1. Introduction
Invasive alien weeds are a major problem in agriculture, reducing crop yields [
1].
Argemone ochroleuca (Sweet) is among the most important economically devastating invasive plant species that affect both agricultural and natural ecosystems [
2]. This weed has been reported to release allelochemicals that affect crops in agricultural fields [
3,
4]. Muche et al. [
4] observed that
A. ochroleuca leaf, stem and root, and water extracts inhibited the growth and germination of three sorghum varieties. Abd-ElGawad et al. [
5] reported that the chemical composition of
A. ochroleuca essential oils had highly oxygenated constituents including mono-, sesqui-, di-terpenoids, carotenoids, and hydrocarbons. These compounds have been associated with the inhibition of the germination and growth of crops [
5]. Few empirical studies have been conducted to assess the impact of
A. ochroleuca on crops in South African agricultural fields, leaving the effects speculative due to the limited research on the germination and growth of locally produced crops.
Soybean (
Glycine max (L.) Merr.) is a high-value leguminous food source and an oil seed crop important for human consumption and animal feeds [
6]. Khojely et al. [
7] reported that in Africa, the soybean is a non-staple and non-native crop with the prospective to become a commercialized crop due to its multiple applications as food, industrial raw material, and feed. Soybean is also considered a significant source of oil and protein [
8] and accounts for about a quarter of global protein and animal feed production [
9]. In South Africa, soybean is mostly cultivated in the Free State, KwaZulu-Natal, Mpumalanga, and Gauteng Provinces, but its production is extended to areas where predominantly maize and crops such as groundnut, sunflower, potato and others are traditionally grown [
10]. The need for soybean is gradually increasing due to an increase in population and societal development [
11]. However, the production of soybean is currently threatened by invasive alien weeds all over the world [
12].
Paul and Begum [
13] reported that the aqueous extracts of
A. mexicana root and leaf could reduce the germination of
Lens culinaris. This conforms to a study by Alagesaboopathi [
14], who reported that
A. mexicana, another
Argemone species, decreased seed germination of
Sorghum bicolor by 18% compared to the 89% germination observed in the control. Dar et al. [
15] also noted that the shoot extracts of
A. ochroleuca inhibited the germination of
Farsetia aegyptia Turra,
Salvia aegyptiaca,
Hordeum vulgare and
Medicago sativa more than their corresponding root extracts. With
A. ochroleuca spreading across Africa at an alarming rate [
2], the understanding of how this invasive species affects economic crops is the starting point in the management decisions [
16]. Currently, there is no information on the impact of
A. ochroleuca on the germination of soybean, one of the most economically important crops belonging to the Leguminosae family, and the information on the phytochemicals present in this plant is inadequate. Therefore, the aim of the current study was to evaluate the TLC profiles of different extracts of
A. ochroleuca and determine the effects of shoot and root extracts on soybean seed germination.
4. Discussion
The current study indicates that the allelopathic effects of water, hexane and acetone extracts obtained from
A. ochroleuca shoots and roots inhibited the germination of soybean seeds, with the highest inhibition observed in the germination bioassay of water extracts. The explanation of this could be that most of the allelopathic active compound concentrations were water-soluble, hence the higher concentrations in water extracts [
24]. Ashrafi et al. [
24] observed that the inhibitory effects of the water-soluble fractions of
Azadirachta indica were the highest, compared to the n-hexane-soluble and acetone-soluble fractions in all germination bioassays of
Amaranthus rotundus,
Cirsium arvense,
Digitaria sanguinalis,
Sinapis arvensis,
Lactuca sativa and
Lolium ultiforum. These findings were also reported by Tanveer et al. [
25], who observed that there were differences in the inhibitory effects of
Euphorbia dracunculoides n-hexane, chloroform, ethyl acetate, 1-butanol and aqueous fractions on the germination and seedling growth of maize and chickpea. Tanveer et al. [
25] reported that hexane fractions had more suppressive effects on the germination of chickpeas and wheat when compared with chloroform, ethyl acetate and 1-butanol fractions. Sultana et al. [
26] attributed the differences in the allelochemical composition of solvents to different compounds in plants with varying polarities and chemical properties affecting their solubility. Lower concentrations of extracts were observed to stimulate germination. This has been attributed to a process called hormesis [
27]. Hormesis is an adaptative response where there is an induction of beneficial effects when the organism is exposed to low dosages of harmful chemical or physical agents [
28]. In hormesis, after a small stress, special proteins responsible for the removal of damage produced by stressors are over-produced, resulting in not only the removal of damage produced by the current stress, but also the removal of the pre-existing damage, which produces a stimulating effect [
29].
