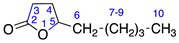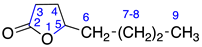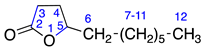Abstract
The challenge of managing invasive weed species continues to affect the agricultural industry, presenting ecological, economic, and agronomic hurdles that lead to over 100 billion USD in annual crop losses globally. One such concern is the management of Agrostis stolonifera L., commonly known as creeping bentgrass, particularly due to its ability to form hybrids. This scenario underscores the urgent need for innovative, effective, and environmentally sustainable herbicides, steering the focus toward natural substances as potential candidates. We report here a promising natural lactone, commonly known as menthalactone, which is derived from Mentha piperita L. Its phytotoxic activity was assessed against the monocot, bentgrass, and a dicot, lettuce (Lactuca sativa L.). Menthalactone displayed outstanding activity against bentgrass and was further evaluated for phytotoxic characteristics. The germination of A. stolonifera seeds was significantly inhibited with an IC50 value of 4.9 ± 1.2 µM. In contrast to bentgrass seeds, Lemna pausicostata L. plants were less responsive to menthalactone treatment, shown by an IC50 of 293.4 ± 70.6 µM. Both species are monocots, and the results suggest that menthalactone might have effects on seed germination but not on the metabolism of green tissues. The susceptibility of three common, obnoxious weed species, i.e., ryegrass (Lilium perenne), barnyard grass (Echinochloa crus-galli (L.) P. Beauv.), and crabgrass (Digitaria sanguinalis (L.) Scop.), to menthalactone was assessed. Menthalactone at 1000 µM completely inhibited the germination of all three species of grasses, while 330 µM inhibited germination by less than 50%. The post-emergence application of menthalactone at 1% did not produce a significant inhibitory effect against ryegrass, barnyard grass, or crabgrass.
1. Introduction
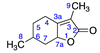
The management of invasive weed species presents major ecological, economic, and agronomic challenges [1]. Their adaptability, ability to form hybrids, and varied ecological impacts make noxious monocot weed species a significant agricultural pest. In the broader context of the agricultural industry, the relentless spread of invasive weeds like barnyard grass (E. crus-galli) significantly impacts rice crop yields and agricultural practices. The adaptability of species like A. stolonifera coupled with their hybridization potential complicate their management and control [2]. This difficulty is magnified by the current crisis faced by the agricultural sector, in which weeds have developed resistance to conventional herbicides, leading to a drastic reduction in effective management tools. Furthermore, the delay in the development and introduction to the market of newly discovered herbicides with novel mechanisms of action over the past two decades has amplified this issue [3]. The status quo of relying on synthetic herbicides, often mere reiterations of older substances, has proven to be a double-edged sword. While these chemicals may offer immediate, potent action against undesirable plants, their long-term impact often includes harmful environmental repercussions, the development of resistant weed strains, and the disruption of ecological balance [1]. This unsustainable trajectory highlights the necessity for a paradigm shift in pest management strategies within the agricultural realm. Amid this, the discovery and utilization of natural products in developing new herbicides offers a viable alternative for novel modes of action [3]. One promising candidate for this approach is menthalactone or mintlactone (3,6-dimethyl-5,6,7,7a-tetrahydro-1-benzofuran-2(4H)-one, CAS Number 13341-72-5), a compound isolated from M. piperita [4]. This compound’s unique structural characteristics, revealed through detailed spectroscopic analysis, contribute to its biological activity, presenting potential beyond its current pharmaceutical applications.
Lactones are an abundant and structurally diverse group of natural products with numerous biological activities. Their unique potential is utilized in many industries and sectors. The characteristic fruit aroma is attributed mainly to γ- and δ-lactones detected as major components, and, therefore, lactones have found particular attention in the food industries as flavoring agents [5]. Also, the pharmaceutical industry intensively exploits these natural products as well as their synthetic analogues which possess anticancer, antibacterial, antifungal, and antimalarial properties [6,7,8,9]. Furthermore, the allelopathic properties of numerous plant lactones, especially sesquiterpenes, are attractive within the agricultural sector as promising solutions for sustainable pest management [5,10].
Peaches (Prunus persica (L.) Batsch), apricots (Prunus armeniaca L.), and nectarines (P. persica var nectarina), representative fruits of the Rosaceae family, are commercially involved in an important industrial market. The pleasant flavor of these fruits attracted the attention of flavorant research early on [11,12,13,14], with a total of 86 peaks in the chromatogram of the tree ripe peach volatiles with gas–liquid chromatography. Major components of the volatiles were mainly identified as γ-and δ-lactones like γ-valerolactone, γ-octalactone, γ-decalactone, δ-decalactone, γ-dodecalactone, and δ-dodecalactone [15]. Several lactones, including γ-caprolactone, γ-octalactone, γ-decalactone, and δ-decalactone, were also reported in the analysis of the volatiles in another peach species [16].
