Abstract
This study investigates the influence of different light qualities, including red, green, blue, purple, and white lights, on the growth, physiological activity, and ornamental characteristics of two Coleus cultivars. Emphasizing the importance of leveraging phenotypic plasticity in plants within controlled environments, using light quality is a practice prevalent in the ornamental industry. The research explores the varied responses of two Coleus cultivars to distinct light spectra. The key findings reveal the efficacy of red light in enhancing shoot and leaf parameters in C. ‘Highway Ruby’, while red and green light exhibit comparable effects on shoot width and leaf dimensions in C. ‘Wizard Jade’. White light-emitting diodes (LEDs), particularly with color temperatures of 4100 K and 6500 K, promote root length growth in the respective cultivars. Moreover, chlorophyll content and remote sensing vegetation indices, including chlorophyll content (SPAD units), the normalized difference vegetation index (NDVI), the modified chlorophyll absorption ratio index (MCARI), and the photochemical reflectance index (PRI), along with the chlorophyll fluorescence, were significantly affected by light qualities, with distinct responses observed between the cultivars. In summary, this study highlights the transformative potential of LED technology in optimizing the growth and ornamental quality of foliage plants like Coleus, setting a benchmark for light quality conditions. By leveraging LED technology, producers and nursery growers access enhanced energy efficiency and unparalleled versatility, paving the way for significant advancements in plant growth, color intensity, and two-tone variations. This presents a distinct advantage over conventional production methods, offering a more sustainable and economically viable approach for increased plant reproduction and growth development. Likewise, the specific benefits derived from this study provide invaluable insights, enabling growers to strategically develop ornamental varieties that thrive under optimized light conditions and exhibit heightened visual appeal and market desirability.
1. Introduction
Coleus are popular plants known for their vibrant foliage, which has a wide range of uses for human consumption [1]. This genus, a member of the mint family (i.e., Lamiaceae), contains more than 300 species, which are mostly annual or perennial herbs that are native to the diverse locales of different countries, such as the Philippines, the Malay Archipelago, Australia, Africa, and the East Indies [2]. Many Coleus species are known to be pharmacologically important, such as C. forskohlii [3], C. amboinicus [4], C. aromaticus [5], and C. blumei [6]. These species are reported to have antibacterial and antifungal properties, holding various potentials for medicinal use. Aside from its medicinal use, it is used as an ornamental plant due to its beautifully prized and wide array of colorful foliage patterns, which may possess combined shades of green, maroon, yellow, pink, red, and white. Because of this, Coleus species have been used as potted plants or bed plants in many gardens, museums, and herbaria all over the world [7].
Because of their multi-colored leaves, Coleus represents an intriguing subject for investigating the influence of the light spectrum on growth dynamics and physiological activity to improve its ornamental value [8]. In fact, one of the interests in breeding ornamental bedding plants is improving and increasing their ornamental quality by producing foliage that have a rich leaf color intensity and unique variegation [9,10]. These improvements may be induced and achieved through the use of the proper light quality. Light is a fundamental environmental factor influencing the growth and physiological responses of plants [11]. The spectral composition of light, characterized by wavelengths ranging from ultraviolet to infrared, plays a pivotal role in regulating various aspects of plant development, including morphogenesis, photosynthesis, and overall productivity, as well as ornamental quality [12].
Recent advancements in horticultural lighting technologies have provided unprecedented opportunities to manipulate the spectral characteristics of light. Light-emitting diodes (LEDs) offer precise control over wavelength outputs, allowing researchers to investigate the effects of specific regions of the spectrum on plant growth and development [13]. Among the visible light spectra, the red, green, and blue monochromatic lights have provided sufficient evidence among other herbaceous crops to enhance growth and development, such as those of basil [14,15], carnations [16], impatiens, and petunia [17]. Likewise, other medicinal succulents, such as those of Orostachys spp. [18], Pachyphytum spp. [19], Peperomia spp. and cultivars [20], and other ornamental foliage plants [21], have found that the use of white LEDs also has profound effects on their growth and quality. For Coleus, it has been reported that exposure to varying light intensities in varieties of this genus increases anthocyanin and chlorophyll amounts, leading to changes in pigmentation [22]. Conversely, there have been reports on the use of light quality for a Coleus hybrid (C. blumei × C. fredericii) comparing blue, green, and red and far-red light, but these were mainly focused on its effect on the circadian rhythm of leaf movement for short-day plants [23]. While other studies on light quality have been focused on its in vitro propagation [24] and the increasing accumulation of certain phenolic compounds [25], limited studies have been found to evaluate the effects of light qualities on the growth and physiological activity of Coleus to increase its ornamental value. Aside from the use of monochromatic red, blue, green, and white LEDs, a composite purple LED light including a far-red wavelength has been recently reported to induce growth and improve the market value of multi-colored ornamental plants with respect to their size, and leaf and flower color, such as in Viola cornuta ‘Penny Red Wing’ [26], which may be applied to the colorful Coleus cultivars.
Although light quality has been previously studied in other Coleus cultivars, these studies have been limited to vegetative propagation methods (i.e., cuttings) and in vitro settings [24]. In contrast, this study aims to determine the seedling growth, developmental, and physiological responses of two Coleus cultivars using sexual propagation (seeds) grown under controlled environments, exploring the effects of different light spectra, including white light with various color temperatures. This approach sheds light on how these responses contribute to variations in growth performance and physiological efficiency, providing a broader understanding of the environmental impacts on Coleus cultivation. The importance of this research extends the benefits to ornamental crop production. Additionally, with the increasing emphasis on sustainable agricultural practices and resource optimization, optimizing light quality for Coleus growth will ultimately contribute to sustainability by maximizing resource efficiency (i.e., water and electricity), and producing high-quality and desirable ornamentals, while simultaneously minimizing energy consumption in indoor cultivation facilities. The present investigation delves into the specific effects of varied light spectra on the growth and physiological activity of two Coleus cultivars, aiming to contribute valuable knowledge and practical insights to the field of plant science.
2. Materials and Methods
2.1. Plant Material
The cultivars used in this study were grown using seeds, namely: Coleus ‘Highway Ruby’ (Sakata Seeds, Yokohama, Japan) and C. ‘Wizard Jade’ (Ball Horticultural, West Chicago, IL, USA). Three seeds were directly sown in individual pots (6.5 × 6.5 × 6.5 cm; width × length × height) filled with a fertilized horticultural substrate (Hanareumsangto, Shinsung Mineral, Goesan-gun, Republic of Korea).
The seeds sown were carefully managed until their germination in a greenhouse with a 25% shading level, which had an average temperature and humidity of 21.4 ± 4.1 °C and 59.3 ± 18.7%, respectively. After the successful germination and full development of the first true leaf, only one plant per pot was left, and the pots were moved to the seedling bed with a light-emitting diode (LED) installed (1.2 × 0.7 × 0.6 m; width × length × height).
2.2. Experimental Design and Treatments
The study was conducted using a randomized complete block design with seven different light quality sources, namely: red (peak at 630 nm), green (peak at 520 nm), blue (peak at 450 nm), purple (peaks at 450 and 650 nm + far-red), 3000 K (peaks at 455 and 600 nm), 4100 K (peaks at 455 and 590 nm), and 6500 K white LEDs (peaks at 450 and 545 nm), respectively (T5 LED, Zhong Shan Jinsung Electronic, Zhōngshān, China) (Figure 1). Each treatment was replicated four times with two plants per replication, thus having eight plants per treatment and a total of 56 plants for all the treatments. For the purple LED, a composite spectrum of purple was emitted from each diode with a far-red wavelength ratio of approximately 17%. The LEDs were 1.2 m long with a 20 W power consumption, which had an AC 220 V rated voltage at 60 Hz frequency.