Weeds compete with other crops for water, nutrients and space, and they release allelochemicals into the environment that inhibit plant growth [
30]. The combined effects of allelochemicals such as fatty acids, fatty acid methyl esters, terpenoids and phenolics that are released have inhibitory effects on plants [
31]. Even though this particular trial did not quantify the allelochemicals, the current study has demonstrated similar decreasing trends of
A. ochroleuca extracts’ effects on the germination and seedling length of soybean seeds. These observed trends were concentration- and plant-part-dependent inhibition responses. This phenomenon is very common to many weed extracts used in crop seed germination [
4,
32,
33]. Nxumalo et al. [
33] reported a concentration-dependent inhibition response of
A. ochroleuca extracts on the germination, seedling length and early growth of millet and maize.
Cassia occidentalis seeds’ response effects were found to be more pronounced at lower concentrations of
Psidium guajava extracts than at higher concentrations [
34]. Muche et al. [
4] also reported the same effects of
A. ochroleuca extracts on the germination and seedling length of
Sorghum bicolor varieties. In the current study,
A. ochroleuca extracts also affected other germination variables such as germination speed and mean daily germination. M’barek et al. [
35] reported the phytotoxic effects of
Tetraclinis articulata on germination speed, even though in their report the allelochemicals had no effects on the final germination percentage. The delay in seed germination can have some important biological and ecological implications, because it affects the ability of the seedling to establish itself in natural conditions, resulting in uneven plant stands [
35].
The current study also found that there were differences in the allelopathic effects of the different plant parts of
A. ochroleuca on the germination of soybean seeds, with extracts from the shoots inhibiting all measured germination variables more when compared to extracts from the roots. Water and acetone shoot and root extracts and hexane shoot extracts had inhibitory effects on the measured germination and seedling length variables, whereas hexane root extracts stimulated germination and seedling length variables. Generally, the distributions of allelopathic active compounds differ with plant organ, both in quantity and quality [
36]. The leaves of
Eucalyptus camaldulensis were recorded by Nasr et al. [
37] to contain the highest total allelochemicals when compared to other plant organs. Ghareib et al. [
38] reported that the allelopathic potential induced by low concentrations of the acetone fraction of
Chenopodium murale stimulated the germination and growth of tomato. According to Muche et al. [
4],
A. ochroleuca weed leaf extracts had more inhibitory effects on
Sorghum bicolor varieties compared to extracts from the roots and stems. Root, stem and leaf aqueous extracts of
A. philoxeroides had different effects on the root length, shoot length and fresh weight of
Z. matrella [
39]. Paul and Begum [
40] explained the high degree of inhibition of the germination and seedling growth of blackgram, rapeseed and wheat using leaf and root extracts of
A. mexicana as being due to the fact that
A. mexicana synthesizes and stores the phytochemicals in leaves and roots.
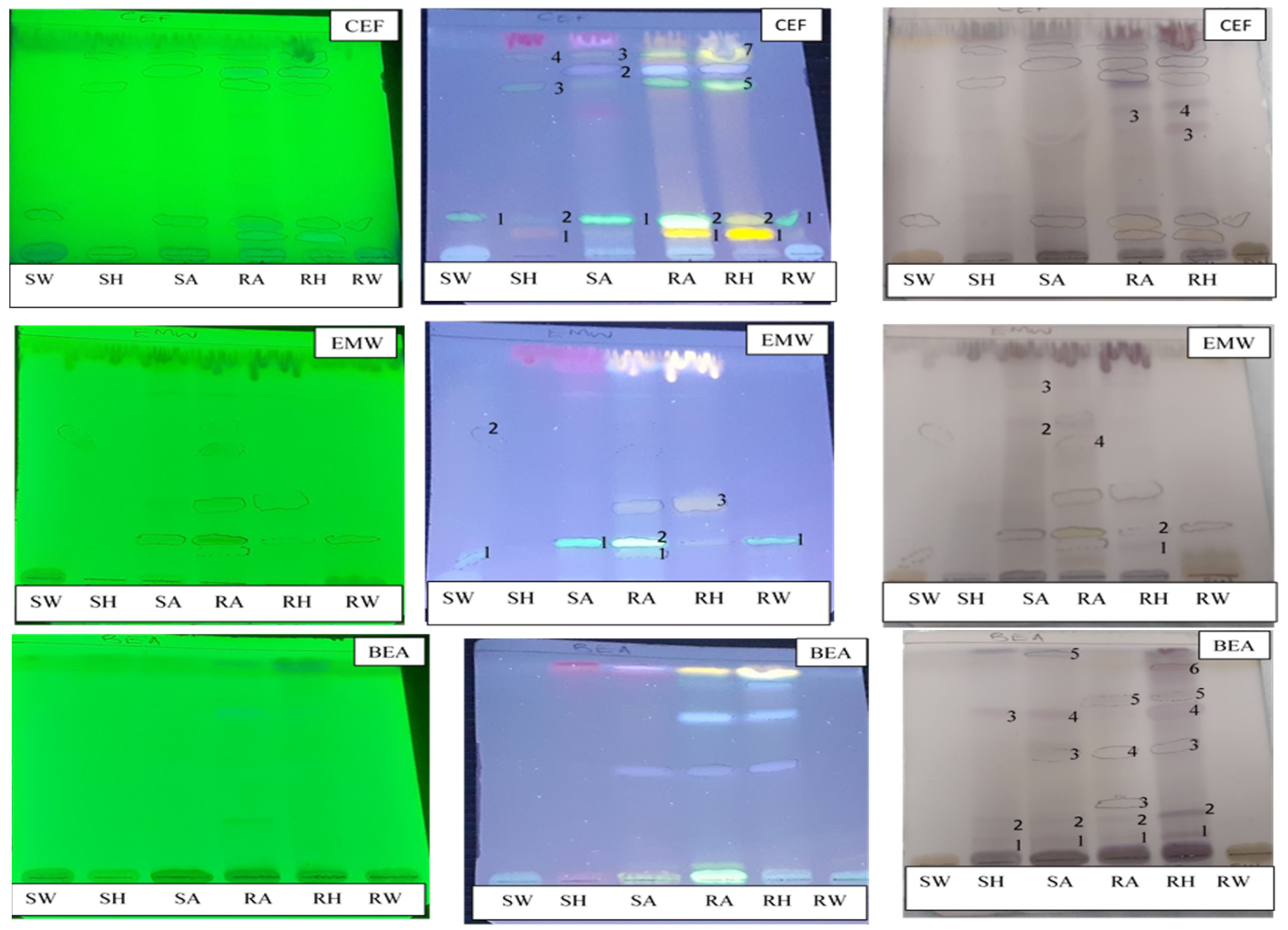
