Abstract
The excessive use of synthetic chemicals in agriculture demands sustainable alternatives to combat crop-affecting microorganisms. Plant-derived secondary metabolites have garnered attention as promising candidates with antimicrobial properties. This study investigates the antimicrobial potential of tobacco plants, specifically non-commercial accessions Nic 1015 (“TI 1341”) and BHmN, recognized for their rich bioactive compounds. Our objectives encompassed the extraction of leaf surface compounds and the assessment of their in vitro antimicrobial activity against crop-damaging microorganisms. Ethanol-based extracts, abundant in diterpenes, were meticulously analyzed. Notably, BHmN contained cis-abienol, while both accessions featured α-CBT diol, as confirmed by thin-layer chromatography (TLC) and high-performance liquid chromatography (HPLC). TLC-Bioautography and microdilution assays unveiled substantial antifungal activity. The growth inhibition percentages correlated with extract concentrations, highlighting the pivotal role of diterpenes. These extracts exhibited pronounced efficacy against Rhizoctonia solani and Stemphylium solani but displayed relatively weaker activity against Sarocladium oryzae. Notably, Nic 1015 extract demonstrated remarkable antifungal activity at a minimal concentration of 78 µg·mL−1, while cis-abienol and sclareol inhibited the growth of Fusarium graminearum and Alternaria alternata. Additionally, the extracts demonstrated in vitro antibacterial activity against common plant culture contaminants, Bacillus licheniformis and Stenotrophomonas maltophilia. In conclusion, the findings underscore the potential of these extracts as effective tools for controlling pathogenic fungi and bacterial contaminants in plant in vitro cultures. Harnessing plant-derived secondary metabolites, especially those from tobacco leaf surface, presents a sustainable and eco-friendly strategy to mitigate the detrimental impact of microorganisms on agricultural crops, promising a greener alternative to synthetic chemical products.
1. Introduction
Plants are vulnerable to diseases caused by microorganisms, resulting in substantial economic losses in agriculturally important crops, many of which are utilized for human and animal nutrition [1]. The most prevalent method for pest control presently involves the use of synthetic pesticides, which, regrettably, have adverse side effects, including the contamination of water, soil, and plant products. This leads to environmental imbalances and human health issues [1,2]. Additionally, it is essential to consider the potential long-term yield reductions resulting from excessive pesticide use [1]. Annually, an estimated 385 million cases of unintentional pesticide poisoning occur globally, with approximately 11 thousand of these cases being fatal. Given the worldwide population of farmers close to 860 million, this equates to 44.8% of farmers being poisoned each year [3].
One promising alternative to address this issue is the utilization of natural products derived from plants within an integrated management approach. This approach aims to maintain high crop yields while minimizing environmental impact and enhancing food safety [4]. The tobacco plant is well-suited for this purpose, being easy to cultivate and rich in powerful active ingredients with high chemical stability and optimal production [5,6,7]. This versatility makes it not only crucial for the tobacco industry but also highly appealing for alternative uses [6,8,9].
An intriguing feature of tobacco crops is the presence of glandular trichomes on the leaf surface, which produce, store, and secrete various compounds. These compounds contribute to organoleptic properties and the plant’s physiological response to the environment, rendering these structures promising cellular biofactories with diverse applications for humanity [6,7,10,11]. Notably, glandular trichomes on tobacco leaves are involved in the production and secretion of compounds, primarily diterpenes, sucrose esters, waxes, small amounts of volatile compounds [7,12,13], and phylloplanins [14]. Given their location on the leaf surface, these compounds serve as a first line of defense against insects and other pathogens [6,14,15].
Phytopathogenic fungi such as Rhizoctonia solani J.G. Kühn, Stemphylium solani Weber, Sarocladium oryzae, Alternaria alternata, and Fusarium graminearum inflict annual losses on crops of global agricultural significance, including tomatoes, potatoes, rice, and others [16,17]. Among these, R. solani stands out as a significant pathogen with a broad host range, infecting plants across more than 32 families and 188 genera [18].
In plant in vitro culture, bacterial contaminants, including Bacillus licheniformis (gram +) and Stenotrophomonas (Xanthomonas) maltophilia (gram −), frequently hinder normal growth, development of micropropagated plants, and in vitro experiments. S. maltophilia has also emerged as an opportunistic human pathogen, resistant to multiple drugs, isolated from various environmental sources, and in patients with cystic fibrosis and pneumonia, among other infections [19]. Incorporating natural products with antimicrobial activity into the culture medium holds the potential for controlling in vitro culture contamination. Despite the documented antimicrobial properties of plant extracts [8,20,21], few reports exist regarding the inhibitory effects of crude extracts from tobacco leaf surfaces on phytopathogenic fungi and contaminating microorganisms in in vitro culture.
Hence, this research endeavors to obtain bioactive plant extracts from Nicotiana tabacum leaf surfaces, enriched with diterpenes and natural compounds, and to evaluate their in vitro antimicrobial properties. The objective is to provide a potential solution for the control of pathogenic fungi in agriculturally significant crops and the management of bacterial contaminants in plant in vitro cultures.
2. Materials and Methods
2.1. Plant Material
Tobacco extracts were obtained from the leaves of three 115-day-old plants, grown in semi-controlled greenhouse conditions in polyethylene bags with a 6 kg capacity. The growth medium comprised a mixture of soil, filter cake, and zeolite in a 3:2:1 ratio (m:m:m), with the use of carbonated sialitic brown soil. Notably, the collected plant material was carefully selected, considering the plants’ robust phytosanitary status and ensuring it was free from abiotic contamination.
2.2. Extraction and Characterization of Crude Ethanolic Extracts from N. tabacum Leaf Surfaces
To obtain crude ethanolic extracts, two tobacco accessions were selected: BHmN and Nic 1015 (“TI 1341”), with and without the presence of cis abienol, respectively. BHmN is a non-commercial Cuban accession, generously provided by the Center for Genetic Engineering and Biotechnology (CIGB) in Havana. This accession is also available in the Cuban Germplasm Bank of Tobacco. Nic 1015 (“TI 1341”) is a non-commercial accession provided by the Germplasm Bank of the Leibniz Institute of Plant Genetics and Crop Plant Research (IPK-Gatersleben) in Germany.
Crude extracts were obtained from the leaf surfaces of three plants using a modified method based on Severson [22], employing commercial ethanol produced nationally as the extraction solvent [23]. The whole leaves were individually immersed in commercial ethanol eight times for two seconds in a container, the solvent used was a 90% ethanol and 10% water mixture (v:v). The ratio of plant material mass: solvent volume was 1:10 (m:v). The resulting extracts were concentrated using a rotary evaporator at 40 °C and then stored at 4 °C until evaluation. In triplicate, they were dried via rotary evaporation at 50 °C in a Speed Vac SC100 Savant, and the dry residue was weighed. The extraction yield was calculated as the total mass of dry extract in milligrams per gram of fresh leaf weight (mg of dry extract·g of FW−1) used in the extraction process for each tobacco accession.