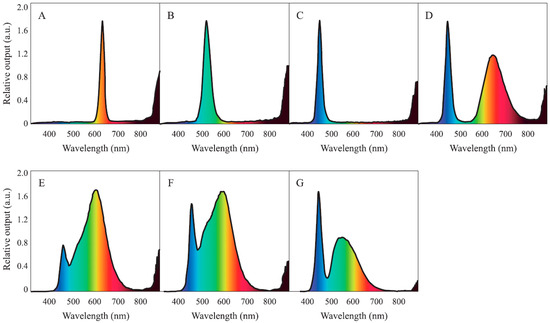
Figure 1.
Relative outputs of seven different light-emitting diode (LED) light sources: (A) red; (B) green; (C) blue; (D) purple; (E) 3000 K white; (F) 4100 K white; and (G) 6500 K white LED lights, respectively.
2.3. Growth Conditions and Experimental Management
The photon flux density (PFD) in the 350–800 nm range was measured using a portable spectroradiometer (SpectraPen mini, Photon Systems Instruments, Drásov, Czech Republic). The distance between the plants and the LEDs was standardized to ensure that the PFD was ≈100 μmol m−2 s−1. To maintain a consistent PFD, the distance between the plants and the LEDs was adjusted every week. To ensure the blocking of light treatments, a blackout curtain made from a black polyethylene shade film was installed on each side of the bed. The temperature and relative humidity were maintained at 20 ± 1 °C and 57.8 ± 16.1%, respectively. The plants were irrigated incrementally with a schedule where they were watered once a week at 1 to 5 weeks after treatment, and twice a week at 6 to 10 weeks after treatment. At 6 weeks after treatment, the Coleus cultivars’ seedlings were watered with 1000 ppm of a liquid fertilizer (N-P-K: 7-10-6, High-Grade S, Hyponex, Osaka, Japan). The plants were irrigated and fertilized by completely submerging the pots in water or a nutrient solution for 3 min or until the substrate was fully drenched.
The experimental set-up was performed at the Plant Physiology Laboratory of the Experimental Greenhouse, Department of Environmental Horticulture, Sahmyook University, Nowon-gu, Seoul, Republic of Korea for a total of 10 weeks.
2.4. Data Gathered
2.4.1. Plant Growth Parameters
Plant growth parameters were measured on all plants, including shoot, leaf, and root data, as well as ground cover. A further analysis was also performed in terms of the fresh weight (FW) and dry weight (DW) of the shoot and root, and their moisture content (MC). The dry weight was measured after hot-air drying the samples in a dry oven (HK-DO135F, Hankook S&I, Hwaseong-si, Republic of Korea) at 85 °C for 24 h. To determine the moisture content, it was calculated using this formula: %MC = [(FW − DW)/FW] × 100.
To determine the leaf pattern area and ratio, photographs of representative leaf samples alongside a ruler as a standard were taken. Leaves were taken from the same location of each plant, specifically 30 cm from the base to the top of the plant. Standardized images were analyzed using ImageJ version 1.51 (National Institutes of Health and Laboratory for Optical and Computational Instrumentation, Madison, WI, USA) to determine the leaf pattern area of the entire leaf. The leaf color ratio was computed using the formula:
Leaf color ratio (%) = (Leaf pattern area/Total leaf area) × 100
2.4.2. Leaf Color Analysis
To measure the CIELAB color space values, two readings were taken on each cultivar to signify the primary (i.e., green) and secondary (i.e., red or white) colors present in each leaf, depicting a two-tone color pattern. For each color pattern, four replications were performed with each replication having two random readings, signifying a total of eight readings per treatment. After setting the spectrophotometer (CM-2600d, Konica Minolta, Tokyo, Japan) to CIELAB D65/10°, the specular component including the (SCI) CIELAB values of the leaf colors were measured and analyzed. Based on the CIELAB values, the Royal Horticultural Society (RHS) values were derived as referenced in the study by Lee et al. [21].
2.4.3. Remote Sensing Vegetation Indices
The chlorophyll content was measured using a portable chlorophyll meter (SPAD-502Plus, Konica Minolta, Tokyo, Japan). The normalized difference vegetation index (NDVI) [27], the photochemical reflectance index (PRI) [28], and the modified chlorophyll absorption ratio index (MCARI) [29] were measured using a portable device that measures vegetation indices (PolyPen RP410, Photon Systems Instruments, Drásov, Czech Republic), and the equations are as follows:
2.4.4. Chlorophyll Fluorescence Analysis
To analyze the physiological responses of two Coleus cultivars, a chlorophyll fluorescence analysis was performed. Using a portable fluorometer (FluorPen FP 110/D, Photon Systems Instruments, Czech Republic), the chlorophyll fluorescence measurements were replicated three times per plant in randomly selected parts where the leaf veins did not pass within the green color parts of the leaves. Prior to the chlorophyll fluorescence indices measurements, the plants were placed in a dark room for about 15 min following the manufacturer’s guidelines. Five chlorophyll fluorescence parameters (Fv/Fm, ΦDo, ABS/RC, DIo/RC, and PIABS) were applied to determine the stress levels and performance indices of the Coleus cultivars. Fv/Fm represents the maximum quantum yield of photosystem II (PSII); ΦDo represents the probability that an absorbed photon is dissipated; ABS/RC represents the absorption (ABS) flux per reaction center (RC); DIo/RC represents the dissipated energy flux per RC; and PIABS represents the performance index (PI) on an absorption basis. The parameters were estimated following the equations described by Strasser et al. [30]:
2.5. Statistical Analysis
All the results, including the plant growth parameters, vegetation indices, CIELAB color space values, and chlorophyll fluorescence data, were analyzed using SAS 9.4 (SAS Institute, Cary, NC, USA). To compare the mean differences between the light quality treatments, Duncan’s multiple range test (DMRT) was used at a p < 0.05 level of significance.
3. Results
3.1. Plant Growth Parameters
For the plant growth parameters, the phenotypic data (i.e., shoot, leaf, and root measurements) and the moisture content analysis were gathered and analyzed and are presented in this section. The results showed that different light qualities significantly affected the plant growth parameters of both the Coleus cultivars as shown in Table 1.

Table 1.
Growth performance of two Coleus cultivars as influenced by different LED light qualities.
For C. ‘Highway Ruby’, plants grown under the monochromatic red light were significantly the tallest (15.0 cm) and widest (15.7 cm) plants, possessing the highest stem diameter (0.5 cm) and number of leaves (24.8 leaves). Likewise, those under the red-light spectrum had the longest (7.7 cm) and widest (4.4 cm) leaves, which ultimately resulted in the widest ground cover (225.6 cm2), which significantly differed from all the other light quality sources (Figure 2A). However, in terms of the root length, the use of the 4100 K white light produced the longest root, measuring 17.0 cm.

Figure 2.
Comparative growth responses of two Coleus cultivars: (A) C. ‘Highway Ruby’; and (B) C. ‘Wizard Jade’ grown under different light qualities: (1) red; (2) green; (3) blue; (4) purple; (5) 3000 K; (6) 4100 K; and (7) 6500 K white LED lights, respectively.
