Evaluating Growth and Physiological Responses of a Medicinal Plant Phyla nodiflora to Salinity
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Salt Treatment
2.3. Biomass Measurement
2.4. Determination of Chlorophyll Content
2.5. Determination of Proline and MDA Contents
2.6. Determination of EL and Ion Content
2.7. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Effects of Salinity on Biomass of Shoots
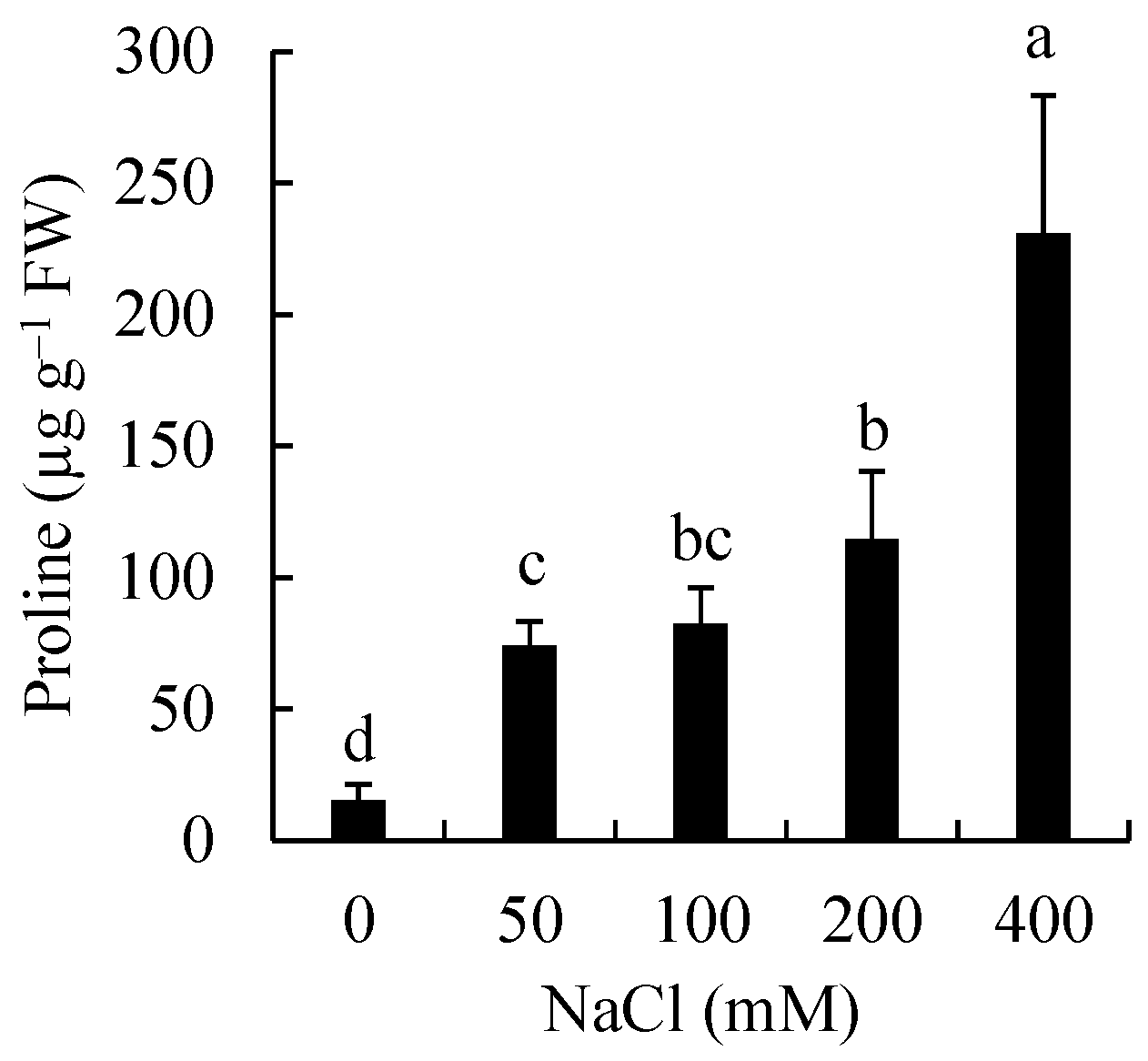
3.2. Effects of Salinity on Proline Accumulation
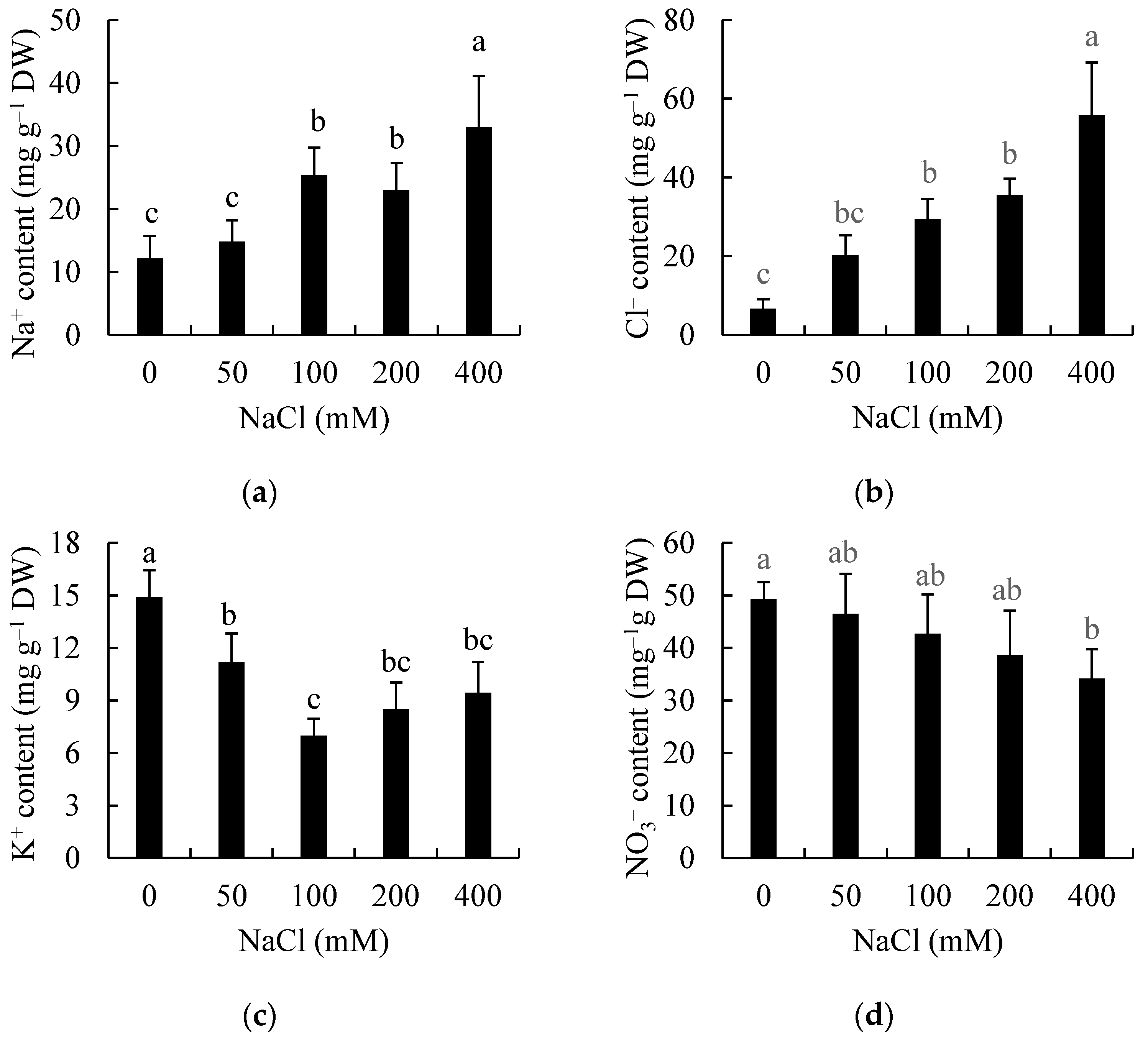
3.3. Effects of Salinity on Ion Accumulation
3.4. Effects of Salinity on Chlorophyll Content
3.5. Effects of Salinity on Lipid Peroxidation and Membrane Damage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abobatta, W. Some physiological mechanisms of salt tolerance in the glycophytes plant: Overview. Acta Sci. Agric. 2018, 2, 154–156. [Google Scholar]
- Panta, S.; Flowers, T.; Lane, P.; Doyle, R.; Haros, G.; Shabala, S. Halophyte agriculture: Success stories. Environ. Exp. Bot. 2014, 107, 71–83. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Caparros, P.; Al-Azzawi, M.J.; Flowers, T.J. Economic uses of salt-tolerant plants. Plants 2023, 12, 2669. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.-K.; Shabala, S. Mechanisms of plant responses and adaptation to soil salinity. Innovation 2020, 1, 100017. [Google Scholar] [CrossRef] [PubMed]
- Safdar, H.; Amin, A.; Shafiq, Y.; Ali, A.; Yasin, R.; Shoukat, A.; Hussan, M.U.; Sarwar, M.I. A review: Impact of salinity on plant growth. Nat. Sci. 2019, 17, 34–40. [Google Scholar]
- Taïbi, K.; Taïbi, F.; Abderrahim, L.A.; Ennajah, A.; Belkhodja, M.; Mulet, J.M. Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. S. Afr. J. Bot. 2016, 105, 306–312. [Google Scholar] [CrossRef]
- Kotagiri, D.; Kolluru, V.C. Effect of salinity stress on the morphology and physiology of five different Coleus species. Biomed. Pharmacol. J. 2017, 10, 1639–1649. [Google Scholar] [CrossRef]