2.3. Separation and Quantification via Thin Layer Chromatography (TLC) and High-Performance Liquid Chromatography (HPLC)
In the analysis using TLC, silica gel plates (Aluminum sheets 60 F254, 7 × 7.5 cm, MERCK) were employed to determine the presence of major compounds within the crude extracts. The extracts were prepared at a concentration of 20 µg·µL−1, and 10 µL of the extracts from selected accessions Nic 1015, BHmN, and Nic 1003 were applied. Nic 1003 served as a reference for the presence of cis-abienol [24]. α-CBT diol (Sc-360746 Lot K 2311, Santa Cruz Biotechnology, Dallas, TX, USA), sclareol (98%, Sigma Aldrich, St. Louis, MO, USA), and cis-abienol (Cat A 107600 Lot 5-YMK-49-2, Toronto Research Chemicals Inc., 2 Brisbane Road, Toronto, ON, Canada) were prepared as standards at a concentration of 10 µg·µL−1. A solvent system of toluene: hexane: methanol (5:5:1) was employed for the chromatographic runs. Subsequent to the run, the plate was dried for 2–3 min at 60 °C, and then developed with a sulfuric Anisaldehyde solution before drying for 5 min at 100 °C [25]. The developed plates were observed under visible light.
For the HPLC quantification of major compounds, a Velos unit coupled to a diode array detector with ultraviolet (UV) capabilities was utilized. A 10 μL sample was injected with three replicates for each extraction. The running solvents used were A: Water + 5% ACN and B: ACN. A Phenomenex Kinetex 2.6 µ XB-C18 100 A, 150 × 4.6 mm column was used at room temperature with a flow rate of 0.5 mL·min−1. The gradient system was as follows: (A:B)—[0–1 min, 50:50; 1–11 min, 0:100; 11–13 min, 0:100, 13–16 min, 50:50]. The UV spectrum was recorded over a range of 200 to 400 nm (Figure A1). Chromatograms were created at 210 nm for α-CBTdiol quantification [26] and at 238 nm for cis-abienol quantification.
2.4. In Vitro Antifungal Activity of Crude Ethanolic Extracts against Phytopathogenic Fungi TLC-Bioautography and Microdilution Test
2.4.1. TLC-Bioautography
In this experiment, direct bioautography was employed [27]. Phytopathogenic fungi, specifically Alternaria alternata (Alt/7), and Fusarium graminearum (Fus/13), obtained from the Phytopathology Laboratory of the Institute of Phytomedicine, University of Hohenheim, Germany, were utilized as the microorganisms. For the antifungal assays, pure monosporic isolates were cultivated in a potato dextrose agar (PDA) culture medium. The TLC was conducted with similar characteristics as described in Section 2.3, utilizing a solvent system of tert-butyl methyl ether: isooctane: methanol (5:5:1) without staining. Subsequently, the TLC plate was coated with a thin layer of PDA at approximately 50 °C. Following this, a conidial suspension of A. alternata (approximately 2.1∙106 conidia·mL−1) or F. graminearum (approximately 1.7∙106 conidia·mL−1) was applied to the plate. The inoculated TLC plates were then transferred to sterile plastic containers, configured as humid chambers, and sealed to maintain 100% relative humidity. The humid chambers were incubated at 25 ± 2 °C in the dark for 3–4 days in an incubation room until visible fungal growth occurred. This allowed for assessing the presence of growth inhibition zones in the fungi.
2.4.2. Microdilution Test
To assess the antifungal activity of the crude extracts, the microdilution test was conducted as described in reference [27]. Three fungi (Rhizoctonia solani (Rh-63), Stemphylium solani (St-46), and Sarocladium oryzae (Sa-52), were used for the evaluation. The assays were performed in a Potato Dextrose (PD) liquid medium, which consisted of a broth prepared with 200 g of potatoes boiled in 1L of water, and 20 g of dextrose, adjusted to a pH of 7. These pathogens were obtained from the Microbial Culture Collection of the Plant Health Research Institute in Havana City, Cuba. The crude extracts were dissolved in dimethyl sulfoxide (DMSO) (3%) at a concentration of 10 µg·µL−1.
The microdilution method was executed in 96-well ELISA plates. Six serial dilutions of the extracts were prepared, ranging from 2.5 µg·µL−1 to 0.078 µg·µL−1. Each well of the plate received 100 µL of the culture medium. Then, 100 µL of the crude extract solution was added, and further serial dilutions were made. Finally, 100 µL of the pathogen suspension was introduced. The ELISA plates were incubated in the dark at 28 ± 2 °C for 120 h, in an incubation room.
Throughout the experiments, several control samples were used:
- (1)
- A sterility control of the culture medium (200 µL of culture medium).
- (2)
- A growth control for each pathogen (100 µL of culture medium and 100 µL of pathogen suspension).
- (3)
- A solvent control using DMSO (100 µL of culture medium, 100 µL of pathogen, and 100 µL of 3% DMSO with serial dilutions).
The quantification of pathogen growth was determined by measuring the absorbance or optical density at 595 nm (OD595 nm) in the spectrophotometer (Erba Lisa Scan II, Mannheim, Germany) with four replicates for each test. Periodic evaluations were performed every 24 h.
The growth inhibition percentage (GIP) was calculated using the following formula [28]:
where: OD595nm Control: Represents the value of the optical density of control 3 (DMSO solvent control).
GIP = ((OD595nm Control − OD595nm Treatment)/OD595nm Control) * 100
OD595nm Treatment: Represents the value of the difference in optical density for each treatment every 24 h, relative to the optical density obtained at the start of the evaluation (zero hour).
The minimum inhibitory concentration (MIC) was determined as the minimum concentration of the extract required to inhibit fungal growth entirely. The maximum mean concentration (IC50) was estimated as the effective concentration of the extract resulting in a GIP of approximately 50%. This estimation was calculated based on the results obtained from concentrations with GIP values both less than and greater than 50%.
2.5. In Vitro Antibacterial Activity of Crude Ethanolic Extracts against Bacterial Contaminants of In Vitro Culture
The evaluation of the antimicrobial activity against bacterial contaminants in in vitro culture, specifically Bacillus licheniformis (Gram +) and Stenotrophomonas maltophilia (Gram −), was conducted using the in vitro diffusion method [27]. These bacteria had been previously isolated, classified, and provided by the Microbial Culture Collection of the Institute of Plant Biotechnology (IBP) in Villa Clara, Cuba. For the assessment, each bacterial aqueous solution (200 µL) was prepared at an OD620 nm equal to 0.1 determined in the spectrophotometer (Erba Lisa Scan II, Mannheim, Germany). These solutions were added to separate Petri dishes in triplicate, and each dish contained a semisolid culture medium known as Luria Bertani (LB). The LB medium comprised 1 L containing 5 g of yeast extract, 10 g of tryptone, 10 g of sodium chloride, and 15 g of agar, with a pH adjusted to 7.
