Optimizing the Genetic Transformation of Coffea arabica Using Agrobacterium tumefaciens
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vitro Culture Conditions
2.2. Agrobacterium Tumefaciens Strains and Vectors
2.3. Agrobacterium Transformation
- Sonication time: Early EC of genotype BI.625 without pre-culturing was sonicated in a suspension of A. tumefaciens LBA4404 containing vector pCambia1301 for 0, 60, 120, and 300 s. After 1 h incubation in the suspension, the early EC was co-cultured for four days in both solid and liquid differentiation media.
- Co-culturing time: Early EC of genotype BK.620 without pre-culturing was sonicated in a suspension of A. tumefaciens EHA105 containing vector pCambia1301 for 300 s. After 1 h incubation in the suspension, the early EC was co-cultured in solid differentiation medium with 100 µM acetosyringone for 0–8 days.
- Pre-culturing time: Early EC of genotype BK.620 was pre-cultured in the dark in solid differentiation medium supplemented with 100 µM acetosyringone for 0–8 days on sterile filter paper, followed by sonication of embryogenic cells for 300 s in a suspension of A. tumefaciens EHA105 containing vector pCambia2301. After 1 h incubation in the suspension, the early EC was co-cultured in a solid differentiation medium for four days.
- Age of EC: Transformation efficiency of the uidA gene was evaluated using early and differentiated EC of genotype BI.625 without pre-culturing, followed by sonication for 300 s of embryogenic cells in a suspension of A. tumefaciens LBA4404 containing vector pCambia1301. After 1 h incubation in the suspension, the EC was co-cultured in a solid differentiation medium for four days.
- Agrobacterium strain: A. tumefaciens strains LBA4404 and EHA105 were evaluated with the vector pCambia1301.
- Transformation vector: Vectors pCambia1301 and pCambia2301, previously introduced into strain LBA4404, were compared.
- Coffee genotype: The early ECs of genotypes BI.625 and BK.620 were co-cultured with A. tumefaciens strain LBA4404 containing the vector pCambia1301. Agrobacterium strains, transformation vectors, and coffee genotypes were evaluated using the same protocol used to evaluate the age of EC (described above, item 4).
2.4. Stable Transformation
2.4.1. Development of Transgenic Somatic Embryos
2.4.2. Histochemical GUS Assay
2.4.3. PCR and Southern Blot Analysis of Transgenic Plants
2.5. Experimental Design and Data Analysis
3. Results
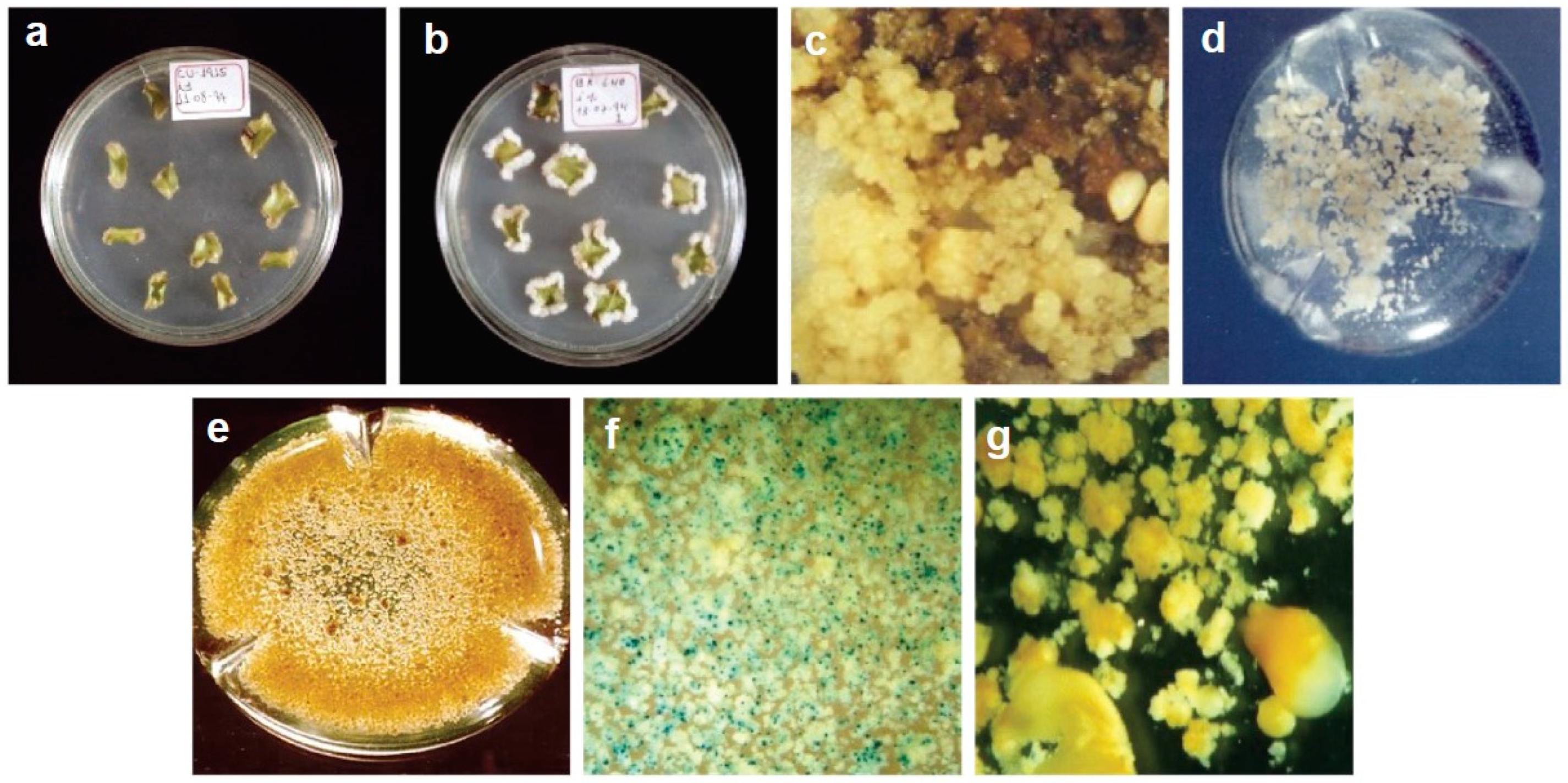
3.1. Age of EC
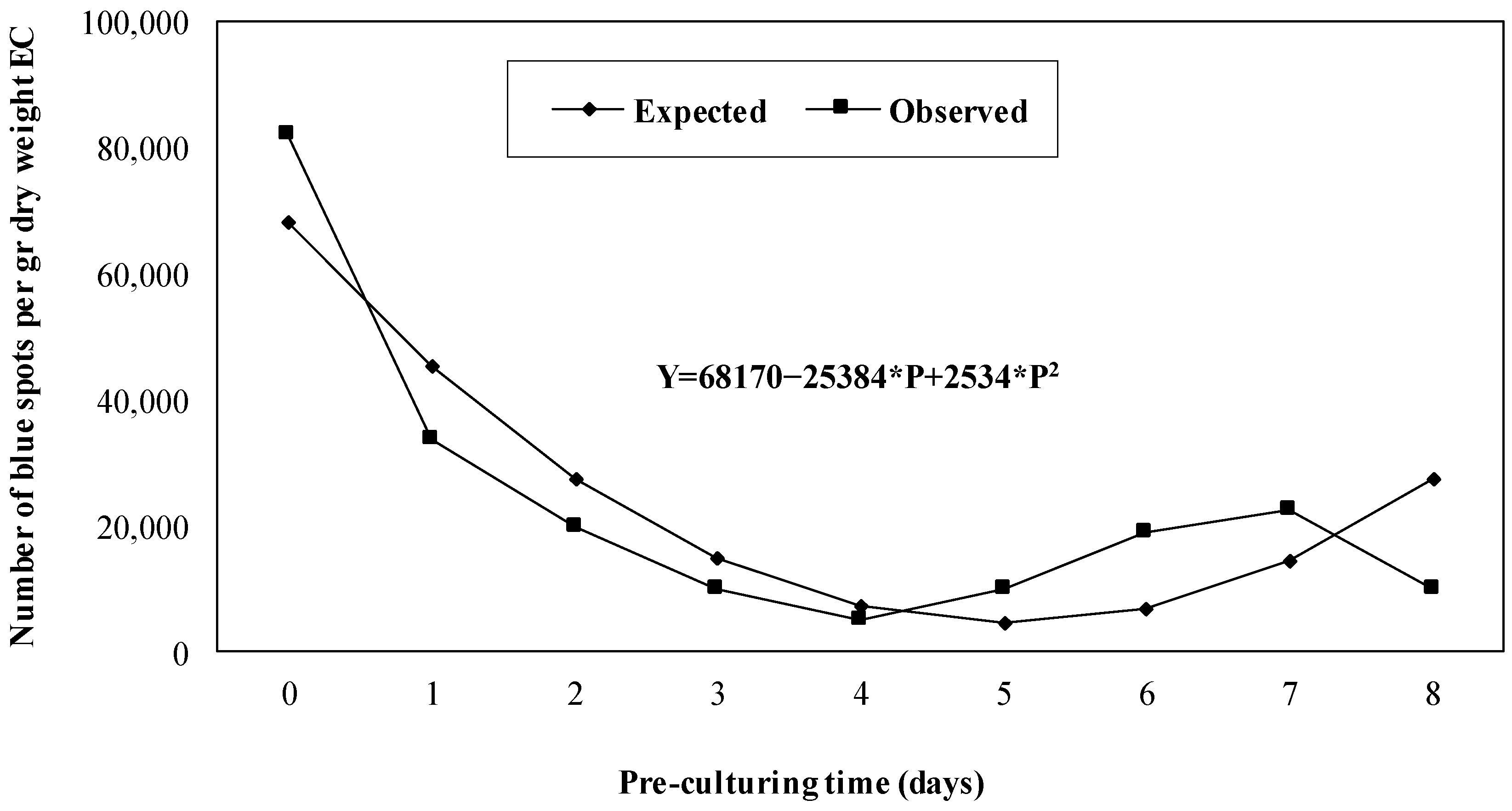
3.2. Pre-Culturing Time
3.3. Agrobacterium Strain, Transformation Vector, and Coffee Genotype
3.4. Sonication Time and Co-Culturing Medium
3.5. Co-Culturing Time