- Khan, M.O.; Irfan, M.; Muhammad, A.; Ullah, I.; Nawaz, S.; Khalil, M.K.; Ahmad, M. A practical and economical strategy to mitigate salinity stress through seed priming. Front. Environ. Sci. 2022, 10, 991977. [Google Scholar] [CrossRef]
- Meng, X.; Zhou, J.; Sui, N. Mechanisms of salt tolerance in halophytes: Current understanding and recent advances. Open Life Sci. 2018, 13, 149–154. [Google Scholar] [CrossRef]
- Gross, C.L.; Fatemi, M.; Julien, M.; McPherson, H.; Van Klinken, R. The phylogeny and biogeography of Phyla nodiflora (Verbenaceae) reveals native and invasive lineages throughout the world. Diversity 2017, 9, 20. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, S.; Chen, T. Verbenaceae. Fl. Taiwan 1998, 127, 421. [Google Scholar]
- Jabeen, M.; Jillani, U.; Chaudhary, B.A.; Uzair, M. Phytochemical and pharmacological studies of Phyla nodiflora (Verbenaceae): A review. Pak. J. Pharm. Res. 2016, 2, 49–54. [Google Scholar]
- Parmar, G.R.; Baile, S.B.; Gohel, K.; Shah, A.; Patel, S.; Seth, A.K. An ethnobotanical and pharmacological review on Phyla nodiflora. Int. J. Pharm. Res. 2020, 12, 3667–3673. [Google Scholar]
- Abbas, T.; Ahmad, I.; Khan, Z.I.; Okla, M.K.; Saleh, I.A.; AbdElgawad, H. Comparative physiological adaptations to industrial pollution stress mediated by melatonin in riparian vegetation and Phyla nodiflora an ornamental plant. Sci. Hortic. 2023, 321, 112367. [Google Scholar] [CrossRef]
- Sahin, U.; Ekinci, M.; Ors, S.; Turan, M.; Yildiz, S.; Yildirim, E. Effects of individual and combined effects of salinity and drought on physiological, nutritional and biochemical properties of cabbage (Brassica oleracea var. capitata). Sci. Hortic. 2018, 240, 196–204. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Senthilkumar, M.; Amaresan, N.; Sankaranarayanan, A.; Senthilkumar, M.; Amaresan, N.; Sankaranarayanan, A. Estimation of malondialdehyde (MDA) by thiobarbituric acid (TBA) assay. In Plant-Microbe Interactions; Springer Protocols Handbooks; Springer: New York, NY, USA, 2021; pp. 103–105. [Google Scholar]