Similarly, C. ‘Wizard Jade’ grown in red light also yielded the tallest plants, measuring 18.4 cm, the thickest stem diameter of 0.9 cm, and the most abundant leaves (34.8 leaves), which significantly differed from the other light treatments (Figure 2B). Along with the red light producing the widest shoot width (24.0 cm), highest leaf length (10.9 cm) and width (7.0 cm), and widest ground cover (579.2 cm2), these did not significantly differ from those plants grown under the green monochromatic light. It could also be noted that the use of 6500 K white light produced the longest root with 31.8 cm, which significantly differed from the other treatments.
As a crucial parameter in providing insight into the effectiveness of different light qualities in water retention and the optimization of growth, a moisture content analysis was performed. The results of the moisture content analysis are shown in Table 2.

Table 2.
Plant weights and moisture content of two Coleus cultivars as influenced by different LED light qualities.
In C. ‘Highway Ruby’, the highest fresh weights of the shoot (6.2 g) and root (0.09 g), along with the highest dry weight of the shoot (0.30 g), were observed under the red light treatment. Similarly, the use of monochromatic red light in C. ‘Wizard Jade’ also resulted in the highest fresh weights of the shoot (16.0 g) and root (0.77 g), and the highest dry weights of the shoot (0.90 g) and root (0.13 g). However, it was noted that the use of white light was found to be comparable with red light when considering the root dry weight of both cultivars.
In the case of C. ‘Highway Ruby’, the moisture content of the shoot was significantly affected by different light qualities, with the highest value found in plants exposed to the monochromatic green light at 96.7%. In C. ‘Wizard Jade’, a higher shoot moisture content was observed under red, green, and blue lights compared with the 3000 K and 4100 K white lights. Meanwhile, the moisture contents under purple and 6500 K lights were comparable with those under lights that exhibited higher moisture levels. However, there were no significant differences in the root moisture content between treatments in both C. ‘Highway Ruby’ and C. ‘Wizard Jade’ cultivars.
In addition, the leaf area ratios of the leaf color patterns was determined (Figure 3). The results suggest that the use of different light qualities significantly affected the leaf area ratios of both cultivars. The highest leaf pattern ratio in C. ‘Highway Ruby’ was observed in those treated with 4100 K white light (59.5%), while those in red (23.4%) and green (23.4%) lights had the lowest leaf area ratio (Figure 3A and Figure 4A).
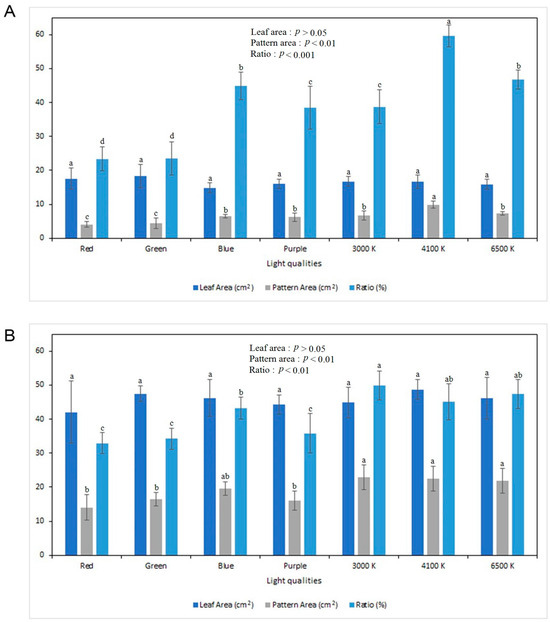
Figure 3.
Leaf area parameters of two Coleus cultivars: (A) C. ‘Highway Ruby’; and (B) C. ‘Wizard Jade’ as influenced by different light qualities. Different lowercase letters indicate significant differences at p < 0.05 based on Duncan’s multiple range test (DMRT), and the same lowercase letters indicate no significant difference.
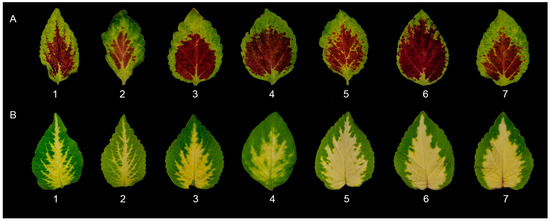
Figure 4.
Representative leaf samples measured for the leaf area parameters of two Coleus cultivars: (A) C. ‘Highway Ruby’; and (B) C. ‘Wizard Jade’ as affected by different light qualities: (1) red; (2) green; (3) blue; (4) purple; (5) 3000 K; (6) 4100 K; and (7) 6500 K white LED lights, respectively.
On the other hand, C. ‘Wizard Jade’ had the highest leaf area ratio in those in 3000 K white light (49.9%), which significantly differed from the other light qualities, while the use of red light resulted in the lowest leaf area ratio of 32.9%, which did not significantly differ from green and purple lights (Figure 3B and Figure 4B).
3.2. Leaf Color Analysis and Remote Sensing Vegetation Indices
3.2.1. Leaf Color Analysis
The two Coleus cultivars were subjected to a CIELAB color space analysis, and the values were converted to show the RHS color values and color groups. The results of the leaf color analysis are shown in Table 3.

Table 3.
CIELAB color space values of two Coleus cultivars as influenced by different LED light qualities.
The leaf color analysis was determined using the CIELAB color space, which defines three color coordinates (L*, a*, and b*) to indicate the degrees of lightness, hue, and saturation of the leaf colors, respectively. In the study of Cabahug et al. [31], it was summarized that the lightness is indicated by the L* value, while the chromaticity coordinates are represented by the a* and b* values. The lightness of the color is represented by the L* color value with a 0 to 100 value range. A higher positive value indicates a lighter color and a lower value indicates a darker color. Positive a* values indicate the red direction, while a negative a* value indicates the green direction. On the other hand, a positive b* value indicates a yellow direction, while a negative value indicates a blue direction.
For C. ‘Highway Ruby’, the CIELAB values of the primary color, which is the green part, indicated no significant differences between treatments, thus a common RHS value and the color group were found to be either 144A or 152B, signifying a ‘yellow-green’ color group. However, the secondary color, which is depicted by a red-maroon color, was significantly affected by different light qualities. The L* value was found to be highest when grown under green light, at 36.8, which significantly differed from the other treatments, while the use of purple light, 3000 K, or 4100 K white lights yielded the highest a* values, indicating a more reddish tone compared with the other treatments. For the b* value, a lighter hue was observed with the use of green light, at 15.3. The variable change in the use of green-colored light indicated a change in the color group separate from the other treatments, which were ‘greyed-purple’ to those being ‘greyed-red’.
An opposite trend was noted for C. ‘Wizard Jade’, wherein significant effects were found on the primary color of the leaf compared with the secondary color. The primary color of the leaf was found to be significantly affected by the use of the light quality spectrum, where the highest L* and a* values were observed in the plants grown under the monochromatic red and green lights, while the b* values were highest in green light alone. However, despite their significant differences, they still belonged to the same color group of ‘yellow-green’. On the other hand, the secondary color, visually presenting as ‘greyed-yellow’, showed no significant differences between treatments and remained within the ‘greyed-yellow’ color group.
3.2.2. Remote Sensing Vegetation Indices
The remote sensing vegetation indices, namely the chlorophyll content (SPAD units), the normalized difference vegetation index (NDVI), the modified chlorophyll absorption ratio index (MCARI), and the photochemical reflectance index (PRI) were analyzed using a portable device with direct contact with the plant. The results of the analyses are shown in Table 4.

Table 4.
Chlorophyll content (SPAD units), normalized difference vegetation index (NDVI), modified chlorophyll absorption ratio index (MCARI), and photochemical reflectance index (PRI) of both the primary and secondary leaf colors of two Coleus cultivars, as influenced by different LED light qualities.
