Humus Forms and Organic Matter Decomposition in the Swiss Alps
Abstract
1. Introduction
2. Material and Methods
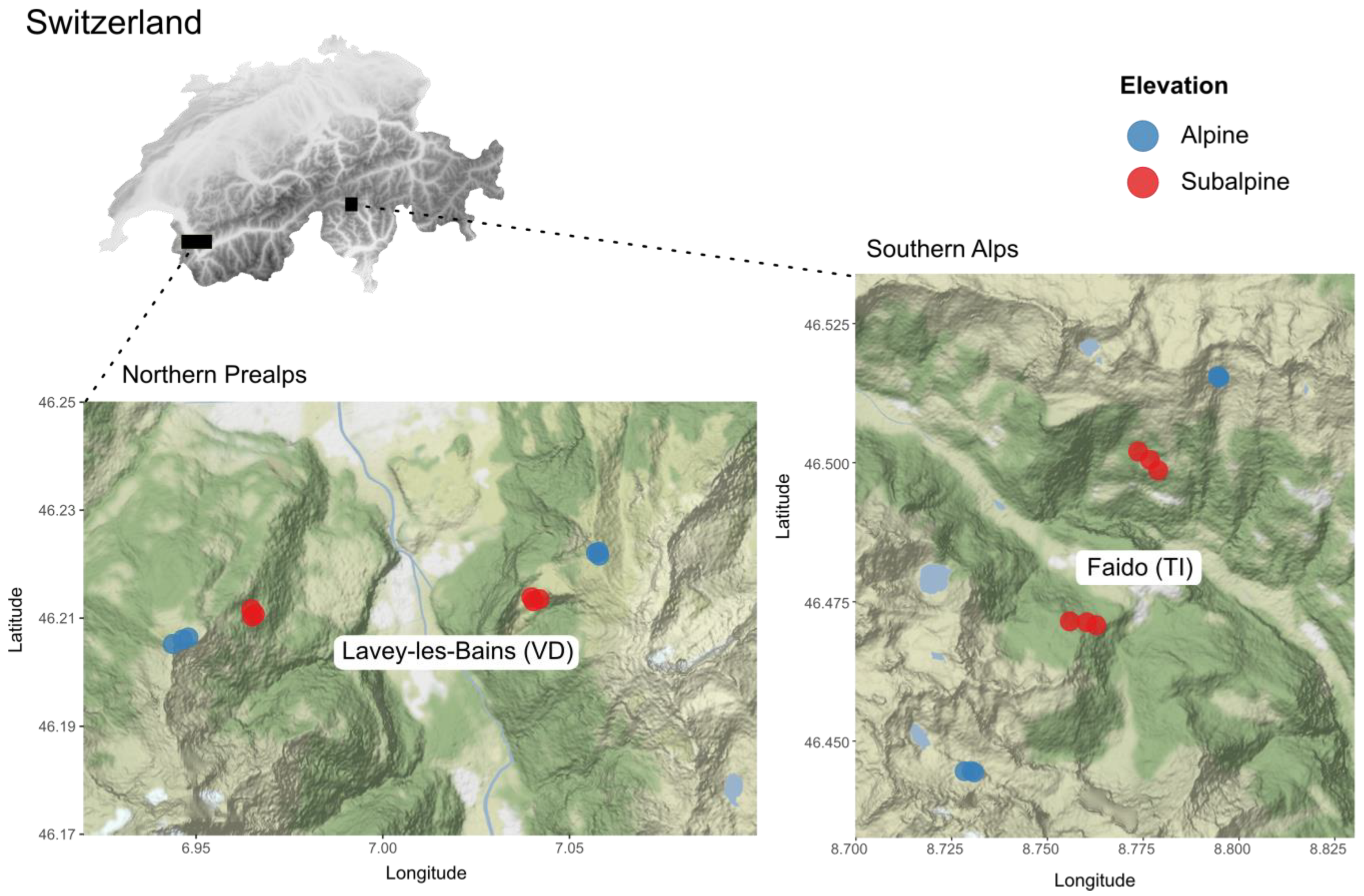
2.1. Study Sites
2.2. Humus Forms and Soils
2.3. Teabag Experiment
2.4. Statistical Analysis
3. Results
3.1. Soils
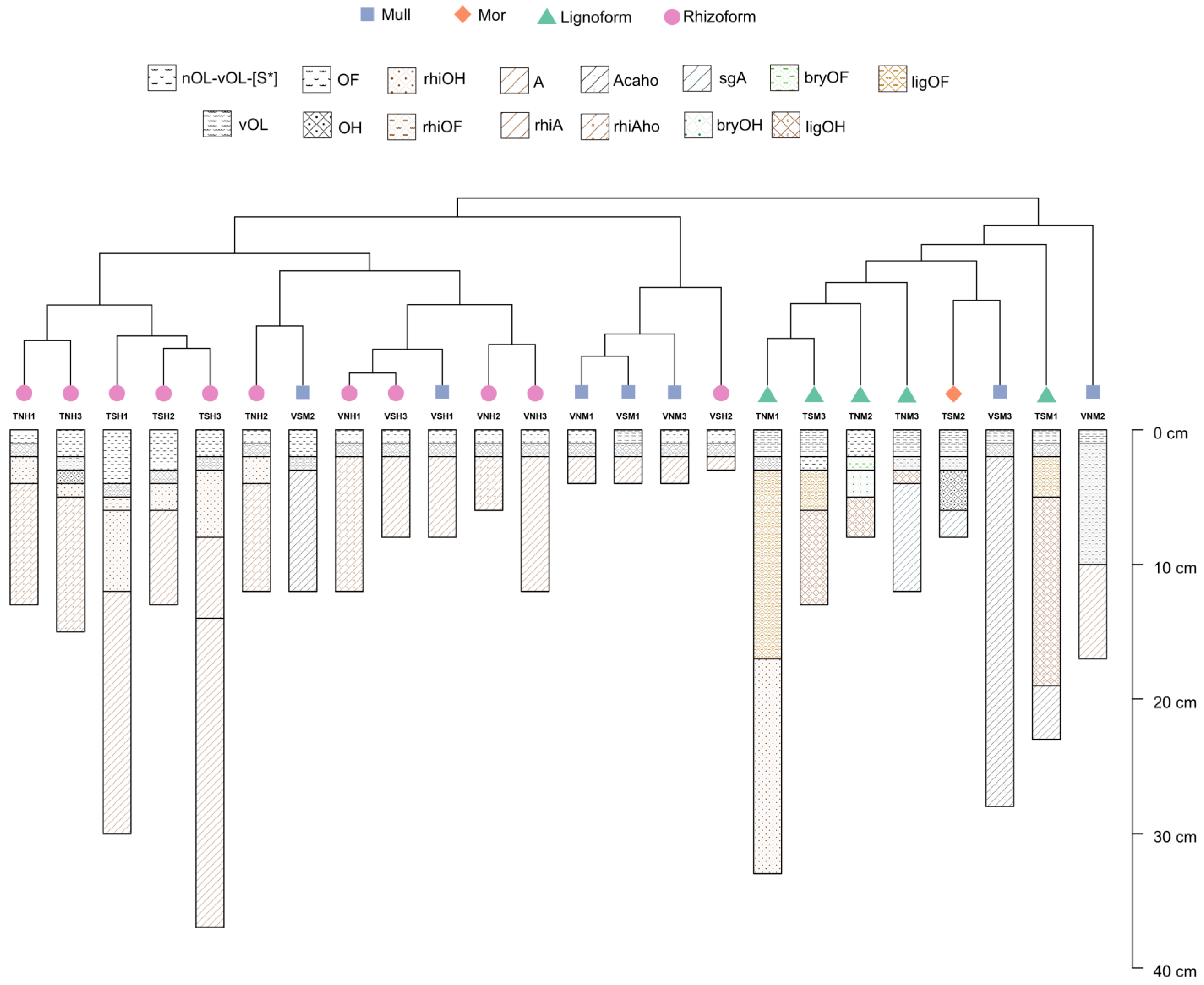
3.2. Terrosystems and Parasystems Distribution
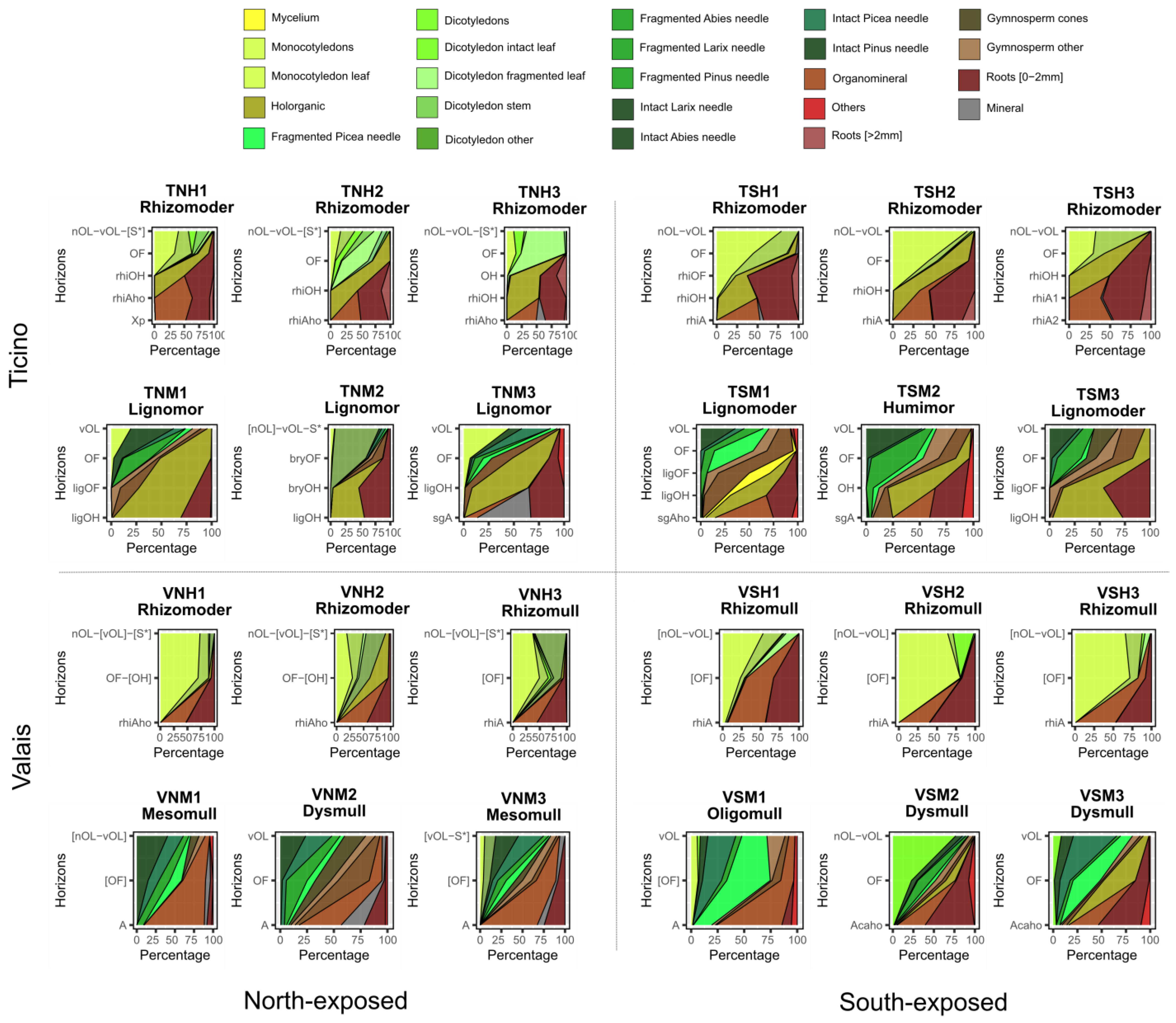
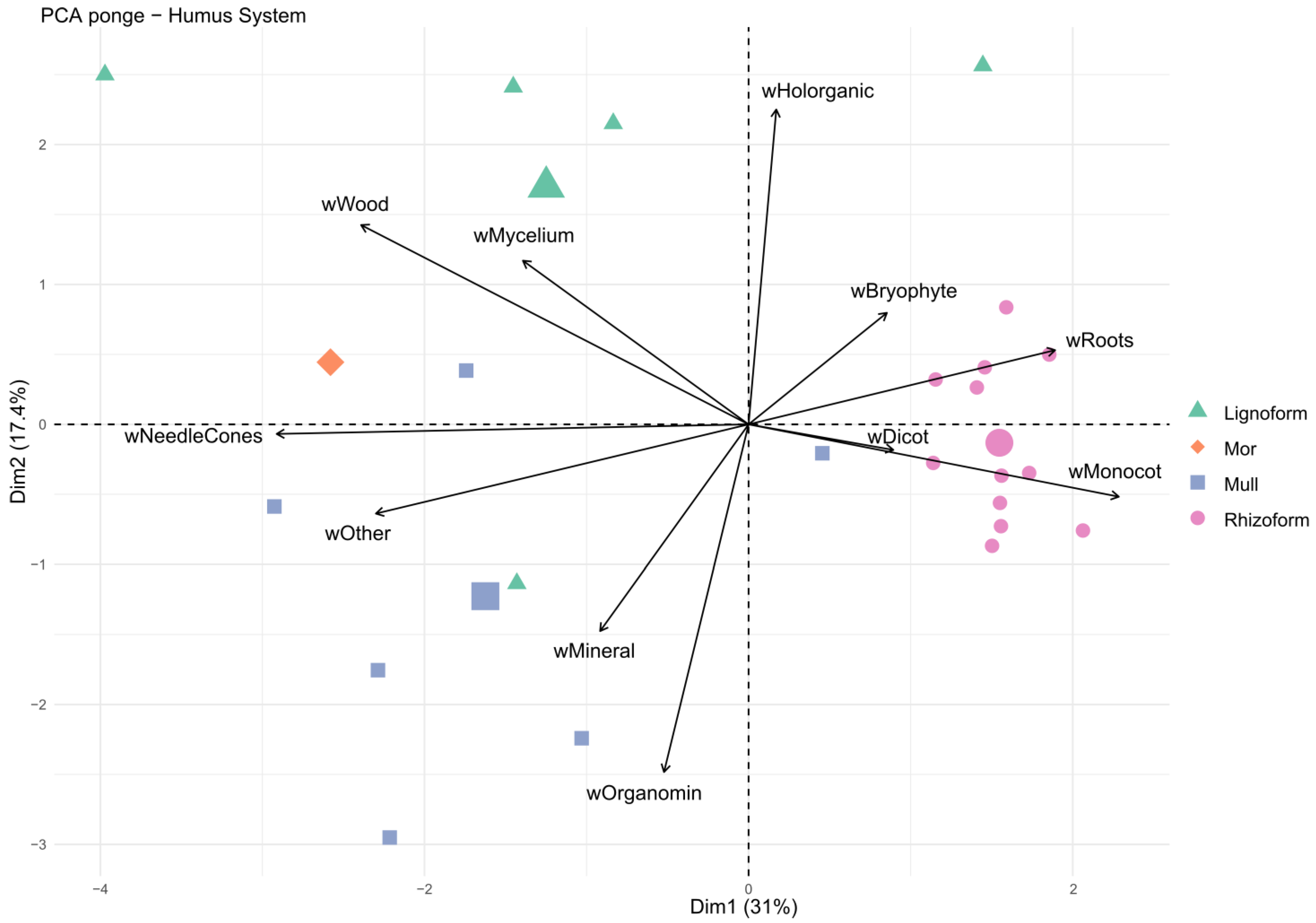
3.3. Humus Forms and Their Morphological Features across Regions
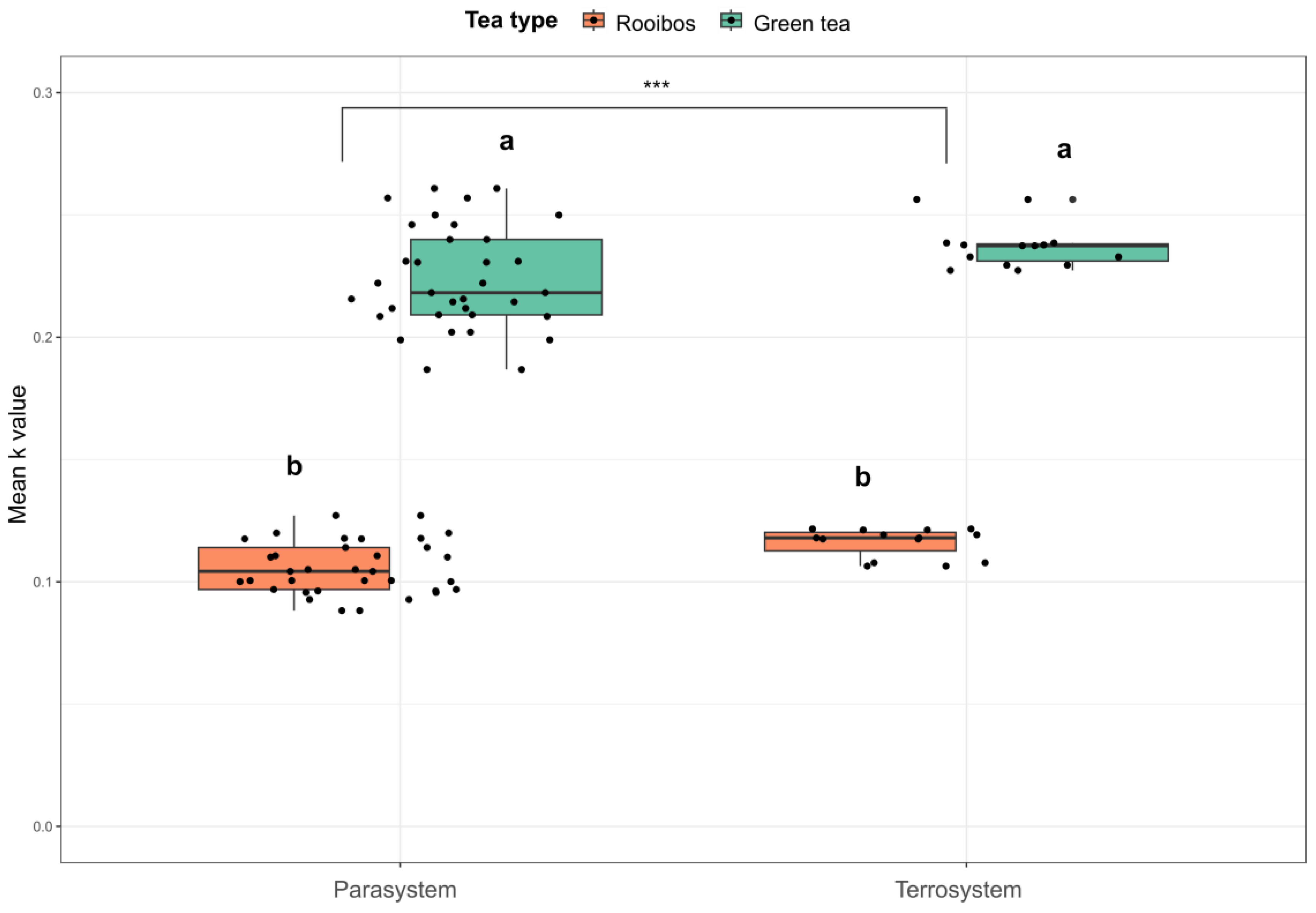
3.4. Tea Litter Decomposition
4. Discussion
4.1. Humus Forms and Soil Pedogenetic Processes
4.2. Alpine Meadows: Humus Homogeneity and Organic Matter Accumulation
4.3. Subalpine Forests: The Organic Matter Dynamics Vary According to the Vegetation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TNH1 to 3 | Ticino North High elevation Plot No. 1 to 3 |
| TSH1 to 3 | Ticino South High elevation Plot No. 1 to 3 |
| TNM1 to 3 | Ticino North Mid elevation Plot No. 1 to 3 |
| TSM1 to 3 | Ticino South Mid elevation Plot No. 1 to 3 |
| VNH1 to 3 | Valais North High elevation Plot No. 1 to 3 |
| VSH1 to 3 | Valais South High elevation Plot No. 1 to 3 |
| VNM1 to 3 | Valais North Mid elevation Plot No. 1 to 3 |
| VSM1 to 3 | Valais South Mid elevation Plot No. 1 to 3 |
Appendix A
Appendix B
Appendix C
References
- Lehmann, J.; Bossio, D.A.; Kögel-Knabner, I.; Rillig, M.C. The concept and future prospects of soil health. Nat. Rev. Earth Environ. 2020, 1, 544–553. [Google Scholar] [CrossRef]