The tests were conducted using the crude ethanolic extracts obtained from the leaf surfaces of the Nic 1015 and BHmN accessions. These extracts were evaluated at a concentration of 10 µg·µL−1 in ethanol, with 50 µL applied, equivalent to 500 µg of crude extract. Sterile paper discs, each having a diameter of 0.6 cm, were impregnated with the extracts. Once the impregnated discs had dried, they were placed in Petri dishes filled with LB medium that had been previously inoculated with the bacteria, with four replicates for each test.
For this assessment, the following controls were considered:
- (1)
- A positive control (Kanamycin 50 µg·µL−1), where 10 µL (equivalent to 500 µg) was used.
- (2)
- A solvent control (commercial Ethanol 90%), with 25 µL applied.
The Petri dishes were incubated at 37 °C for 24 h. The inhibition of bacterial growth was determined by measuring the diameter and radius of the observed circumference (inhibition zone) using a millimeter ruler. The area of inhibition was calculated using the formula: IA = πr2; where: IA: Inhibition Area (cm2), r: Radius of the inhibition circumference (cm).
2.6. Statistical Analysis
Statistical data processing was carried out using the Statistical Package for Social Sciences (SPSS) utility (version 20 for Windows, SPSS Inc, Chicago, Illinois, USA). The data pertaining to the growth control of each pathogen were treated as a mono-factorial experiment, involving more than two levels. The evaluation of the extracts was handled as a bifactorial experiment, comparing the extracts and their respective concentrations concerning the GIP at different time points. Data were subjected to the required transformation, and parametric tests, specifically bifactorial ANOVA, were applied. In cases where the ANOVA revealed significant differences at p ≤ 0.05, a Tukey HSD post hoc test was performed. Before conducting the statistical analyses, it was verified that the data from each treatment met the assumptions of normal distribution and homogeneity of variances. This was assessed through the Kolmogorov-Smirnov and Levene tests, respectively, at p ≤ 0.05. The results obtained through the disk diffusion method were processed as mono-factorial experiments, with more than two levels for each independent bacterium. Further details of the statistical treatment for each dataset can be found in the respective figures and tables of results.
3. Results
3.1. Obtaining Ethanolic Crude Extracts of Selected Tobacco Accessions
Crude ethanolic extracts from tobacco leaf surfaces were obtained, yielding 5.02 mg·g of fresh weight (FW−1) for the Nic 1015 accession and 2.6 mg·g of FW−1 for the BHmN accession. In the separation system employed, the compound α-CBTdiol (Rf: 0.03) was identified, along with another band with a slightly higher Rf of 0.05, potentially indicating traces of the isomer β-CBTdiol. Both exhibited an intense gray-green coloration for the Nic 1015 accession, with the intensity of the stain notably higher than that observed for the BHmN accession (as illustrated in Figure 1).

Figure 1.
Composition of crude ethanolic extracts of tobacco accessions. (a) Thin layer chromatography developed with sulfuric Anisaldehyde solution and observed under visible light showing the accessions: BH: BHmN, 1015: Nic 1015, 1003: Nic 1003, CBT: α-CBTdiol standard, Ab: cis Abienol. The compounds α CBTdiol and cis-abienol are indicated with arrows. (b) Chromatograms of the extracts of the accessions Nic 1015 (above) and BHmN (below), the region from 8 to 16 min showing the majority peaks of interest corresponding to (1): α-CBTdiol, (2): β-CBTdiol, (3): cis Abienol, (4): α-CBT ol, (5): β-CBT ol.
Cis Abienol (Rf: 0.51) was detected, characterized by an intense violet coloration typical of terpenes when stained with anisaldehyde [25]. Cis abienol was identified in the Nic 1003 and BHmN accessions but was not present in the Nic 1015 accession, as previously documented in the characterization of these accessions [24,29]. Furthermore, the accessions exhibited other compounds, each displaying distinctive colors such as blue, violet, yellow, gray, dark green, and pink.
The majority of peaks corresponding to five diterpenoids of interest were successfully observed, with the following retention times: 9.8 min for α-CBTdiol (peak 1), 10.48 min for β-CBTdiol (peak 2), 14.54 min for cis abienol (peak 3), 14.75 min for α-CBTol (peak 4), and 14.88 min for β-CBTol (peak 5). The spectra of compounds 1, 2, 4, and 5 corresponded to cembranoid-type diterpenes, characterized by an absorption maximum at approximately 210 nm (Figure A1a). These spectral characteristics were consistent with previous chromatograms [24] (Figure 1b). The spectrum of compound 3 exhibited an absorption maximum at 238 nm (Figure A1b), indicating its distinct nature compared to the other diterpenoids.
Liquid chromatography confirmed the presence of distinct diterpene profiles for the Nic 1015 and BHmN accessions of tobacco. For Nic 1015, the profile consisted of the presence of CBT diol isomers, small amounts of CBT ol isomers, and notably, the absence of cis abienol. In contrast, BHmN exhibited a characteristic profile, including the presence of CBT diol isomers, small amounts of CBT ol isomers, and the presence of cis abienol, which was consistent with the profile of the Nic 1003 accession. The evaluated accessions, Nic 1015 and BHmN, exhibited the two most common diterpene profiles found on the leaf surface of tobacco (N. tabacum), as previously documented [24]. Notably, Nic 1015 displayed a higher yield of α-CBT diol (4.42 mg·g of FW−1) compared to BHmN (1.78 mg·g of FW−1). BHmN, on the other hand, yielded cis abienol at 0.2 mg·g of FW−1. The quantitative analysis of the major diterpenes, α-CBTdiol, and cis abienol, underscored the significant differences between the evaluated accessions.
3.2. In Vitro Antifungal Activity of Crude Ethanolic Extracts against Phytopathogenic Fungi
The antifungal activity was assessed through TLC-Bioautography, which revealed the presence of several active antifungal compounds for each of the crude ethanolic extracts obtained from the evaluated accessions (Figure 2). Of note, the extract from the Nic 1015 accession displayed a prominent zone of inhibition in close proximity to the application point, with other zones of inhibition also observed.

Figure 2.
TLC-Bioautography to detect active antifungal substances in crude ethanolic extracts of N. tabacum accessions with the solvent system tert-butyl metyl ether (TBME): isooctane: methanol (5:5:1; v:v:v) and against fungi: (a) Fusarium graminearum and (b) Alternaria alternata. Sc: esclareol standard, CBT: α-CBTdiol standard, Ab: cis Abienol standard, 15: Nic 1015, BH: BHmN. Arrows indicate the inhibition zones of fungal growth corresponding to the compounds sclareol and cis abienol.
