Abstract
Microalgae-based wastewater treatment offers an eco-friendly opportunity for simultaneous nutrient recovery and biomass generation, aligning with the circular bioeconomy concept. This approach aims to utilize the nutrients of potato industry wastewater (PIW) for algal growth while mitigating the environmental impact of this industrial byproduct. This study focused on cultivating three cyanobacterial strains, Anabaena oryzae, Nostoc muscorum, and Spirulina platensis, in PIW and synthetic media for 30 days to assess feasibility. Growth performance was monitored by measuring chlorophyll content, dry weight (DW), optical density (OD), and pH at 3-day intervals. The high-performing cyanobacterial biomass from the laboratory findings was formulated into a biofertilizer, which was then evaluated in a controlled greenhouse experiment on celery and lettuce plants. The biofertilizer replaced conventional NPK mineral fertilizers at different levels (25%, 50%, and 75%), while a control group received 100% chemical fertilizer. The results showed favourable growth of all three cyanobacteria strains and their mixture in PIW throughout the experiment. The mixed cyanobacteria followed by Spirulina platensis exhibited the highest growth rates, achieving chlorophyll contents of 3.75 and 2.30 µg·mL−1, DWs of 1.79 g·L−1 and 1.63 g·L−1, and ODs of 0.41 and 0.38, respectively, surpassing the other treatments. The formulated biofertilizers, Spi-PIW (Spirulina platensis + potato industry wastewater) and Cyano-PIW (mixed culture+ potato industry wastewater), significantly enhanced plant height, root and stem lengths, and the number of leaves per plant in celery and lettuce compared to the control group. These biofertilizer treatments also improved chlorophyll contents, as well as macro- and micronutrient levels, in the two crops. Additionally, the application of these biofertilizers improved certain sandy soil properties, i.e., pH, total organic matter, total nitrogen, phosphorus, and potassium. In conclusion, utilizing PIW as a substrate for cultivating cyanobacteria strains and producing high-quality liquid bio-organic fertilizers holds potential for reducing recommended NPK fertilizer doses by 25–50% in celery and lettuce growth, providing an environmentally friendly approach.
1. Introduction
In recent years, there has been a growing interest in bioremediation technologies that utilize industrial waste as culture media for microorganisms. This approach enables the complete biodegradation of organic compounds and the production of new products with added value. Additionally, using waste products as medium components reduces overall production costs [1]. Egypt is a significant producer and consumer of potatoes, with a total annual production of 5 million tons and a market value of USD 250 million. It is among the top 20 potato producers globally and was the largest producer and exporter of potatoes in Africa in 2019 [2]. However, the potato processing industry generates a substantial amount of wastewater, posing potential water pollution problems. Processing 1 ton of potatoes can result in the production of 7 m3 of potato wastewater [3]. This wastewater contains high concentrations of biodegradable components such as starch and proteins, and chemical oxygen demand (COD) levels can reach up to 10,000 mg·L−1. It also contains significant amounts of total suspended solids, volatile suspended solids, and total nitrogen, which can reach levels of 9700, 9500, and 224 mg·L−1, respectively [4]. Additionally, potato wastewater contains mineral compounds, predominantly potassium and phosphorus, with protein fractions including patatins and complex proteins [5]. Improper management of potato wastewater can pose risks to the environment, including microbial contamination and soil damage. Previously, raw potato wastewater was used for irrigation, enriching soil with nitrogen compounds. Although this method allowed for wastewater treatment, it had drawbacks such as soil clogging and water eutrophication. Full-scale aerobic treatment of potato industry wastewater has been used for decades; however, it has several notable disadvantages. It requires substantial energy to maintain adequate oxygen levels in the treatment tanks, leading to significant operational costs. Additionally, these processes generate large quantities of sludge, resulting in additional costs and logistical challenges for management and disposal. The mechanical and aeration systems in aerobic treatment plants also require regular maintenance, adding to the overall cost and complexity of the treatment process. With increasing environmental regulations, the cost of sludge disposal has risen substantially, making options such as landfill disposal or incineration more expensive and regulated. Unpleasant odours can be produced by aerobic treatment plants, particularly if there are issues with the aeration system or if the sludge is not handled promptly. Managing a full-scale aerobic treatment system is complex, requiring skilled operators and continuous monitoring to ensure optimal performance and compliance with environmental standards. While aerobic treatment is effective at reducing organic pollutants, significant environmental footprints are created by the energy consumption and sludge production, potentially offsetting some of the treatment’s environmental benefits. These disadvantages highlight the need for more sustainable and cost-effective alternatives [6]. This study focuses on microalgae-based phycoremediation, which offers several significant advantages. The purification of food processing wastewater using microalgae provides a sustainable solution for the bioeconomy, as wastewater from the food industry serves as a lesstoxic growth medium for microalgae biomass production. Beneficial microbes, such as cyanobacteria, can enhance agricultural productivity and reduce greenhouse gas emissions [7,8]. Nitrogen fixation and the production of bioactive compounds that promote crop growth, protect against pathogens, and improve soil nutrient status are some of the capabilities exhibited by cyanobacteria [9]. Additionally, cyanobacteria are effective in phycoremediation, comprehensively removing contaminants such as heavy metals, pesticides, crude oil, and various organic compounds, as opposed to traditional aerobic treatments [10]. The cultivation of cyanobacteria in potato processing wastewater achieves three goals: wastewater recycling, the production of cost-effective algal protein biomass, and the utilization of cyanobacterial culture filtrate, which is rich in plant growth regulators and other bioagents, for biofertigation. This approach capitalizes on wastewater as a resource, converting waste into valuable biomass that can be used as biofertilizer, thereby closing the loop in waste management and resource utilization.
The objective of this study was to explore the potential of phycoremediation as a cost-effective and environmentally friendly approach for recycling potato industry effluents. The aim was to use these effluents as a substrate for cyanobacterial biomass development, facilitating the production of high-quality biofertilizers. The effectiveness of these biofertilizers was evaluated by assessing the growth parameters of celery and lettuce in sandy soil under greenhouse conditions.
2. Materials and Methods
2.1. Potato Industry Wastewater (PIW): Collection, Primary Treatment, and Analysis
During the peak potato processing season in 2020, samples of raw wastewater from the potato industry (PIW) were collected from Chipsy Food Industries—October Plant (coordinates: 29.9492° N, 30.8878° E) in 6th October City, Giza, Egypt, by averaging several samples taken throughout the full production cycle. To remove suspended solids, the wastewater underwent primary treatment using sedimentation. Screening was initially employed, but the wastewater still contained residual organic solids. These solids were further eliminated through gravitational settling in a sedimentation tank for a period of 2 to 3 h [11]. The analysis of the wastewater followed standard methods outlined for the examination of water and wastewater [12].
2.2. Isolation, Purification, Characterization, and Molecular Identification of Cyanobacterial Strains
Three strains of cyanobacteria, namely, Nostoc muscorum isolate HSSASE1, Anabaena oryzae isolate HSSASE6, and Spirulina platensis isolate HSSASE5, were obtained from the Department of Microbiology at the Soils, Water and Environment Research Institute (SWERI), Agricultural Research Center (ARC), in Giza, Egypt. The isolation process involved specific locations and media for each strain. For the nitrogen-fixing cyanobacterial strains, Nostoc muscorum isolate HSSASE1 and Anabaena oryzae isolate HSSASE6, the isolation was carried out from the rice rhizosphere in Sahl El-Hussinia, El-Sharkia Governorate, Egypt. Two enrichment cultures were prepared for the development of different cyanobacteria as follows: approximately 2 g of soil was transferred into sterile 250 mL conical flasks containing 90 mL of nitrogen-free BG110 liquid medium to develop heterocyst cyanobacteria (specialized N2-fixing cells), following the method described by Rippka et al. [13]. And pure Spirulina isolate, originated from Soda Lake in Wadi El Natrun, Beheira Governorate, Egypt, was obtained by inoculating 250 mL aliquots of Zarrouk’s liquid medium [14] in 500 mL screw bottles with 10 mL of a 5-day-old culture followed by incubation for 10 days at 25± 2 °C under constant light conditions (600–800 lux) provided by a 36 W white fluorescent bulb. Subsequently, the streaking method on BG110 or Zarrouk’s agarized medium was used to obtain pure cultures of Nostoc muscorum, Anabaena oryzae, and Spirulina platensis. The plates were incubated at 25 ± 2 °C under continuous illumination (600 lux), and colonies were collected and examined under a microscope. Colonies composed of cyanobacterial cells were preserved on slants containing BG110 or Zarrouk’s medium. To determine the morphological characteristics of the isolates, microscopic observations were conducted at various growth stages. Microscopic examination took place over a period of 3–4 weeks of incubation at 30 °C. Successive transfers and purification processes were performed as outlined by Ferris and Hirsch [15] and Roger and Kulasooriya [16]. The isolated cyanobacteria were preserved on slants containing BG110 or Zarrouk’s solid medium. The maintenance of the cyanobacterial isolates involved their growth in BG110 and Zarrouk’s liquid media under photoautotrophic conditions using cool-white fluorescent lamps at a light intensity of 400–500 lux and incubation at 30 °C. Purification from heterotrophic bacteria was achieved using antibiotics (nystatin and cycloheximide) according to Ferris and Hirsch [15], along with purification using UV radiation for 30 min as described by Garcia-Pichel et al. [17]. The morphological characterization identification of the cyanobacterial isolates was based on morphological characteristics observed through microscopic analysis at different growth stages in nitrogen-free BG110 [13] and Zarrouk’s media. Desikachary [18], Rippka et al. [13], and Bergey’s Manual of Systematic Bacteriology [19] were used as references for the general morphological characterization and measurements. The molecular-technique identification of the cyanobacteria isolates was carried out by targeting the 16s region, and the accession numbers obtained from GenBank are listed in Table 1 [20]. The characteristics of the cyanobacteria culture suspensions in the logarithmic growth phase are presented in Table 1.