In C. ‘Highway Ruby’, the highest chlorophyll content was found in 6500 K white light (14.5 SPAD units), which was comparable with that of blue (14.0 SPAD units) and 4100 K white lights (12.5 SPAD units). On the other hand, C. ‘Wizard Jade’ had the highest chlorophyll content in the cultivar grown under the blue light (29.5 SPAD units), which did not significantly differ from those grown in 6500 K white light (26.9 SPAD units).
Aside from the chlorophyll content, the primary leaf color of C. ‘Highway Ruby’ was significantly affected by the use of different light qualities, particularly NDVI and PRI. The highest NDVI was found in plants grown under 6500 K white light, with a value of 0.51, which was significantly different from the other treatments, while the use of red and green lights resulted in the highest PRI values of 0.003 and −0.003, respectively. On the other hand, the secondary leaf color was significantly affected by light treatments for the NDVI and MCARI parameters, with the highest values observed when treated with blue light.
The vegetation indices were significantly affected by the different light qualities in both the primary and secondary leaf colors of the C. ‘Wizard Jade’ cultivar. In the primary leaf color, the highest NDVI and PRI were observed in plants treated with blue light, showing values of 0.73 and 0.03, respectively. Meanwhile, the MCARI values were higher in monochromatic lights of green and red, and 3000 K white light, with 0.94, 0.89, and 0.94, respectively, and these did not significantly differ from each other. The results of the secondary leaf color analysis revealed that the use of red light resulted in the highest NDVI (0.07) and MCARI values (0.15), while PRI was highest with those under green and 3000 K white lights, showing a value of −0.01.
3.3. Chlorophyll Fluorescence Analysis
The chlorophyll fluorescence parameters of two Coleus cultivars, namely C. ‘Highway Ruby’ and C. ‘Wizard Jade’, were investigated under different light qualities. The results showed that different light qualities significantly affected all the parameters, as shown in Table 5.

Table 5.
Chlorophyll fluorescence parameters of two Coleus cultivars as influenced by different LED light qualities.
The maximum quantum yield of photosystem II (PSII) (Fv/Fm) was found to be highest in blue (0.846) and 6500 K white (0.842) lights in C. ‘Highway Ruby’, and in C. ‘Wizard Jade’, the use of blue (0.819) light consistently showed highest in this parameter. According to the standards for determining stress in plants, an Fv/Fm value between 0.780–0.840 indicates a healthy and unstressed plant, while values below 0.780 suggest potential stress or suboptimal conditions [20]. However, Strasser et al. [30] suggest that values less than 0.850 could also indicate stress. For PIABS in C. ‘Highway Ruby’, plants exposed to blue and 6500 K white lights recorded the highest values. Similarly, in C. ‘Wizard Jade’, blue light consistently yielded the highest values. Conversely, the probability that an absorbed photon is dissipated (ΦDo), the absorption flux per reaction center (ABS/RC), as well as the dissipated energy flux per reaction center (DIo/RC), were found to be highest in plants grown under green light for both cultivars.
4. Discussion
For the past decade, the plant industry has used the phenotypic plasticity of plants to its advantage, wherein plants are subjected to controlled environments for enhanced variations [32,33]. Among these conditions, the use of light quality improves plant growth, photomorphogenesis, and ornamental quality, which are deemed desirable [12]. The versatility of LED technology, characterized by energy efficiency, durability, compact design, extended lifespan, and minimal heat generation, presents promising possibilities as a primary and supplementary lighting system. It provides a distinct advantage compared with conventional production methods [34]. In this study, the growth responses and physiological activity of Coleus cultivars subjected to different light qualities were investigated.
Although it has been generally reported that plants suffer from poor development under monochromatic light as they are impacted by photosynthetic imbalances, numerous studies have been conducted recognizing their potential in variegated and green-colored plants [14,18,19,22,24]. Among the growth parameters, the use of red light produced the highest shoot and leaf parameters for C. ‘Highway Ruby’. This high growth is caused by the active phytochrome A, B, C, and D photoreceptors, which are mediated by and responsive to red light, that are responsible for leaf expansion [35,36]. Through these phytochrome receptors, vegetative growth is improved by inducing membrane properties and gene expression [37], thus promoting shoot and leaf growth. Likewise, the abovementioned phytochromes are also linked to stomatal opening [38] and development [39], improving photosynthetic efficiency. Red light significantly enhances photosynthetic yield by optimally aligning with the peak absorption wavelengths of chlorophyll a and b around 660 nm, thereby maximizing electron excitation and overall photosynthetic efficiency [14,16,18,19]. Aside from this, the use of red light increases the cell division rate leading to leaf-area expansion [40]. These results lead to the high fresh and dry weights or biomass accumulation of Coleus cultivars. Red light has been reported to increase net photosynthetic rates and expected increase in transpiration leading to a higher biomass and secondary metabolite accumulation [41]. Reviews by Denotes-Mainard et al. [42] also revealed that red light regulates a wide range of biological processes, including phytochrome expression, the development of vegetative architecture, and mineral nutrition. Various characteristics are considered when choosing ornamental plants based on purpose or location; larger leaf ornamentals are preferred for landscaping, while compact plants are more suitable for potted arrangements [43]. In cases where the market demands larger-sized plants, red light can be used to accelerate growth and increase plant size. On the other hand, if shorter and compact plants are preferred, the use of purple and white lights may be a better option. In C. ‘Wizard Jade’, red light is maintained to produce the tallest plants, widest stem diameter, and highest number of leaves. However, the use of green light on this cultivar had similar effects to red light, which produced the widest shoots, leaf length and width, and ground cover.
While red light promotes the responses from phytochrome, green light also plays a crucial role in enhancing plant growth through various mechanisms. Although plants appear green because they reflect a significant amount of green light, around 85 percent of the green light is absorbed or transmitted through the leaf, benefiting the lower leaves and deeper parts of the plant. Additionally, pigments other than chlorophyll, such as carotenoids, absorb green light and contribute to photosynthesis. Wang and Folta [44] noted that green light influences gene expression, enhancing adaptability to low-light environments and affecting shade-avoidance responses, which contributes to overall plant growth. Bian et al. [45] further demonstrated that exposure to green light enhances the activity of oxidative enzymes and promotes the expression of photosynthetic genes, such as LHCb and PsbA, in lettuce. Experiments, including those conducted at agricultural universities, show that incorporating green light can modify growth patterns, such as reducing seedling extension growth or, at higher intensities, promoting extension growth similar to far-red radiation [44,45,46]. Therefore, while the impact of green light varies with intensity and light combinations, its role in plant growth is significant. Further research on the manipulation of light durations and combinations of red and green lights for Coleus cultivars could provide additional insights into optimizing growth conditions.
However, root parameters were observed to be higher in different color temperatures of white LEDs with 4100 K for C. ‘Highway Ruby’ and 6500 K for C. ‘Wizard Jade’. White lights have been reported to increase root productivity and root biomass, attributed to the intercellular CO2 partial pressure [47]. Aside from this, the use of white lights rapidly enhances root growth by elevating sucrose translocation from the shoot to the root organs [48]. These results were similarly observed in studies where the use of various temperature levels of white lights produced increased root growth, such as in Artemisia annua [49], Orostachys japonica [18], Pachyphytum [19], and tobacco [50]. Having desirable plant characteristics for outdoor and indoor gardens, where soil media containers are shallow [51], the use of white LEDs may be considered a viable option for developing or preparing Coleus cultivars for these types of garden scaping where root growth and anchorage are of great importance [52].