Additionally, the sclareol and cis abienol standards exhibited clear zones of fungal growth inhibition, thereby demonstrating their antifungal activity against the microorganisms.
It is worth mentioning that while the sclareol standard was not detectable under ultraviolet light, its existence was substantiated by the zones of fungal growth inhibition observed for Fusarium graminearum and Alternaria alternata, underscoring its pronounced antifungal activity. A similar antifungal behavior was observed for both sclareol and cis abienol against the two fungi (Figure 2).
The growth curve for Rhizoctonia solani demonstrated active growth up to 48 h, after which its behavior remained similar until the end of the evaluation (Figure 3a). Notably, the extracts exhibited a more substantial influence on the inhibition of fungal growth at 24 and 48 h (Figure 3b,c), aligning with the peak growth and development phase of the fungus (Figure 3a).

Figure 3.
Growth Inhibition Percentage (GIP) of the fungus Rhizoctonia solani testing different concentrations of crude ethanolic extracts from two N. tabacum accessions (Nic 1015 and BHmN). (a) Growth of R. solani expressed in absorbance (Abs) at 595 nm; (b) GIP after 24 h of incubation. (c) GIP after 48 h of incubation; (d) GIP after 72 h of incubation. Means with different letters have statistically significant differences (a) One-way ANOVA n = 4 and (b–d) ANOVA, Tukey HSD, p ≤ 0.05, n = 4 with bifactor statistical treatment comparing concentrations and tobacco accessions, the data were transformed according to y’ = 2 × arcsine (y/100)0.5.
The BHmN accession exhibited consistent GIP at all evaluated time points for the highest concentrations of the extract. In contrast, Nic 1015 displayed its most significant inhibitory effect at 24 h, which subsequently decreased at 48 and 72 h. Therefore, its primary effect seemed to be associated with the initiation and formation of the initial fungal hyphal structures.
Nic 1015 proved to be more effective in inhibiting the growth of R. solani, achieving a maximum GIP of 65.9% at the highest concentration, surpassing the effect of BHmN (30.2%). For BHmN, the MIC was determined to be 0.156 µg·µL−1. In contrast, for Nic 1015, the lowest concentration evaluated did not reach the MIC, suggesting that even lower concentrations of the extract might exert an inhibitory effect on R. solani. Subsequent experiments will explore concentrations below 0.078 µg·µL−1 to pinpoint the exact MIC.
In the case of S. solani similar to R. solani, an exponential growth of the fungus was observed up to 48 h (Figure 4a), and then it remained without growing significantly until the end of the evaluation. At all times evaluated, the influence of the extracts on growth inhibition was observed (Figure 4b–d).

Figure 4.
Growth Inhibition Percentage (GIP) of the fungus Stemphylium solani testing different concentrations of crude ethanolic extracts from two N. tabacum accessions (Nic 1015 and BHmN). (a) Growth of S. solani expressed in absorbance (Abs) at 595 nm; (b) GIP after 24 h of incubation. (c) GIP after 48 h of incubation; (d) GIP after 72 h of incubation. Means with different letters have statistically significant differences (a) One-way ANOVA n = 4 and (b–d) ANOVA, Tukey HSD, p ≤ 0.05, n = 4 with bifactor statistical treatment comparing concentrations and tobacco accessions, the data were transformed according to y’ = 2 × arcsine (y/100)0.5.
Nic 1015 shows a higher inhibitory effect (60%) than BHmN (23%) for the highest concentration evaluated. Nic 1015 with concentrations of 2.5 µg·µL−1 manages to inhibit more than 50%. The MIC for Nic 1015 is 0.625 µg·µL−1 and for BHmN it is 1.25 µg·µL−1.
Sarocladium oryzae showed higher growth during evaluation than the fungi R. solani and S. solani with exponential growth up to 96 h, growth after 96 h did not show significant differences (Figure 5a). At 24 h, the highest concentrations for both extracts showed growth inhibition, with the Nic 1015 extract (60%) standing out at the highest concentration (Figure 5b). Only Nic 1015 showed inhibition with the highest concentrations at 48 and 72 h (Figure 5c,d). Because the inhibition of 0.625 µg·µL−1 of the Nic 1015 extract was not significant, the MIC is 1.25 µg·µL−1, similar to BHmN extract.
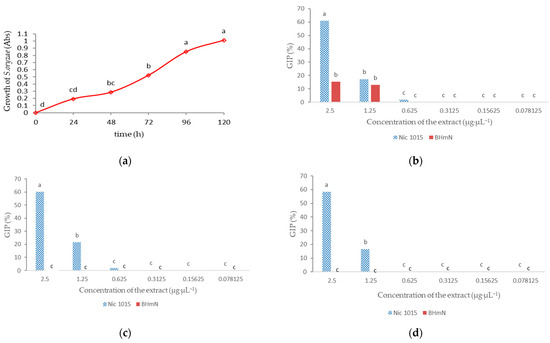
Figure 5.
Growth Inhibition Percentage (GIP) of the fungus Sarocladium oryzae testing different concentrations of crude ethanolic extracts of two N. tabacum accessions (Nic 1015 and BHmN). (a) Growth of S. oryzae expressed in absorbance (Abs) at 595 nm; (b) GIP after 24 h of incubation. (c) GIP after 48 h of incubation; (d) GIP after 72 h of incubation. Means with different letters have statistically significant differences (a) One-way ANOVA n = 4 and (b–d) ANOVA, Tukey HSD, p ≤ 0.05, n = 4 with bifactor statistical treatment comparing concentrations and tobacco accessions, the data were transformed according to y’ = 2 × arcsine (y/100)0.5.
In general, the ethanolic extracts of tobacco in this experiment were more active against R. solani, followed by S. solani and less active against S. oryzae, with a greater influence in the first 24 h of incubation. The GIP were similar for R. solani and S. solani (Figure 3 and Figure 4), perhaps due to the fact that they are fungi of the same species. In all cases the inhibitory activity correlated to the extract concentration for both accessions. In addition, the growth inhibition was higher under the influence of Nic 1015 extracts compared to BHmN extracts in all the cases (Figure 3, Figure 4 and Figure 5). These results also emerging after analyzing the values of IC50 and MIC as a summary for each tobacco accessions and fungi evaluated (Table 1). All the indicators IC50 and MIC are higher for Nic 1015 extracts compared to BHmN extracts.

Table 1.
Values estimated of IC50 and MIC to the growth inhibition of fungi caused by the ethanolic leaf surface extract of tobacco accessions Nic 1015 and BHmN. * No values represent no growth inhibition at the concentrations tested.