- Zanella, A.; Berg, B.; Ponge, J.-F.; Kemmers, R.H. Humusica 1, article 2: Essential bases—Functional considerations. Appl. Soil Ecol. 2018, 122, 22–41. [Google Scholar] [CrossRef]
- Blouin, M. Chemical communication: An evidence for co-evolution between plants and soil organisms. Appl. Soil Ecol. 2018, 123, 409–415. [Google Scholar] [CrossRef]
- Veen, G.F.; Fry, E.L.; Ten Hooven, F.C.; Kardol, P.; Morriën, E.; De Long, J.R. The role of plant litter in driving plant-soil feedbacks. Front. Environ. Sci. 2019, 7, 168. [Google Scholar] [CrossRef]
- Zanella, A.; Ponge, J.-F.; Gobat, J.-M.; Juilleret, J.; Blouin, M.; Aubert, M.; Chertov, O.; Rubio, J.L. Humusica 1, article 1: Essential bases–Vocabulary. Appl. Soil Ecol. 2018, 122, 10–21. [Google Scholar] [CrossRef]
- Ponge, J.-F.; Jabiol, B.; Gégout, J.-C. Geology and climate conditions affect more humus forms than forest canopies at large scale in temperate forests. Geoderma 2011, 162, 187–195. [Google Scholar] [CrossRef]
- Bonifacio, E.; Falsone, G.; Petrillo, M. Humus forms, organic matter stocks and carbon fractions in forest soils of northwestern Italy. Biol. Fertil. Soils 2011, 47, 555–566. [Google Scholar] [CrossRef]
- Ponge, J.-F. Humus forms in terrestrial ecosystems: A framework to biodiversity. Soil Biol. Biochem. 2003, 35, 935–945. [Google Scholar] [CrossRef]
- Marklein, A.R.; Winbourne, J.B.; Enders, S.K.; Gonzalez, D.J.X.; van Huysen, T.L.; Izquierdo, J.E.; Light, D.R.; Liptzin, D.; Miller, K.E.; Morford, S.L.; et al. Mineralization ratios of nitrogen and phosphorus from decomposing litter in temperate versus tropical forests. Glob. Ecol. Biogeogr. 2016, 25, 335–346. [Google Scholar] [CrossRef]