In this study, we introduce the characteristics and phytotoxic capabilities of menthalactone isolated from M. piperita along with ten other natural lactones that were selected for a preliminary structure activity relationship (SAR) evaluation. The possibility of a selective herbicide that targets monocot species without affecting other beneficial flora could significantly advance sustainable agriculture.
2. Experimental Section
2.1. General Experimental Procedures
The 1H and 13C NMR spectra of the pure compounds 1–11 were recorded in CDCl3 on a Bruker 400/500 MHz spectrometer operating at 400, or 500 MHz for 1H NMR and 100 or 150 MHz for 13C NMR. Chemical shift (δ) values are presented in ppm and in reference to the residual solvent signals of CDCl3 at 7.25/70.2. Coupling constants (J) are reported in Hz.
LC analysis of 1–11 was conducted using an Agilent 1100 HPLC system (Santa Clara, CA, USA), an RP-C18 column (150 × 4.6 mm; particle size 5 μm; Luna) with column oven temperature set at 25 °C, and a gradient system of eluent water (A) and acetonitrile (B). The gradient conditions were 0–2 min (5% B), 2–5 min (5% B→50% B), 5–10 min (50% B→100% B), 10–15 min (100% B). The flow rate of the solvent was 1.0 mL/min, and the injection volume was 25 μL. All analyses were carried out at wavelengths of 220/254 nm with run times of 15 min. HPLC-grade acetonitrile and water were used as solvents. Acetic acid was added as a modifier to achieve a final concentration of 0.1% in each solvent.
Other common chromatographic techniques, such as thin-layer chromatography (TLC) on precoated silica gel G254 aluminum plates and silica gel flash column chromatography, were also engaged in the purification of the synthesized compounds.
2.2. Chemicals
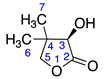
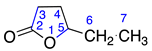
Menthalactone (1) was purchased from Sigma Aldrich (Burlington, MA, USA) with purity ≥ 99%. γ-nonalactone (3), δ-tetradecalactone (4), δ-undecalactone (5), γ-octalactone (6), and γ-valerolactone (7) were purchased from TCI AMERICA (Portland, OR, USA) with purity ≥ 98%. δ-dodecalactone (2), δ-hexalactone (8), γ-undecalactone (9), D-(-)-pantolactone (10), and γ-hexalactone (11) were purchased from Thermo Fisher Scientific (Waltham, MA, USA) with purity ≥ 98%. All compounds were authenticated using LC/MS and NMR data.
2.2.1. Menthalactone (1)
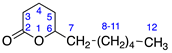
Identified from M. piperita and purchased commercially from Sigma Aldrich (Burlington, MA, USA) with purity ≥ 99% and authenticated with LC and NMR analysis. 1H-NMR (400 MHz, CDCl3): δ =0.73 (m, 1H, H-6), 0.85 (m, 3H, H-8), 1.60 (s, 3H, H-9), 13C-NMR (100 MHz, CDCl3): δ = 7.46 (C-9), 20.56 (C-8), 24.74 (C-6), 28.96 (C-4), 33.84 (C-5), 41.36 (C-7), 79.24 (C-7a), 118.58 (C-3), 162.08 (C-3a), 174.15 (C=O); (Supplementary Materials S1–S3).
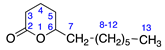
2.2.2. δ-Dodecalactone (2)
Identified from peach (P. persica) and purchased commercially from Thermo Fisher Scientific (Waltham, MA, USA) with purity ≥ 98% and authenticated with LC and NMR analysis. 1H-NMR (400 MHz, CDCl3): δ = 0.89 (m, 3H, H-13), 1.11 (m, 10H, H-8-12), 1.36 (m, 2H, H-7), 1.67 (m, 4H, H-4,5), 2.31 (2H, H-3), 4.11 (m, 1H, H-6); 13C-NMR (100 MHz, CDCl3): δ = 13.89 (C-13), 18.30 (C-4), 22.45 (C-12), 24.47 (C-8), 27.62 (C-5), 29.00 (C-3), 29.22 (C-9), 29.27 (C-10), 31.60 (C-11), 35.69 (C-7), 80.33 (C-6), 171.70 C=O); (Supplementary Materials S4–S6).
2.2.3. γ-Nonalactone (3)
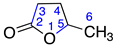
Identified from peach (P. persica) and purchased commercially from TCI AMERICA (Portland, OR, USA) with purity ≥ 98%. 1H-NMR (400 MHz, CDCl3): δ = 0.74 (bt, 3H, H-10), 1.20 (m, 6H, H-7-9), 1.56 (m, 2H, H-6), 2.18 (td, 1H, H-4), 2.37 (m, 2H, H-3), 4.33 (m, 1H, H-5); 13C-NMR (100 MHz, CDCl3): δ = 13.80 (C-10), 22.32 (C-9), 24.79 (C-7), 27.87 (C-3), 28.70 (C-4), 31.28 (C-8), 35.39 (C-6), 80.92 (C-5), 177.16 (C=O); (Supplementary Materials S7–S9).