In the case of Nic 1015 the IC50 was approximately between 1.5 and 2.0 µg·µL−1 (Table 1). The lowest values were found in the first 48 h against R. solani. In subsequent experiments, concentrations higher than 2.5 µg·µL−1 will be evaluated in order to find the concentration of Nic 1015 extracts capable of inhibiting fungal growth by 100%, fungicidal and fungistatic concentrations. The results showed no IC50 for the accession BHmN with the three fungi evaluated. Concentrations higher than 2.5 µg·µL−1 should be evaluated to find the IC50 for BHmN extracts.
3.3. In Vitro Antibacterial Activity of Plant Extracts against Bacterial Contaminants of In Vitro Culture
The extracts of both tobacco accessions at the evaluated concentration achieved growth inhibition for the bacteria Bacillus licheniformis and Stenotrophomonas maltophilia with a slightly lower inhibition (Table 2). Kanamycin alone, used as an indicator of growth inhibition in vitro, caused significantly greater inhibition compared to the extracts.

Table 2.
Growth Inhibition of the bacteria Bacillus licheniformis and Stenotrophomonas maltophilia by the crude extracts of the tobacco accessions Nic 1015 and BHmN using the disk diffusion method. The radius and the diameter of the inhibition zone are measured. Means with different letters have statistically significant differences (One-way ANOVA, Tukey HSD, p ≤ 0.05, n = 4).
The ethanol used to obtain and dissolve the extracts did not show growth inhibition, which shows that the behavior of the extracts is inherent to their chemical composition. The use of ethanol is effective for these experiments, also because in this test the total evaporation of the solvent from the sample is achieved, which avoids any direct reaction of ethanol with the microorganism.
4. Discussion
4.1. Diterpenoids Composition of Ethanolic Crude Extracts
Figure 1 vividly illustrates the chemical diversity exhibited by the ethanolic extracts, primarily attributed to the genetic diversity of the tobacco accessions [7,12,13,30]. The identification and quantification of secondary metabolites of interest contribute significantly to characterizing these non-commercial tobacco accessions. This knowledge can serve as a valuable resource for obtaining these metabolites, as well as becoming an integral part of genetic improvement schemes and strategies. Many of these metabolites play pivotal roles in conferring resistance to specific diseases and enhancing the organoleptic properties of tobacco [6,7,15,24,26].
The Cuban accession BHmN, known for its utility in monoclonal antibody production, is particularly noteworthy. BHmN possesses the capability to produce diterpenes of interest on the leaf surface, encompassing both cembranoid types (e.g., α-CBTdiol) and labdanoid types (e.g., cis Abienol) (Figure 1). An intriguing prospect arises when considering the integration of both processes—extracting crude extracts from the leaf surface and producing plantibodies. This integration could pave the way for the initial extraction of active crude extracts rich in commercially valuable diterpenes, while concurrently generating plantibodies without the interference of major leaf surface compounds. Subsequent studies are warranted to evaluate the efficacy of this proposal.
4.2. Antifungal Activities of Plants Crude Extracts and Diterpenoids Obtained from the Leaf Surface of Tobacco
The use of TLC-Bioautography revealed that the presence of cis Abienol, which is also found in the BHmN accession, effectively inhibits the growth of fungi like Fusarium graminearum and Alternaria alternata. This compound contributes to the observed antifungal activity in the crude extracts derived from these accessions.
The presence of various compounds that exhibit inhibition zones is intriguing and holds promise for identifying new antifungal agents of natural origin. To explore this further, it would be beneficial to isolate and identify these other antifungal compounds. This can be achieved through methods such as scraping the TLC plate or obtaining fractions of these crude extracts.
The extract from Nic 1015 demonstrates remarkable antifungal activity, with a MIC of 78 µg·mL−1 for Rhizoctonia solani and a lighter MIC of 625 µg·mL−1 for Stemphylium solani (Table 1). In line with the classification of antimicrobial activities based on concentration, extracts or compounds exhibiting activity at concentrations below 100 µg·mL−1 are considered to have high activity, while those within the range of 100 to 500 µg·mL−1 are deemed moderate, and those ranging from 500 to 1000 µg·mL−1 are considered light. Anything above 1000 µg·mL−1 is categorized as slightly active [2].
The inhibitory effects on mycelial growth, particularly the observed MIC, in the ethanolic extracts of Nic 1015 and BHmN are indeed noteworthy. These effects are within a range similar to those seen in ethanolic extracts from Citrus spp. leaves [31], even though the chemical compositions of these extracts differ. Ethanolic extracts of Citrus spp. leaves inhibited the mycelial growth of S. solani, with MIC values ranging in some cases from 0.039 µg·µL−1 to 2.5 µg·µL−1 and higher than 40 µg·µL−1, as determined by phenol content.
The fact that various plant extracts, with distinct chemical profiles, show comparable MIC values suggests the possibility of common or analogous action mechanisms against fungal growth. While the chemical composition of phenolic compounds differs from diterpenes, it is possible that their mechanisms of action may exhibit similarities in certain instances [32]. Moreover, it is important to note that the extracts obtained are crude and comprise a mixture of substances. It is worth mentioning that the crude extracts of Nic 1015 are known to contain high levels of diterpenoids and the terpenoid compounds and essential oils have been associated with antifungal activity in other plant species [31,32].
In various in vitro tests, certain plant extracts have demonstrated the ability to impede the growth of S. oryzae. This inhibitory effect extends to mycelium growth, biomass production, spore germination, and germ tube length. These observed effects were primarily attributed to the presence of essential oils, tannins, and phenolic compounds [33].
The inhibition of mycelial growth observed in this study is likely attributed to the presence of terpenes, which are the predominant compounds in tobacco leaf exudates. Additionally, crude extracts contain various secondary metabolites known for their diverse biological activities, such as fungicidal, antibacterial, and insecticidal properties [6,8,14,15,24,26]. Terpenes comprise one of the largest and most diverse families of natural substances, displaying a wide range of chemical structures and biological activities [34].
The ethanolic extracts obtained from Nic 1015 are particularly rich in α-CBT diol, a cembrane-type diterpene. It constitutes the majority of the compounds in tobacco leaf exudates, comprising 88% of the total dry mass of the extract. In BHmN, although to a slightly lesser extent (68%), it remains a significant component. Previous research has confirmed that cembranoid diterpenes, present in hexanic extracts of tobacco varieties at a concentration of 5 µg·µL−1, serve as effective antifungal agents [8]. Furthermore, other chemical constituents derived from ethanolic extracts of N. tabacum flowers, many of which are terpenic in nature, have exhibited antifungal activity against various fungi, including Valsa mali var. mali, Alternaria porri, and Botrytis cinerea [21].
In a separate study, researchers examined ethanolic extracts from three typical Saudi Arabian plants, collecting samples from various plant parts. These extracts exhibited in vitro antifungal activity against Alternaria alternata, Fusarium oxysporum, and Rhizoctonia solani, demonstrating effectiveness at different concentrations [35].
