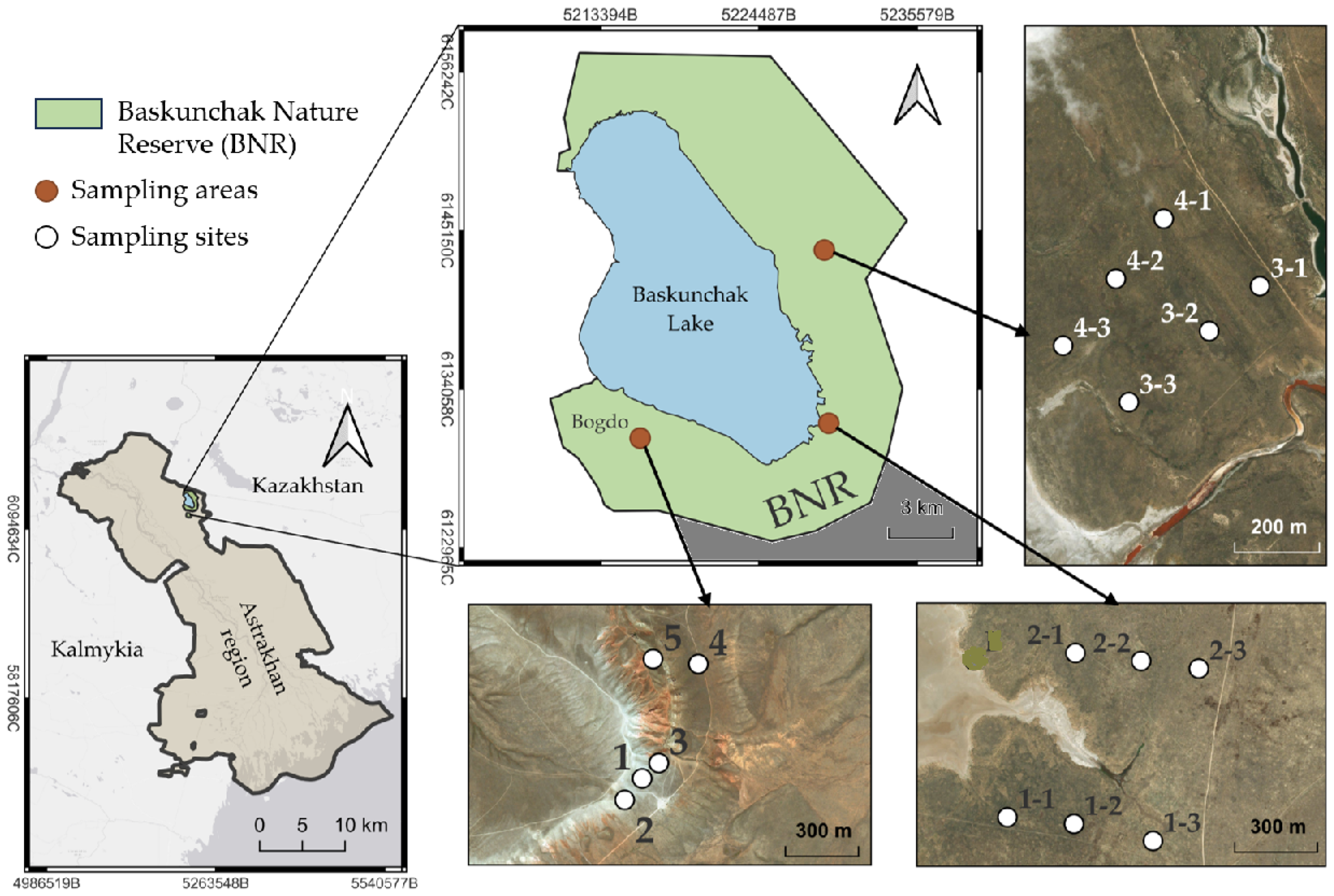
3.1. Soils
The comparison was performed between a steppe zone (BNR) and the Big Bogdo mountain. The latter is the only peak at the Caspian lowland situated in the close vicinity of the salt-lake, is characterized by outcrops of rocks from the Triassic period (the period of the mountain formation), and is the edge of a constantly growing (about 1 mm per year) salt dome covered by sandstones, lime stones, and clay, whose height does not exceed 150 m [
36].
High soil variability, low organic matter content (humus concentration is 0.66–1.45%), and the presence of easily soluble salts and gypsum are typical for this area [
27,
29].