Table 1.
GenBank accession numbers and diverse characterization parameters of cyanobacterial species under investigation.
2.3. Preparation of Cyanobacterial Inocula and Optimization of Growth Conditions
Three different liquid growth media for the individual and mixed culture development of cyanobacterial strains were used in this study. The two N-fixing strains, Nostoc muscorum and Anabaena oryzae, were separately maintained on nitrogen-free BG110 medium [13]. Meanwhile, the non-N2-fixing strain Spirulina platensis was grown on Zarrouk medium [14]. The nitrate-based medium BG11-NO3, containing 8.8 mM NaNO3 and supplemented with 100 mM NaHCO3, was used for growing mixed cultures of cyanobacteria, including Nostoc muscorum, Anabaena oryzae, and Spirulina platensis, as described by Kamennaya et al. [21]. The cultures were incubated in a growth chamber with continuous shaking (150 rpm) under continuous illumination with Philips Florescent 40-W cool-white fluorescent lamps at a relatively low light intensity (400–500 lux) and incubation at 27 ± 2 °C for 30 days to be used as inocula for laboratory experiments.
2.4. Formulation of Cyanobacteria–PIW-Based Fertilizers
The cyanobacteria strains, either individually or in a mixture of Spirulina platensis, Nostoc muscorum, and Anabaena oryzae at a 1:1:1 ratio, were inoculated into sterilized 500 mL conical flasks containing 200 mL of both synthetic media (as control treatments) and PIW medium at a rate of 20% (Vinoculum/Vmedia). The PIW treatments that contained Spirulina platensis and the mixed culture of cyanobacteria were supplemented with 10 mM NaHCO3 [21]. The cultures were then incubated at a temperature of 27 ± 2 °C with continuous shaking at 150 rpm under continuous illumination with Philips Florescent 40-W cool-white fluorescent lamps at a relatively low light intensity (400–500 lux). The growth of the algal biomass was monitored at three-day intervals throughout the 30-day experimental period. Initial characterization and monitoring of cyanobacteria growth parameters, including optical density, dry weight, chlorophyll, and pH, were performed following the procedures outlined by Vonshak and Richmond [22] and APHA [12]. The cyanobacterial treatment(s) that exhibited superior growth in the PIW medium were scaled up to a 50 L sterilized photobioreactor (PBR) under batch operation with continuous aeration for a duration of 30 days. The upscaled biomass was then used in a potted greenhouse experiment. The details of the treatments are provided in Table 2.

Table 2.
Growth culture media for cyanobacterial strains and abbreviations of experimental treatments.
2.5. Soil Sample Collection and Comprehensive Analysis
The soil used for the cultivation of celery and lettuce in the greenhouse was obtained from the Ismailia Governorate, Egypt. Physical, chemical, and mechanical properties of the soil (Table 3) were determined at the Soil, Water and Environmental Institute (SWERI), Agricultural Research Center (ARC), Giza, Egypt. The soil particle size distribution was determined according to Piper [23]. Soil EC and pH were measured using a soil–water extract at a ratio of 1:2.5, as described by Richards [24]. Cation exchange capacity (CEC) was determined according to Hissing’s method reported by Piper [23]. Soil chemical analyses were carried out according to AOAC [25] and SOC (soil organic C), following the method of Walkley and Black [26]. The physiochemical characteristics of the soil are shown in Table 3.

Table 3.
Mechanical and chemical analyses of Ismailia sandy soil.
2.6. Celery and Lettuce Transplanting
During the summer season of 2020, a pot experiment was conducted at the Algae and Hydroponics Production Greenhouse at the Giza Research Station, Agricultural Research Center (ARC), Egypt. The experiment utilized a randomized block design with five replications and focused on the cultivation of celery (Apium graveolens L.) and lettuce (Lactuca sativa L.). Twenty-day-old seedlings were purchased from a local Egyptian farm and transplanted into 15 cm diameter polyethylene pots, with each pot containing 2 kg of soil. The pots were irrigated every two days, with each pot receiving 1/4 L of water. Fertilizers, including commercial NPK mineral fertilizers (ammonium sulphate 100 Kg/acre, superphosphate 100 Kg/acre, and potassium sulphate 50 Kg/acre) at different levels (0, 25, 50, and 100%), as well as cyanobacteria–PIW biofertilizer, were applied 15 days after seeding and at 25- and 35-day intervals. The biofertilizer, derived from cyanobacteria grown on PIW, was applied through fertigation by diluting 5 mL of each fertilizer in 2 L of irrigation water. The plants were harvested after 50 days. Plants growth parameters, including number of leaves, plant height (cm), and fresh and dry weight (g), were determined. The macro- and micronutrients in plants leaves were determined using the official methods of analysis [25]. The total chlorophyll content of leaves (mg·g−1 fresh weight) was measured by spectrophotometry, using the modified method of Arnon by Jia et al. [27]. The experiment consisted of three groups: a control group without any fertilizers, a group treated with NPK chemical fertilizers at different rates, and a group treated with cyanobacteria–PIW biofertilizers. The specific treatments were as follows:
| T1 = 100% NPK | T2 = 50% NPK |
| T3 = 25% NPK | T4 = 75% NPK+ Spi-PIW |
| T5 = 50% NPK+ Spi-PIW | T6 = 25% NPK + Spi-PIW |
| T7= 75% NPK + Cyano-PIW | T8 = 50% NPK +Cyano-PIW |
| T9 = 25% NPK + Cyano-PIW | T10 = Control without any fertilizers |
2.7. Statistical Analysis
The results were subjected to analysis of variance (ANOVA), and significant means were compared with Duncan’s multiple range test method using the SPSS 13.0 statistical package program [28].
3. Results
3.1. Assessment of Cyanobacterial Growth Performance in Potato Industry Wastewater (PIW) versus Standard Synthetic Media
To investigate the potential of potato industry wastewater (PIW) for algal cultivation, three cyanobacterial strains (Anabaena oryzae, Nostoc muscorum, and Spirulina platensis) were grown in PIW and synthetic media for 30 days. The initial analysis of the PIW sample indicated a slightly acidic pH; high electrical conductivity; elevated COD and BOD contents; and the presence of nitrogen, phosphorus, potassium, and other minerals.
A subsequent sedimentation treatment significantly improved water quality, reducing various parameters like pH, electrical conductivity, salinity, TSS, COD, and BOD by an average of 25.04, 28.46, 25.00, 28.49, 8.78, and 23.14%. However, total nitrogen remained unchanged, and essential nutrients for algal growth like Ca, Fe, Cu, NO3-N, NO2-N, K, and P showed reductions ranging from 6.24% to 33.33%. This nutrient deficiency in pretreated PIW compared to synthetic media (Table 4) likely influenced the growth performance of the cyanobacterial strains, which was monitored by measuring chlorophyll content, dry weight, optical density, and pH at 3-day intervals, as highlighted in Figure 1. The PIW medium demonstrated favourable conditions for the growth of all tested cyanobacterial strains and their mixture, and the growth reached its peak between days 27 and 30. The chlorophyll content progressively increased throughout the cultivation period, with Spi-PIW reaching the highest value of 2.43 μg·mL−1 and Cyano-PIW attaining 3.75 μg·mL−1. Notably, Spi-PIW and Cyano-PIW exhibited higher biomass dry weights (DWs) of 1.63 g·L−1 and 1.80 g·L−1, respectively. The optical density measurements indicated significant cell growth, as evidenced by the highest values recorded on the 30th day for Spi-PIW (0.35) and Cyano-PIW (0.41), which were notably distinct from the other treatments. The pH values of microalgal cultures in PIW medium displayed variations ranging from 7.04 to 10.12, influenced by the time from the initial phase to the stationary phase, depending on the algal species. However, the highest values (pH > 9) were observed with Spirulina platensis.