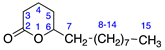
2.2.4. δ-Tetradecalactone (4)
Identified from peach (P. persica) and purchased commercially from TCI AMERICA (Portland, OR, USA) with purity ≥ 98%. 1H-NMR (400 MHz, CDCl3): δ = 0.79 (m, 3H, H-15), 1.13 (m, 14H, H-8-14), 1.41 (m, 2H, H-7), 1.81 (m, 4H, H-4,5), 2.39 (2H, H-3), 4.19 (m, 1H, H-6); 13C-NMR (100 MHz, CDCl3): δ = 14.04 (C-15), 18.44 (C-4), 22.61 (C-14), 24.88 (C-8), 27.74 (C-5), 29.24 (C-12), 29.37 (C-11), 29.41 (C-10), 29.44 (C-9), 35.80 (C-7), 80.55 (C-6), 171.94 (C=O); (Supplementary Materials S10–S12).
2.2.5. δ-Undecalactone (5)
Identified from peach (P. persica) and purchased commercially from TCI AMERICA (Portland, OR, USA) with purity ≥ 98%. 1H-NMR (400 MHz, CDCl3): δ = 0.68 (m, 3H, H-12), 1.09 (m, 8H, H-8-11), 1.34 (m, 2H, H-7), 1.49 (m, 4H, H-4,5), 2.31 (2H, H-3), 4.09 (m, 1H, H-6); 13C-NMR (100 MHz, CDCl3): δ = 13.87 (C-12), 18.30 (C-4), 22.38 (C-11), 24.72 (C-8), 27.62 (C-5), 28.92 (C-3), 29.27 (C-9), 31.60 (C-10), 35.68 (C-7), 80.33 (C-6), 171.73 C=O); (Supplementary Materials S13–S15).
2.2.6. γ-Octalactone (6)
Identified from peach (P. persica) and purchased commercially from TCI AMERICA (Portland, OR, USA) with purity ≥ 98%. 1H-NMR (400 MHz, CDCl3): δ = 0.70 (bt, 3H, H-9), 1.20 (m, 4H, H-7-8), 1.46 (m, 2H, H-6), 2.13 (m, 1H, H-4), 2.30 (m, 2H, H-3), 4.27 (m, 1H, H-5); 13C-NMR (100 MHz, CDCl3): δ = 13.72 (C-9), 22.25 (C-8), 27.20 (C-7), 27.82 (C-3), 28.64 (C-4), 35.06 (C-6), 80.85 (C-5), 177.10 (C=O); (Supplementary Materials S16–S18).
2.2.7. γ-Valerolactone (7)
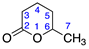
Identified from peach (P. persica) and purchased commercially from TCI AMERICA (Portland, OR, USA) with purity ≥ 98%. 1H-NMR (400 MHz, CDCl3): δ = 1.00 (bd, 3H, H-6), 1.46, 2.00 (m, 2H, H-4), 2.14 (m, 2H, H-3), 4.25 (q, 1H, H-5); 13C-NMR (100 MHz, CDCl3): δ = 20.05 (C-6), 28.62 (C-3), 29.24 (C-4), 76.85 (C-5), 176.87 (C=O); (Supplementary Materials S19–S21).
2.2.8. δ-Hexalactone (8)
Identified from peach (P. persica) and purchased commercially from Thermo Fisher Scientific (Waltham, MA, USA) with purity ≥ 98% and authenticated with LC and NMR analysis. 1H-NMR (400 MHz, CDCl3): δ = 1.00 (d, 3H, H-7), 1.17, 1.56 (m, 4H, H-4,5), 2.14 (2H, H-3), 4.11 (m, 1H, H-6); 13C-NMR (100 MHz, CDCl3): δ = 18.13 (C-7), 21.32 (C-4), 28.85 (C-5), 29.18 (C-3), 76.45 (C-6), 171.45 C=O); (Supplementary Materials S22–S24).
2.2.9. γ-Undecalactone (9)
Identified from peach (P. persica) and purchased commercially from Thermo Fisher Scientific (Waltham, MA, USA) with purity ≥ 98% and authenticated with LC and NMR analysis. 1H-NMR (400 MHz, CDCl3): δ = 0.70 (m, 3H, H-12), 1.13 (m, 10H, H-7-11), 1.66 (m, 2H, H-6), 2.16 (m, 1H, H-4), 2.33 (m, 2H, H-3), 4.30 (m, 1H, H-5); 13C-NMR (100 MHz, CDCl3): δ = 13.88 (C-12), 22.45 (C-11), 25.11 (C-7), 27.87 (C-3), 28.65 (C-4), 28.99 (C-9) 29.19 (C-10), 31.58 (C-8), 35.42 (C-6), 80.85 (C-5), 177.05 (C=O); (Supplementary Materials S25–S27).