Soil alkalinity (pH > 7.5) is characteristic of arid and semi-arid regions, and highly calcareous soils. High soil pH creates the conditions of decreased heavy metals accumulation affecting solubility, bioavailability, and translocation [
36]. High pH levels are known to be beneficial for trace element absorption [
37], encouraging their higher bioavailability, compared to acidic soil conditions [
36], also referring to soil Se [
37].
According to the analysis, the mean pH was significantly higher (9.4) at the Big Bogdo mountain compared to most of the BNR parts (8.1). The coefficients of variation of this parameter did not exceed 5% within each territory (
Supplement Table S1).
On the contrary, variations in soil salinity at the territory were much greater: from 0.280 to 0.980 g kg
−1 at Big Bogdo mountain up to 0.223–5.652 g kg
−1 at the BNR area (
Table 3).
The analysis of acid-soluble mineral content also revealed high variations in soil Cu, Zn, Fe, Cr, Sr, and Pb levels, especially remarkable at the Big Bogdo mountain (CV = 33.6–70.6%), less pronounced at the territory of the BNR (CV 11.5–30.8%) (
Supplement Table S2). The complexity of soil relief of the Big Bogdo mountain indicates the uneven distributions of mineral elements (
Supplement Table S2).
Overall, the elemental analysis of 17 soil samples revealed the existence of several highly significant correlations between Zn, Cu, Se, Cr, Pb, and Mn (
p < 0.001) (
Table 4). Similar significant Zn, Cu, and Cr correlations were previously recorded in different urban soils of Belgrade using acid extraction of the elements [
38].
The results express only the peculiarities of reactive soluble elements, providing the opportunity to evaluate the element amounts potentially available for plant absorption [
39,
40]. A diluted nitric acid extraction used in the present investigation was adopted as a standard to extract geochemically reactive elements in soil, allowing for excluding the inert fraction incorporated in minerals, organic matter, and oxides [
39]. The obtained reactive concentrations are considered “potentially available” for plants [
40], reflecting their geochemical association in soils and sediment-forming minerals.
The obtained data indicate that negative correlations were recorded only between Pb and Zn, Cr, Se, and between Mn and Zn, Cr, Cu, and Sr. No significant correlations between the analyzed elements and soil salinity were recorded (
Table 4).
3.2. Plants
To date, 588 higher vascular plant species belonging to 77 families have been identified at the Baskunchak Nature Reserve [
41]. The main territory of the Reserve is occupied by wormwood desert and fescue-feather grass steppes. Among the species inhabiting the Baskunchak Nature Reserve territory, representatives of Asteraceae, Poaceae, Fabaceae, and Lamiaceae families account for 14.6, 10.0, 6.0, and 3.1% out of the total identified species [
30]. Due to low precipitation levels (
Figure 2) and intensive fluctuations of temperature, most plants inhabiting the Baskunchak Nature Reserve show extremely short periods of active growth and development. Therefore, the obtained results refer to the end-of-May period, corresponding to the highest increase in biomass for most grasses. Notably, the studied area is characterized by widespread pterophitic and psammophitic plants that are able to survive in sandy and stony soils due to a powerful root system. They are characterized by small aerial extensions and limited height. Slow-growing taproot turfy herbaceous plants and subshrubs and small-turf grasses predominate.
Plant resistance to stresses greatly depends on genetic peculiarities, nutrient availability, antioxidant status, and hormonal regulation [
42]. The elemental analysis of the Baskunchak Nature Reserve plant collection showed high species variability of the content of macro- (
Table 5), toxic (
Table 6), and microelements (
Table 7), suggesting different strategies of plant defense against environmental hazards.
Among the species examined, two contrasting groups of plants can be indicated: Artemisia representatives, and plants belonging to Poaceae family. Indeed, while A. taurica and A. lerchiana accumulated the highest levels of ash and minerals including Na, K, Al, Ni, V, Cu, Zn, and the lowest of Si, Poaceae species showed an opposite frame. Particularly, ash, K, Ni, V, Cu, and Zn levels in Poaceae plants were 2–10 times lower than in wormwood, whereas a 25–32 times lower sodium concentration and 14–30 times higher Si one were recorded.