It is worth noting that the active compounds in our research, primarily terpenes, possess a distinctive advantage when compared to other plant extracts used for similar purposes. These compounds can be easily isolated from the leaf surface through straightforward washes with the appropriate solvent. This attribute contributes to the practicality and convenience of utilizing these compounds as potential antifungal agents.
In this investigation it was also demonstrated that the use of DMSO in the low concentrations used is suitable for in vitro tests of antimicrobial activity, in the cases in which it is necessary to dissolve the extract and prepare different concentrations to be applied to the microorganism and in this way evaluate its effect on its in vitro growth (Figure 3, Figure 4 and Figure 5). DMSO is the safest compared to other solvents to detect the presence of bioactive compounds and phytotoxic effects [36].
It is highly likely that the primary mechanism through which these ethanolic extracts affect the development of the evaluated fungi is by directly influencing the fungal mycelium. This is supported by prior observations involving cembranoid-type diterpenes, which have been shown to induce variations in the ergosterol content of the cell membrane in the apple pathogenic fungus Valsa mali [8]. As a result, the internal membrane of the hyphae thickens, leading to deformation and abnormal growth of the mycelium, resulting in surface depression and hyphal adhesion. Additionally, when Valsa mali is treated with cembranoids, it exhibits a decomposed cell wall, disordered intracellular composition, including broken vacuoles and degraded organelles, as well as an increased intracellular cavity, ultimately causing deformation of the fungus and inhibiting mycelial growth [8]. Another possible mechanism of action could involve interference with the cell division process of the fungus. In previous studies, it has been demonstrated that α-CBT diol, with an IC50 of 0.018 µg·µL−1, alters the expression of genes associated with redox processes, iron binding, coenzymes, and other vital reactions in Valsa mali, affecting its multiplication and growth [20]. These findings strongly suggest that the extracts contain antifungal compounds, potentially making them suitable for controlling these pathogens.
4.3. Antibacterial Activities of Plants Crude Extracts Obtained from the Leaf Surface of Tobacco
The results in this investigation indicate an antibacterial activity for these non-pathogenic plant bacteria, although with less inhibition than that shown for Xanthomonas campestris and Pectobacterium carotovorum at the same amount of extract (500 µg) [23]. Other ethanolic extracts of Acacia aroma leaves and flowers [37] and other plants [38] showed activity against S. maltophilia. Some authors obtained inhibition of S. maltophilia isolates in a MIC range of 64 µg·mL−1 to 512 µg·mL−1 of the compound epigallocatechin-3-gallate, the most abundant polyphenol found in green tea (Camellia sinensis) [19]. Chloroformic extracts from Semecarpus anacardium also showed inhibition of B. licheniformis by the agar diffusion disk method [37]. All these bacteria are Gram −, except B. licheniformis, so it seems that the mechanism of the antibacterial activity of these extracts against these bacteria might not specifically involve the composition of the cell wall of the bacteria, which is based on the thickness of peptidoglycan and that determines its classification in the Gram test. Thus, the antibacterial activity of these extracts appears to be of a broad bacterial spectrum. It is possible that the extracts cause a thickening of the bacterial cell wall through interference in bacterial cell division, as occurs with terpene compounds that act in this way against a bacterium of the same genus, Bacillus cereus [39]. Kanamycin is one of the commonly used antibiotics for in vitro susceptibility testing assays [23] and gentamicin and ampicillin have been used for S. maltophilia [38]. Other trials that consider the application of these extracts in in vitro culture media should be developed in the future to verify their efficiency in controlling bacterial contamination.
The extracts at the evaluated concentrations can generally be classified as active, based on the classification of biological activity within a concentration range of <5000 ppm (5 µg·µL−1) [40]. The relationship between the chemical structure and the biological activity of these compounds is not yet well understood. However, it has been observed that the antimicrobial activity of these compounds is influenced by several structural factors [41], including:
- (1)
- Number of Hydroxyl Groups: Compounds with more hydroxyl groups may exhibit higher antimicrobial activity.
- (2)
- Degree of Unsaturation: The presence of double bonds or macrocyclic structures in the compounds can also influence their activity.
- (3)
- Type and Position of Substituents: The type and location of chemical substituents on the compound’s structure can affect its antimicrobial properties.
The compounds of interest in this study, including CBTdiols, sclareol, and cis abienol, contain two hydroxyl groups, as shown in Figure A1a and reference [22]. This structural feature could be related to their higher activity against fungi and bacteria when compared to CBTols. The extracts in this study contain a higher proportion of CBTdiols, even more than cis abienol, while CBTols are present in lower quantities (as mentioned in Section 3.1). As a result, the accession Nic 1015, which has a higher content of CBTdiols (with a macrocyclic structure and two hydroxyl groups), is demonstrating higher overall antimicrobial activity.
The findings suggest that the structural characteristics of the compounds in the extracts, particularly the presence of two hydroxyl groups, may contribute to their antimicrobial properties. Further research is needed to fully understand the structure-activity relationship of these compounds.
The search for a natural product with a broad spectrum of action against microorganisms is a significant challenge and a primary objective in this field of research. One potential solution could be to explore the combination of extracts with antimicrobial activity against various microorganisms. This approach might yield a product with a wide range of effectiveness. Using a combination of these extracts could offer an alternative means of controlling several microorganisms simultaneously. Moreover, the compounds within the mixture, originating from the same plant species, could potentially exhibit additive effects, enhancing the overall antimicrobial properties of the product.
5. Conclusions
The crude ethanolic extracts obtained from the leaf surfaces of both accessions, Nic 1015 and BHmN, serve as sources of diterpene α-CBT diol. However, only the BHmN accession contains cis Abienol. Both extracts have demonstrated antifungal activity against fungi like Alternaria alternata and Fusarium graminearum, as confirmed through TLC-Bioautography. Furthermore, they’ve exhibited antifungal activity against Rhizoctonia solani, Stemphylium solani, and Sarocladium oryzae, as determined by a microdilution test, with the level of antimicrobial activity correlating with the concentration of the extract. This correlation suggests that diterpene content, particularly as the predominant compounds, plays a significant role in the observed antimicrobial activity. Both extracts have shown similar antibacterial activity against bacterial contaminants in in vitro cultures, namely Bacillus licheniformis (Gram+) and Stenotrophomonas maltophilia (Gram-). These results indicate the potential utility of ethanolic extracts obtained from the leaf surfaces of Nicotiana tabacum L. accessions Nic 1015 and BHmN as effective antimicrobial agents. It is proposed to explore the potential of a combination product derived from these selected extracts in future studies, with the aim of enhancing their effectiveness in controlling tested phytopathogenic microorganisms and in vitro culture contaminants.