The CIELAB color space in ornamental research helps to calculate and compare treatments in the metrics of lightness, chroma, hue, and color across condition changes [53]. It was revealed that the secondary color of C. ‘Highway Ruby’ was significantly affected by light qualities. In particular, green light significantly gave the highest L* and b* values resulting in a ‘greyed-red’ color, which differed from the other treatments that displayed ‘greyed-purple’. Because the red-type leaf coloration and pattern colors of plants increase the ornamental value in landscape gardening and the horticultural market [54,55], the use of green light may be detrimental for Coleus cultivars, while the use of red, blue, purple, and white LEDs, particularly 6500 K, were found to increase or intensify secondary color patterns. Among plant pigments, anthocyanins are flavonoids appearing in red-to-blue hues and are considered to be powerful antioxidants [56]. Previous studies reported that green light has minimal effects in promoting anthocyanin levels in microgreens [57], cabbage seedlings [58], and petunia flowers [59], which explains a lighter color in green light than under the other light spectra in Coleus cultivars. Likewise, it has minimal-to-no effect on the expression of the anthocyanin biosynthetic gene chalcone synthase, which is involved in the central flavonoid pathway [60,61]. On the other hand, the C. ‘Wizard Jade’ cultivar subjected to green light lightened the primary color, as indicated by higher L*, a*, and b* values, indicating a more yellowish hue. It has been reported that green light is less effective in increasing photosynthetic efficiency and activity [62]. The study by Liu and van Iersel [63] explained that green light provides the lowest photosynthetic efficiency due to its low absorbance. This is already established, as green light is least absorbed by green leaves, hence the green appearance of plants [64].
Based on the leaf area ratio and pattern analysis, the results suggest that the use of white light, especially at 4100 K, increased the secondary pattern color area (p < 0.01), leading to a higher pattern ratio (p < 0.001) in C. ‘Highway Ruby’. Likewise, in C. ‘Wizard Jade’, the use of white light at 3000 K significantly gave the highest percentage leaf color ratio (p < 0.01). A previous study suggests that white LEDs increased the purple leaf area or patterns while creating more compact plants [65], which was found to be similar to the results of this study. The literature suggests that the use of lights that increase light absorption in the green and yellow parts of the spectrum would result in increased purple colors and leaf pattern areas [66]. Results of chlorophyll content of both Coleus cultivars showing the highest SPAD units under 6500 K white light for C. ‘Highway Ruby’, which was not significantly different from the results under blue, purple, and 4100 K lights. For C. ‘Wizard Jade’, blue light and 6500 K white light produced similar results. As prominently known, red and blue lights play an important role in photosynthesis and chlorophyll content [67]. However, the use of cool white light has also been reported to increase chlorophyll content in rice [68], Chlorella vulgaris cells [69], potato tubers [70], and cucumber seedlings [71]. Williams et al. [72] indicated that plants are most effective in carrying out photosynthetic processes under white light, which leads to an increase in chlorophyll content.
In C. ‘Highway Ruby’, 6500 K white light resulted in the highest NDVI, while red and green lights showed the highest PRI values for the primary leaf color. For the secondary leaf color, blue light showed the highest NDVI values, and, along with blue and 6500 K white lights, produced the highest MCARI values. In C. ‘Wizard Jade’, the use of blue light had the highest NDVI and PRI values for the primary leaf color, while the use of red had the highest NDVI and MCARI values for the secondary leaf color. The NDVI serves as a straightforward visual metric indicator and gauges vegetation levels, wherein its values span from +1 to −1, with higher values indicating healthier and more photosynthetically active vegetation [73]. Aside from NDVI, MCARI determines the leaf chlorophyll concentration and ground reflectance [74], while PRI is a non-destructive tool to estimate the xanthophyll pigment interconversion [75]. Given the variations in the remote sensing vegetation indices caused by different pigments, it is plausible that various leaf colors would show distinct performances. This was demonstrated in the study by Sims and Gamon [76], which covered diverse leaf structural characteristics across different species or varieties.
The use of chlorophyll fluorescence is a newer technique and is more non-destructive (or non-invasive) than older biomass analysis methods [26,77]. The results of the chlorophyll fluorescence parameters suggest that the use of blue and 6500 K white lights consistently produced the highest rates of Fv/Fm and PIABS in C. ‘Highway Ruby’. In contrast, for C. ‘Wizard Jade’, blue light exclusively yielded the highest PIABS values. Both parameters are used to evaluate the photosystem II (PSII) efficiency and performance index in plants [78]. The values of both parameters in Coleus cultivars, excluding those exposed to green light in the C. ‘Wizard Jade’ cultivar (0.710), ranged from 0.776 to 0.846, which is considered to be within the range indicative of plants experiencing minimal or no stress [20]. This indicates that plants did not undergo stress under various light qualities, except for C. ‘Wizard Jade’ grown under green light. Meanwhile, the use of green light considerably had higher ΦDo, ABS/RC, and DIo/RC values in both cultivars. ΦDo denotes the likelihood of absorbed photons being dispersed; ABS/RC represents the absorption flux per reaction center; and DIo/RC shows the movement in the number of reaction centers existing in a reduced state within the photosystem II [79]. It was also suggested that a higher number of these parameters (i.e., ΦDo, ABS/RC, and DIo/RC) would indicate a higher inactivity of the reaction centers. The use of green light is considered less effective in driving photosynthesis compared with the other light qualities [80,81]. The inefficiency of green light absorption by chlorophyll is due to the fact that chlorophyll pigments have lower absorption peaks in the green region. As a result, a substantial portion of incident green light fails to be captured for use in the light-dependent reactions of photosynthesis [81]. The high biomass using red light, despite similarly high values in these parameters in green light, may be due to compensatory mechanisms, light utilization efficiency, and wavelength-specific effects associated with red light [42,82].
It is, likewise, important to recognize that chlorophyll fluorescence, while a valuable tool for assessing photosynthetic and physiological efficiency, including determining stress responses, does not constitute overall growth trends or future stress conditions. Thus, chlorophyll florescence data should incorporate a broader range of growth monitoring techniques. This approach would account for any discrepancies observed in chlorophyll florescence or growth parameters and provide a more holistic understanding of plant health and development.
5. Conclusions
This study highlights the significant impact of light quality, particularly red, green, blue, purple, and white light-emitting diode (LED) lights, on the growth, physiological activity, and ornamental characteristics of two Coleus cultivars. The results underscore the importance of leveraging phenotypic plasticity in plants within controlled environments to achieve enhanced morphological variations, a practice widely adopted in the ornamental industry. The key findings include the notable effects of red light, which demonstrated favorable outcomes for the shoot and leaf parameters, ground cover, and biomass accumulation in C. ‘Highway Ruby’. Similarly, red and green lights had positive effects on shoot width, leaf length and width, and ground cover in C. ‘Wizard Jade’. White LED lights, specifically wavelengths of 4100 K for ‘Highway Ruby’ and 6500 K for ‘Wizard Jade’, were advantageous for root length growth. A biomass analysis revealed that red light contributed to the highest shoot and root weight and moisture content, likely due to enhanced physiological activity. Regarding color and chlorophyll content, C. ‘Highway Ruby’ exhibited a consistent primary color across treatments, while C. ‘Wizard Jade’ showed significant effects on the primary leaf color based on light qualities. Different light qualities significantly impacted the chlorophyll content and remote sensing vegetation indices, with specific lights favoring various parameters. A chlorophyll fluorescence analysis indicated that blue light consistently produced the highest rates of Fv/Fm and PIABS in both cultivars, suggesting high PSII efficiency with minimal stress. Additionally, in C. ‘Highway Ruby’, the highest values for both parameters were observed under 6500 K white LED light. Conversely, green light exposure suggested reduced activity of the reaction centers, highlighting the inefficiency of green light absorption by chlorophyll. The study underscores the potential of LED technology, characterized by energy efficiency and versatility, in optimizing ornamental plant growth and quality. These findings provide valuable insights into the ornamental industry, offering a distinct advantage over conventional production methods. Producers and nursery growers can strategically use these results as a benchmark for assessing light quality conditions conducive to optimizing the growth, color intensity, and two-tone variations of Coleus and related ornamental plants. Moving forward, the authors plan to incorporate photosynthetic gas exchange measurements alongside stomatal conductance to provide a more comprehensive understanding of the physiological responses to different light qualities. The integration of advanced LED technology in horticulture not only paves the way for more sustainable and efficient production methods but also opens new avenues for achieving unparalleled aesthetic qualities in ornamental plants. By continuing to explore and refine these light-based strategies, we can push the boundaries of plant phenotypic expression, transforming the future of ornamental horticulture.