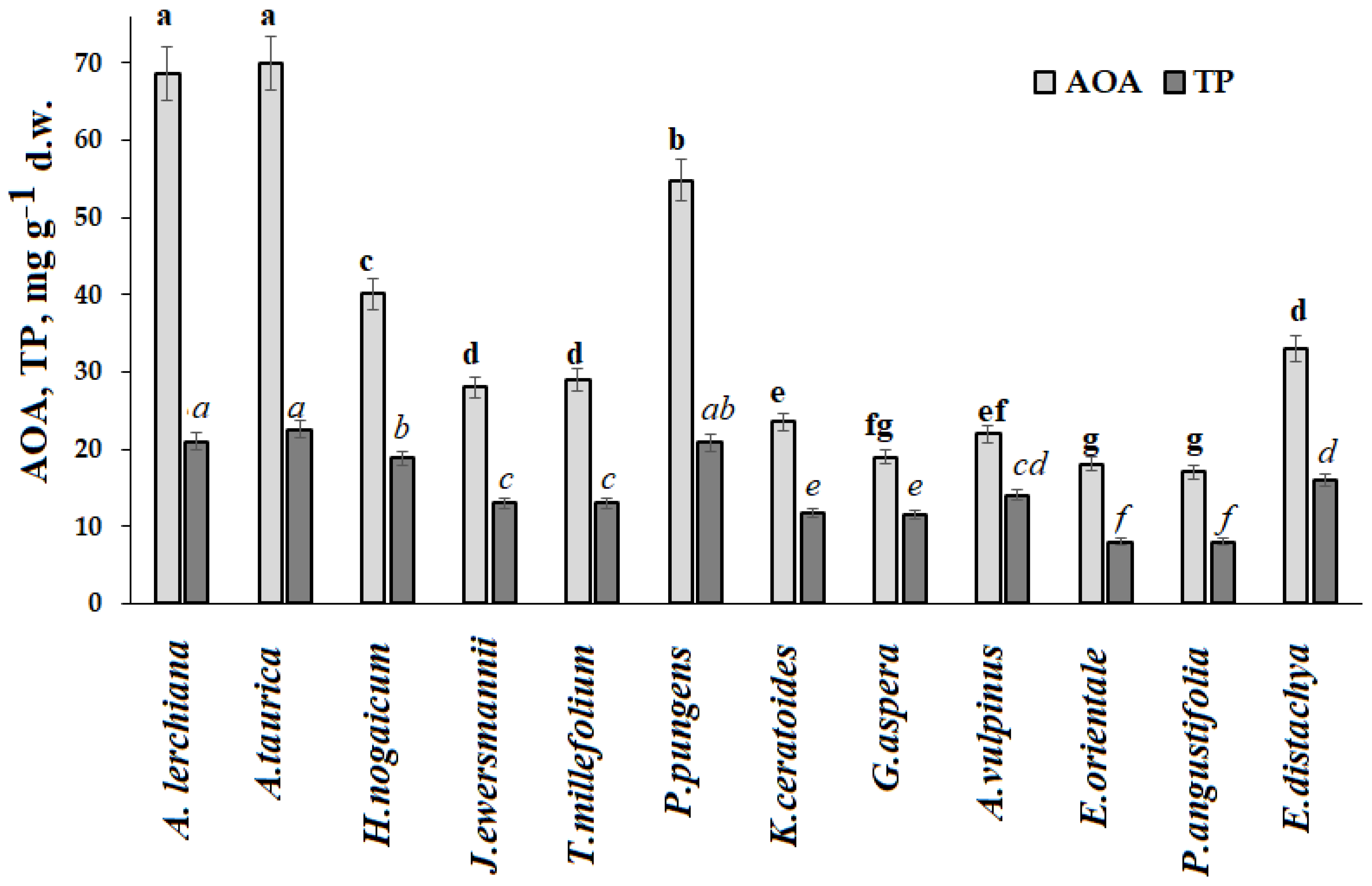
Furthermore, the highest antioxidant status was typical for Asteraceae plants and the lowest for Poaceae species (
Figure 3). In this respect,
Artemisia species may be considered as an appropriate example of plants with powerful antioxidant defense strategy provided by secondary metabolites (polyphenols, essential oil) and minerals. Indeed, the ability of these plants to accumulate high concentrations of potassium makes it easy to tolerate high salinity and high Na levels, activate enzymes, promote the rapid accumulation of carbohydrates, and improve osmoregulation and water transport, protecting against oxidative stress via the induction of the secondary metabolite synthesis, particularly polyphenols [
43]. It is worth mentioning that among the examined plant species,
A. taurica and
A. lerchiana showed an unusual ability to accumulate high levels of Na, contrary to the root salt exclusion mechanism especially typical for most of halophytes and Poaceae representatives [
44]. Low levels of Na in other Baskunchak plants may also relate to the ability of halophytes to secrete salt via salt gland [
44], as previously reported in several
Artemisia species [
45] and
Eremopyrum orientale, belonging to Poaceae family [
46].