Table 4.
Comparison of raw and pretreated PIW composition with algal synthetic media (Zarrouk and BG110).
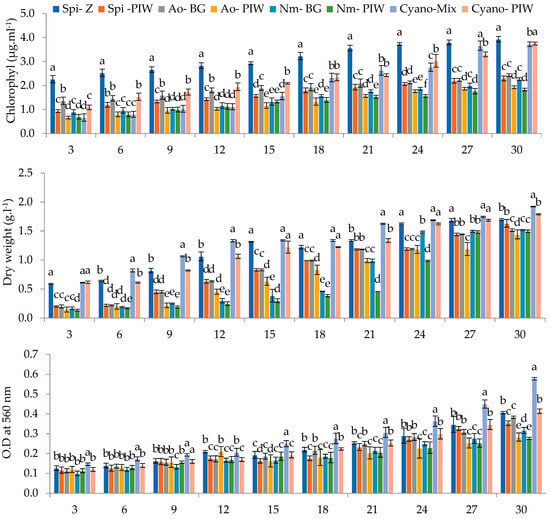
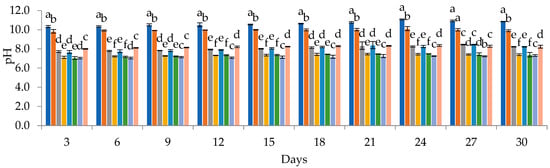
Figure 1.
Chlorophyll contents (μg·mL−1), DWs (g·L−1), ODs, and pHs of cyanobacteria and their mixture in pretreated PIW and synthetic media (a, b, c, …, f: values with different letters within the same column are significantly different at p < 0.05).
The successful growth of Spirulina platensis, Anabaena oryzae, Nostoc muscorum, and their mixed culture was confirmed in both PIW and synthetic standard media through microscopic examination, as depicted in Figure 2. The strains exhibited distinct characteristics, shapes, and growth densities, consistent with previous studies [29,30].
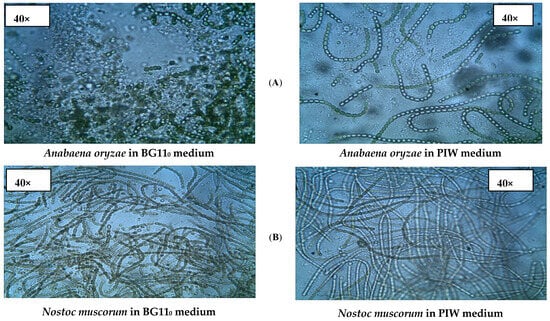
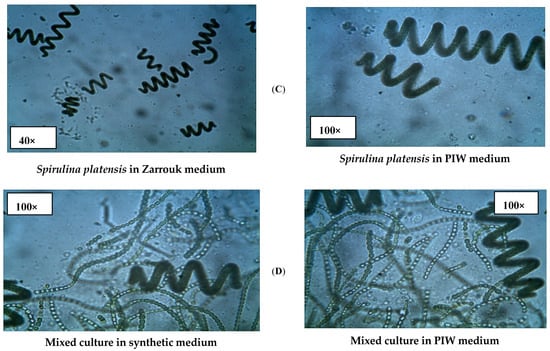
Figure 2.
Light micrographs of cyanobacterial growth in synthetic growth media and in PIW using 40 and 100× objectives of a compound light microscope. (A) Anabaena oryzae; (B) Nostoc muscorum; (C) Spirulina platensis; (D) Mixed culture.
Anabaena oryzae (Figure 2A) appeared as filamentous, soft, straight, gelatinous, and green. Nostoc muscorum (Figure 2B) appeared as bluish-green, gelatinous, and tightly packed, with oblong, irregular, filamentous cells and the presence of heterocysts. Spirulina platensis (Figure 2C) appeared as microscopic blue-green algae, characterized by spiral filaments and cylindrical, multicellular trichomes arranged in an open helix.
Moreover, Figure 2D provides evidence of the robust growth and distinctive characteristics exhibited by each strain in the presence of other species, confirming the homogeneous growth of cyanobacteria in their mixed culture. This observation indicates successful coexistence and interaction among the different strains.
3.2. Characterization of Cyanobacteria–PIW-Based Biofertilizers
The assessment of cyanobacterial growth performance in PIW medium led to the identification of Spirulina platensis (Spi-PIW) and the mixed culture (Cyano-PIW), distinguished by their superior growth characteristics, as highly promising candidates for biofertilizer development. Chemical composition analysis of these biofertilizers revealed abundant carbohydrates, proteins, and lipids, as well as essential macro- and micronutrients, including NPK, Fe, Zn, Cu, and Mn. A comprehensive documentation of their chemical composition is presented in Table 5. These findings validate the potential of Spi-PIW and Cyano-PIW as bio-organic fertilizers.

Table 5.
Chemical composition of cyanobacteria–PIW-based biofertilizers.
3.3. Effect of Cyanobacteria–PIW-Based Biofertilizers on Celery and Lettuce Growth Parameters and Chlorophyll Content
Application of Spi-PIW or Cyano-PIW biofertilizers resulted in significant enhancements in growth parameters and chlorophyll contents in both celery and lettuce plants, as depicted in Table 6 and Table 7, respectively.

Table 6.
Effect of different fertilizers on some growth parameters and chlorophyll content of celery.

Table 7.
Effect of different fertilizers on some growth parameters and chlorophyll content of lettuce.
In the case of celery (Table 6), treatments T7 (75% NPK + Cyano-PIW) and T8 (50% NPK + Cyano-PIW) exhibited notable improvements across all measured growth parameters and in leaf chlorophyll content. These treatments demonstrated substantially increased plant height (47.66 cm and 84.00 cm, respectively), root height (17.00 cm and 17.70 cm, respectively), fresh weight (135.30 and 134.00 g·plant−1, respectively), and dry weight (5.71 and 5.42 g·plant−1, respectively), as well as leaf count (59.30 and 58.66 leaves·plant−1, respectively), compared to the full-dose NPK chemical fertilizer control treatment T1 (with values of 47.66 cm, 17.70 cm, 130.00 g·plant−1, 5.18 g·plant−1, and 57.66 leaves·plant−1, respectively). Furthermore, T7 and T8 demonstrated chlorophyll content levels of 2.86 mg·g−1 fresh weight and 2.76 mg·g−1 fresh weight, respectively, compared to the chlorophyll content of T1, which was 2.96 mg·g−1 fresh weight.
In the case of lettuce (Table 7), treatment T7 (75% NPK + Cyano-PIW) exhibited statistically similar values (p > 0.05) for plant height (42.70 cm), root height (11.66 cm), fresh weight (289.33 g·plant−1), dry weight (15.33 g·plant−1), and number of leaves (17.00 leaves·plant−1) compared to the complete dose of the recommended chemical fertilizer for lettuce T1, with values of 44.00 cm, 12.66 cm, 285.00 g·plant−1, 12.66 g·plant−1, and 17.33 leaves·plant−1, respectively. Additionally, T7 demonstrated a comparable chlorophyll content in leaves (3.60 mg·g−1 fresh weight) compared to T1’s value of 3.63 mg·g−1 fresh weight.
3.4. Impact of Cyanobacteria–PIW Biofertilizers on Macro- and Micronutrient Contents in Celery and Lettuce
The application of various treatments exerted a significant impact on the nitrogen, phosphorus, and potassium (NPK) concentrations in both the leaves and roots of celery and lettuce plants, as evidenced in Table 8 and Table 9, respectively. Compared to the mineral control fertilizer 100% NPK (T1), which yielded the highest significant percentages of N, P, and K in both celery and lettuce plants, the treatment of 75% NPK+ Cyano-PIW (T7) resulted in concentrations of N, P, and K that were significantly comparable to those of the control treatment. Moreover, treatments T4 (75% NPK + Spi-PIW) and T8 (50% NPK + Cyano-PIW) notably enhanced the potassium (2.70%) and phosphorus (0.35%) contents in the roots of celery plants, respectively (Table 8). Furthermore, T8 (50% NPK + Cyano-PIW) significantly elevated the nitrogen content in the leaves and roots of lettuce plants (Table 9).