Author Contributions
Conceptualization, B.G.P., J.H.L. and S.Y.N.; methodology, J.H.L. and S.Y.N.; formal analysis, B.G.P. and S.Y.N.; investigation, B.G.P., E.J.S. and E.A.K.; writing—original draft preparation, B.G.P., J.H.L., E.J.S. and E.A.K.; writing—review and editing, B.G.P., J.H.L. and S.Y.N.; project administration, S.Y.N. All authors have read and agreed to the published version of the manuscript.
Funding
This paper was supported by the Sahmyook University Research Fund in 2023.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, M.; Coneva, V.; Robbins, K.R.; Clark, D.; Chitwood, D.; Frank, M. Quantitative dissection of color patterning in the foliar ornamental Coleus. Plant Physiol. 2021, 187, 1310–1324. [Google Scholar] [CrossRef]
- Lebowitz, R.J. The genetics and breeding of Coleus. Plant Breed. Rev. 1985, 3, 343–360. [Google Scholar]
- Kavitha, C.; Rajamani, K.; Vadivel, E. Coleus forskohlii: A comprehensive review on morphology, phytochemistry and pharmacological aspects. J. Med. Plants Res. 2010, 4, 278–285. [Google Scholar]
- Malathi, R.; Cholarajan, A.; Karpagam, K.; Jaya, K.R.; Muthukumaran, P. Antimicrobial studies on selected medicinal plants (Coleus amboinicus, Phyla nodiflora and Vitex negundo). Asian J. Pharm. Technol. 2011, 1, 53–55. [Google Scholar]
- Rout, O.P.; Acharya, R.; Mishra, S.K.; Sahoo, R. Pathorchur (Coleus aromaticus): A review of the medicinal evidence for its phytochemistry and pharmacology properties. Int. J. Appl. Biol. Pharma Technol. 2012, 3, 348–355. [Google Scholar]
- Khattak, M.M.A.K.; Taher, M.; Abdulrahman, S.; Abu Bakar, I.; Damanik, R.; Yahaya, A. Anti-bacterial and anti-fungal activity of Coleus leaves consumed as breast-milk stimulant. Nutr. Food Sci. 2013, 43, 582–590. [Google Scholar] [CrossRef]
- Duke, J.A.; Godwin, M.J.; Cellier, D.U. Handbook of Medicinal Herbs, 1st ed.; CRC Press: Boca Raton, FL, USA, 2002; pp. 210–215. [Google Scholar]
- Buddhika, W.M.C.; Srikrishnah, S.; Sutharsan, S. Effects of different levels of shade on the growth and quality characters of Coleus (Plectranthus scutellarioides) var. “Chocolate Covered Cherry”. J. Agric. Sci. Crop Res. 2020, 1, 101. [Google Scholar]
- Paton, A.; Mwanyambo, M.; Culham, A. Phylogenetic study of Plectranthus, Coleus and allies (Lamiaceae): Taxonomy, distribution and medicinal use. Bot. J. Linn. Soc. 2018, 188, 355–376. [Google Scholar] [CrossRef]
- Albert, N.W.; Davies, K.M.; Lewis, D.H.; Zhang, H.; Montefiori, M.; Brendolise, C.; Boase, M.R.; Ngo, H.; Jameson, P.E.; Schwinn, K.E. A conserved network of transcriptional activators and repressors regulates anthocyanin pigmentation in eudicots. Plant Cell 2014, 26, 962–980. [Google Scholar] [CrossRef]
- Fitter, A.H.; Hay, R.K. Environmental Physiology of Plants; Academic Press: London, UK, 2012; pp. 34–50. [Google Scholar]
- Paradiso, R.; Proietti, S. Light-quality manipulation to control plant growth and photomorphogenesis in greenhouse horticulture: The state of the art and the opportunities of modern LED systems. J. Plant Growth Reg. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Mitchell, C.A.; Dzakovich, M.P.; Gomez, C.; Lopez, R.; Burr, J.F.; Hernández, R.; Kubota, C.; Currey, C.J.; Meng, Q.; Runkle, E.S.; et al. Light-emitting diodes in horticulture. Hortic. Rev. 2015, 43, 1–88. [Google Scholar]
- Lin, K.H.; Huang, M.Y.; Hsu, M.H. Morphological and physiological response in green and purple basil plants (Ocimum basilicum) under different proportions of red, green, and blue LED lightings. Sci. Hortic. 2021, 275, 109677. [Google Scholar] [CrossRef]
- Hosseini, A.; Zare Mehrjerdi, M.; Aliniaeifard, S.; Seif, M. Photosynthetic and growth responses of green and purple basil plants under different spectral compositions. Physiol. Mol. Biol. Plants 2019, 25, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Manivannan, A.; Soundararajan, P.; Park, Y.G.; Wei, H.; Kim, S.H.; Jeong, B.R. Blue and red light-emitting diodes improve the growth and physiology of in vitro-grown carnations ‘green beauty’ and ‘purple beauty’. Hortic. Environ. Biotechnol. 2017, 58, 12–20. [Google Scholar] [CrossRef]
- Wollaeger, H.M.; Runkle, E.S. Growth of impatiens, petunia, salvia, and tomato seedlings under blue, green, and red light-emitting diodes. HortScience 2014, 49, 734–740. [Google Scholar] [CrossRef]
- Lee, J.H.; Soh, S.Y.; Kim, H.J.; Nam, S.Y. Effects of LED light quality on the growth and leaf color of Orostachys japonica and O. boehmeri. J. Biol. Environ. Control 2022, 31, 104–113. [Google Scholar] [CrossRef]
- Lee, J.H.; Nam, S.Y. Vegetative propagation of six Pachyphytum species as influenced by different LED light qualities. Korean J. Hortic. Sci. Technol. 2023, 41, 237–249. [Google Scholar] [CrossRef]
- Shin, E.J.; Lee, J.H.; Nam, S.Y. Changes in growth, visual qualities, and photosynthetic parameters in Peperomia species and cultivars under different color temperatures of white lighting conditions. J. Agric. Life Environ. Sci. 2023, 35, 307–321. [Google Scholar]
- Lee, J.H.; Cabahug, R.A.M.; You, N.H.; Nam, S.Y. Chlorophyll fluorescence and growth evaluation of ornamental foliage plants in response to light intensity levels under continuous lighting conditions. Flower Res. J. 2021, 29, 153–164. [Google Scholar] [CrossRef]
- Nguyen, P.; Dal Cin, V. The role of light on foliage colour development in coleus (Solenostemon scutellarioides (L.) Codd). Plant Physiol. Biochem. 2009, 47, 934–945. [Google Scholar] [CrossRef]
- Halaban, R. Effects of light quality on the circadian rhythm of leaf movement of a short-day plant. Plant Physiol. 1969, 44, 973–977. [Google Scholar] [CrossRef]
- Cho, K.H.; Laux, V.Y.; Wallace-Springer, N.; Clark, D.G.; Folta, K.M.; Colquhoun, T.A. Effects of light quality on vegetative cutting and in vitro propagation of Coleus (Plectranthus scutellarioides). HortScience 2019, 54, 926–935. [Google Scholar] [CrossRef]
- Dörr, O.S.; Zimmermann, B.F.; Kögler, S.; Mibus, H. Influence of leaf temperature and blue light on the accumulation of rosmarinic acid and other phenolic compounds in Plectranthus scutellarioides (L.). Environ. Exp. Bot. 2019, 167, 103830. [Google Scholar] [CrossRef]