Furthermore, high levels of Cu and Zn in
Artemisia species indicate the importance of these microelements in plant adaptability due to the participation of these elements in various redox reactions and antioxidant enzymes [
15]. Particularly, Zn is an essential element participating in plant development, antioxidant status, and tolerance to drought and salinity [
16], being a cofactor of many enzymes, such as Zn-superoxide dismutase, carboxypeptidase, and carbonic anhydrase. Increased plant ability to accumulate high concentrations of Zn may also relate to plant exudates, containing organic acids, phenolics, carbohydrates, amino acids, sugars, and polysaccharides, which are able to decrease pH and improve Zn extraction ability [
47]. Moreover, benefits to plant growth in stress conditions have been reported with reference to V [
17] and Ni [
48].
The high occurrence of Poaceae plants at the territory of the Baskunchak Nature Reserve indirectly indicates their high adaptability based on a different strategy. Indeed, these species show an exclusion mechanism that prevents the accumulation of Na and toxic elements in the aboveground part of plants [
49].
Poa angustifolia and
E. orientale demonstrated the ability to avoid the absorption of many elements (Na, V, Ni, Al, Zn, Cu, Mn, B, Li, Fe, Cu, Co), which is consistent with the known adaptation feature of Poaceae representatives [
50,
51]. However, the most typical skill of Poaceae plants is their ability to accumulate high levels of silicon. The latter element is known to enhance growth, photosynthesis and carbon assimilation, phytohormone biosynthesis, and plant tolerance to environmental stresses, including drought, high salinity, extreme temperature and insolation, nutrient deficiency and metal toxicity, and pathogen and herbivory attack [
52]. Morphological changes caused by SiO
2 deposition improve stem rigidity and leaf erectness of plants [
53].
It is worth mentioning that neither
Artemisia species nor Poaceae representatives accumulate significant amounts of the powerful natural antioxidant Se [
37]. Contrarily, about 50% of the tested plants had significant concentrations of this element, which may participate in plant adaptability to stress conditions. The latter protection mechanism could involve plant species belonging to the Fabaceae family, such as
J. ewersmannii,
H. nogaicum,
T. millefolium,
P. pungens, and
K. ceratoides, with relatively high Se levels (
Table 7).
More complex adaptability mechanisms may take place in other plant species. Indeed, high antioxidant activity was recorded in
H. nogaicum,
P. pungens, and
E. distachya.
Tanacetum millefolium showed high Si content.
Jurinea ewersmannii Wge accumulated unusually high levels of Ca.
Ephedra displayed rather high antioxidant status (
Figure 3) and accumulated the highest levels of Co, Fe, Pb, and Sr and the lowest of Mo, K, Na, and Cu. Contrary to literature reports [
25],
Ephedra was not among the plants with high B accumulation at the territory of the Baskunchak Nature Reserve. According to literature reports,
E. intermedia also demonstrated a high ability to accumulate Sr and Fe [
54].
Both the Fabaceae species studied accumulated extremely low concentrations of V in leaves. The latter fact is not in contrast with V participation in nitrogen fixation via V-dependent nitrogenases of legumes, as the process proceeds exclusively in roots.
High pH and low heavy metal contents in soils result in low concentrations of the latter in the Baskunchak plants (
Table 6). Nevertheless, among toxic elements, Al mostly accumulated in Asteraceae representatives and
Ephedra distachya. Arsenic predominated in Asteraceae plants, including
Phlomis pungens and
Ephedra distachya. The lowest levels of chromium were recorded in Fabaceae species and
K. ceratoides, and its concentration was the highest in
Phlomis punhems. The leaves of the latter plant, grown in a primrose clay at the Bogdo mountain, accumulated up to 2.2 mg Cr kg
−1, which was 2–7 times higher than Cr accumulation levels in other plants. Nevertheless, it is worth highlighting that Cr predominantly accumulates in plant roots but not leaves [
55]. Therefore, the obtained data may reflect both differences in translocation of the element from roots to shouts and species variations.