The TLC analysis identified different classes of compounds from
A. ochroleuca hexane and acetone extracts, which include flavonoids, lactones, phenolic acids, alkaloids, saponins and anthracene derivatives. Water extract did not elute many compounds as compared to hexane and acetone; their highly suppressive effects could mean that the compounds present in water are phytotoxic. The activities of non-polar compounds and polar ones from hexane and acetone could be attributed to the presence of the compounds shown in TLC plates. Cheng and Cheng [
41] reported that the allelochemicals produced by plants exhibiting allelopathy include phenolics, terpenoids, and alkaloids, but water-soluble phenolic compounds have been established to play a major role in the growth suppression of associated plants. Phytochemicals such as phenolics, alkaloids, steroids, terpenes, saponins, and quinones have allelopathic effects on the growth and development of certain plant species [
42]. According to Sasikumar et al. [
43], the allelopathic effects of
Eucalyptus globulus can be attributed to volatile terpenes and phenolic acids, and have been reported to be responsible for the inhibitory effects on the germination and seedling growth of various crops. Ghimire et al. [
44] reported that alfalfa-derived phenolic compounds and saponins exhibit phytotoxicity effects on the growth of
Digitaria ciliaris,
Chenopodium album,
Amaranthus lividus,
Portulaca oleracea and
Commelina communis. Phenolic acids have been identified and isolated from many allelopathic plants, and the role of phenolic compounds in inducing allelopathic abilities is well-established [
45]. According to Movafeghi et al. [
46], alkaloids, steroids, flavonoids, anthraquinones, amino acids, and polysaccharides were isolated from the seeds, leaves, flowers, stems, and roots of
Peganum harmala, a weed reported to have phytotoxic effects on plants. Shao et al. [
47] reported that alkaloids isolated from the seeds of
Peganum harmala exerted significant inhibitory activity on lettuce, amaranth, wheat, and ryegrass seed germination. Synowiec et al. [
48] reported that the essential oils of
Achillea millefolium,
Acorus calamus,
Carum carvi,
Chamomilla recutita,
Foeniculum vulgare,
Lavandula angustifolia,
Melissa officinalis,
Mentha piperita,
Salvia officinalis,
Solidago canadensis,
Tanacetum vulgare and
Thymus vulgaris inhibited the germination of
Amaranthus retroflexus,
Avena fatua,
Bromus secalinus and
Centaurea cyanus, which have been reported as notorious weeds that affect the germination of economically important crops (
Avena sativa,
Brassica napus and
Zea mays). Allelopathic phytochemicals are released from donor plants as volatiles, roots exudates, or foliage leachates, and contain secondary metabolites such as flavonoid phenolics [
49], ketones, aldehydes, terpenoids, lactones, cinnamic acid, and quinines [
50]. When these compounds are excreted into the rhizosphere, neighboring plants absorb them through the uptake of sap [
51], and interfere with the physiologic and biosynthetic machinery of the receiver plant [
52,
53]. Given the most recent developments in allelochemistry, which provides a physiologically and ecologically solid explanation for plant invasion, it is hypothesized that the phytotoxicity of the applied extract is responsible for the negative response of seeds or seedlings [
15].