3.6. Regeneration of Transgenic Plants and Molecular Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Food and Agriculture Organization of the United Nations, Statistics Division. 2024. Available online: https://faostat.fao.org (accessed on 26 July 2024).
- International Coffee Organization. Coffee Development Report 2022. 2022. Available online: www.ico.org/documents/cy2022-23/annual-review-2021-2022-e.pdf (accessed on 24 June 2024).
- Davis, A.P.; Rakotonasolo, F. Six new species of coffee (Coffea) from northern Madagascar. Kew Bull. 2021, 76, 497–511. [Google Scholar] [CrossRef]
- International Coffee Organization. Coffee Market Report. 2023. Available online: www.ico.org/documents/cy2022-23/cmr-1222-e.pdf (accessed on 24 April 2024).
- Scalabrin, S.; Magris, G.; Liva, M.; Vitulo, N.; Vidotto, M.; Scaglione, D.; Del Terra, L.; Ruosi, M.R.; Navarini, L.; Pellegrino, G.; et al. A chromosome-scale assembly reveals chromosomal aberrations and exchanges generating genetic diversity in Coffea arabica germplasm. Nat. Commun. 2024, 15, 463. [Google Scholar] [CrossRef] [PubMed]
- Federación Nacional de Cafeteros de Colombia. Estadísticas Cafeteras. 2024. Available online: https://federaciondecafeteros.org/wp/estadisticascafeteras (accessed on 24 April 2024).
- Federación Nacional de Cafeteros de Colombia. Sistema de Información Cafetero de Colombia. 2024. Available online: https://sica.cafedecolombia.com (accessed on 24 April 2024).