- Tran, D.Q.; Konishi, A.; Cushman, J.C.; Morokuma, M.; Toyota, M.; Agarie, S. Ion accumulation and expression of ion homeostasis-related genes associated with halophilism, NaCl-promoted growth in a halophyte Mesembryanthemum crystallinum L. Plant Prod. Sci. 2020, 23, 91–102. [Google Scholar] [CrossRef]
- Gulzar, S.; Khan, M.A.; Ungar, I.A. Salt tolerance of a coastal salt marsh grass. Commun. Soil Sci. Plant Anal. 2003, 34, 2595–2605. [Google Scholar] [CrossRef]
- Said-Al Ahl, H.; Omer, E. Medicinal and aromatic plants production under salt stress. A review. Herba Pol. 2011, 57, 72–87. [Google Scholar]
- De Almeida Cartaxo, P.H.; da Silva, D.G.; Araújo, J.R.E.S.; da Silva, J.H.B.; Targino, V.A.; da Silva Xavier, L.M.; Neto, F.P.; de Oliveira, A.B.; da Silva, A.M. Salinity and medicinal plants: Challenges and strategies for production. Sci. Electron. Arch. 2022, 15, 8–15. [Google Scholar]
- González, L.; González-Vilar, M. Determination of relative water content. In Handbook of Plant Ecophysiology Techniques; Springer: Berlin/Heidelberg, Germany, 2001; pp. 207–212. [Google Scholar]
- Munns, R.; Passioura, J.B.; Colmer, T.D.; Byrt, C.S. Osmotic adjustment and energy limitations to plant growth in saline soil. New Phytol. 2020, 225, 1091–1096. [Google Scholar] [CrossRef]
- Adolf, V.I.; Jacobsen, S.-E.; Shabala, S. Salt tolerance mechanisms in quinoa (Chenopodium quinoa Willd.). Environ. Exp. Bot. 2013, 92, 43–54. [Google Scholar] [CrossRef]
- Flowers, T.J.; Munns, R.; Colmer, T.D. Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Ann. Bot. 2015, 115, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Amiri, B.; Assareh, M.; Rasouli, B.; Jafari, M.; Arzani, H.; Jafari, A. Effect of salinity on growth, ion content and water status of glasswort (Salicornia herbacea L.). Casp. J. Environ. Sci. 2010, 8, 79–87. [Google Scholar]
- Hniličková, H.; Hnilička, F.; Orsák, M.; Hejnák, V. Effect of salt stress on growth, electrolyte leakage, Na+ and K+ content in selected plant species. Plant Soil Environ. 2019, 65, 90–96. [Google Scholar] [CrossRef]