- Muller, P.E. Studier over Skovjord, som bidrag til skovdyrkningens theori. I. Om bogemuld og bogrmor paa sand og ler. Tidsskr Skovbr 1879, 3, 1–124. [Google Scholar]
- Muller, P.E. Studier over Skoyjord. II Om Muld og Mor i Egeskove og paa Heder. Tidsskr Skovbr 1884, 7, 1–232. [Google Scholar]
- Green, R.N.; Trowbridge, R.L.; Klinka, K. Towards a taxonomic classification of humus forms. For. Sci. 1993, 39, a0001–z0002. [Google Scholar] [CrossRef]
- Brêthes, A.; Brun, J.-J.; Jabiol, B.; Ponge, J.; Toutain, F. Classification of Forest Humus Forms: A French Proposal; EDP Sciences: Les Ulis, France, 1995; pp. 535–546. [Google Scholar]
- Baize, D.; Girard, M.-C. Référentiel Pédologique 2008; Editions Quae: Versailles, France, 2009. [Google Scholar]
- Jabiol, B.; Zanella, A.; Ponge, J.-F.; Sartori, G.; Englisch, M.; van Delft, B.; de Waal, R.; Le Bayon, R.-C. A proposal for including humus forms in the World Reference Base for Soil Resources (WRB-FAO). Geoderma 2013, 192, 286–294. [Google Scholar] [CrossRef]
- Chertov, O.G.; Nadporozhskaya, M.A. Humus Forms in Forest Soils: Concepts and Classifications. Eurasian Soil Sci. 2018, 51, 1142–1153. [Google Scholar] [CrossRef]
- Tatti, D.; Fatton, V.; Sartori, L.; Gobat, J.-M.; Le Bayon, R.-C. What does ‘lignoform’ really mean? Appl. Soil Ecol. 2018, 123, 632–645. [Google Scholar] [CrossRef]
- Zanella, A.; Ponge, J.-F.; Fritz, I.; Pietrasiak, N.; Matteodo, M.; Nadporozhskaya, M.; Juilleret, J.; Tatti, D.; Le Bayon, R.-C.; Rothschild, L.; et al. Humusica 2, article 13: Para humus systems and forms. Appl. Soil Ecol. 2018, 122, 181–199. [Google Scholar] [CrossRef]