Table 8.
Impact of PIW biofertilizers on macronutrient contents in celery leaves and roots.

Table 9.
Impact of PIW biofertilizers on macronutrient contents in lettuce leaves and roots.
The effect of PIW–cyanobacteria-based biofertilizers on micronutrients in celery and lettuce was investigated (Table 10 and Table 11, respectively). The findings revealed that the recommended fully NPK mineral fertilizers (T1) and T7 (75% NPK + Cyano-PIW) exhibited significantly higher concentrations of micronutrients (Mn, Fe, Zn, and Cu) in the leaves and roots of both celery and lettuce compared to the other treatments. Furthermore, the T8 treatment (50% NPK+ Cyano-PIW) showed a considerable increase in certain microelements, such as manganese in the roots and zinc in the leaves. Furthermore, this treatment demonstrated enhanced levels of iron and copper in both the leaves and roots of celery (Table 10).

Table 10.
Effect of biofertilizers on micronutrients (mg/Kg) in celery leaves and roots.

Table 11.
Impact of biofertilizers on micronutrient levels (mg/kg) in lettuce leaves and roots.
In the case of lettuce, T8 (50% NPK + Cyano-PIW) significantly increased the Mn and Zn contents in leaves and roots, as well as the Fe content in leaves (p > 0.05). It is worth noting that T4 (75% NPK+ Spi-PIW) demonstrated a significant increase (p > 0.05) in Mn contents in both the leaves and roots of lettuce (Table 11).
3.5. Impact of Cyanobacteria–PIW Biofertilizers on Some Chemical Properties of Sandy Soil following Celery and Lettuce Harvesting
The application of PIW–cyanobacteria-based biofertilizers had a significant influence on the chemical properties of sandy soil after the harvest of celery and lettuce, as outlined in Table 12 and Table 13, respectively. Across both crops, treatments incorporating different levels of chemical fertilizers (0%, 50%, 75%, and 100% NPK, serving as the control group) resulted in a notable increase in soil pH values (p < 0.05) in sandy soil (Table 12). In contrast, the utilization of PIW-Spi or PIW-Mix biofertilizers led to a considerable decrease in soil pH compared to the control group. This decrease in pH values was attributed to reduced mineral fertilization and increased application of formulated Cyano-PIW. Particularly noteworthy were treatments T7 (75% NPK + Cyano-PIW), T8 (50% NPK + Cyano-PIW), and T9 (25% NPK + Cyano-PIW), which exhibited the most pronounced reductions in soil pH for both celery and lettuce.

Table 12.
Impact of PIW biofertilizers on some sandy soil characteristics following celery and lettuce harvesting.

Table 13.
Impact of PIW biofertilizers on available macronutrients in sandy soil post-celery and lettuce harvesting.
The implementation of various treatments to celery and lettuce crops had a significant impact on the electrical conductivity (EC) of sandy soil, as presented in Table 12. The control group exhibited the highest EC values, while treatment T9 (25% NPK + Cyano-PIW) demonstrated the lowest EC values for celery. For lettuce, treatments T7 (75% NPK + Cyano-PIW) and T9 (25% NPK + Cyano-PIW) displayed the lowest EC values. The cation exchange capacity (CEC) of the soil after the harvest of celery and lettuce (Table 12) showed significant variation due to the different treatments applied in this study. Treatments T7 (75% NPK + Cyano-PIW) and T8 (50% NPK + Cyano-PIW) exhibited the highest CEC values, which were significantly different (p < 0.05) from those of the other treatments, for both crops. Conversely, the control treatment had the lowest CEC value, which was also significantly different (p < 0.05) from those of the other treatments.
Spi-PIW and Cyano-PIW biofertilizers demonstrated a remarkable impact on the improvement of soil organic matter (SOM) and soil organic carbon (SOC) levels, as indicated by the statistical significance of the results (p < 0.05). Particularly noteworthy was the application of 50% NPK fertilizer in combination with Cyano-PIW (T8), which resulted in the most substantial increase in soil organic carbon compared to the other treatments and the control, for both crops. The increase in soil organic carbon ranged from 57.38% to 171.31% for celery and from 33.33% to 145.83% for lettuce, with all results showing statistical significance (p < 0.05). These findings highlight the effectiveness of the 50% NPK + Cyano-PIW treatment in significantly enhancing soil organic carbon content, indicating its potential as a beneficial approach for promoting soil fertility and crop growth.
Regarding the availability of NPK in sandy soil following the harvest of celery and lettuce, Table 13 illustrates the effects of various treatments. The results indicated that the application of PIW biofertilizers significantly increased the levels of available nitrogen and phosphorus in sandy soil after celery harvest. Treatments T7 (75% NPK + Cyano-PIW), T8 (50% NPK + Cyano-PIW), and T9 (25% NPK + Cyano-PIW) exhibited significant increases (p < 0.05) in available nitrogen and phosphorus compared to the other treatments.
Conversely, following lettuce harvest, there was no significant rise (p < 0.05) in available nitrogen levels compared to the control group. However, treatments T7 and T8 exhibited a noteworthy increase in available phosphorus. Regarding available potassium, both celery and lettuce crops displayed a significant elevation (p < 0.05) in sandy soil contents for treatments T7 and T8, respectively (refer to Table 13).
4. Discussion
In this study, two experiments were conducted to explore the potential of phycoremediation by utilizing potato industry effluents as a substrate for the growth of cyanobacteria strains, namely, Spirulina platensis, Nostoc muscorum, and Anabaena oryzae. The aim was to evaluate the feasibility of this approach as a cost-effective and eco-friendly method for recycling potato industry effluents and promoting the production of biofertilizers of superior quality. In another experiment, the resulting biofertilizers from the first experiment were evaluated by assessing the growth parameters of celery and lettuce in sandy soil under greenhouse conditions. Cyanobacterial biomass is considered highly promising due to its diverse applications in biofuels, biofertilizers, and useful food ingredients. In this study, the growth and biomass productivity of cyanobacteria using PIW medium were evaluated in both small 500 mL laboratory flasks and an upscaled 50 L photobioreactor (PBR). The growth pattern exhibited an initial brief adaptation period followed by an exponential phase characterized by mixotrophy, as evidenced by monitored growth parameters. The mixotrophic condition was maintained throughout the entire experiment, demonstrating that cyanobacteria can be produced mixotrophically in a closed system with improved contamination control from other microbes and optimal culture conditions to increase biomass yield [31]. Although the systems used in this study were previously sterilized, bacteria were observed alongside the microalgae strains. Nevertheless, the cyanobacteria strains continued to grow without interruption for several days, even as the aerobic microbe count, observed via microscopy, decreased. This indicates that, under the studied conditions, microalgal growth might be capable of controlling bacterial presence. Previous studies have also demonstrated that cyanobacteria strains, when part of a consortium competing with other microalgae, exhibit biocidal activity against bacteria. However, the high COD, nitrogen, and phosphorus contents of PIW may lead to adverse effects, such as a reduction in the dissolved oxygen (DO) content in water, resulting in limited mixotrophy conditions [32]. Indeed, the dark-brown color of the PIW can prevent light penetration, resulting in nearly heterotrophic conditions—particularly observed inside the scaled-up batch PBR (50 L). This means that the autotrophic metabolism of microalgae does not contribute enough to supply the oxygen needed for mixotrophic growth [33]. While a higher aeration rate can improve various aspects, such as mass transfer, gas exchange, and liquid agitation, it is important to note that it can also impose mechanical stress on algae cells [34]. Hence, initially, a high aeration rate was not employed during continuous cultivation when the cell density was low. However, on the ninth day, the daily aeration rate was increased significantly to 0.25 vvm (10 times greater), leading to an enhanced removal of chemical oxygen demand (COD) and ammonium. Subsequently, a sudden decline in both NH4+ and COD levels was observed, suggesting that the microalgae’s heterotrophic growth and aerobic respiration were responsible for the assimilation of organic compounds. These findings align with similar observations documented in previous studies [35,36]. The concentration of the PIW effluent was optimized with the addition of mineral carbon sources such as NaHCO3, which was found to be essential for supporting cyanobacterial growth. An optimal concentration of 0.5 g/L NaHCO3 was determined to be the most effective for promoting cyanobacterial growth [37]. Autotrophic growth of photosynthetic microorganisms necessitates a source of inorganic carbon, typically supplied as CO2. There are several methods for delivering CO2 to a cultivation system: (1) pumping air, (2) pumping air enriched with concentrated CO2 (typically 5%), or (3) utilizing bicarbonate salts. However, the concentration of CO2 in atmospheric air is extremely low (approximately 0.04%), so that a large volume of air is required for aeration, leading to high energy consumption associated with air pumping [38]. These costs are linked to technological challenges in capturing, compressing, transporting, temporarily storing, and preventing CO2 loss [39] and account for up to 50% of biomass production costs [40]. An alternative approach is the utilization of bicarbonate salts as a carbon source, employing a bicarbonate-based integrated carbon capture and algae production system (BICCAPS) [39]. Bicarbonate salts exhibit higher solubility in water compared to CO2 (e.g., NaHCO3 solubility > 90 g L−1 at 25 °C), and their use efficiency would be expected to surpass that of CO2 [38]. The use of aqueous bicarbonate solutions for algal cultivation should result in lower costs compared to CO2, which necessitates energy-intensive compression [39]. De Farias et al. [41] investigated the cyanobacterium Synechococcus PCC 7002 and discovered its ability to accumulate substantial amounts of bicarbonate and produce a high biomass yield (reaching 6 g L−1 of dry cell weight and a maximum productivity of 1.12 g L−1 day−1 at 22 g L−1 of sodium bicarbonate). However, substrate inhibition was observed at concentrations of 44 and 88 g L−1. Despite its potential for biomass production, the range of bicarbonate concentrations (5.5–88 g L−1) still limited the carbohydrate content achieved, which was approximately 25% instead of the 50% reported in the literature.