The mineral composition of individual plants within a species may vary considerably depending on the habitat location reflecting the phenotypic adaptation development. On the other hand, the ratio between different elements is less affected by this geographic variation due to plant ability to absorb and accumulate nutrients selectively, providing a balance of intracellular concentrations to optimize metabolism, protein synthesis, and tissue production [
56].
3.3. K/Na, Ca/Sr, Ca/Na, and Fe/Mn Ratio
In the conditions of the Baskunchak Nature Reserve, the K/Na ratio characterizing the adaptability of plants to high salinity levels was the highest in
Krascheninnikova ceratoides,
Ephedra distachya, and
Glycyrrhiza aspera, reaching 151.4–224.4, and the lowest in two
Artemisia species (
A. lerchiana and
A. taurica) and
Tanacetum millifolium (6.21–11.2;
Table 8); the latter three plants demonstrated the ability to accumulate high levels of Na without growth inhibition. High K/Na and Ca/Na ratios are essential for normal plant metabolism [
57].
In conditions of environmental gypsum excess, the Ca/Sr ratio is an important parameter of plant adaptability. In this respect, all the tested Asteraceae representatives in addition to
Krascheninnikova ceratoides (Amaranthaceae) showed the highest Ca/Sr values (201.4–338.5). These observations were consistent with the similar high Ca/Sr ratio detected in a collection of
Artemisia species grown at Nikitsky Botanic Garden, Crimea [
45].
The Ca/Sr ratio in plants depends on their concentration in soil, the ability of plants to discriminate between Ca and Sr, and environmental factors either stimulating or inhibiting Sr bioavailability. Indeed, high variations in the plant Ca/Sr ratio were recorded in Turkey in the vicinity of a silver-mining area [
58]. Strontium bioavailability was shown to be greatly improved in the presence of calcium carbonate [
59]. Furthermore, plants may greatly differ in the ability to distribute Sr between leaves and roots [
60]. In this respect, it may be highlighted that plants at the Big Bogdo mountain contain significantly lower levels of Sr (33.5 ± 13.3; 17.2–49.8 mg kg
−1 d.w.), compared to the steppe zone of the Reserve (55.6 ± 27.7; 25.2–99.6 mg kg
−1 d.w). Relatively high variations in Sr content within each area may reflect uneven distribution of Sr, Ca, and, in particular, gypsum deposits.
Despite the low bioavailability of Fe in soils with high pH, the Fe/Mn ratio in most plants tested was high and reached 2.1–7.7, while extraordinary ratios (13.5–15.3) were recorded in
Ephedra distachya and
Phlomis pungens. Taking into account that either an Fe/Mn ratio of 1.5–2.5 is considered physiologically adequate for plants [
57] or the Fe/Mn antagonism is well documented, the presented data allow to reveal the existence of significant plant Fe uptake [
57]. Furthermore, the Fe/Mn ratio positively correlated with the Ca/Na one (r = 0.559,
p < 0.04), suggesting a positive correlation of Ca and Na with Fe and Mn assimilation in plants in semi-desert conditions. To date, no relationships between Ca/Na and Fe/Mn ratio have been revealed.
According to the literature data [
61], high levels of Ca promote the increase in K/Na ratio in plants, improving their resistance to salinity stress. The present results showed a significant positive correlation between Ca/Na and K/Na ratios (r = 0.605,
p < 0.04). Gypsum is known to regulate the replacement of sodium (Na
+) with calcium (Ca
2+) on clay surfaces, thereby increasing the Ca
2+/Na
+ ratio in the soil solution. Intracellularly, Ca
2+ also promotes a higher K
+/Na
+ ratio. Moreover, the presence of sulfur (S) in gypsum improves plant growth through the increased production of phytohormones, amino acids, glutathione, and osmoprotectants, which are vital elicitors in plant responses to salinity stress.
3.4. Relationship Between Elements in Plants
Differently from most investigations, where the relationship between mineral elements in plants have been assessed only within a single species in different environmental conditions, in this research, 12 plant species belonging to 6 families and grown at the territory of the Baskunchak Nature Reserve were examined. Significant correlations (
p < 0.01) have arisen between macroelements (Na, K, Ca), essential microelements (Cu, Fe, Zn, Co), and beneficial elements (V, Al) (
Table 9). The detected correlations between elements are supposed to reflect similar element accumulation efficiency in the tested plants.