- Jang, I.T.; Lee, J.H.; Shin, E.J.; Nam, S.Y. Evaluation of growth, flowering, and chlorophyll fluorescence responses of Viola cornuta cv. Penny Red Wing according to spectral power distributions. J. People Plants Environ. 2023, 26, 335–349. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring vegetation systems in the Great Plains with ERTS. NASA Spec. Publ. 1974, 351, 309. [Google Scholar]
- Gamon, J.; Serrano, L.; Surfus, J.S. The photochemical reflectance index: An optical indicator of photosynthetic radiation use efficiency across species, functional types, and nutrient levels. Oecologia 1997, 112, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Daughtry, C.S.; Walthall, C.L.; Kim, M.S.; De Colstoun, E.B.; McMurtrey, J.E., III. Estimating corn leaf chlorophyll concentration from leaf and canopy reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis: Mechanism, Regulation and Adaptation, 1st ed.; Yunus, M., Pathre, U., Mohanty, P., Eds.; Taylor and Francis: London, UK, 2000; pp. 445–483. [Google Scholar]
- Cabahug, R.A.M.; Soh, S.Y.; Nam, S.Y. Effects of light intensity on the growth and anthocyanin content of Echeveria agavoides and E. marcus. Flower Res. J. 2017, 25, 262–269. [Google Scholar] [CrossRef]
- Kelly, S.A.; Panhuis, T.M.; Stoehr, A.M. Phenotypic plasticity: Molecular mechanisms and adaptive significance. Compr. Physiol. 2011, 2, 1417–1439. [Google Scholar]
- Ilyas, M.; Liu, Y.Y.; Shah, S.; Ali, A.; Khan, A.H.; Zaman, F.; Yucui, Z.; Saud, S.; Adnan, M.; Ahmed, N.; et al. Adaptation of functional traits and their plasticity of three ornamental trees growing in urban environment. Sci. Hortic. 2021, 286, 110248. [Google Scholar] [CrossRef]
- Trivellini, A.; Toscano, S.; Romano, D.; Ferrante, A. LED lighting to produce high-quality ornamental plants. Plants 2023, 12, 1667. [Google Scholar] [CrossRef]
- Franklin, K.A.; Praekelt, U.; Stoddart, W.M.; Billingham, O.E.; Halliday, K.J.; Whitelam, G.C. Phytochromes B, D, and E act redundantly to control multiple physiological responses in Arabidopsis. Plant Physiol. 2003, 131, 1340–1346. [Google Scholar] [CrossRef] [PubMed]
- Devlin, P.F.; Robson, P.R.; Patel, S.R.; Goosey, L.; Sharrock, R.A.; Whitelam, G.C. Phytochrome D acts in the shade-avoidance syndrome in Arabidopsis by controlling elongation growth and flowering time. Plant Physiol. 1999, 119, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Shacklock, P.; Read, N.; Trewavas, A. Cytosolic free calcium mediates red light-induced photomorphogenesis. Nature 1992, 358, 753–755. [Google Scholar] [CrossRef]
- Wang, F.F.; Lian, H.L.; Kang, C.Y.; Yang, H.Q. Phytochrome B is involved in mediating red light-induced stomatal opening in Arabidopsis thaliana. Mol. Plant 2010, 3, 246–259. [Google Scholar] [CrossRef]
- Casson, S.A.; Franklin, K.A.; Gray, J.E.; Grierson, C.S.; Whitelam, G.C.; Hetherington, A.M. Phytochrome B is required for light-mediated systemic control of stomatal development. Curr. Biol. 2009, 19, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Park, S.; Oh, M. Growth and cell division of lettuce plants under various ratios of red to far-red light-emitting diodes. Hortic. Environ. Biotechnol. 2015, 56, 186–194. [Google Scholar] [CrossRef]
- Karimi, M.; Ahmadi, N.; Ebrahimi, M. Red LED light promotes biomass, flowering and secondary metabolites accumulation in hydroponically grown Hypericum perforatum L. (cv. Topas). Ind. Crops Prod. 2022, 175, 114239. [Google Scholar] [CrossRef]
- Demotes-Mainard, S.; Péron, T.; Corot, A.; Bertheloot, J.; Le Gourrierec, J.; Pelleschi-Travier, S.; Crespel, L.; Morel, P.; Huché-Thélier, L.; Boumaza, R.; et al. Plant responses to red and far-red lights, applications in horticulture. Environ. Exp. Bot. 2016, 121, 4–21. [Google Scholar] [CrossRef]
- Moosavi-Nezhad, M.; Alibeigi, B.; Estaji, A.; Gruda, N.S.; Aliniaeifard, S. Growth, biomass partitioning, and photosynthetic performance of chrysanthemum cuttings in response to different light spectra. Plants 2022, 11, 3337. [Google Scholar] [CrossRef]
- Wang, Y.; Folta, K.M. Contributions of green light to plant growth and development. Am. J. Bot. 2013, 100, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Yang, Q.; Li, T.; Cheng, R.; Barnett, Y.; Lu, C. Study of the beneficial effects of green light on lettuce grown under short-term continuous red and blue light-emitting diodes. Physiol. Plant. 2018, 164, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Runkle, E. Growing plants with green light. GPNMag. Mich. State Univ. 2017, 20, 58. [Google Scholar]
- Kim, S.H.; Park, J.H.; Kim, E.J.; Lee, J.M.; Park, J.W.; Kim, Y.S.; Kim, G.R.; Lee, J.S.; Lee, E.P.; You, Y.H. White LED lighting increases the root productivity of Panax ginseng CA Meyer in a hydroponic cultivation system of a plant factory. Biology 2023, 12, 1052. [Google Scholar] [CrossRef]
- Nagel, K.A.; Schurr, U.; Walter, A. Dynamics of root growth stimulation in Nicotiana tabacum in increasing light intensity. Plant Cell Environ. 2006, 29, 1936–1945. [Google Scholar] [CrossRef]
- Liu, C.; Guo, C.; Wang, Y.; Ouyang, F. Effect of light irradiation on hairy root growth and artemisinin biosynthesis of Artemisia annua L. Process Biochem. 2002, 38, 581–585. [Google Scholar] [CrossRef]
- Walter, A.; Nagel, K. Root growth reacts rapidly and more pronounced than shoot growth towards increasing light intensity in tobacco seedlings. Plant Sign. Behav. 2006, 1, 225–226. [Google Scholar] [CrossRef]
- Irawati, E.B. Plant characteristics for green wall system. Proc. Int. Conf. Sci. Eng. 2020, 3, 77–80. [Google Scholar] [CrossRef]
- Muksan; Singh, D.; Wesley, C.J. Performance of ornamental plants in different media composition for outdoor vertical gardening: Experimental investigation. Int. J. Plant Soil. Sci. 2023, 35, 2204–2210. [Google Scholar]
- Fairchild, M.; Berns, R. Image color-appearance specification through extension of CIELAB. Color. Res. Appl. 1993, 18, 178–190. [Google Scholar] [CrossRef]