- Molina, D.; Moncada, M.P.; Cortina, H.; Benavides, P. Searching for a coffee variety with antibiosis effect to Hypothenemus hampei Ferrari (Coleoptera: Curculionidae). Euphytica 2022, 218, 97. [Google Scholar] [CrossRef]
- Etienne, H.; Breton, D.; Breitler, J.-C.; Bertrand, B.; Déchamp, E.; Awada, R.; Marraccini, P.; Léran, S.; Alpizar, E.; Campa, C.; et al. Coffee somatic embryogenesis: How did research, experience gained and innovations promote the commercial propagation of elite clones from the two cultivated species? Front. Plant Sci. 2018, 9, 1630. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Hernández, H.A.; Galaz-Ávalos, R.M.; Quintana-Escobar, A.O.; Pech-Hoil, R.; Collí-Rodríguez, A.M.; Salas-Peraza, I.Q.M. In vitro conversion of Coffea spp. somatic embryos in SETIS™ bioreactor system. Plants 2023, 12, 3055. [Google Scholar] [CrossRef]
- Avila-Victor, C.M.; Ordaz-Chaparro, V.M.; Arjona-Suárez, E.d.J.; Iracheta-Donjuan, L.; Gómez-Merino, F.C.; Robledo-Paz, A. In vitro mass propagation of coffee plants (Coffea arabica L. var. Colombia) through indirect somatic embryogenesis. Plants 2023, 12, 1237. [Google Scholar] [CrossRef]
- Leroy, T.; Henry, A.M.; Royer, M.; Altosaar, I.; Frutos, R.; Duris, D.; Philippe, R. Genetically modified coffee plants expressing the Bacillus thuringiensis cry1Ac gene for resistance to leaf miner. Plant Cell Rep. 2000, 19, 382–389. [Google Scholar] [CrossRef]
- Perthuis, B.; Vassal, J.M.; Fenouillet, C.; Leroy, T. Cry1Ac insecticidal protein levels in genetically modified Coffea canephora Pierre coffee plants were negatively correlated with the growth speed in a field experiment. Euphytica 2014, 202, 373–383. [Google Scholar] [CrossRef]
- Albuquerque, É.V.; Bezerra, C.A.; Romero, J.V.; Valencia, J.W.; Valencia-Jiménez, A.; Pimenta, L.M.; Barbosa, A.E.; Silva, M.C.; Meneguim, A.M.; Sá, M.E.; et al. Seed-specific stable expression of the α-AI1 inhibitor in coffee grains and the in vivo implications for the development of the coffee berry borer. Trop. Plant Biol. 2015, 8, 98–107. [Google Scholar] [CrossRef]
- Valencia-Lozano, E.; Cabrera-Ponce, J.L.; Gómez-Lim, M.A.; Ibarra, J.E. Development of an efficient protocol to obtain transgenic coffee, Coffea arabica L., expressing the Cry10Aa toxin of Bacillus thuringiensis. Int. J. Mol. Sci. 2019, 20, 5334. [Google Scholar] [CrossRef]
- Valencia-Lozano, E.; Cabrera-Ponce, J.L.; Noa-Carrazana, J.C.; Ibarra, J.E. Coffea arabica L. resistant to coffee berry borer (Hypothenemus hampei) mediated by expression of the Bacillus thuringiensis Cry10Aa protein. Front. Plant Sci. 2021, 12, 765292. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, M.E.; Wang, X.Y.; Escalona, M.; Yan, L.; Huang, L.F. Somatic embryogenesis of Arabica coffee in temporary immersion culture: Advances, limitations, and perspectives for mass propagation of selected genotypes. Front. Plant Sci. 2022, 13, 994578. [Google Scholar] [CrossRef]
- Salaün, C.; Lepiniec, L.; Dubreucq, B. Genetic and molecular control of somatic embryogenesis. Plants 2021, 10, 1467. [Google Scholar] [CrossRef] [PubMed]
- Campos, N.A.; Panis, B.; Carpentier, S.C. Somatic embryogenesis in coffee: The evolution of biotechnology and the integration of omics technologies offer great opportunities. Front. Plant Sci. 2017, 8, 1460. [Google Scholar] [CrossRef] [PubMed]
- Staritsky, G. Embroid formation in calllus tissues of Coffea. Acta Bot. Neerl. 1970, 19, 509–514. [Google Scholar] [CrossRef]