Notably, similar highly significant V-Al-Fe-Co interactions were recorded previously in a collection of
Artemisia species at the Nikitsky Botanic Garden [
45], while Fe-Co correlation was confirmed in a couch grass [
62]. Taking into account the specific environmental conditions at the Baskunchak Nature Reserve, the mentioned elements may participate in plant protection against stresses in semi-desert areas.
Cobalt is a transition metal located in the fourth line of the periodic table, a neighbor of Fe, and it is an essential element for Fabaceae plants which improves N
2 fixation [
63]. The same ionic radius of Co
2+ and Fe
2+ (both 0.76 Å) may explain the significant correlation between these two elements and the known replacement of iron by cobalt in the active sites of enzymes.
The high Fe-Al correlation may be attributed to the similar ionic radius of the hydrated ions of Fe and Al or the same charge [
64]. Fe and Al hydroxides are considered strong primary sorbents of V in soils [
65]; precisely, Fe and Al (hydr)oxides are the main compounds determining the mobility of V in soil. Vanadium bioavailability increases in soils with high pH [
66].
3.5. Soil–Plant Interaction
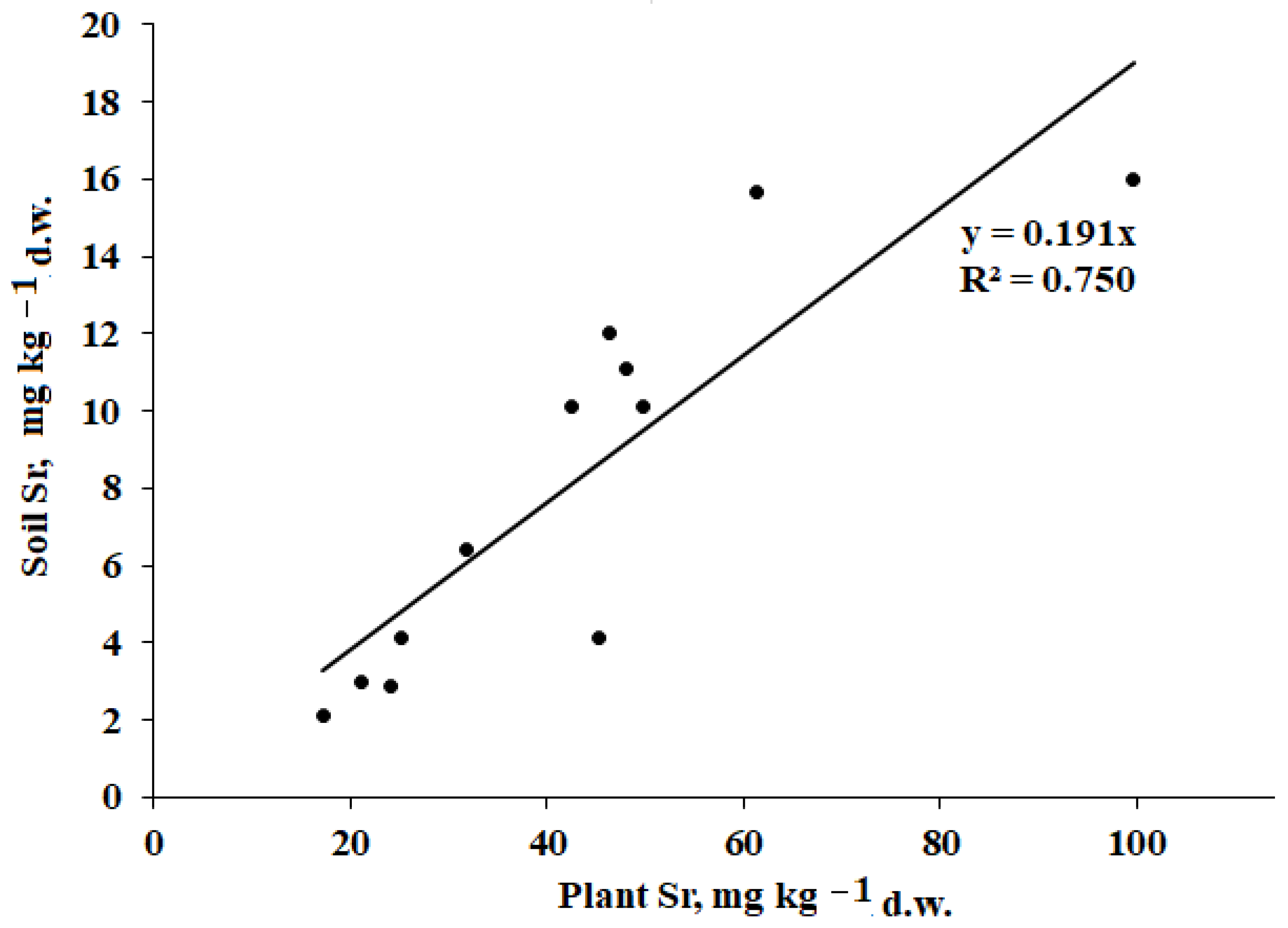
The relationship between mineral element content in plants and soils depends on soil characteristics, level of salinity, climate, and plant species differences. Using a singular extraction technique with diluted HNO
3, only the significant correlation between plant and soil Sr was revealed (
Figure 4).