2.2.10. D-(-)-Pantolactone (10)
Identified from Nicotiana tabacum L. and purchased commercially from Thermo Fisher Scientific (Waltham, MA, USA) with purity ≥ 98% and authenticated with LC and NMR analysis. 1H-NMR (400 MHz, CDCl3): δ = 1.02 (m, 3H, H-6), 1.17 (m, 3H, H-7), 3.96 (m, 2H, H-5), 4.16 (s, 1H, H-3); 13C-NMR (100 MHz, CDCl3): δ = 18.85 (C-6), 22.73 (C-7), 40.76 (C-4), 75.61 (C-3), 76.50 (C-5), 178.21 (C=O); (Supplementary Materials S28–S30).
2.2.11. γ-Hexalactone (11)
Identified from peach (P. persica) and purchased commercially from Thermo Fisher Scientific (Waltham, MA, USA) with purity ≥ 98% and authenticated with LC and NMR analysis. 1H-NMR (400 MHz, CDCl3): δ = 0.98 (bt, 3H, H-7), 1.73 (m, 2H, H-6), 2.31 (m, 2H, H-4), 2.51 (m, 2H, H-3), 4.41 (m, 1H, H-5); 13C-NMR (100 MHz, CDCl3): δ = 9.44 (C-7), 27.50 (C-6), 28.51 (C-4), 28.87 (C-3), 31.28 (C-8), 82.20 (C-5), 177.31 (C=O); (Supplementary Materials S31–S33).
2.3. Phytotoxicity Bioassays
2.3.1. The Pre-Emergence Selection Test of Extracts and Pure Compounds
The phytotoxic activities of 70% EtOH extracts of many plant species including M. piperita, Ipomoea batatas (L.) Lam., Paeonia suffruticosa Andrews, Platanus occidentalis L., Glycyrrhiza glabra L., Laurus nobilis L., Allium sativum L., and the pure compounds 1–11 were assessed against the seeds of monocot bentgrass (A. stolonifera—Penncross variety, Turf-Seed Inc., Hubbard, OR, USA) and dicot lettuce (L. sativa—Iceberg A crisphead cultivar, Burpee Seeds, Warminster, PA, USA) according to published methods [1]. The lettuce and bentgrass seeds were surface sterilized with 2.5% (v/v) sodium hypochlorite solution for 10 min, rinsed with deionized water, and dried in a sterile environment. Each well of a 24-well plate with a filter paper disk (Whatman Grade 1, Cytiva, Marlborough, MA, USA) contained five lettuce seeds or 10 mg of bentgrass seeds. Extracts were dissolved in acetone to make 10 mg/mL of stock solution and pure compounds were dissolved in acetone to a concentration of 10 mM. Water was added to achieve the final concentration of acetone of 10%. The 200 μL of extract or 1 mM compound solution was transferred to each well; then, the plate was sealed with Parafilm. The concentration of 1 mM acifluorfen was used as a positive control (CP) while wells with negative controls contained only water (negative control (C)) or water and 10% acetone (solvent control (CS)). The plates were incubated for seven days in a Percival Scientific CU-36L5 (Perry, IA, USA) growth chamber under 16 h day and 8 h night conditions at 24 °C and 120 μmol∙m−2∙s−1 average photosynthetically active radiation (PAR). A qualitative estimation of the phytotoxicity was scored based on the germination assessment where a rating of zero stands for no effect and five for maximum effect, i.e., no germination of the seeds. Each condition was repeated in three wells.
2.3.2. A Dose Response Bioassay of Lemna paucicostata
A bioassay to test the phytotoxicity of pure compounds against duckweed (L. paucicostata) was carried out using the method by Michel et al. [17]. The replicate series tests were conducted using non-pyrogenic polystyrene and sterile six-well plates (CoStar 3506, Corning Incorporated, Corning, NY, USA). Each well contained 4950 μL of Hoagland’s media and 50 μL of water, or the solvent, or the compound dissolved in acetone. Each well was inoculated with two three-frond plants of similar size and incubated in the Percival Scientific CU-36L5 growth chamber at 24 °C under 16 h of light and 8 h of dark conditions and 120 μmol∙m−2∙s−1 PAR. Each treatment was replicated three times. The surface area of the fronds was determined with an image analyzer, the LabScanalyzer (LemnaTec, Aachen, Germany). The half-maximal inhibitory concentration (IC50) values, expressed as a percentage of frond growth area, were determined with support of the drc package using the R 4.2.1 software [18].
2.3.3. A Dose Response Bioassay of A. stolonifera
The conditions of the germination test are described in Section 2.3.1. (Pre-emergence selection test of extracts and pure compounds). The only difference was that seeds were exposed to different concentrations of the same compounds: 1000 μM, 330 μM, 33 μM, 10 μM, 3.3 μM, 1 μM, 0.33 μM, solvent and negative controls. The plates were transferred to the Percival Scientific CU-36L5 growth chamber at 24 °C under 16 h of light and 8 h of dark conditions and 120 μmol∙m−2∙s−1 PAR. After 7 days the number of the germinated seeds was counted. The IC50 value was determined based on the germination rate using R 4.2.1 software with the drc package.