- Sondahl, M.R.; Sharp, W.R. High frecuency induction of somatic embryos in cultured leaf explants of Coffea arabica L. Z. Pflanzenphysiol. 1977, 81, 395–408. [Google Scholar] [CrossRef]
- Molina, D.; Aponte, M.E.; Cortina, H.; Moreno, L.G. The effect of genotype and explant age on somatic embryogenesis of coffee. Plant Cell Tissue Organ Cult. 2002, 71, 117–123. [Google Scholar] [CrossRef]
- Arimarsetiowati, R.; Daryono, B.S.; Astuti, Y.T.M.; Prastowo, E.; Semiarti, E. Regeneration and development of Coffea arabica L. plants through indirect somatic embryogenesis. Coffee Sci. 2023, 18, e182078. [Google Scholar] [CrossRef]
- Ibrahim, M.S.D.; Hartati, R.S.; Rubiyo, R.; Reflinur, R.; Purwito, A.; Sudarsono, S. Exploring indirect somatic embryogenesis and somaclonal variation for propagation of three Coffea arabica L. cultivars. Chil. J. Agric. Res. 2024, 84, 15–27. [Google Scholar] [CrossRef]
- Avila-Victor, C.M.; Arjona-Suárez, E.d.J.; Iracheta-Donjuan, L.; Valdez-Carrasco, J.M.; Gómez-Merino, F.C.; Robledo-Paz, A. Callus type, growth regulators, and phytagel on indirect somatic embryogenesis of coffee (Coffea arabica L. var. Colombia). Plants 2023, 12, 3570. [Google Scholar] [CrossRef]
- Ribas, A.F.; Dechamp, E.; Champion, A.; Bertrand, B.; Combes, M.-C.; Verdeil, J.-L.; Lapeyre, F.; Lashermes, P.; Etienne, H. Agrobacterium-mediated genetic transformation of Coffea arabica L. is greatly enhanced by using established embryogenic callus cultures. BMC Plant Biol. 2011, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Shilo, S.; Tripathi, P.; Melamed-Bessudo, C.; Tzfadia, O.; Muth, T.R.; Levy, A.A. T-DNA-genome junctions form early after infection and are influenced by the chromatin state of the host genome. PLoS Genet. 2017, 13, e1006875. [Google Scholar] [CrossRef] [PubMed]
- Gelvin, S.B. Plant DNA repair and Agrobacterium T-DNA integration. Int. J. Mol. Sci. 2021, 22, 8458. [Google Scholar] [CrossRef] [PubMed]
- Canche-Moo, R.L.R.; Ku-Gonzalez, A.; Burgeff, C.; Loyola-Vargas, V.M.; Rodriguez-Zapata, L.C.; Castaño, E. Genetic transformation of Coffea canephora by vacuum infiltration. Plant Cell Tissue Organ Cult. 2006, 84, 373–377. [Google Scholar] [CrossRef]
- Ogita, S.; Uefuji, H.; Choi, Y.; Hatanaka, T.; Ogawa, M.; Yamaguchi, Y. Genetic modification of coffee plants. J. Plant Biotechnol. 2002, 3, 91–94. [Google Scholar]
- Ogita, S.; Uefuji, H.; Morimoto, M.; Sano, H. Application of RNAi to confirm theobromine as the major intermediate for caffeine biosynthesis in coffee plants with potential for construction of decaffeinated varieties. Plant Mol. Biol. 2004, 54, 931–941. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays tobacco tissues cultures. Physiol. Plant. 1962, 15, 472–497. [Google Scholar] [CrossRef]
- Trick, H.N.; Finer, J.J. SAAT: Sonication-assisted Agrobacterium-mediated transformation. Transgenic Res. 1997, 6, 329–336. [Google Scholar] [CrossRef]
- Trick, H.N.; Finer, J.J. Sonication-assisted Agrobacterium-mediated transformation of soybean [Glycine max (L.) Merrill] embryogenic suspension culture tissue. Plant Cell Rep. 1998, 17, 482–488. [Google Scholar] [CrossRef]