The statistically significant correlation between plant and soil Sr levels suggests similar conditions of Sr absorption by plants at the territory of the Baskunchak Nature Reserve. Plants accumulate stronthium through Ca
2+ channels as a free divalent ion and to a lesser extent as a complex with low-weight organic compounds [
67,
68]. Contrary to Ca, Sr is not essential to plants and is able to replace Ca and co-precipitate with Ca in soils [
69]; the intensity of its accumulation depends on the competition with Ca, absorption on organic chelators [
70], plant exudates, and soil electric conductivity [
71].
The present results revealed a positive correlation between plant Sr levels and soil salinity (TSS; r = 0.763,
p < 0.001), in accordance with the Yasuda et al. reports [
71], which is the most significant among the plant–soil interactions examined, in agreement with a significant correlation between the values of Sr content in plants and soils (
Figure 4). Moreover, a weak effect of soil salinity on the content of Co, Fe, and Pb in plants was recorded (r = 0.627,
p < 0.02; r = 0.606,
p < 0.03; r = 0.548,
p < 0.05, respectively).
Notably, the effect of salinity on plant mineral composition has not been fully understood, and, indeed, it may consist of either an increase or decrease in plant element accumulation ability [
72]. According to numerous investigations, salinity inhibits plant Se accumulation [
72,
73]. The present results did not demonstrate any relationship between soil salinity and plant Se content, which indirectly indicates a significant role of species differences in the ability to accumulate Se [
37] and a beneficial effect of gypsum on plant growth. However, it is worth highlighting that complex soil fractionation is necessary to reveal other relationships between soil and plant elements (Cu, Zn, Mn, Pb, and Se) [
74]. Furthermore, soil–plant interaction is greatly governed by genetic factors, so that lack of significant correlations between soil/plant elements in all the plants tested is not unexpected. In this respect, it is necessary to mention a positive Cr soil–plant correlation (r = 0.986,
p < 0.001) relevant to Asteraceae plants of the Big Bogdo mountain. Nevertheless, further investigations are needed to confirm the mentioned relationship.
3.6. Biological Concentration Factor (BCF)
The elements analyzed in soil samples allowed us to evaluate the coefficients of biological accumulation or a biological concentration factor (BCF) of only 8 elements: Sr, Zn, Pb, Fe, Cu, Mn, Cr, and Se (
Table 9). All the mentioned elements, except Sr, Pb, and Cr, are known to participate in plant antioxidant defense. Indeed, Mn is a plant-essential element, which is involved in photosynthesis and acts as a cofactor in several enzymes with antioxidant properties [
75]. Copper is another essential element which also plays an important role in photosynthesis, respiration, antioxidant activity, protein metabolism, carbohydrate distribution, and nitrogen fixation, etc. [
76,
77]. Zinc is a constituent of many enzymes affecting gene regulation and activation, protein synthesis, and carbohydrate metabolism, providing the enhancement of membrane stability [
16,
47,
78]. Though Se is not an essential element in plants, it is actively involved in alleviating both biotic and abiotic environmental stresses via improvement of antioxidant defense and enhancement of water uptake by plants [
37,
79]. High Se BCFs were recorded in 6 species (
T. millefolium,
Jurinea ewersmannii,
H. nogaicum,
G aspera,
P. pungens,
K. ceratoides) (
Table 10). In the latter list, the first three representatives belong to Asteraceae family, the fourth one to Fabaceae family, and
K. ceratoides to Amaranthaceae, which include plant species/accumulators of Se [
45,
72,
80,
81]. Nevertheless, the ability of these plants to accumulate Se has never been studied before.
A bio-concentration factor exceeding 1 was recorded for Mn only in one species (T. millefolium), for Cu and Zn in all Asteraceae species.