2.3.4. The Pre-Emergence Test with Selected Weed Species
The seeds were surface sterilized with 2.5% (v/v) sodium hypochlorite solution for 10 min, rinsed thoroughly four times with deionized water, and dried in a sterile environment. The 24 sterilized seeds were placed on sterile paper filters (Whatman Grade 1, Cytiva, Marlborough, MA, USA) into Petri dish plates. Three plates per treatment were utilized. Four milliliters of the solution were transferred onto the plate. Acetochlor was used as a control herbicide. The sealed-with-Parafilm® plates were placed in the growth chamber, Percival Scientific CU-36L5. Since the selected weed species varied in germinability, their germination rates were measured at different time points, viz., DAT7 ryegrass (L. perenne), DAT10 barnyard grass (E. crus-galli), and DAT14 crabgrass (D. sanguinalis). Two concentrations of menthalactone were tested, 1000 µM and 330 µM. The experiment was repeated twice.
2.3.5. The Pre-Emergence and Post-Emergence Bioassay with Selected Weed Species in Soil
10 × 10 cm pots were filled with soil (Miracle-Gro® Potting Soil Mix, Scotts Miracle-Gro, Marysville, OH, USA) and watered from the bottom tray. When the soil was moist 24 seeds of monocot weed (ryegrass, crabgrass, and barnyard grass) or 20 mg of bentgrass seeds were sown. The trays with pots were placed in a growth chamber with established growth conditions at 16 h light and 8 h night at a temperature of 20 °C. Twenty-four hours later seeds were sprayed with a solution of 1% acetone and 0.5% Tween® 20 with 3% menthalactone or with 2 mM acetochlor as a positive control or with a solution containing only 1% acetone and 0.5% Tween 20 as a negative control.
2.4. Postemergence Application against Sorghum halepense L. Pers., Lolium multiflorum L., and E. crus-galli
2.4.1. Seeds and Growth of Plants
Barnyardgrass (E. crus-galli) seeds were obtained from Dr. David Gealy (USDA Dale Bumpers National Rice Research Center, Stuttgart, Arkansas); Italian ryegrass (L. multiflorum) seeds were purchased from Scotts Turf Builders Grass Seed, Marysville, OH; and Johnson grass (S. halepense) seeds were purchased from Azlin Seed Service. For all experiments, plots were set up using 10 cm diameter plastic pots in trays without drainage holes and watered as needed from the bottom throughout the course of the experiments. Plants were grown in a greenhouse at ambient temperatures with supplemental lighting to provide 16 h daylight per day. Pots were each densely seeded with 500 mg of seeds per pot.
2.4.2. Postemergence Activity Assay, Calculations, and Statistics
Spraying of treatments and controls was performed when plants reached 6 cm in height. Test solutions consisted of a water control containing 0.3% surfactant (Southern AG Surfactant for Herbicides, a.i. alkyl aryl polyoxyethylene glycol), glyphosate (2%), and a test sample of 1% menthalactone (+0.3% surfactant). Surfactant was used as an aid to dissolve menthalactone. All treatments were performed in triplicate. Photos were taken on day 0 and day 7. Image areas were determined on day 0 and day 7 as described below and a percentage change in area determined for each individual plant. The plant growth was assessed through analysis with ImageJ 1.53e software. Triplicates were averaged and the standard error of the mean was determined and reported in Table 1. One-way ANOVA with post hoc Tukey HSD Test was performed. Different letters in columns indicate significant differences.

Table 1.
Phytotoxic activity of selected natural lactones.
3. Results
3.1. Bioassay-Guided Identification of Phytotoxic Components—Pre-Emergence Bioassay
In the preliminary study, the phytotoxic activity of several extracts originating from various plant species was assessed. Their germination inhibition potential was tested against lettuce and A. stolonifera seeds. The extract from M. piperita was the most effective one as it entirely inhibited emerging bentgrass seeds at a concentration of 1 mg/mL. The other extracts did not suppress germination or cause visible morphological/phenotypical changes in emerging seedlings in either tested species compared to a negative control (Figure 1). The M. piperita extract exhibited a selective phytotoxicity against monocots, while dicot lettuce did not respond notably to the treatment with any phenotypical alterations.
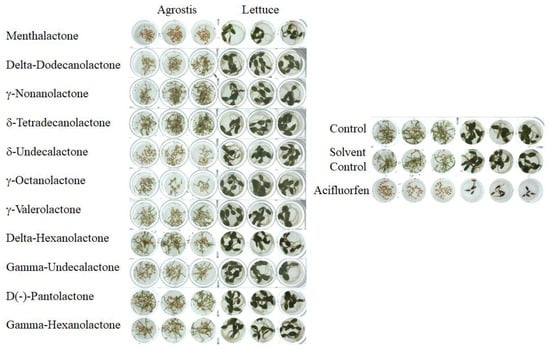
Figure 1.
The results of the pre-emergence bioassay evaluating phytotoxicity of lactones against seeds of A. stolonifera and L. sativa. The seeds germinated in the presence of 1000 µM concentration of compounds. Acifluorfen (1000 µM) was used as the positive control.