- Jefferson, R.A. Assaying chimeric genes in plants: The GUS gene fusion system. Plant Mol. Biol. Rep. 1987, 5, 387–405. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L.A. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Noir, S.; Patheyron, S.; Combes, M.C.; Lashermes, P.; Chalhoub, B. Construction and characterisation of a BAC library for genome analysis of the allotetraploid coffee species (Coffea arabica L.). Theor. Appl. Genet. 2004, 109, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Lin, L.; Luo, H.; Zhou, S.; Zhu, Y.; Wang, X.; Miao, L.; Wang, H.; Zhang, P. Recent progress in the regeneration and genetic transformation system of cucumber. Appl. Sci. 2022, 12, 7180. [Google Scholar] [CrossRef]
- Arroyo, A.; Ku, A.; Canche, R.; Quiroz, F.; Loyola, V.; Rodríguez, L.; Burgeff, C.; Súarez, V.; Castaño, E. Expression of WUSCHEL in Coffea canephora causes ectopic morphogenesis and increases somatic embryogenesis. Plant Cell Tissue Organ Cult. 2008, 94, 171–180. [Google Scholar] [CrossRef]
- Mishra, M.K.; Slater, A. Recent advances in the genetic transformation of coffee. Biotechnol. Res. Int. 2012, 2012, 580857. [Google Scholar] [CrossRef]
- Gordon, J.E.; Christie, P.J. The Agrobacterium Ti plasmids. Microbiol. Spectr. 2014, 2, 295–313. [Google Scholar] [CrossRef]
- Martínez, N.; Dávila, C.; Morales, J.; Castro, K.; Martínez, N.; López, H.; Villalobos, E. 6-benzylaminopurine induces somatic embryogenesis in the staminodia of new genotypes of Theobroma cacao L. from the Papaloapan Basin of Mexico and differs from that of T. bicolor Bonpl. Plant Cell Tissue Organ Cult. 2024, 157, 64. [Google Scholar] [CrossRef]
- Udayabhanu, J.; Huang, T.; Xin, S.; Cheng, J.; Hua, Y.; Huang, H. Optimization of the transformation protocol for increased efficiency of genetic transformation in Hevea brasiliensis. Plants 2022, 11, 1067. [Google Scholar] [CrossRef]
- Srivastava, J.; Datta, S.; Mishra, S.P. Development of an efficient Agrobacterium-mediated transformation system for chickpea (Cicer arietinum). Biologia 2017, 72, 153–160. [Google Scholar] [CrossRef]
- Sadhu, S.K.; Jogam, P.; Gande, K.; Banoth, R.; Suprasanna, P.; Peddaboina, V. Optimization of different factors for an Agrobacterium-mediated genetic transformation system using embryo axis explants of chickpea (Cicer arietinum L.). J. Plant Biotechnol. 2022, 49, 61–73. [Google Scholar] [CrossRef]
- Da Silva, M.L.; Paim Pinto, D.L.; Passos, A.B.; Marcelino-Guimaräes, F.C.; Bandini Rossi, A.; Krause, W.; de Carvalho, I.; Batista, D.; Rocha, D.; Otoni, W. Novel and efficient transformation of wild passion fruit (Passiflora cincinnata Mast.) using sonication-assisted Agrobacterium-mediated transformation. In Vitro Cell. Dev. Biol. Plant 2021, 57, 380–386. [Google Scholar] [CrossRef]
- Gelvin, S.B. Agrobacterium-mediated plant transformation: The biology behind the “gene-jockeying” tool. Microbiol. Mol. Biol. Rev. 2003, 67, 16–37. [Google Scholar] [CrossRef] [PubMed]
- Pazour, G.J.; Das, A. Characterization of the VirG binding site of Agrobacterium tumefaciens. Nucleic Acids Res. 1990, 18, 6909–6913. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vargas-Guevara, C.; Vargas-Segura, C.; Villalta-Villalobos, J.; Pereira, L.F.P.; Gatica-Arias, A.A. simple and efficient agroinfiltration method in coffee leaves (Coffea arabica L.): Assessment of factors affecting transgene expression. 3 Biotech 2018, 8, 471. [Google Scholar] [CrossRef] [PubMed]
- Moreno, G.; Castillo, J. La variedad Colombia una variedad de café con resistencia a la roya (Hemileia vastatrix Berk y Br.). Boletín Técnico Cenicafé 1984, 9, 1–37. [Google Scholar]