- Zanella, A.; Ponge, J.-F.; Briones, M.J.I. Humusica 1, article 8: Terrestrial humus systems and forms—Biological activity and soil aggregates, space-time dynamics. Appl. Soil Ecol. 2018, 122, 103–137. [Google Scholar] [CrossRef]
- Golchin, A.; Baldock, J.A.; Oades, J.M. A model linking organic matter decomposition, chemistry, and aggregate dynamics. In Soil Processes and the Carbon Cycle; CRC Press: Boca Raton, FL, USA, 2018; pp. 245–266. [Google Scholar]
- Hellwig, N.; Gómez-Brandón, M.; Ascher-Jenull, J.; Bardelli, T.; Anschlag, K.; Fornasier, F.; Pietramellara, G.; Insam, H.; Broll, G. Humus forms and soil microbiological parameters in a mountain forest: Upscaling to the slope scale. Soil Syst. 2018, 2, 12. [Google Scholar] [CrossRef]
- Witzgall, K.; Vidal, A.; Schubert, D.I.; Höschen, C.; Schweizer, S.A.; Buegger, F.; Pouteau, V.; Chenu, C.; Mueller, C.W. Particulate organic matter as a functional soil component for persistent soil organic carbon. Nat. Commun. 2021, 12, 4115. [Google Scholar] [CrossRef]
- Ponge, J.-F.; Chevalier, R. Humus Index as an indicator of forest stand and soil properties. For. Ecol. Manag. 2006, 233, 165–175. [Google Scholar] [CrossRef]
- Schaefer, M.; Schauermann, J. The soil fauna of beech forests: Comparison between a mull and a moder soil. Pedobiologia 1990, 34, 299–314. [Google Scholar]
- Ponge, J.-F.; Zanella, A.; Sartori, G.; Jabiol, B. Terrestrial Humus Forms: Ecological Relevance and Classification. 2010. Available online: https://hal.science/hal-00521337/document (accessed on 14 June 2023).