3.2. Phytotoxicity of Selected Lactones
Based on the excellent phytotoxic activity of menthalactone, ten other natural lactones were selected for a primary bioassay (Table 1, Figure 1). These compounds, especially δ-undecalactone and γ-octalactone, maintained a relatively good selectivity towards monocots with no activity against the dicot (Figure 1).
3.3. Dose–Response Analysis of Menthalactone against A. stolonifera and L. pausicostata
Since, compared to the other compounds we tested, menthalactone displayed a very good activity against the monocot bentgrass, it was selected for tests to elucidate its phytotoxic characteristics in more detail. The germination of A. stolonifera seeds in various concentrations of menthalactone was significantly inhibited and the determined IC50 value was 4.9 ± 1.2 µM. In contrast to the results above, the L. pausicostata plants had a lesser response to menthalactone with a half-maximal inhibitory concentration equal to 293.4 ± 70.6 µM. Acifluorfen was used as the positive control in both bioassays (Table 2).

Table 2.
Germination inhibition of A. stolonifera and L. pausicostata in menthalactone and in the control.
3.4. Phytotoxicity Bioassay In Vitro against Selected Weeds
The susceptibility of three common obnoxious weed species, i.e., ryegrass (L. perenne), barnyard grass (E. crus-galli), and crabgrass (D. sanguinalis), to menthalactone was assessed. While a 1000 µM concentration of menthalactone completely hampered the germination of all three species of grass, 330 µM was less effective, inhibiting germination at less than 50% (Figure 2 and Figure 3).
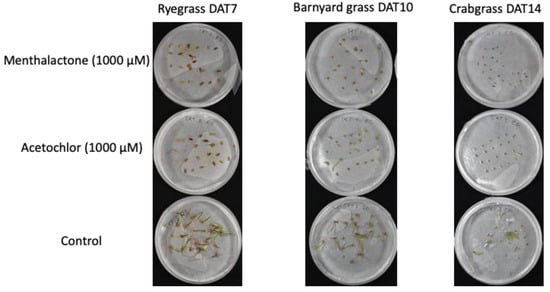
Figure 2.
Pre-emergence effect of menthalactone on seeds of selected weed species.
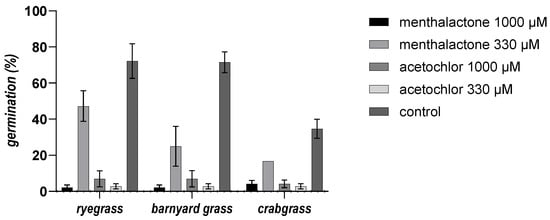
Figure 3.
The germination percentage (±SE) of ryegrass, barnyard grass, and crabgrass exposed to menthalactone and acetochlor with 1000 and 330 µM concentrations.
3.5. Pre-Emergence Bioassay in Soil
The evaluation of the menthalactone’s pre-emergence activity against selected weed seeds sown in pots with soil did not validate its phytotoxic properties. The treated seeds germinated with identical speed as control and seedlings did not display any physical distortions.
3.6. Post-Emergence Application against Sorghum halepense, Lolium multiflorum, and E. crus-galli
In the phytotoxicity assessment of 1% menthalactone against young seedlings of Johnson grass, rye grass, and barnyard grass, no substantial growth inhibition was noted (Table 3).

Table 3.
Postemergence application of the effectiveness of water, glyphosate, and menthalactone against S. halepense, L. multiflorum, and E. crus-galli at day 7.
4. Discussion
Menthalactone is a fragrance and flavor ingredient with reported uses in many cosmetics and personal care products. Its other biological activities are not well studied.
A. stolonifera and L. pausicostata, both monocots, have been proven to be sensitive to menthalactone, implying that this compound negatively affects seed germination. Furthermore, the results indicated that menthalactone does not dramatically impact the metabolism of the green tissues of duckweed or sprayed grass seedlings. Pre-emergent herbicides can be defined as those which inhibit germination following their application on soil and absorption by seeds and emerging seedling’s parts, i.e., hypocotyls, cotyledons, and coleoptiles [19]. Our results strongly suggest that menthalactone has pre-emergent activity―possibly specific targeting and biochemical properties that are favored by emerging seedlings. Grasses are usually a better target for pre-emergence herbicides due to their phenotype allowing compounds to penetrate seedling more easily, although the level of vulnerability depends on monocot species [19]. We observed this phenomenon as bentgrass appeared to be the most sensitive of the group of grasses tested.
In all of the bioassays conducted in vitro, menthalactone displayed a very good level of bioactivity, although, in the soil experiments, even a 1% treatment did not significantly inhibit germination.