The analysis of PIW (Table 4) demonstrated its abundance in essential nutrients and microelements, highlighting its potential as a nutrient-rich resource. Notably, nitrogen and phosphorus—crucial macronutrients for microalgal proliferation and biomass synthesis—were identified within PIW. The nutrient composition of PIW suggests its capability to adequately support the growth of cyanobacteria. This finding aligns with previous research by Hwang et al. [42], indicating that microalgae can flourish in contaminated waters containing requisite nutrients, thus serving as a growth medium while concurrently aiding biological remediation by removing and recycling nitrogen and phosphorus from these environments. Such observations can be attributed to microalgae’s adeptness in utilizing diverse trophic modes for nutrition. Photoautotrophic algae harness energy and carbon from CO2 and sunlight, contributing to CO2 sequestration and the generation of valuable bioproducts. Heterotrophic microalgae draw their carbon and energy from organic nutrients in wastewater, yielding biomass for biorefineries. Mixotrophic microalgae, conversely, exploit both CO2 and organic carbon in wastewater for energy and carbon, fostering biomass production for various applications [8,9].
The growth parameters of cyanobacteria across various culture media indicated superior growth in standard media compared to PIW (Figure 1). This variance in growth could be attributed to the deficiency of specific nutrient sources in PIW when contrasted with standard media. These findings align with prior research [43,44,45], which has linked reduced biomass production to the diminished nutritional content of growth media. When aiming for successful microalgae cultivation, various factors influencing cyanobacterial biomass productivity and composition must be considered. Key factors include nutrient availability, pH levels, light intensity, and temperature [12,44,46,47]. The pH of the medium plays a pivotal role in cultivation. During microalgal cultures, pH tends to increase due to the photosynthetic depletion of carbon dioxide from the culture medium [48]. The photosynthetic process of CO2 fixation leads to a gradual pH elevation owing to the accumulation of OH-. Our findings are consistent with those of El-Nahhal [49], who noted that the majority of cyanobacteria exhibit optimal growth within the pH range of 6.5–10. Furthermore, the tendency of pH to rise is correlated with photosynthetic activity, indicating that pH increases where photosynthetic activity is heightened [12,24]. Electrical conductivity (EC) stands out as a critical factor in the selection of a suitable culture medium for microalgae cultivation, as emphasized by Hwang et al. [42]. The PIW samples scrutinized in this study displayed a BOD/COD range of 0.266–0.316, indicating relatively low levels of biodegradable toxic and organic compounds. Nevertheless, the presence of a high load of undesirable organic matter necessitates the treatment of PIW. The assessment of chlorophyll content serves as a common method to gauge the physiological condition and growth of microalgae. In this investigation, the notable chlorophyll content observed in the microalgae could be attributed to the presence of phosphorus and magnesium in the PIW utilized as a culture medium. Phosphorus and magnesium serve as indispensable elements for chlorophyll synthesis, and their presence likely contributes to the vivid blue-green hue exhibited by the algae [50,51].
The microscopic results (Figure 2) provided further evidence of the growth of Spirulina platensis, Anabaena oryzae, and Nostoc muscorum, both individually and in a mixture, in the PIW media compared to the control (synthetic media). A. oryzae exhibited filamentous shapes and a bluish-green colour. The vegetative cells had cylindrical shapes, while the heterocysts were spherical and located internally or terminally within the filament. Nostoc muscorum, on the other hand, displayed filamentous growth and a brown-coloured culture. The vegetative cells varied in shape from barrel to cylindrical. Heterocysts were present as internal and terminal structures within the filament, and their shapes ranged from spherical to ovoid [52]. Spirulina platensis was observed as spiral filaments with cylindrical, multicellular trichomes arranged in an open-helix structure [53]. Furthermore, in the consortium culture of the three strains, it was observed that each strain maintained its distinct morphological characteristics, which were consistent with their growth in the synthetic medium. These findings were supported by other growth indicators.
The growth of cyanobacteria in PIW media and the subsequent production of biofertilizer formulations in monoculture (Spi-PIW) and polyculture (Cyano-PIW), as depicted in Table 5, supports the findings of Shurin et al. [54]. Their research emphasized the potential benefits of polycultures in enhancing biological characteristics and resource utilization. In line with this, the polyculture formulation (Cyano-PIW) demonstrated higher biomass and nitrogen fixation capabilities compared to the monoculture formulation (Spi-PIW).
Additionally, the study by Godwin et al. [55] on culturing multiple species of microalgae showed that while the presence of multiple species may not enhance any single function, it can improve the overall resilience of an algae culture when faced with external factors. This suggests that more complex multispecies agricultural communities of microalgae may utilize resources more efficiently, leading to increased biomass and pollutant removal [56,57].
Therefore, the successful growth and biofertilizer production observed in the PIW media, particularly in the polyculture formulation, align with the concept of utilizing polycultures to enhance biological characteristics, resource utilization, and overall resilience in microalgae communities. These findings have significant implications for the development of sustainable and efficient agricultural practices.
The study findings demonstrated that the application of cyanobacteria–PIW biofertilizers had a positive impact on some growth parameters of celery and lettuce (Table 6 and Table 7, respectively), surpassing the control group and even outperforming the treatments with chemical fertilizers. This indicates that the biofertilizers effectively provided the essential nutrients necessary for optimal plant growth [58]. The use of cyanobacteria–PIW biofertilizers is preferable to chemical fertilizers due to their organic nature and their ability to aid in soil nutrient retention and deliver nutrients to plants over time through capillary action. Cyanobacteria, in addition to nitrogen fixation, produce extracellular products such as growth promoters, vitamins, beneficial enzymes, and minerals. These bioactive substances contribute to improved soil fertility, enhance soil biological processes, and release growth-promoting substances and vitamins [59,60]. The positive results observed in the study align with the findings reported by Sanaa et al. [61], who also noted increased biomass weight in plants treated with cyanobacteria biofertilizer compared to unfertilized plants. They attributed this effect to the activities of algal enzymes like nitrogenase and nitrate reductase, as well as the production of amino acids, peptides, and other plant growth stimulants. Overall, the use of cyanobacteria–PIW biofertilizers has demonstrated their effectiveness in supplying essential nutrients, promoting optimal plant growth, and potentially enhancing soil fertility. These findings highlight the potential of biofertilizers derived from cyanobacteria as valuable tools in sustainable agricultural practices. Significant variations in chlorophyll content were observed among the different treatments in celery and lettuce plants (Table 6 and Table 7, respectively). The application of the T7 and T8 treatments resulted in a significant increase in chlorophyll content in both crops compared to the other treatments and even the full-dose chemical fertilization treatment (T1). Previous studies have indicated that cyanobacteria suspensions contain unique bioactive compounds, including plant growth regulators, which can reduce transpiration and senescence while increasing the chlorophyll content of leaves [62].