- Casarin, T.; Freitas, N.C.; Pinto, R.T.; Breitler, J.-C.; Zebral Rodrigues, L.A.; Marraccini, P.; Etienne, H.; Cardamone, L.E.; Carvalho, A.; Vilela, L. Multiplex CRISPR/Cas9-mediated knockout of the phytoene desaturase gene in Coffea canephora. Sci. Rep. 2022, 12, 17270. [Google Scholar] [CrossRef]
- Duan, X.; Zheng, L.; Sun, J.; Liu, W.; Wang, W.; An, H. Co-culturing on dry filter paper significantly increased the efficiency of Agrobacterium-mediated transformations of maize immature embryos. Physiol. Mol. Biol. Plants 2019, 25, 549–560. [Google Scholar] [CrossRef]
- Anwaar, S.; Jabeen, N.; Ahmad, K.S.; Shafique, S.; Irum, S.; Ismail, H.; Ullah, S.; Tahir, A.; Mehmood, N.; Gleason, M.L. Cloning of maize chitinase 1 gene and its expression in genetically transformed rice to confer resistance against rice blast caused by Pyricularia oryzae. PLoS ONE 2024, 19, e0291939. [Google Scholar] [CrossRef]
- Nanasato, Y.; Konagaya, K.I.; Okuzaki, A.; Tsuda, M.; Tabei, Y. Improvement of Agrobacterium-mediated transformation of cucumber (Cucumis sativus L.) by combination of vacuum infiltration and co-cultivation on filter paper wicks. Plant Biotechnol. Rep. 2013, 7, 267–276. [Google Scholar] [CrossRef]
- Jogam, P.; Sandhya, D.; Alok, A.; Shekhawat, M.S.; Peddaboina, V.; Singh, K.; Allini, V.R. Agrobacterium-mediated genetic transformation and cloning of candidate reference genes in suspension cells of Artemisia pallens Wall. ex DC. 3 Biotech 2022, 12, 194. [Google Scholar] [CrossRef]
- Molina, D.; Zamora, H.; Blanco-Labra, A. An inhibitor from Lupinus bogotensis seeds effective against aspartic proteases from Hypothenemus hampei. Phytochemistry 2010, 71, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Molina, D.; Patino, L.; Quintero, M.; Cortes, J.; Bastos, S. Effects of the aspartic protease inhibitor from Lupinus bogotensis seeds on the growth and development of Hypothenemus hampei: An inhibitor showing high homology with storage proteins. Phytochemistry 2014, 98, 69–77. [Google Scholar] [CrossRef] [PubMed]




| Factor | Average Number of Blue Spots per gr Dry Weight EC | |
|---|---|---|
| Age of EC | ||
| Early | 229,535 ± 9626 a | |
| Differentiated | 65,769 ± 5340 b | |
| A. tumefaciens strain | ||
| LBA4404 | 264,877 ± 12,176 a | |
| EHA105 | 99,759 ± 4270 b | |
| Co-culturing medium | ||
| Solid | 167,526 ± 80,923 a | |
| Liquid | 62,434 ± 54,765 b | |
| Transformation vector | ||
| pCambia1301 | 164,199 ± 8894 a | |
| pCambia2301 | 146,344 ± 9150 b | |
| Genotype | ||
| BK.620 | 172,437 ± 87,017 a | |
| BI.625 | 182,955 ± 87,810 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina, D.; Acuña, R. Optimizing the Genetic Transformation of Coffea arabica Using Agrobacterium tumefaciens. Int. J. Plant Biol. 2024, 15, 1250-1265. https://doi.org/10.3390/ijpb15040086
Molina D, Acuña R. Optimizing the Genetic Transformation of Coffea arabica Using Agrobacterium tumefaciens. International Journal of Plant Biology. 2024; 15(4):1250-1265. https://doi.org/10.3390/ijpb15040086
Chicago/Turabian StyleMolina, Diana, and Ricardo Acuña. 2024. "Optimizing the Genetic Transformation of Coffea arabica Using Agrobacterium tumefaciens" International Journal of Plant Biology 15, no. 4: 1250-1265. https://doi.org/10.3390/ijpb15040086
APA StyleMolina, D., & Acuña, R. (2024). Optimizing the Genetic Transformation of Coffea arabica Using Agrobacterium tumefaciens. International Journal of Plant Biology, 15(4), 1250-1265. https://doi.org/10.3390/ijpb15040086