One of the reasons that nature keeps seeds in a dormant state is that a pause is needed for the environment to reach optimal conditions that are favorable for successful germination and maturation to the seed propagation stage. Dormancy delay times are controlled by an array of phytochemicals. Such molecules can be endogenic and biosynthesized within a seed or exogenic. An interesting example is a naturally occurring in plant-derived smoke trimethylbutenolide (TMB) which efficiently inhibits seed germination by increasing the ABA activity and reducing the enzymatic activity of hydrolases, e.g., amylases [20]. Moreover, the current findings revealed that Ras-kinase participates in the dormancy regulation as a mediator of TMB-dependent signal transduction [21]. Based on the data above and our results for the inhibitory attributes of menthalactone, we selected a set of volatile lactones biosynthesized by ripe fruits with similar chemical structures to menthalactone and TMB for further study. To our surprise the phytotoxic activity of the new lactones was significantly lower compared to menthalactone. One of the possible explanations for this could be the age and storage of the seeds used in our experiments. The inhibitory activity of TMB depends on how long a seed has been mature for (only freshly maturated seeds are sensitive to TMB exposure) [21].
A recent study has addressed the genotoxic potential of menthalactone, in vitro and in vivo. The in vivo data were positive for the livers of female mice but negative for male mice. However, the in vitro, in vivo micronucleus tests, and 3D skin comet/micronucleus tests revealed no chromosomal destruction [22]. Due to such variations in the data, the safe use of menthalactone in fragrances cannot be proven, and, in fact, many chemical companies have stopped producing this product and it is currently rarely found in the market.
5. Conclusions
Menthalactone, a natural lactone derived from M. piperita, demonstrated remarkable activity against the monocot, bentgrass. The germination of A. stolonifera seeds was significantly inhibited with an IC50 value of 4.9 ± 1.2 µM. In contrast to the bentgrass seeds, another monocot, L. pausicostata, did not respond as strongly to menthalactone with an IC50 of 293.4 ± 70.6 µM. These results suggest that menthalactone has a damaging effect on seed germination but does not effectively impact the metabolism of green tissues. The susceptibility of three common obnoxious weed species, i.e., ryegrass (L. perenne), barnyard grass (E. crus-galli), and crabgrass (D. sanguinalis), to menthalactone was assessed. Menthalactone at 1000 µM entirely hindered the germination of all three species of grass seeds, while 330 µM was less effective with a less than 50% inhibition of germination. Post-emergence application of menthalactone at 1% did not have a significant impact on ryegrass, barnyard grass, or crabgrass.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijpb15020025/s1, NMR spectra of the isolated compound 1 and generated compound 1–11.
Author Contributions
Conceptualization, M.A.I., A.S., Z.M.A.I., J.B.-H., C.L.C., J.V.C. and I.A.K.; methodology, M.A.I., A.S., M.O., Z.M.A.I. and J.B.-H.; software, M.O., J.B.-H. and M.A.I.; validation, M.A.I., C.L.C. and J.B.-H.; formal analysis, A.S., M.O., Z.M.A.I., J.B.-H. and M.A.I.; investigation A.S., M.O., Z.M.A.I., J.B.-H. and M.A.I.; resources, J.B.-H., I.A.K., C.L.C. and M.A.I.; data curation, M.A.I., C.L.C. and J.B.-H.; writing—original draft preparation, A.S., M.O., J.B.-H. and M.A.I.; writing—review and editing, M.A.I., M.O., A.S., J.B.-H. and C.L.C.; supervision, J.B.-H. and M.A.I.; funding acquisition, I.A.K., C.L.C. and M.A.I. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by "Discovery and Development of Natural Product-Based Pesticides and Pharmaceuticals" funded by the United States Department of Agriculture, Agricultural Research Service, Specific Cooperative Agreement No. 58-6060-1-001.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data relevant to the paper will be available from the authors upon request.
Acknowledgments
The authors are thankful to Jeff Solomon and Hadi Obaji for proofreading the final version of the manuscript. A. Soltani is thankful to NCNPR and USDA-ARS for such a training opportunity.
Conflicts of Interest
The authors declare no competing financial interests.