Consistent with the present study, previous research has also demonstrated the effectiveness of cyanobacteria biofertilizers on various crops such as pea [63], rice [64], tomato [65], and common bean [66]. The concentrations of NPK (as shown in Table 8 and Table 9) and micronutrients (Table 10 and Table 11) in the leaves and roots of celery and lettuce were significantly influenced by the type of fertilizer used. Compared to the 100% chemical fertilizer treatment, both the T7 and T8 treatments exhibited significantly higher concentrations of NPK and micronutrients comparable to those of the mineral control (T1) and higher than those of the other treatments. These findings align with the results reported by Menamo and Wolde [67], who observed significant increases in leaf number, leaf area, leaf length, fresh leaf weight, leaf dry weight, and root dry weight of lettuce when treated with cyanobacteria biofertilizer compared to the control group. They reported increases of 159.5%, 112.4%, 80.8%, 48%, 137.5%, and 110%, respectively. Moreover, the incorporation of cyanobacteria biofertilizer into the soil led to significant increases in lettuce plant tissue concentrations of P, Zn, and Fe of38.54%, 18.95%, and 105.57%, respectively, compared to the control. Additionally, the application of liquid cyanobacteria resulted in a 33.3% increase in N concentration in the lettuce tissue compared to the control. In our previous study, we concluded that one-quarter to one-half of the recommended dose of mineral NPK fertilizers could be saved for celery growth by using the Bio-Mix product made from cyanobacteria and olive milling wastewater [10], which serves as a promising eco-friendly bio-organic fertilizer. We found that applying Bio-Mix biofertilizers combined with 25% and 50% of the recommended NPK dose resulted in the most significant increases (p < 0.05) in plant height, root and stem lengths, number of leaves per plant, total chlorophyll content, as well as total macro- and micronutrient content in celery compared to the control group.
Moreover, we observed significant improvements in certain sandy soil properties after harvest, such as pH, soil organic matter, soil organic carbon, total nitrogen, phosphorus, and potassium, when using Bio-Mix in25% and 50% treatments [68]. This notable enhancement could be attributed to the efficiency of cyanobacteria biofertilizers and their ability to supply plant nutrients, thus improving soil fertility, biological processes in the soil, and the release of growth-promoting substances and vitamins. These findings underscore the potential benefits of incorporating the 50% NPK + Cyano-PIW treatment as an effective approach for enhancing soil fertility and promoting the growth of crops. The significant improvements in soil organic carbon and soil organic matter achieved through the application of these biofertilizers indicate their potential to contribute positively to sustainable agricultural practices and the overall productivity of agricultural systems. Cyanobacteria biofertilizers have been widely recognized for their ability to enhance soil fertility and promote plant growth. They are efficient in fixing atmospheric nitrogen and converting it into plant-available forms, such as ammonia and nitrates [9]. This process not only increases nitrogen availability to plants but also improves soil nitrogen content, contributing to enhanced soil fertility [69].
The significant improvements in soil organic carbon and soil organic matter achieved through the application of cyanobacteria biofertilizers highlight their potential contribution to sustainable agricultural practices and overall productivity. Cyanobacteria biofertilizers are recognized for their ability to enhance soil fertility and promote plant growth by efficiently fixing atmospheric nitrogen and converting it into plant-available forms, such as ammonia and nitrates [70].
This study has shown that the application of Spi-PIW or Cyano-PIW in combination with reduced NPK mineral fertilizers (at 25% and 50%) results in significant improvements in various sandy soil properties post-harvest. These improvements include enhancements in pH, total organic matter, total nitrogen, phosphorus, and potassium levels. The effectiveness of cyanobacteria biofertilizers stems from their unique ability to supply essential nutrients to plants, fostering a symbiotic relationship that boosts nitrogen content within soil, which is crucial for optimal plant growth.
Moreover, cyanobacteria biofertilizers facilitate the release of growth-promoting substances and vitamins into soil, acting as stimulants for plant growth, enhancing root development, and improving nutrient uptake efficiency. This comprehensive enhancement of biological processes within soil leads to improved soil health and increased nutrient availability for plants [71].
The findings underscore the potential of integrating the 50% NPK + Cyano-PIW or Spi-PIW treatments as an effective strategy to enhance soil fertility and promote crop growth. By adopting this approach, farmers can align their practices with sustainable agriculture principles, aiming for long-term soil health and productivity.
5. Conclusions
The study demonstrated the effectiveness of using cyanobacteria strains (Spirulina platensis, Nostoc muscorum, and Anabaena oryzae) for phycoremediation of potato industry effluents, producing high-quality biofertilizers. The experiments confirmed that potato industry wastewater (PIW) serves as a nutrient-rich growth medium for cyanobacteria, supporting significant biomass production and nitrogen fixation, especially in polyculture formulations. Cyanobacteria–PIW biofertilizers improved growth parameters, chlorophyll contents, and nutrient concentrations in celery and lettuce, outperforming chemical fertilizers and controls. These biofertilizers also enhanced soil fertility, organic carbon, and organic matter, contributing to soil health and sustainability. The findings underscore the potential of cyanobacteria-based phycoremediation and biofertilizers as cost-effective, eco-friendly tools for wastewater treatment, nutrient recycling, and soil enrichment. Implementing these practices can reduce environmental pollution, conserve resources, and promote resilient agricultural systems. This approach aligns with sustainable and environmentally conscious food production and soil management strategies, highlighting the importance of integrating cyanobacteria-based technologies into agriculture.
Author Contributions
Conceptualization, S.S.M.M.; methodology, S.R., N.M.M.E. and A.A.A.; software, G.A.E.-C.; validation, S.S.M.M., A.S.E.-H. and A.S.S.; formal analysis, S.S.M.M., A.S.E.-H., A.S.S. and G.A.E.-C.; investigation, S.S.M.M. and A.S.E.-H.; resources, S.S.M.M., S.R. and N.M.M.E.; data curation, S.S.M.M., A.S.E.-H., G.A.E.-C. and A.S.S.; writing—original draft preparation, S.S.M.M.; writing—review and editing, S.S.M.M.; visualization, S.S.M.M. and A.S.E.-H.; supervision, S.S.M.M. and A.S.E.-H.; project administration, S.S.M.M.; funding acquisition, authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available on request to the corresponding author’s email with appropriate justification.
