Monitor Microbial Colonization on Monument Surfaces. J. Microbiol. Methods 2001, 44, 1–11. [Google Scholar] [CrossRef]
- Fernando, P.-Q.; Gregorio, L.-M.M.; Alejandro, B.-F.; Lourdes, V.-N.; Teófilo, S.-C. Comunidad de hongos filamentosos en suelos del Agroecosistema de K’iphak’iphani, Comunidad Choquenaira-Viacha. J. Selva Andina Res. Soc. 2017, 8, 2–25. [Google Scholar] [CrossRef]
- Samuels, G.J.; Hebbar, P.K. Trichoderma: Identification and Agricultural Applications, 1st ed.; American Phytopathological Society: Saint Paul, MN, USA, 2015; ISBN 978-0-89054-484-6. [Google Scholar]
- Chaverri, P.; Castlebury, L.A.; Overton, B.E.; Samuels, G.J. Hypocrea/Trichoderma: Species with Conidiophore Elongations and Green Conidia. Mycologia 2003, 95, 1100–1140. [Google Scholar] [CrossRef] [PubMed]
- Barnett, H.L.; Hunter, B.B. Illustrated Genera of Imperfect Fungi, 4th ed.; APS Press: Saint Paul, MN, USA, 1998; ISBN 978-0-89054-192-0. [Google Scholar]
- Seifert, K.A.; Morgan-Jones, G.; Gams, W.; Kendrick, B. The Genera of Hyphomycetes; CBS-KNAW Fungal Biodiversity Centre: Utrecht, The Netherlands, 2011; ISBN 978-90-70351-85-4. [Google Scholar]
- Rashmi, M. A Worldwide List of Endophytic Fungi with Notes on Ecology and Diversity. Mycosphere 2019, 10, 798–1079. [Google Scholar] [CrossRef]
- Asman, A.; Baharuddin; Rosmana, A.; Ariska. Diversity of Fungal Community Associated with Cacao (Theobroma cacao L.) Top Clones from Sulawesi, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2020, 486, 012171. [Google Scholar] [CrossRef]
- Talontsi, F.M.; Dittrich, B.; Schüffler, A.; Sun, H.; Laatsch, H. Epicoccolides: Antimicrobial and Antifungal Polyketides from an Endophytic Fungus Epicoccum sp. Associated with Theobroma cacao. Eur. J. Org. Chem. 2013, 2013, 3174–3180. [Google Scholar] [CrossRef]
- Villavicencio Vásquez, M.; Espinoza Lozano, R.; Sosa Del Castillo, D.; Pérez Martínez, S. Hongos Endófitos Foliares Como Candidatos a Biocontroladores Contra Moniliophthora spp. de Theobroma cacao (Malvaceae) En Ecuador. Acta Biol. Colomb. 2018, 23, 235–241. [Google Scholar] [CrossRef]
- Ten Hoopen, G.M.; Rees, R.; Aisa, P.; Stirrup, T.; Krauss, U. Population Dynamics of Epiphytic Mycoparasites of the Genera Clonostachys and Fusarium for the Biocontrol of Black Pod (Phytophthora palmivora) and Moniliasis (Moniliophthora roreri) on Cocoa (Theobroma cacao). Mycol. Res. 2003, 107, 587–596. [Google Scholar] [CrossRef]
- Becker, K.; Stadler, M. Recent Progress in Biodiversity Research on the Xylariales and Their Secondary Metabolism. J. Antibiot. 2021, 74, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Tigrero-Vaca, J.; Maridueña-Zavala, M.G.; Liao, H.-L.; Prado-Lince, M.; Zambrano-Vera, C.S.; Monserrate-Maggi, B.; Cevallos-Cevallos, J.M. Microbial Diversity and Contribution to the Formation of Volatile Compounds during Fine-Flavor Cacao Bean Fermentation. Foods 2022, 11, 915. [Google Scholar] [CrossRef]
- Sanchez-Capa, M.; Viteri-Sanchez, S.; Burbano-Cachiguango, A.; Abril-Donoso, M.; Vargas-Tierras, T.; Suarez-Cedillo, S.; Mestanza-Ramón, C. New Characteristics in the Fermentation Process of Cocoa (Theobroma cacao L.) “Super Árbol” in La Joya de Los Sachas, Ecuador. Sustainability 2022, 14, 7564. [Google Scholar] [CrossRef]
- Pérez-Jaramillo, J.E.; Carrión, V.J.; Bosse, M.; Ferrão, L.F.V.; De Hollander, M.; Garcia, A.A.F.; Ramírez, C.A.; Mendes, R.; Raaijmakers, J.M. Linking Rhizosphere Microbiome Composition of Wild and Domesticated Phaseolus vulgaris to Genotypic and Root Phenotypic Traits. ISME J. 2017, 11, 2244–2257. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Yue, W.; Jiao, S.; Kim, H.; Lee, Y.-H.; Wei, G.; Song, W.; Shu, D. Plant Domestication Shapes Rhizosphere Microbiome Assembly and Metabolic Functions. Microbiome 2023, 11, 70. [Google Scholar] [CrossRef] [PubMed]
- Jaimez, R.E.; Barragan, L.; Fernández-Niño, M.; Wessjohann, L.A.; Cedeño-Garcia, G.; Sotomayor Cantos, I.; Arteaga, F. Theobroma cacao L. Cultivar CCN 51: A Comprehensive Review on Origin, Genetics, Sensory Properties, Production Dynamics, and Physiological Aspects. PeerJ 2022, 10, e12676. [Google Scholar] [CrossRef] [PubMed]
- Toloza-Moreno, D.L.; Yockteng, R.; Zuñiga, J.I.P.; Castillo, C.S.; Caro-Quintero, A. Implications of Domestication in Theobroma cacao L. Seed-Borne Microbial Endophytes Diversity. Microb. Ecol. 2024, 87, 108–123. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).