- Andreetta, A.; Ciampalini, R.; Moretti, P.; Vingiani, S.; Poggio, G.; Matteucci, G.; Tescari, F.; Carnicelli, S. Forest humus forms as potential indicators of soil carbon storage in Mediterranean environments. Biol. Fertil. Soils 2011, 47, 31–40. [Google Scholar] [CrossRef]
- Bayranvand, M.; Akbarinia, M.; Salehi Jouzani, G.; Gharechahi, J.; Alberti, G. Dynamics of humus forms and soil characteristics along a forest altitudinal gradient in Hyrcanian forest. Iforest-Biogeosci. For. 2021, 14, 26. [Google Scholar] [CrossRef]
- Lehmann, J.; Hansel, C.M.; Kaiser, C.; Kleber, M.; Maher, K.; Manzoni, S.; Nunan, N.; Reichstein, M.; Schimel, J.P.; Torn, M.S. Persistence of soil organic carbon caused by functional complexity. Nat. Geosci. 2020, 13, 529–534. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A.C. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Marland, G.; West, T.O.; Schlamadinger, B.; Canella, L. Managing soil organic carbon in agriculture: The net effect on greenhouse gas emissions. Tellus B Chem. Phys. Meteorol. 2003, 55, 613–621. [Google Scholar] [CrossRef]
- van Breemen, N. Soils as biotic constructs favouring net primary productivity. Geoderma 1993, 57, 183–211. [Google Scholar] [CrossRef]
- Zanella, A.; Bolzonella, C.; Lowenfels, J.; Ponge, J.-F.; Bouché, M.; Saha, D.; Kukal, S.S.; Fritz, I.; Savory, A.; Blouin, M. Humusica 2, article 19: Techno humus systems and global change–conservation agriculture and 4/1000 proposal. Appl. Soil Ecol. 2018, 122, 271–296. [Google Scholar] [CrossRef]
- Bednorz, F.; Reichstein, M.; Broll, G.; Holtmeier, F.-K.; Urfer, W. Humus forms in the forest-alpine tundra ecotone at Stillberg (Dischmatal, Switzerland): Spatial heterogeneity and classification. Arct. Antarct. Alp. Res. 2000, 32, 21–29. [Google Scholar] [CrossRef]
- Ascher, J.; Sartori, G.; Graefe, U.; Thornton, B.; Ceccherini, M.T.; Pietramellara, G.; Egli, M. Are humus forms, mesofauna and microflora in subalpine forest soils sensitive to thermal conditions? Biol. Fertil. Soils 2012, 48, 709–725. [Google Scholar] [CrossRef]
- Kozlova, A.; Martynova, N.; Perfiliev, D.; Ludwig, U. Chemodestructive Fractionation of Humus as an Indicator of the Functional State of Soil Organic Matter. In E3S Web of Conferences 2023; EDP Sciences: Les Ulis, France, 2023; Volume 371, p. 06010. [Google Scholar]
- Mora, J.L.; Molina-Clerencia, M.; Girona-García, A.; Martí-Dalmau, C.; Badía-Villas, D. Factors controlling the buildup of humus and particulate organic matter in European beech and Scots pine stands at their southernmost distribution limits (Moncayo Massif, Spain). Geoderma 2021, 401, 115211. [Google Scholar] [CrossRef]
- Ponge, J.-F. Etude écologique d’un humus forestier par l’observation d’un petit volume, premiers résultats. I. La couche L1 d’un moder sous pin sylvestre. Rev. D’écol. Biol. Sol 1984, 21, 161–187. [Google Scholar]
- Semeraro, S.; Kipf, P.; Le Bayon, R.-C.; Rasmann, S. Solar radiation explains litter degradation along alpine elevation gradients better than other climatic or edaphic parameters. Front. Microbiol. 2023, 14, 1152187. [Google Scholar] [CrossRef]
- Binz, A.; Thommen, E. Flore de la Suisse; F. Rouge: Lausanne, Switzerland, 1941. [Google Scholar]
- Lauber, K.; Wagner, G. Flora Helvetica; Verlag Paul Haupt: Bern, Switzerland, 2001. [Google Scholar]
- IUSS. World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Zanella, A.; Ponge, J.-F.; Jabiol, B.; Sartori, G.; Kolb, E.; Le Bayon, R.-C.; Gobat, J.-M.; Aubert, M.; De Waal, R.; Van Delft, B. Humusica 1, article 5: Terrestrial humus systems and forms—Keys of classification of humus systems and forms. Appl. Soil Ecol. 2018, 122, 75–86. [Google Scholar] [CrossRef]
- Bernier, N.; Ponge, J.-F. Humus form dynamics during the sylvogenetic cycle in a mountain spruce forest. Soil Biol. Biochem. 1994, 26, 183–220. [Google Scholar] [CrossRef]
- Jabiol, B.; Baize, D. Guide Pour la Description des Sols; Editions Quae: Versailles, France, 2011. [Google Scholar]
- Jahn, R.; Blume, H.P.; Asio, V.B.; Spaargaren, O.; Schad, P. Guidelines for Soil Description; FAO: Rome, Italy, 2006. [Google Scholar]
- Burke, I.C.; Yonker, C.M.; Parton, W.J.; Cole, C.V.; Flach, K.; Schimel, D.S. Texture, climate, and cultivation effects on soil organic matter content in US grassland soils. Soil Sci. Soc. Am. J. 1989, 53, 800–805. [Google Scholar]
- Allen, S.E.; Grimshaw, H.M.; Parkinson, J.A.; Quarmby, C. Chemical Analysis of Ecological Materials; Blackwell Scientific Publications: Hoboken, NJ, USA, 1974. [Google Scholar]
- Ciesielski, H.; Sterckeman, T.; Santerne, M.; Willery, J.P. Determination of cation exchange capacity and exchangeable cations in soils by means of cobalt hexamine trichloride. Effects of experimental conditions. Agronomie 1997, 17, 1–7. [Google Scholar]
- Keuskamp, J.A.; Dingemans, B.J.J.; Lehtinen, T.; Sarneel, J.M.; Hefting, M.M. Tea Bag Index: A novel approach to collect uniform decomposition data across ecosystems. Methods Ecol. Evol. 2013, 4, 1070–1075. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, version 3.5.1; R Core Team: Vienna, Austria, 2013; Available online: https://www.r-project.org/ (accessed on 14 June 2023).