Author Contributions
Conceptualization, Y.C., J.Q.-G.; Methodology, Y.C., C.L., J.S., A.E.-H., E.R. and J.Q.-G.; Formal analysis, Y.C., C.L., E.Y.-P. and J.Q.-G.; Investigation, Y.C., C.L., R.V., E.Y.-P. and J.Q.-G.; Data curation, Y.C.; Writing—original draft, Y.C., M.E.M.-M. and J.Q.-G.; Writing—review & editing, Y.C., E.O.-D., M.E.M.-M. and J.Q.-G.; Visualization, Y.C., C.L., J.S., A.E.-H., R.V., E.Y.-P., E.O.-D., M.E.M.-M., E.R. and J.Q.-G.; Supervision, E.O.-D. and J.Q.-G.; Project administration, J.Q.-G.; Funding acquisition, M.E.M.-M. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank the National Project “Obtaining bioactive plant extracts, rich in secondary metabolites for controlling pests and diseases of agricultural importance crops”, the Food Security Center (FSC) of Germany for supporting part of the research carried out.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors very appreciated the collaboration and important contribution of Grace Ngatia, Geeisy Cid, Rolando Morán, Néstor Mora and the Chemistry laboratory of the Phytomedicine Institute, University of Hohenheim, Stuttgart, Germany.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Figure A1.
Absorption spectra of main diterpenoids of tobacco leaf surface obtained by HPLC (a): α- CBT diol in accession Nic 1015 showing the maximum absorption close to 210 m and compound structure. (b): cis Abienol in accession Nic 1003 showing maximum absorption of 238 nm and compound structure.
References
- López-Dávila, E.; Martínez, Y.; Romero, O. Characteristics and adverse consequences on human health of agrochemicals used in Cuban agriculture. Rev. Cuba. De Salud Pública 2022, 48, e2810-7. [Google Scholar]
- Nava-Pérez, E.; García-Gutiérrez, C.; Camacho-Báez, J.R.; Vázquez-Montoya, E.L. Biopesticides: An option for the biological pest control. Rev. Ra Ximhai 2012, 8, 17–29. [Google Scholar] [CrossRef]
- Boedeker, W.; Watts, M.; Clausing, P.; Marquez, E. The global distribution of acute unintentional pesticide poisoning: Estimations based on a systematic review. BMC Public Health 2020, 20, 1875. [Google Scholar] [CrossRef]
- Pérez-Consuegra, N.; Montano-Pérez, M. Los plaguicidas altamente peligrosos en Cuba. IPEN/ACTAF/RAPAL. La Habana. Ed. Agroecol. 2021, 56. [Google Scholar]
- Cen, X.; Ding, W.; Ding, J. Preliminary studies on the activity of crude extracts from Nicotiana tabacum stems against Tetranychus cinnabar inus. Plant Prot. 2009, 35, 156–158. [Google Scholar]
- Jassbi, A.R.; Zare, S.; Asadollahi, M.; Schuman, M.C. Ecological Roles and Biological Activities of Specialized Metabolites from the Genus Nicotiana. Chem. Rev. 2017, 117, 12227–12280. [Google Scholar] [CrossRef]
- Lewis, R.S. Nicotiana tabacum L.: Tobacco. In Medicinal, Aromatic and Stimulant Plants; Springer: Cham, Switzerland, 2020; pp. 345–375. [Google Scholar]
- Duan, S.; Du, Y.; Hou, X.; Yan, N.; Dong, W.; Mao, X.; Zhang, Z. Chemical Basis of the Fungicidal Activity of Tobacco Extracts against Valsa mali. Molecules 2016, 21, 1743. [Google Scholar] [CrossRef]
- Ruiz, Y.; Ramos, P.L.; Soto, J.; Rodríguez, M.; Carlos, N.; Reyes, A.; Fuentes, A. The M4 insulator, the TM2 matrix attachment region, and the double copy of the heavy chain gene contribute to the enhanced accumulation of the PHB-01 antibody in tobacco plants. Transgenic Res. 2020, 29, 171–186. [Google Scholar] [CrossRef]
- Uzelac, B.; Stojičić, D.; Budimir, S. Glandular Trichomes on the Leaves of Nicotiana tabacum: Morphology, Developmental Ultrastructure, and Secondary Metabolites. In Plant Cell and Tissue Differentiation and Secondary Metabolites. Reference Series in Phytochemistry; Ramawat, K.G., Ekiert, H.M., Goyal, S., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Tissier, A. Plant secretory structures: More than just reaction bags. Curr. Opin. Biotechnol. 2018, 49, 73–79. [Google Scholar] [CrossRef]
- Tissier, A. Glandular trichomes: What comes after expressed sequence tags. Plant J. 2012, 70, 51–68. [Google Scholar] [CrossRef]
- Sallaud, C.; Giacalone, C.; Topfer, R.; Goepfert, S.; Bakaher, N.; Rosti, S.; Tissier, A. Characterization of two genes for the biosynthesis of the labdane Z-abienol in tobacco (Nicotiana tabacum). Plant J. 2012, 72, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, R.W.; Bass, W.T.; Houtz, R.L.; Wagner, G.J. Phylloplanins of Tobacco Are Defensive Proteins Deployed on Aerial Surfaces by Short Glandular Trichomes. Plant Cell 2005, 17, 1851–1861. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Gomi, K.; Kaku, H.; Abe, H.; Seto, H.; Nakatsu, S.; Neya, M.; Kobayashi, M.; Nakaho, K.; Ichinose, Y.; et al. Identification of Natural Diterpenes that Inhibit Bacterial Wilt Disease in Tobacco, Tomato and Arabidopsis. Plant Cell Physiol. 2012, 53, 1432–1444. [Google Scholar] [CrossRef]
- Cedeño, L.; Carrero, C.; Ruíz, R.; Fermín, G.; Pino, H.; Quintero, K. Primer reporte de Stemphylium solani en Lisiantus. Fitopatol. Venez. 2011, 24, 38–41. [Google Scholar]
- Giraldo, A.; Gené, J.; Sutton, D.A.; Madrid, H.; de Hoog, G.S.; Cano, J.; Decock, C.; Crous, P.W.; Guarro, J. Phylogeny of Sarocladium (Hypocreales). Persoonia 2015, 34, 10–24. [Google Scholar] [CrossRef]
- Emara, A.R.; Ibrahim, H.M.; Masoud, S.A. The role of storage on Mancozeb fungicide formulations and their antifungal activity against Fusarium oxysporum and Rhizoctonia solani. Arab. J. Chem. 2021, 14, 103322. [Google Scholar] [CrossRef]