Acknowledgments
The authors express their gratitude to the Department of Microbiology at the Soil, Water, and Environment Research Institute (SWERI), Agricultural Research Center (ARC), for providing the microalgae strains, access to the algae laboratory, greenhouse facilities, and necessary equipment.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kot, A.M.; Pobiega, K.; Piwowarek, K.; Kieliszek, M.; Błażejak, S.; Gniewosz, M.; Lipińska, E. Biotechnological Methods of Management and Utilization of Potato Industry Waste—A Review. Potato Res. 2020, 63, 431–447. [Google Scholar] [CrossRef]
- Rabia, A.H.B.; Mohamed, A.A.; Abdelaty, E.F.; Shahin-Sara, F.; Yacout Dalia, M.M. Investigating Adaptation Strategies Developed by Potato Farmers to Cope with Climate Change Impacts in Egypt. Alex. Sci. Exch. J. 2021, 42, 871–881. [Google Scholar] [CrossRef]
- Bzducha-Wróbel, A.; Błażejak, S.; Molenda, M.; Reczek, L. Biosynthesis of β (1,3)/(1,6)-glucans of cell wall of the yeast Candida utilis ATCC 9950 strains in the culture media supplemented with deproteinated potato juice water and glycerol. Eur. Food Res. Technol. 2015, 240, 1023–1034. [Google Scholar] [CrossRef]
- Miedzianka, J.; Pęksa, A.; Pokora, M.; Rytel, E.; Tajner-Czopek, A.; Kita, A. Improving the properties of fodder potato protein concentrate by enzymatic hydrolysis. Food Chem. 2014, 159, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.K.; Hung, Y.-T.; Lo, H.H.; Yapijakis, C. (Eds.) Handbook of Industrial and Hazardous Wastes Treatment, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar] [CrossRef]
- Kowalczewski, P.; Celka, K.; Białas, W.; Lewandowicz, G. Antioxidant activity of potato juice. Acta Sci. Pol. Technol. Aliment. 2012, 11, 175–181. [Google Scholar] [PubMed]
- Hussain, F.; Shah, S.Z.; Ahmad, H.; Abubshait, S.A.; Abubshait, H.A.; Laref, A.; Manikandan, A.; Kusuma, H.S.; Iqbal, M. Microalgae an ecofriendly and sustainable wastewater treatment option: Biomass application in biofuel and biofertilizer production: A review. Renew. Sustain. Energy Rev. 2021, 137, 110603. [Google Scholar] [CrossRef]
- Ummalyma, S.B.; Sahoo, D.; Pandey, A. Resource recovery through bioremediation of wastewaters and waste carbon by microalgae: A circular bioeconomy approach. Environ. Sci. Pollut. Res. 2021, 28, 58837–58856. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.S.; Kumar, A.; Rai, A.N.; Singh, D.P. Cyanobacteria: A Precious Bio-resource in Agriculture, Ecosystem, and Environmental Sustainability. Front. Microbiol. 2016, 7, 529. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, S.S.; El-Hassanin, A.S.; Rashad, S.; El-Chaghaby, G.A. Microalgae growth in effluents from olive oil industry for biomass production and decreasing phenolics content of wastewater. Egypt. J. Aquat. Biol. Fish. 2019, 23, 359–365. [Google Scholar] [CrossRef]
- Hung, Y.T.; Lo, H.H.; Awad, A.; Salman, H. Potato wastewater treatment. In Handbook of Industrial and Hazardous Wastes Treatment; CRC Press: Boca Raton, FL, USA, 2004; pp. 894–951. [Google Scholar]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005; p. 1220. [Google Scholar]
- Rippka, R.; Deruelles, J.; Waterburg, J.B.; Herdman, M.; Stanier, R.Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteira. J. Gen. Microbiol. 1979, 111, 1–16. [Google Scholar]
- Zarrouk, C. Contribution á l’étuded’unecyanophycée. Influence de Divers Facteurs Physiques et Chimiques sur la Croissance et la Photosynthése de Spirulina maxima (Setch. Et Gardner) Geitler. Ph.D. Thesis, University of Paris, Paris, France, 1966. [Google Scholar]
- Ferris, M.J.; Hirsch, C.F. Method for isolation and purification of cyanobacteria. Appl. Environ. Microbiol. 1991, 57, 1448–1452. [Google Scholar] [CrossRef] [PubMed]
- Roger, P.A.; Kulasooriya, S.A. Blue Green Algae and Rice; The International Rice Research Institute: Los Banos, Philippines, 1980. [Google Scholar]
- Garcia-Pichel, F.; Sherry, N.D.; Castenholz, R.W. Evidence for an ultraviolet sunscreen role of the extracellular pigment scytonemin in the terrestrial cyanobacterium Chlorogloeopsis sp. Photochem. Photobiol. 1992, 56, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Desikachary, T.V. Cyanophyta. In ICAR Monograph on Algae; ICAR: New Delhi, India, 1959. [Google Scholar]
- Herdman, M.; Castenholz, R.W.; Iteman, I.; Waterbury, J.B.; Rippka, R. The Cyanobacteria: Subsection 1 (Formerly Chroococcales Wettstein 1924, emend. Rippka, Deruelles, Waterbury. Herdman and Stanier 1979). In Bergey’s Manual of Systematic Bacteriology, 2nd ed.; Boone, D.R., Castenholz, W.R., Eds.; Springer: New York, NY, USA, 2001; pp. 493–514. [Google Scholar]
- Senousy, H.H.; Hamoud, Y.A.; Abu-Elsaoud, A.M.; Mahmoud Al zoubi, O.; Abdelbaky, N.F.; Zia-ur-Rehman, M.; Usman, M.; Soliman, M.H. Algal Bio-Stimulants Enhance Salt Tolerance in Common Bean: Dissecting Morphological, Physiological, and Genetic Mechanisms for Stress Adaptation. Plants 2023, 12, 3714. [Google Scholar] [CrossRef] [PubMed]
- Kamennaya, N.A.; Ahn, S.; Park, H.; Bartal, R.; Sasaki, K.A.; Holman, H.Y.; Jansson, C. Installing extra bicarbonate transporters in the cyanobacterium Synechocystis sp. PCC6803 enhances biomass production. Metab. Eng. 2015, 29, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Vonshak, A.; Richmond, A. Mass production of the blue-green algae Spirulina: An overview. Biomass 1988, 15, 233–247. [Google Scholar] [CrossRef]
- Piper, C.S. Soil and Plant Analysis; Interscience Publishers, Inc.: New York, NY, USA, 1950. [Google Scholar]
- Richards, L.A. Diagnosis and Improvement of Saline and Alkaline Soils; Agriculture Handbook No. 60; US Department of Agriculture: Washington, DC, USA, 1954.
- AOAC. Methods of Soil Analysis, 12th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2005. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil. Sci. 1934, 37, 29–37. [Google Scholar] [CrossRef]
- Jia, M.; Li, D.; Colombo, R.; Wang, Y.; Wang, X.; Cheng, T.; Zhu, Y.; Yao, X.; Xu, C.; Ouer, G.; et al. Quantifying Chlorophyll Fluorescence Parameters from Hyperspectral Reflectance at the Leaf Scale under Various Nitrogen Treatment Regimes in Winter Wheat. Remote Sens. 2019, 11, 2838. [Google Scholar] [CrossRef]
- SPSS. Tutorial for SPSS-13.0 Software, version 13.0; SPSS Inc.: Chicago, IL, USA, 2004. [Google Scholar]
- Balasooriya, B.L.W.K. Culture Collection of Cyanobacteria and Microalgae at Department of Biotechnology, Wayamba University of Sri Lanka; Neth Win Printers: Kandy, Sri Lanka, 2019; ISBN 978-624-5327-00-3. [Google Scholar]
- Abdel-Hamid, M.S.; Hamouda, R.A.E.-F.; El-Aal, H.A.; Badawy, G.A. Distinctive Application of the Consortium of Chlorella vulgaris and Anabaena oryzae toward Different Planting Dates and Climate Change on Jerusalem Artichoke Yield. J. Plant Growth Regul. 2022, 41, 479–493. [Google Scholar] [CrossRef]
- Ende, S.S.W.; Noke, A. Heterotrophic microalgae production on food waste and by-products. J. Appl. Phycol. 2019, 31, 1565–1571. [Google Scholar] [CrossRef]
- Lois-Milevicich, J.; Casá, N.; Alvarez, P.; Mateucci, R.; Busto, V.; de Escalada, M. Chlorella vulgaris biomass production using brewery wastewater with high chemical oxygen demand. J. Appl. Phycol. 2020, 32, 2773–2783. [Google Scholar] [CrossRef]
- Cai, T.; Park, S.Y.; Li, Y. Nutrient recovery from wastewater streams by microalgae: Status and prospects. Renew. Sustain. Energy Rev. 2013, 19, 360–369. [Google Scholar] [CrossRef]
- Mohamadnia, S.; Tavakoli, O.; Faramarzi, M.A. Production of fucoxanthin from the microalga Tisochrysis lutea in the bubble column photobioreactor applying mass transfer coefcient. J. Biotechnol. 2022, 348, 47–54. [Google Scholar] [CrossRef]
- Perez-Garcia, O.; Escalante, F.M.E.; de-Bashan, L.E.; Bashan, Y. Heterotrophic cultures of microalgae: Metabolism and potential products. Water Res. 2011, 45, 11–36. [Google Scholar] [CrossRef] [PubMed]
- Don, C.D.Y.A.; Babel, S. Effects of organic loading on bioelectricity and micro-algal biomass production in microbial fuel cells using synthetic wastewater. J. Water Process Eng. 2021, 39, 101699. [Google Scholar]