- Wickham, H.; Chang, W. Package ‘ggplot2’: Create Elegant Data Visualisations Using the Grammar of Graphics, Version 2.1.0. 2016. Available online: https://cran.r-project.org/src/contrib/Archive/ggplot2/ (accessed on 14 June 2023).
- Beaudette, D.E.; Roudier, P.; O’Geen, A.T. Algorithms for quantitative pedology: A toolkit for soil scientists. Comput. Geosci. 2013, 52, 258–268. [Google Scholar] [CrossRef]
- Maechler, M. Finding groups in data: Cluster analysis extended Rousseeuw et al. R Package Version 2019, 2, 242–248. [Google Scholar]
- Dray, S.; Dufour, A.-B. The ade4 Package: Implementing the Duality Diagram for Ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar] [CrossRef]
- Oksanen, J. Package ‘Vegan’: Community Ecology Package. 2010. Available online: http://vegan.r-forge.r-project.org/ (accessed on 14 June 2023).
- Chersich, S. Pedogenesis: Humus forms and soils under spruce forest by a morphological approach. Appl. Soil Ecol. 2018, 123, 581–587. [Google Scholar] [CrossRef]
- Duchaufour, P. Pédologie: Tome 1: Pédogenèse et Classification; Elsevier Masson: Paris, France, 1977. [Google Scholar]
- Weil, R.; Brady, N. The Nature and Properties of Soils, 15th ed.; Pearson Education: London, UK, 2017. [Google Scholar]
- Gobat, J.-M.; Guenat, C. Sols et Paysages. Types de Sols, Fonctions et Usages en Europe Moyenne; PPUR: Lausanne, Switzerland, 2019. [Google Scholar]
- Edwards, C.A.; Arancon, N.Q.; Bohlen, P.J.; Hendrix, P. Biology and Ecology of Earthworms; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Veen, G.F.; Sundqvist, M.K.; Wardle, D.A. Environmental factors and traits that drive plant litter decomposition do not determine home-field advantage effects. Funct. Ecol. 2015, 29, 981–991. [Google Scholar] [CrossRef]
- Wallenstein, M.D.; Haddix, M.L.; Ayres, E.; Steltzer, H.; Magrini-Bair, K.A.; Paul, E.A. Litter chemistry changes more rapidly when decomposed at home but converges during decomposition–transformation. Soil Biol. Biochem. 2013, 57, 311–319. [Google Scholar] [CrossRef]
- Austin, A.T.; Vivanco, L.; González-Arzac, A.; Pérez, L.I. There’s No Place Like Home? An Exploration of the Mechanisms behind Plant Litter–Decomposer Affinity in Terrestrial Ecosystems. New Phytol. 2014, 204, 307–314. [Google Scholar] [CrossRef]
- Lin, H.; Li, Y.; Bruelheide, H.; Zhang, S.; Ren, H.; Zhang, N.; Ma, K. What drives leaf litter decomposition and the decomposer community in subtropical forests–the richness of the above-ground tree community or that of the leaf litter? Soil Biol. Biochem. 2021, 160, 108314. [Google Scholar] [CrossRef]
- Rosenfield, M.V.; Keller, J.K.; Clausen, C.; Cyphers, K.; Funk, J.L. Leaf traits can be used to predict rates of litter decomposition. Oikos 2020, 129, 1589–1596. [Google Scholar] [CrossRef]