- Vidigal, P.G.; Müsken, M.; Becker, K.A.; Häussler, S.; Wingender, J.; Steinmann, E.; Kehrmann, J.; Gulbins, E.; Buer, J.; Rath, P.M.; et al. Effects of Green Tea Compound Epigallocatechin-3-Gallate against Stenotrophomonas maltophilia Infection and Biofilm. PLoS ONE 2014, 9, e92876. [Google Scholar] [CrossRef]
- Yan, N.; Du, Y.; Liu, X.; Zhang, H.; Liu, Y.; Shi, J.; Xue, S.J.; Zhang, Z. Analyses of effects of α-cembratrien-diol on cell morphology and transcriptome of Valsa mali var. mali. Food Chem. 2017, 214, 110–118. [Google Scholar] [CrossRef]
- Yang, C.; Xie, S.; Ni, L.; Du, Y.; Liu, S.; Li, M.; Yang, X.K. Chemical Constituents from Nicotiana tabacum L. and Their Antifungal Activity. Nat. Prod. Commun. 2021, 16, 1–5. [Google Scholar] [CrossRef]
- Severson, R.F.; Arrendale, R.F.; Chortyk, O.T.; Johnson, A.W.; Jackson, D.M.; Gwynn, G.R.; Chaplain, J.F.; Stephenson, M.G. Quantitation of the major cuticular components from green leaf of different tobacco types. J. Agric. Food Chem. 1984, 32, 566–570. [Google Scholar] [CrossRef]
- Capdesuñer, Y.; Rivas, M.; Rodríguez, E.; Gallo, M.; Quiñones-Galvez, J.; Yanes, E.; Hernández, M. In vitro antibacterial effect of tobacco leaf exudates against two bacterial plant pathogens. Rev. Colomb. De Biotecnol. 2015, 17, 91–100. [Google Scholar] [CrossRef][Green Version]
- Capdesuñer, Y.; García-Brizuela, J.; Mock, H.P.; Hernández, K.V.; Hernández de la Torre, M.; Santiesteban-Toca, C.E. Accessing to the Nicotiana tabacum leaf antimicrobial activity: In-silico and in-vitro investigations. Plant Physiol. Biochem. 2019, 139, 591–599. [Google Scholar] [CrossRef]
- Wagner, H.; Bladt, S. Plant drug analysis: A Thin Layer Chromatography Atlas (Segunda Ed.); Springer: Berlin/Heidelberg, Germany, 1996; ISBN 3-540-58676-8. [Google Scholar]
- Feng, Y.; Jia, H.; Guan, H.; Zhang, W.; Zhou, Y.; Liu, K.; Wang, Y.; Li, Q.; Chen, W.; Sohail, M.A.; et al. Extraction, purification and anti-TMV effects of α (β)-2,7,11-cembratriene-4,6-diol from tobacco leaves. bioRxiv 2021. [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Terras, F.R.; Schoofs, H.M.; De Bolle, M.F.; Van Leuven, F.; Rees, S.B.; Vanderleyden, J.; Cammue, B.P.; Broekaert, W.F. Analysis of two novel classes of plant antifungal proteins from radish (Raphanus sativus L.) seeds. J. Biol. Chem. 1992, 267, 15301–15309, ISSN: 0021-9258. [Google Scholar] [CrossRef] [PubMed]
- Capdesuñer, Y.; Rivas, M.; Quiñones-Galvez, J.; Gallo, M.; Rodríguez, E.; Pérez, J.L.; Yanes-Paz, E.; Hernández, M. Análisis comparativo de indicadores químicos de la hoja y diterpenos de exudados foliares de Nicotiana tabacum L. Cultiv. Trop. 2016, 37, 127–135. [Google Scholar]
- Cui, H.; Zhang, S.T.; Yang, H.J.; Ji, H.; Wang, X.J. Gene expression profile analysis of tobacco leaf trichomes. BMC Plant Biol. 2011, 11, 76. [Google Scholar] [CrossRef]
- Iglesias, D.; Ojito-Ramos, K.; Linares, C.; Portal, O. Actividad antifúngica in vitro de extractos de hojas de Citrus spp. frente a Stemphyllium solani Weber. Cent. Agrícola 2017, 44, 5–12. [Google Scholar]
- Arif, T.; Bhosale, J.D.; Kumar, N.; Mandal, T.K.; Bendre, R.S.; Lavekar, G.S.; Dabur, R. Natural products—Antifungal agents derived from plants. J. Asian Nat. Prod. Res. 2009, 11, 621–638. [Google Scholar] [CrossRef]
- Meera, T.; Balabaskar, P. Antifungal activity of botanicals against Sarocladium oryzae causing rice sheath rot disease. Int. J. Food Agric. Vet. Sci. 2012, 2, 121–127. [Google Scholar]
- Kutchan, T.M.; Gershenzon, J.; Møller, B.L.; Gang, D.R. Natural products. In Biochemistry and Molecular Biology of Plants, 2nd ed.; Buchanan, B.B., Gruissem, W., Jones, R.L., Eds.; John Wiley & Sons, Ltd.: Oxford, UK, 2015; pp. 1133–1134. [Google Scholar]
- Al-Askar, A.A. In vitro antifungal activity of three Saudi plant extracts against some phytopathogenic fungi. J. Agric. Chem. Biotechnol. 2012, 3, 277–284. [Google Scholar]
- Joshi, D.R.; Adhikari, N. An Overview on Common Organic Solvents and Their Toxicity. J. Pharm. Res. Int. 2019, 28, 1–18. [Google Scholar] [CrossRef]
- Kapoor, A.; Kaur, G.; Kaur, R. Antimicrobial activity of different herbal plants extracts: A review. World J. Pharm. Pharm. Sci. 2015, 4, 422–459. [Google Scholar]
- Oskay, M.; Oskay, D.; Kalyoncu, F. Activity of Some Plant Extracts Against Multi-Drug Resistant Human Pathogens. Iran. J. Pharm. Res. 2009, 8, 293–300. [Google Scholar]
- Dos Santos, E.C.G.; Donnici, C.L.; da Silva Camargos, E.R.; de Rezende, A.A.; de Aguiar Andrade, E.H.; Soares, L.A.L.; de Macêdo Farias, L.; de Carvalho, M.A.R.; das Graças Almeida, M. Effects of Copaifera duckei Dwyer oleoresin on the cell wall and cell division of Bacillus cereus. J. Med. Microbiol. 2013, 62, 1032–1037. [Google Scholar] [CrossRef]
- Mesa, V.A.M.; Marín, P.; Ocampo, O.; Calle, J.; Monsalve, Z. Fungicidas a partir de extractos vegetales: Una alternativa en el manejo integrado de hongos fitopatógenos. Rev. De Investig. Agropecu. 2019, 45, 23–30. [Google Scholar]
- Wang, J.; Xu, K.; Zhang, J.; Ren, G.; Yang, X.; Zhang, Z.; Zhang, Y.; Xiao, Y.; Du, Y. Systematic activity-oriented separation and structure-activity relationship of tobacco cembranoids. Ind. Crops Prod. 2021, 173, 114136. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).