- Johnson, T.J.; Zahler, J.D.; Baldwin, E.L.; Zhou, R.; Gibbons, W.R. Optimizing cyanobacteria growth conditions in a sealed environment to enable chemical inhibition tests with volatile chemicals. J. Microbiol. Methods 2016, 126, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Markou, G.; Vandamme, D.; Muylaert, K. Microalgal and cyanobacterial cultivation: The supply of nutrients. Water Res. 2014, 65, 186–202. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.; O’Fallon, J.V.; Chen, S. Bicarbonate produced from carbon capture for algae culture. Trends Biotechnol. 2011, 29, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Chisti, Y. Constraints to commercialization of algal fuels. J. Biotechnol. 2013, 167, 201–214. [Google Scholar] [CrossRef] [PubMed]
- De Farias Silva, C.; Gris, B.; Sforza, E.; La Rocca, N.; Bertucco, A. Effects of Sodium Bicarbonate on Biomass and Carbohydrate Production in Synechococcus PCC 7002. Chem. Eng. Trans. 2016, 49, 241–246. [Google Scholar]
- Hwang, J.-H.; Church, J.; Lee, S.-J.; Park, J.; Lee, W.H. Use of microalgae for advanced wastewater treatment and sustainable bioenergy generation. Environ. Eng. Sci. 2016, 33, 882–897. [Google Scholar] [CrossRef]
- Mostafa, S.S.; Shalaby, E.A.; Mahmoud, G.I. Cultivating Microalgae in Domestic Wastewater for Biodiesel Production. Not. Sci. Biol. 2012, 4, 56–65. [Google Scholar] [CrossRef]
- Brar, A.; Kumar, M.; Pareek, N. Comparative appraisal of biomass production, remediation, and bioenergy generation potential of microalgae in dairy wastewater. Front. Microbiol. 2019, 10, 678. [Google Scholar] [CrossRef] [PubMed]
- Zayadan, B.K.; Sadvakasova, A.K.; Usserbayeva, A.A.; Bolatkhan, K.; Baizhigitova, A.M.; Akmukhanova, N.R.; Sidorov, R.A.; Sinetova, M.A.; Los, D.A. Waste-free technology of wastewater treatment to obtain microalgal biomass for biodiesel production. Int. J. Hydrogen Energy 2017, 42, 8586–8591. [Google Scholar] [CrossRef]
- Moustafa, S.; El Shimi, H. Phycoremediation of Olive Wastewater for Sustainable Production. Int. J. ChemTech Res. 2016, 9, 567–579. [Google Scholar]
- Paraskeva, P.; Diamadopoulos, E. Technologies for Olive Mill Wastewater (OMW) Treatment: A Review. J. Chem. Technol. Biotechnol. 2006, 81, 1475–1485. [Google Scholar] [CrossRef]
- Dolganyuk, V.; Belova, D.; Babich, O.; Prosekov, A.; Ivanova, S.; Katserov, D.; Patyukov, N.; Sukhikh, S. Microalgae: A Promising Source of Valuable Bioproducts. Biomolecules 2020, 10, 1153. [Google Scholar] [CrossRef] [PubMed]
- El-Nahhal, Y.; Hamms, S. Effects of Bromacil, malathion and thiabendazole on Cyanobacteria mat growth. Int. J. Appl. Sci. Res. Rev. 2017, 4, 1. [Google Scholar] [CrossRef]
- Das, N.; Chandran, P. Microbial degradation of petroleum hydrocarbon contaminants: An overview. Biotechnol. Res. Int. 2011, 2011, 941810. [Google Scholar] [CrossRef] [PubMed]
- Shruthi, M.S.; Rajashekhar, M. Effect of salinity and growth phase on the biochemical composition of two diatom species isolated from estuarine waters near Mangalore, West Coast of India. Int. J. Adv. Life Sci. 2014, 7, 135–142. [Google Scholar]
- Rajaniemi, P.; Hrouzek, P.; Kastovska, K.; Willame, R.; Rantala, A.; Hoffmann, L.; Komarek, J.; Sivonen, K. Phylogenetic and morphological evaluation of the genera Anabaena, Aphanizomenon, Trichormus and Nostoc (Nostocales, Cyanobacteria). Int. J. Syst. Evol. Microbiol. 2005, 55, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Wuang, S.C.; Khin, M.C.; Chua, P.Q.D.; Luo, Y.D. Use of Spirulina biomass produced from treatment of aquaculture wastewater as agricultural fertilizers. Algal Res. 2016, 15, 59–64. [Google Scholar] [CrossRef]
- Shurin, J.B.; Abbott, R.L.; Deal, M.S.; Kwan, G.T.; Litchman, E.; McBride, R.C.; Mandal, S.; Smith, V.H. Industrial-strength ecology: Trade-offs and opportunities in algal biofuel production. Ecol. Lett. 2013, 16, 1393–1404. [Google Scholar] [CrossRef] [PubMed]
- Godwin, C.M.; Hietala, D.C.; Lashaway, A.R.; Narwani, A.; Savage, P.E.; Cardinale, B.J. Ecological Stoichiometry Meets Ecological Engineering: Using Polycultures to Enhance the Multifunctionality of Algal Biocrude Systems. Environ. Sci. Technol. 2017, 51, 11450–11458. [Google Scholar] [CrossRef] [PubMed]
- Bell, T.A.S.; Doig, L.; Peyton, B.M.; Gerlach, R.; Fields, M.W. Contributions of the microbial community to algal biomass and biofuel productivity in a wastewater treatment lagoon system. Algal Res. 2019, 39, 101461. [Google Scholar] [CrossRef]
- Mhedhbi, E.; Khelifi, N.; Foladori, P.; Smaali, I. Real-Time Behavior of a Microalgae–Bacteria Consortium Treating Wastewater in a Sequencing Batch Reactor in Response to Feeding Time and Agitation Mode. Water 2020, 12, 1893. [Google Scholar] [CrossRef]
- Kumar, D.; Nikhil, K. Effect of FYM, NPK and Algal fertilizers on the Growth and Biomass of Vetiver Grass [Vetiveriazizanioides L. Nass]. Int. J. Eng. Appl. Sci. 2016, 3, 257695. [Google Scholar]
- Jaiswal, A.; Das, K.; Koli, D.K.; Pabbi, S. Characterization of cyanobacteria for IAA and siderophore production and their effect on rice seed germination. Int. J. Curr. Microbiol. Appl. Sci. 2018, 5, 212–222. [Google Scholar]
- Aly, M.H.A.; Abd El-All, A.A.M.; Mostafa, S.S.M. Enhancement of sugar beet seed germination, plant growth performance and biochemical compounds as contributed by algal extracellular products. J. Agric. Sci., Mansoura Univ. 2008, 33, 8429–8448. [Google Scholar]
- Burjus, S.; Jawad, A.; Al-Ani, N. Effect of Two Species of Cyanobacteria as Biofertilizers on Characteristics and Yield of Chickpea Plant. Iraqi J. Sci. 2014, 55, 685–696. [Google Scholar]
- Ibraheem, I.B.M.; Abdel-Raouf, N.; Hammouda, O.; AbdelWahab, N. The potential for using culture filtrate of chroococcus minutes as fungicial agent against phytopathogenic Pythium sp. Egypt. J. Phycol. 2008, 9, 99–114. [Google Scholar] [CrossRef]
- Osman, M.E.H.; El-Sheekh, M.M.; El-Naggar, A.H.; Gheda, S.F. Effect of two species of cyanobacteria as biofertilizers on some metabolic activities, growth, and yield of pea plant. Biol. Fertil. Soils 2010, 46, 861–875. [Google Scholar] [CrossRef]
- Mahmoud, Y.I.; Mostafa, S.S.M.; Mohamed, F.M. Comparative Evaluation of Biological Treatments and Mineral NPK on Rice Productivity in Alkaline-Saline Soil. Middle East J. Agric. Res. 2015, 4, 735–744. [Google Scholar]
- Abuye, F.; Achamo, B. Potential Use of Cyanobacterial Bio-fertilizer on Growth of Tomato Yield Components and Nutritional Quality on Grown Soils Contrasting pH. J. Biol. 2016, 6, 54–62. [Google Scholar]
- Hegazi, A.Z.; Mostafa, S.S.M.; Ahmed, H.M.I. Influence of different cyanobacterial application methods on growth and seed production of common bean under various levels of mineral nitrogen fertilization. Nat. Sci. 2010, 88, 183–194. [Google Scholar]
- Menamo, M.; Wolde, Z. Effect of cyanobacteria application as biofertilizer on growth, yield and yield components of Romaine lettuce (Lactuca sativa L.) on soil of Ethiopia. Am. Sci. Res. J. Eng. Technol. Sci. 2013, 4, 50–78. [Google Scholar]
- Rashad, S.; El-Hassanin, A.S.; Mostafa, S.S.M.; El-Chaghaby, G.A. Cyanobacteria cultivation using olive milling wastewater for bio-fertilization of celery plant. Glob. J. Environ. Sci. Manag. 2019, 5, 167–174. [Google Scholar]
- Mostafa, S.; El-Taweel, A.A.; Aly, A.A. Use Efficiency of Cyanobacteria and Olive Vegetation Water (Cyano/Ovw) Biofertilizer for Olive Trees under Different Mineral NPK Levels. Egypt. J. Hort. 2016, 43, 77–107. [Google Scholar]
- Bhagat, R.; Gupta, S.; Singh, D.P.; Singh, D.; Kumar, P. Cyanobacteria: A potential biofertilizer for sustainable agriculture. In Plant-Microbe Interactions in Agro-Ecological Perspectives; Springer: Singapore, 2020; pp. 275–296. [Google Scholar]
- Abiven, S.; Menasseri, S.; Chenu, C.; Torn, M.S. Identifying mechanisms of soil organic matter dynamics: A critical review using compartmental analysis. Soil. Biol. Biochem. 2009, 41, 1613–1629. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).