- Badía-Villas, D.; Girona-García, A. Soil humus changes with elevation in Scots pine stands of the Moncayo Massif (NE Spain). Appl. Soil Ecol. 2018, 123, 617–621. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, W.; He, X. Temporal dynamics of abiotic and biotic factors on leaf litter of three plant species in relation to decomposition rate along a subalpine elevation gradient. PLoS ONE 2013, 8, e62073. [Google Scholar]
- Bian, H.; Li, C.; Zhu, J.; Xu, L.; Li, M.; Zheng, S.; He, N. Soil moisture affects the rapid response of microbes to labile organic C addition. Front. Ecol. Evol. 2022, 10, 857185. [Google Scholar] [CrossRef]
- Matteodo, M.; Grand, S.; Sebag, D.; Rowley, M.C.; Vittoz, P.; Verrecchia, E.P. Decoupling of topsoil and subsoil controls on organic matter dynamics in the Swiss Alps. Geoderma 2018, 330, 41–51. [Google Scholar] [CrossRef]
- A’Bear, A.D.; Jones, T.H.; Kandeler, E.; Boddy, L. Interactive effects of temperature and soil moisture on fungal-mediated wood decomposition and extracellular enzyme activity. Soil Biol. Biochem. 2014, 70, 151–158. [Google Scholar] [CrossRef]
- Didion, M.; Repo, A.; Liski, J.; Forsius, M.; Bierbaumer, M.; Djukic, I. Towards harmonizing leaf litter decomposition studies using standard tea bags—A field study and model application. Forests 2016, 7, 167. [Google Scholar]
- Duddigan, S.; Shaw, L.J.; Alexander, P.D.; Collins, C.D. Chemical underpinning of the tea bag index: An examination of the decomposition of tea leaves. Appl. Environ. Soil Sci. 2020, 2020, 6085180. [Google Scholar] [CrossRef]
- Hellwig, N.; Tatti, D.; Sartori, G.; Anschlag, K.; Graefe, U.; Egli, M.; Gobat, J.-M.; Broll, G. Modeling spatial patterns of humus forms in montane and subalpine forests: Implications of local variability for upscaling. Sustainability 2018, 11, 48. [Google Scholar] [CrossRef]
- Zhu, M.; Feng, Q.; Qin, Y.; Cao, J.; Zhang, M.; Liu, W.; Deo, R.C.; Zhang, C.; Li, R.; Li, B. The role of topography in shaping the spatial patterns of soil organic carbon. Catena 2019, 176, 296–305. [Google Scholar]
- Hyde, K.D.; Fryar, S.; Tian, Q.; Bahkali, A.H.; Xu, J. Lignicolous freshwater fungi along a north–south latitudinal gradient in the Asian/Australian region; can we predict the impact of global warming on biodiversity and function? Fungal Ecol. 2016, 19, 190–200. [Google Scholar]
- Riley, R.; Salamov, A.A.; Brown, D.W.; Nagy, L.G.; Floudas, D.; Held, B.W.; Levasseur, A.; Lombard, V.; Morin, E.; Otillar, R. Extensive sampling of basidiomycete genomes demonstrates inadequacy of the white-rot/brown-rot paradigm for wood decay fungi. Proc. Natl. Acad. Sci. USA 2014, 111, 9923–9928. [Google Scholar]
- Fukasawa, Y.; Matsukura, K. Decay stages of wood and associated fungal communities characterise diversity–decomposition relationships. Sci. Rep. 2021, 11, 8972. [Google Scholar]
- Desie, E.; Van Meerbeek, K.; De Wandeler, H.; Bruelheide, H.; Domisch, T.; Jaroszewicz, B.; Joly, F.-X.; Vancampenhout, K.; Vesterdal, L.; Muys, B. Positive feedback loop between earthworms, humus form and soil pH reinforces earthworm abundance in European forests. Funct. Ecol. 2020, 34, 2598–2610. [Google Scholar] [CrossRef]