References
- Stefanski, F.S.; Camargo, A.F.; Scapini, T.; Bonatto, C.; Venturin, B.; Weirich, S.N.; Ulkovski, C.; Carezia, C.; Ulrich, A.; Michelon, W.; et al. Potential use of biological herbicides in a circular economy context: A sustainable approach. Front. Sustain. Food Syst. 2020, 4, 521102. [Google Scholar] [CrossRef]
- Macbryde, B. White Paper: Perspective on Creeping Bentgrass, Agrostis stolonifera L.; USDA/APHIS/BRS: Riverdale, MD, USA, 2006. [Google Scholar]
- Dayan, F.E.; Owens, D.K.; Duke, S.O. Rationale for a natural products approach to herbicide discovery. Pest Manag. Sci. 2012, 68, 519–528. [Google Scholar] [CrossRef]
- Shigeto, A.; Wada, A.; Kumazawa, K. Identification of the novel odor active compounds “p-menthane lactones” responsible for the characteristic aroma of fresh peppermint leaf. Biosci. Biotechnol. Biochem. 2020, 84, 421–427. [Google Scholar] [CrossRef]
- Tamura, H.; Appel, M.; Richling, E.; Schreier, P. Authenticity assessment of γ-and δ-decalactone from Prunus fruits by gas chromatography combustion/pyrolysis isotope ratio mass spectrometry (GC-C/P-IRMS). J. Agric. Food Chem. 2005, 53, 5397–5401. [Google Scholar] [CrossRef] [PubMed]
- Sartori, S.K.; Diaz, M.A.N.; Diaz-Munoz, G. Lactones: Classification, synthesis, biological activities, and industrial applications. Tetrahedron 2021, 84, 132001. [Google Scholar] [CrossRef]
- Dhyani, P.; Sati, P.; Sharma, E.; Attri, D.C.; Bahukhandi, A.; Tynybekov, B.; Szopa, A.; Sharifi-Rad, J.; Calina, D.; Suleria, H.A.R.; et al. Sesquiterpenoid lactones as potential anti-cancer agents: An update on molecular mechanisms and recent studies. Cancer Cell Int. 2022, 22, 305. [Google Scholar] [CrossRef] [PubMed]
- Wedge, D.E.; Galindo, J.C.G.; Macıas, F.A. Fungicidal activity of natural and synthetic sesquiterpene lactone analogs. Phytochemistry 2000, 53, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Bridgford, J.L.; Xie, S.C.; Cobbold, S.A.; Pasaje, C.F.A.; Herrmann, S.; Yang, T.; Gillett, D.L.; Dick, L.R.; Ralph, S.A.; Dogovski, C.; et al. Artemisinin kills malaria parasites by damaging proteins and inhibiting the proteasome. Nat. Commun. 2018, 9, 3801. [Google Scholar] [CrossRef] [PubMed]
- Scavo, A.; Mauromicale, G. Crop allelopathy for sustainable weed management in agroecosystems: Knowing the present with a view to the future. Agronomy 2021, 11, 2104. [Google Scholar] [CrossRef]
- Takeoka, G.R.; Flath, R.A.; Guentert, M.; Jennings, W. Nectarine volatiles: Vacuum steam distillation versus headspace sampling. J. Agric. Food Chem. 1988, 36, 553–560. [Google Scholar] [CrossRef]
- Takeoka, G.R.; Flath, R.A.; Mon, T.R.; Teranishi, R.; Guentert, M. Volatile constituents of apricot (Prunus armeniaca). J. Agric. Food Chem. 1990, 38, 471–477. [Google Scholar] [CrossRef]
- Horvat, R.J.; Chapman, G.W., Jr.; Robertson, J.A.; Meredith, F.I.; Scorza, R.; Callahan, A.M.; Morgens, P. Comparison of the volatile compounds from several commercial peach cultivars. J. Agric. Food Chem. 1990, 38, 234–237. [Google Scholar] [CrossRef]
- Narain, N.; Hsieh, T.C.Y.; Johnson, C.E. Dynamic headspace concentration and gas chromatography of volatile flavor components in peach. J. Food Sci. 1990, 55, 1303–1307. [Google Scholar] [CrossRef]
- Do, J.Y. Isolation, Identification, and Comparison of the Volatiles of Peach (Prunus persica L., Cultivar, Gleason Early Elberta) Fruit as Related to Harvest Maturity and Artificial Ripening. In All Graduate Theses and Dissertations; Utah State University: Logan, UT, USA, 1968; p. 5104. [Google Scholar]
- Jennings, W.G.; Sevenants, M.R. Volatile Esters of Bartlett Pear. III. J. Food Sci. 1964, 29, 158–163. [Google Scholar] [CrossRef]
- Michel, A.; Johnson, R.D.; Duke, S.O.; Scheffler, B.E. Dose-response relationships between herbicides with different modes of action and growth of Lemna paucicostata: An improved ecotoxicological method. Environ. Toxicol. Chem. Int. J. 2004, 23, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Dayan, F.E.; Romagni, J.G.; Duke, S.O. Investigating the mode of action of natural phytotoxins. J. Chem. Ecol. 2000, 26, 2079–2094. [Google Scholar] [CrossRef]
- Krähmer, H.; Walter, H.; Jeschke, P.; Haaf, K.; Baur, P.; Evans, R. What makes a molecule a pre-or a post-herbicide–how valuable are physicochemical parameters for their design? Pest Manag. Sci. 2021, 77, 4863–4873. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Plačková, L.; Kulkarni, M.G.; Doležal, K.; Van Staden, J. Role of smoke stimulatory and inhibitory biomolecules in phytochrome-regulated seed germination of Lactuca sativa. Plant Physiol. 2019, 181, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Kim, E.; Choi, S.; Kim, D.; Hong, W.; Choi, J.; Choi, H.; Kim, J.; Sable, G.A.; Markkandan, K.; et al. A Raf-like kinase is required for smoke-induced seed dormancy in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2021, 118, e2020636118. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, Y.; Moustakas, H.; Api, A.M.; Smith, B.; Williams, G.; Greim, H.; Eisenbrand, G.; Dekant, W. Assessment of the genotoxic potential of mintlactone. Food Chem. Toxicol. 2022, 159, 112659. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).