In this respect, the possibility of element participation in plant protection against environmental stresses in semi-desert conditions is the most pronounced in Asteraceae representatives that are able to actively accumulate Zn, Cu, and Se, while, among other species, only K. ceratoides and P. pungens showed similar ability.
In conditions of high pH and low soil organic matter, Cr is present in soil predominantly in a mobile toxic Cr(VI) form [
55,
82]. In this respect, the exclusion of toxic Cr (BCF < 1 in the aerial plant parts) was recorded in most of the species examined, which may be partly connected to the main accumulation of this element in roots and active reduction in Cr(VI) to Cr(III) [
83]. Only three species,
J. ewersmannii,
A. Taurica, and
P. pungens, displayed BCF values > 1 at extremely low soil Cr contents. Among the latter species,
P. pungens also showed a remarkable ability to accumulate Zn, Cu, and Se, which supposedly participate in plant defense.
Pb exclusion was recorded only in half of the species investigated. The BCF value of Pb decreased according to the following sequence: Ephedra distachya > Artemisia lerchiana > Eremopyrum orientale > Astragalus vulpinus > Poa angustifolia.
Among Fe accumulators, it is worth highlighting the high accumulation abilities of E. distachya, H. nogaicum, and A. lerchiana.
Despite the linear correlation between soil–plant Sr levels, the distribution of the parameters suggests significant differences in the BCF among the plants tested. Unexpectedly, the broad BCF range of values, from 4.5 in
Astragalus vulpinus to 11 in
G. aspera, was in accordance with the detected effect of species variability and the external factors [
84]. In the conditions of the Bogdinsko-Baskunchak Nature Reserve, the BCF values of Sr were the highest (8–11) in
G. aspera,
P. pungens, and
T. millefolium, which may be beneficial in phytoremediation of radioactive Sr with similar behavior as stable Sr [
84]. A positive correlation between Sr levels in plants and soils at the Baskunchak Nature Reserve suggests high availability of this element in semi-desert conditions at the beginning of summer (25–28 May) with sufficient water supply. Indeed, high soil pH and low organic matter content enhances Sr bioavailability [
84].
The high Se, Zn, Cu, and other element concentrations in plant tissues may relate to phenotypic adaptability, leading, in certain cases, to the formation of genotypic changes. In this respect, high Se levels in plant tissues may reflect not only a phenotypic answer of plants to environmental stress but also changes in the expression of genes, encoding Se transport proteins, and the assimilation of selenate, encoding enzymes for the conversion of selenocysteine into non-toxic methylated forms or volatile derivatives [
85]. Among the plant families examined, Amaranthaceae, Asteracea, and Fabaceae reportedly contain Se accumulators [
86]. A high BCF value for
Phlomis pungens Wielld, the representative of the Lamiaceae family, has been recorded in the present research for the first time. The mineral composition of the examined species has never been studied previously, which indicates the need of further investigations in plant genetics.
3.7. Principal Component Analysis
The behavior of the 12 sampled plant species is shown in
Figure 5. The five species belonging to Asteraceae family (
Artemisia lerchiana L.,
Artemisia taurica L.,
Helichrysum nogaicum L.,
Jurinea ewersmannii Wge., and
Tanacetum millefolium L.) demonstrated an affinity for the accumulation of the elements Ca, V, Li, Ni, Na, Zn, Cu, Mn, K, Mg, B, Cd, and Mo and a higher Ca/Sr ratio than the other species examined.
The species belonging to the Lamiaceae family (Phlomis pungens L.) are graphically represented at the opposite end of the spectrum compared to the species of Asteraceae, regarding the accumulation of the aforementioned elements, with a slight affinity referring to the higher Ca/Na and K/Na ratios.
The two Fabaceae species (Glycyrrhiza aspera L. and Astragalus vulpinus L.) exhibited a common trend to accumulate Se and P, with a greater propensity for Se in the case of Glycyrrhiza aspera L. and P in Astragalus vulpinus L.
Furthermore, the species belonging to the Poaceae family (Eremopyrum orientale L. and Poa angustifolia L.) showed the highest K/Na ratio.
Finally, Ephedra distachya L. (Ephedraceae) and Krascheninnikova ceratoides L. (Amaranthaceae) exhibited contrasting behavior in the accumulation of mineral elements. Indeed, the first species was characterized by a higher relative accumulation of Cr and Sr, and greater Ca/Na and Fe/Mn ratios, whereas the latter showed a higher accumulation of P and Mo.