- Lindahl, B.D.; de Boer, W.; Finlay, R.D. Disruption of root carbon transport into forest humus stimulates fungal opportunists at the expense of mycorrhizal fungi. ISME J. 2010, 4, 872–881. [Google Scholar] [CrossRef]
- Salmon, S. Changes in humus forms, soil invertebrate communities and soil functioning with forest dynamics. Appl. Soil Ecol. 2018, 123, 345–354. [Google Scholar] [CrossRef]
- Yudina, A.; Kuzyakov, Y. Dual nature of soil structure: The unity of aggregates and pores. Geoderma 2023, 434, 116478. [Google Scholar] [CrossRef]
- Daebeler, A.; Petrová, E.; Kinz, E.; Grausenburger, S.; Berthold, H.; Sandén, T.; Angel, R. Pairing litter decomposition with microbial community structures using the Tea Bag Index (TBI). Soil 2022, 8, 163–176. [Google Scholar] [CrossRef]
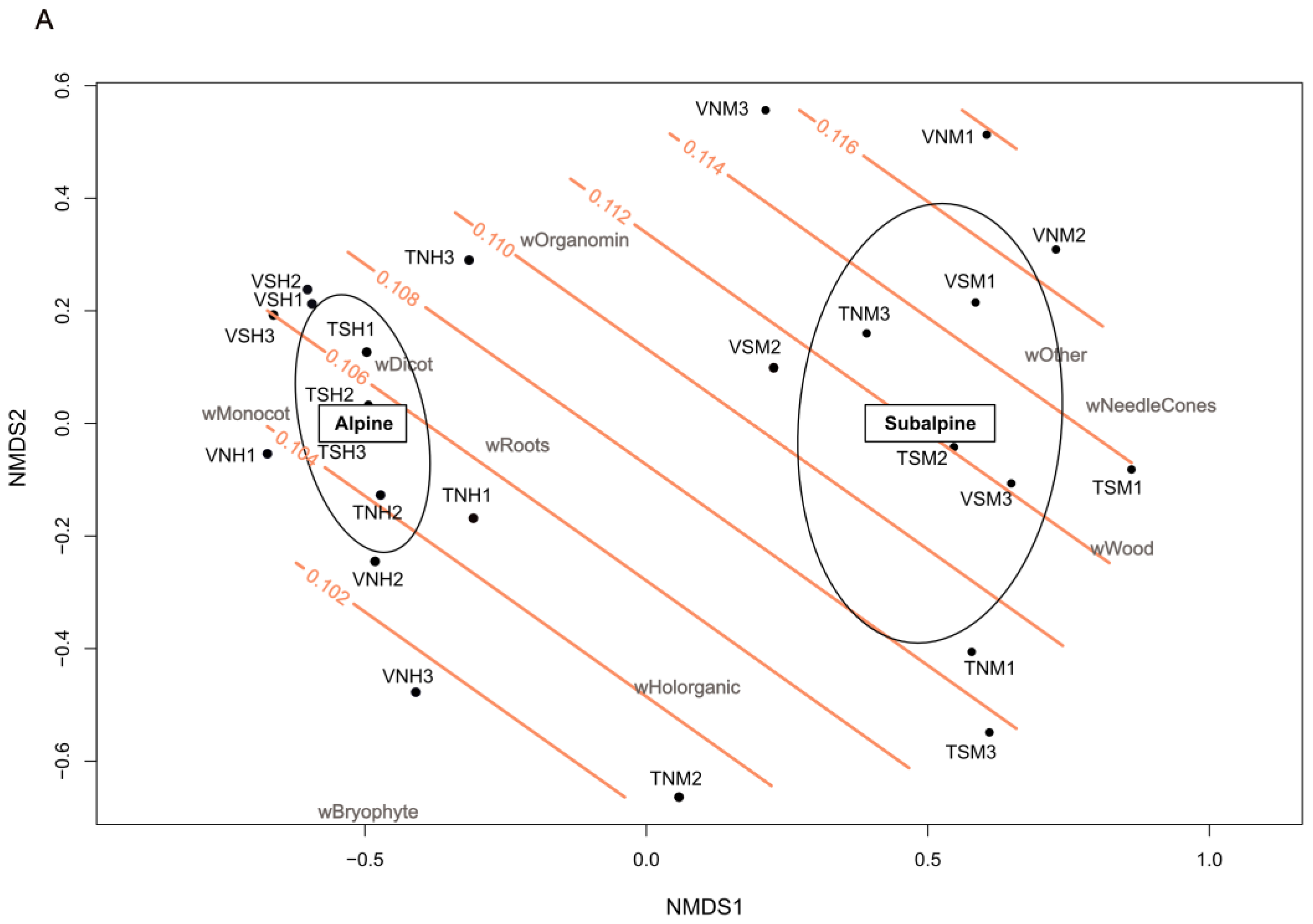
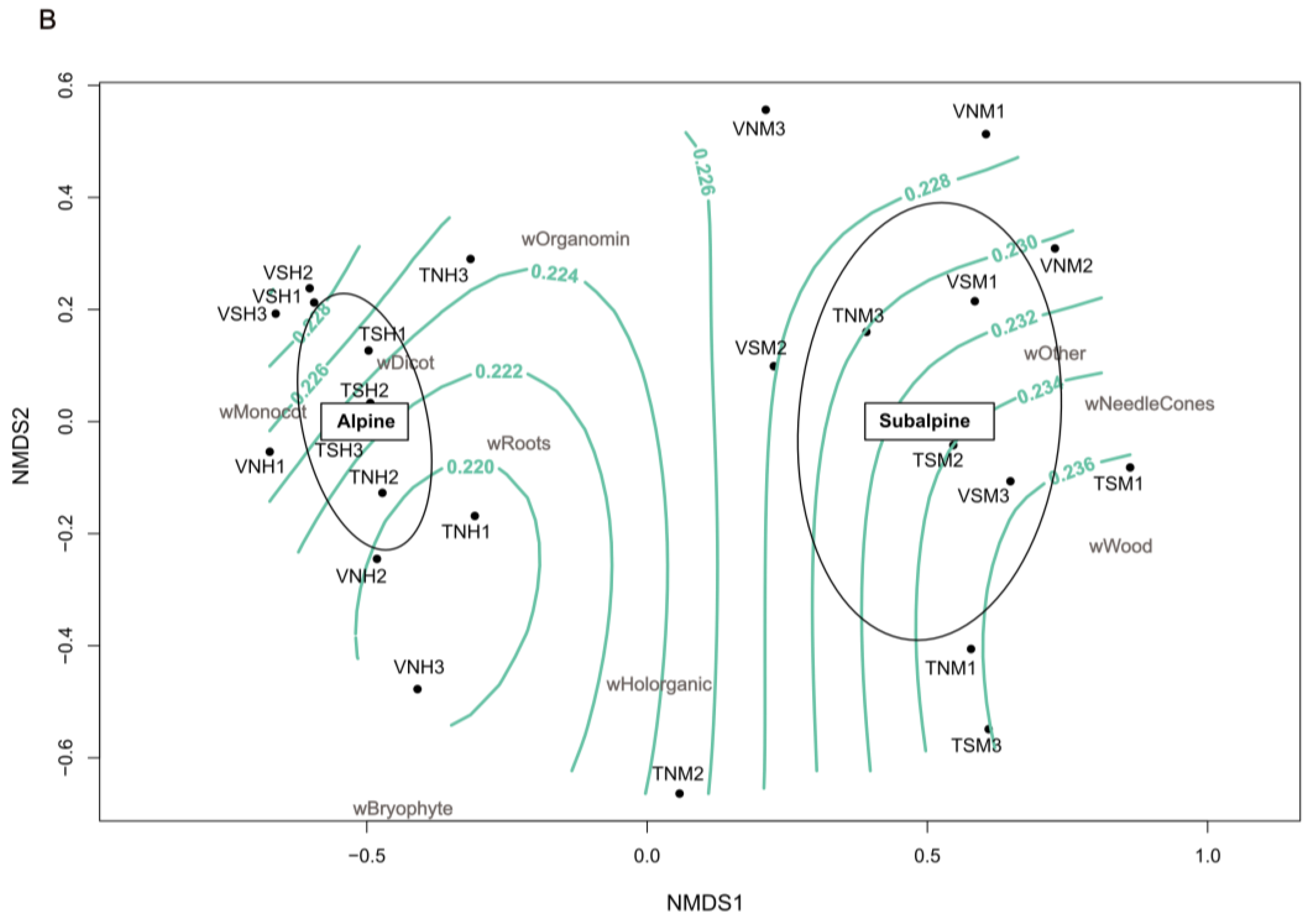
| Group Number | Group Name Abbreviation | Subgroup Content |
|---|---|---|
| 1 | Monocot | Monocotyledon |
| 2 | Monocotyledon.leaf | |
| 3 | Monocotyledon.stem | |
| 4 | Monocotyledons | |
| 5 | Dicot | Dicotyledon.fragmented.leaf |
| 6 | Dicotyledon.intact.leaf | |
| 7 | Dicotyledon.other | |
| 8 | Dicotyledon.skeletonized.leaf | |
| 9 | Dicotyledon.stem | |
| 10 | Dicotyledons | |
| 11 | NeedleCones | Fragmented.Abies.needle |
| 12 | Fragmented.Larix.needle | |
| 13 | Fragmented.Picea.needle | |
| 14 | Fragmented.Pinus.needle | |
| 15 | Gymnosperm.cones | |
| 16 | Gymnosperm.other | |
| 17 | Gymnosperm.twig | |
| 18 | Intact.Abies.needle | |
| 19 | Intact.Larix.needle | |
| 20 | Intact.Picea.needle | |
| 21 | Intact.Pinus.needle | |
| 22 | Roots | Roots [>2 mm] |
| 23 | Roots [0–2 mm] | |
| 24 | Wood | Wood |
| 25 | Bryophyte | Bryophyte |
| 26 | Holorganic | Holorganic |
| 27 | Organomin | Organomin |
| 28 | Mineral | Mineral |
| 29 | Mycelium | Mycelium |
| 30 | Other | Fauna, feces and undetermined holorganic matter |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semeraro, S.; Fazzari, M.; Kipf, P.; Rasmann, S.; Le Bayon, R.-C. Humus Forms and Organic Matter Decomposition in the Swiss Alps. Int. J. Plant Biol. 2023, 14, 729-745. https://doi.org/10.3390/ijpb14030054
Semeraro S, Fazzari M, Kipf P, Rasmann S, Le Bayon R-C. Humus Forms and Organic Matter Decomposition in the Swiss Alps. International Journal of Plant Biology. 2023; 14(3):729-745. https://doi.org/10.3390/ijpb14030054
Chicago/Turabian StyleSemeraro, Sarah, Maud Fazzari, Pascal Kipf, Sergio Rasmann, and Renée-Claire Le Bayon. 2023. "Humus Forms and Organic Matter Decomposition in the Swiss Alps" International Journal of Plant Biology 14, no. 3: 729-745. https://doi.org/10.3390/ijpb14030054
APA StyleSemeraro, S., Fazzari, M., Kipf, P., Rasmann, S., & Le Bayon, R.-C. (2023). Humus Forms and Organic Matter Decomposition in the Swiss Alps. International Journal of Plant Biology, 14(3), 729-745. https://doi.org/10.3390/ijpb14030054