- Pan, L.; Li, J.; Yin, H.; Fan, Z.; Li, X. Integrated physiological and transcriptomic analyses reveal a regulatory network of anthocyanin metabolism contributing to the ornamental value in a novel hybrid cultivar of Camellia japonica. Plants 2020, 9, 1724. [Google Scholar] [CrossRef]
- Wang, D. Seasonal color matching method of ornamental plants in urban landscape construction. Open Geosci. 2021, 13, 594–605. [Google Scholar] [CrossRef]
- Sudhakar, P.; Latha, P.; Reddy, P.V. Plant pigments. In Phenotyping Crop Plants for Physiological and Biochemical Traits, 1st ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 121–127. [Google Scholar]
- Carvalho, S.D.; Folta, K.M. Green light control of anthocyanin production in microgreens. Acta Hortic. 2016, 1134, 13–18. [Google Scholar] [CrossRef]
- Mizuno, T.; Amaki, W.; Watanabe, H. Effects of monochromatic light irradiation by led on the growth and anthocyanin contents in leaves of cabbage seedlings. Acta Hortic. 2011, 907, 179–184. [Google Scholar] [CrossRef]
- Katz, A.; Weiss, D. Light regulation of anthocyanin accumulation and chalcone synthase gene expression in Petunia flowers. Israel J. Plant Sci. 1999, 47, 225–229. [Google Scholar] [CrossRef]
- Lalusin, A.; Ohta, M.; Fujimura, T. Temporal and spatial expression of genes involved in anthocyanin biosynthesis during sweet potato (Ipomoea batatas [L.] Lam.) root development. Int. J. Plant Sci. 2006, 167, 249–256. [Google Scholar] [CrossRef]
- Honda, C.; Kotoda, N.; Wada, M.; Kondo, S.; Kobayashi, S.; Soejima, J.; Zhang, Z.; Tsuda, T.; Moriguchi, T. Anthocyanin biosynthetic genes are coordinately expressed during red coloration in apple skin. Plant Physiol. Biochem. 2002, 40, 955–962. [Google Scholar] [CrossRef]
- Lee, J.; Kang, W.; Park, K.; Son, J. Spectral dependence of electrical energy-based photosynthetic efficiency at single leaf and canopy levels in green- and red-leaf lettuces. Hortic. Environ. Biotechnol. 2017, 58, 111–118. [Google Scholar] [CrossRef]
- Liu, J.; Van Iersel, M.W. Photosynthetic physiology of blue, green, and red light: Light intensity effects and underlying mechanisms. Front. Plant Sci. 2021, 12, 328. [Google Scholar] [CrossRef]
- McCree, K.J. The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agric. Meteorol. 1971, 9, 191–216. [Google Scholar] [CrossRef]
- Klug, T.; Assumpção, C.; Magro, L.; Wieth, A.; Bender, R.; Flôres, S.; Rios, A. Evaluation of growth and phenolic compounds profile of purple lettuce under indoor cultivation. Brazil J. Food Res. 2021, 11, 62–76. [Google Scholar] [CrossRef]
- Ouzounis, T.; Fretté, X.; Ottosen, C.; Rosenqvist, E. Spectral effects of LEDs on chlorophyll fluorescence and pigmentation in Phalaenopsis ‘Vivien’ and ‘Purple Star’. Physiol. Plant 2015, 154, 314–327. [Google Scholar] [CrossRef]
- Manivannan, A.; Soundararajan, P.; Halimah, N.; Ko, C.H.; Jeong, B.R. Blue LED light enhances growth, phytochemical contents, and antioxidant enzyme activities of Rehmannia glutinosa cultured in vitro. Hortic. Environ. Biotechnol. 2015, 56, 105–113. [Google Scholar] [CrossRef]
- Hamdani, S.; Khan, N.; Perveen, S.; Qu, M.; Jiang, J.G.; Zhu, X. Changes in the photosynthesis properties and photoprotection capacity in rice (Oryza sativa) grown under red, blue, or white light. Photosynth. Res. 2018, 139, 107–121. [Google Scholar] [CrossRef]
- Kondzior, P.; Tyniecki, D.; Butarewicz, A. Influence of color temperature of white LED diodes and illumination intensity on the content of photosynthetic pigments in Chlorella vulgaris algae cells. Proceedings 2019, 16, 46. [Google Scholar] [CrossRef]
- Kozukue, N.; Tsuchida, H.; Mizuno, S. Effect of light intensity, duration, and photoperiod on chlorophyll and glycoalkaloid production by potato tubers. J. Jpn. Soc. Hortic. Sci. 1993, 62, 669–673. [Google Scholar] [CrossRef][Green Version]
- Su, N.; Wu, Q.; Shen, Z.; Xia, K.; Cui, J. Effects of light quality on the chloroplastic ultrastructure and photosynthetic characteristics of cucumber seedlings. Plant Grow. Reg. 2014, 73, 227–235. [Google Scholar] [CrossRef]
- Williams, M.; Waters, E.; Golbek, M.; Wormington, J. Effect of different shades of light on photosynthesis. J. Introd. Biol. Investig. 2015, 2. [Google Scholar]
- Pettorelli, N. The Normalized Difference Vegetation Index, 1st ed.; Oxford University Press: New York, NY, USA, 2013; pp. 70–79. [Google Scholar]
- Nagler, P.L.; Daughtry, C.S.T.; Goward, S.N. Plant litter and soil reflectance. Remote Sens. Environ. 2000, 71, 229. [Google Scholar] [CrossRef]
- Magney, T.S.; Vierling, L.A.; Eitel, J.U.; Huggins, D.R.; Garrity, S.R. Response of high frequency photochemical reflectance index (PRI) measurements to environmental conditions in wheat. Remote Sens. Environ. 2016, 73, 84–97. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Habibi, G. Effects of high light and chilling stress on photosystem II efficiency of Aloe vera L. plants probing by chlorophyll a fluorescence measurements. Iran. J. Sci. Technol. Trans. A Sci. 2019, 43, 7–13. [Google Scholar] [CrossRef]
- Kitajima, M.; Butler, W.L. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim. Biophys. Acta Bioenerg. 1975, 376, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Strasser, R.J.; Govindjee, G. Greening of peas: Parallel measurements of 77 K emission spectra, OJIP chlorophyll a fluorescence, period four oscillation of the initial fluorescence level, delayed light emission, and P700. Photosynthetica 1999, 37, 365–392. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Liu, W.; Gan, Y.; Wu, Y. Biomass allocation, compensatory growth and internal C/N balance of Lolium perenne in response to defoliation and light treatments. Polish J. Ecol. 2016, 64, 485–499. [Google Scholar] [CrossRef]
- Nishio, J.N. Why are higher plants green? Evolution of the higher plant photosynthetic pigment complement. Plant Cell Environ. 2002, 23, 539–548. [Google Scholar] [CrossRef]
- Shin, E.J.; Lee, J.H.; Nam, S.Y. Evaluation of growth, vegetation indices, and photosynthesis of Cichorium intybus L. seedlings as affected by LED light qualities in a closed nursery facility. Hortic. Sci. Technol. 2024, 42, 350–364. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).



