- Kumar, S.; Li, G.; Yang, J.; Huang, X.; Ji, Q.; Liu, Z.; Ke, W.; Hou, H. Effect of salt stress on growth, physiological parameters, and ionic concentration of water dropwort (Oenanthe javanica) cultivars. Front. Plant Sci. 2021, 12, 660409. [Google Scholar] [CrossRef]
- Gong, D.; Wang, G.; Si, W.; Zhou, Y.; Liu, Z.; Jia, J. Effects of salt stress on photosynthetic pigments and activity of ribulose-1, 5-bisphosphate carboxylase/oxygenase in Kalidium foliatum. Russ. J. Plant Physiol. 2018, 65, 98–103. [Google Scholar] [CrossRef]
- Yang, J.; Zheng, W.; Tian, Y.; Wu, Y.; Zhou, D. Effects of various mixed salt-alkaline stresses on growth, photosynthesis, and photosynthetic pigment concentrations of Medicago ruthenica seedlings. Photosynthetica 2011, 49, 275–284. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. The response of salinity stress-induced A. tricolor to growth, anatomy, physiology, non-enzymatic and enzymatic antioxidants. Front. Plant Sci. 2020, 11, 559876. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, A.C.; Vo, T.C.; Bui, T.D.; Van, T.-T.H.; Tran, D.Q. Evaluating Growth and Physiological Responses of a Medicinal Plant Phyla nodiflora to Salinity. Int. J. Plant Biol. 2024, 15, 187-197. https://doi.org/10.3390/ijpb15010015
Pham AC, Vo TC, Bui TD, Van T-TH, Tran DQ. Evaluating Growth and Physiological Responses of a Medicinal Plant Phyla nodiflora to Salinity. International Journal of Plant Biology. 2024; 15(1):187-197. https://doi.org/10.3390/ijpb15010015
Chicago/Turabian StylePham, Anh Cong, Tuan Chau Vo, Thang Duc Bui, Thi-Thao Hien Van, and Dan Quang Tran. 2024. "Evaluating Growth and Physiological Responses of a Medicinal Plant Phyla nodiflora to Salinity" International Journal of Plant Biology 15, no. 1: 187-197. https://doi.org/10.3390/ijpb15010015
APA StylePham, A. C., Vo, T. C., Bui, T. D., Van, T.-T. H., & Tran, D. Q. (2024). Evaluating Growth and Physiological Responses of a Medicinal Plant Phyla nodiflora to Salinity. International Journal of Plant Biology, 15(1), 187-197. https://doi.org/10.3390/ijpb15010015






