Screening of the Most Effective Media for Bioprospecting Three Indigenous Freshwater Microalgae Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Cultivation and Morphometric Analysis
2.2. Screening of Cultivation Media for Maximum Biomass, Lipids, and Carotenoids
2.3. Biomass Estimation
2.4. Quantification of Lipids
2.5. Pigment Estimation
2.6. Microscopic Examination of Chlorophyll, Lipid, and Lipophilic Compounds
2.7. Pigments Quantification
2.8. TLC Analysis
2.9. Statistics
3. Results and Discussion
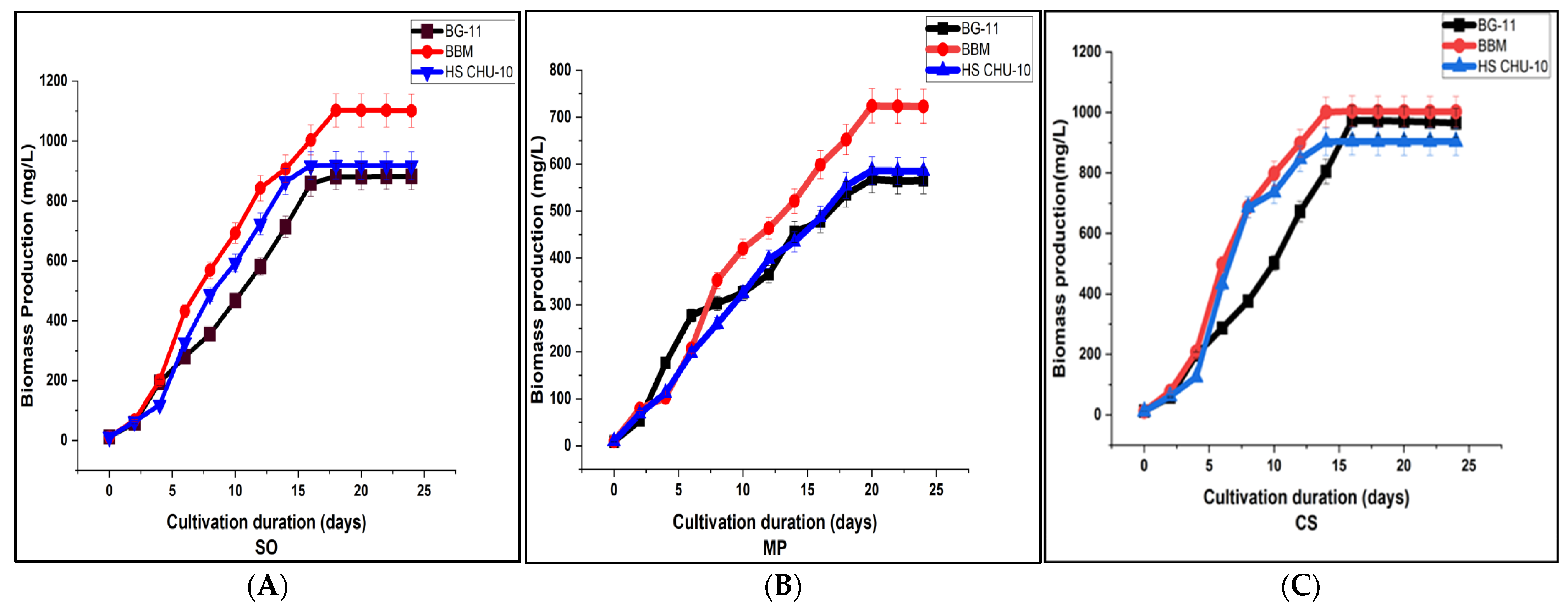
3.1. Biomass Analysis
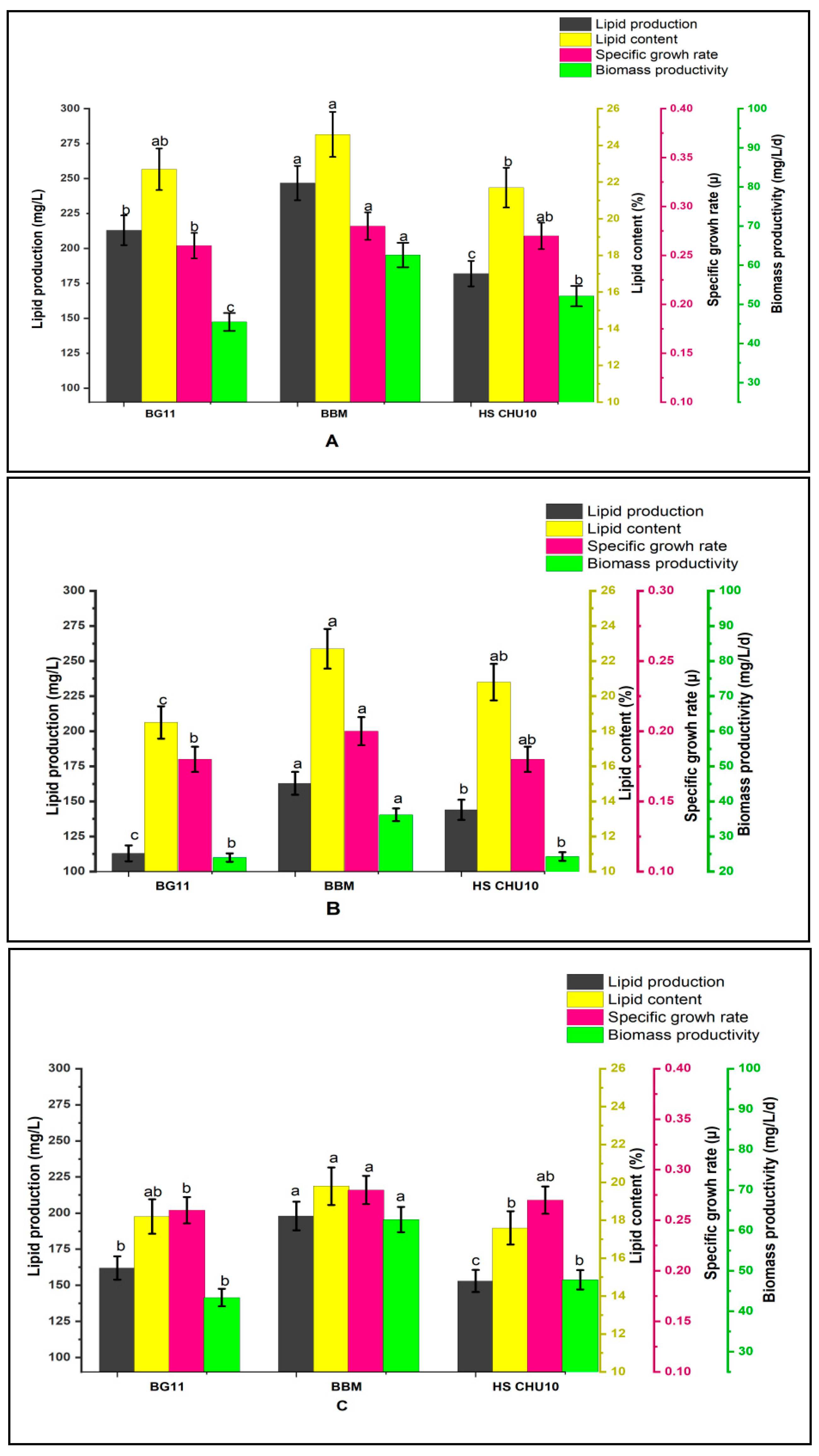
3.2. Lipids Analysis
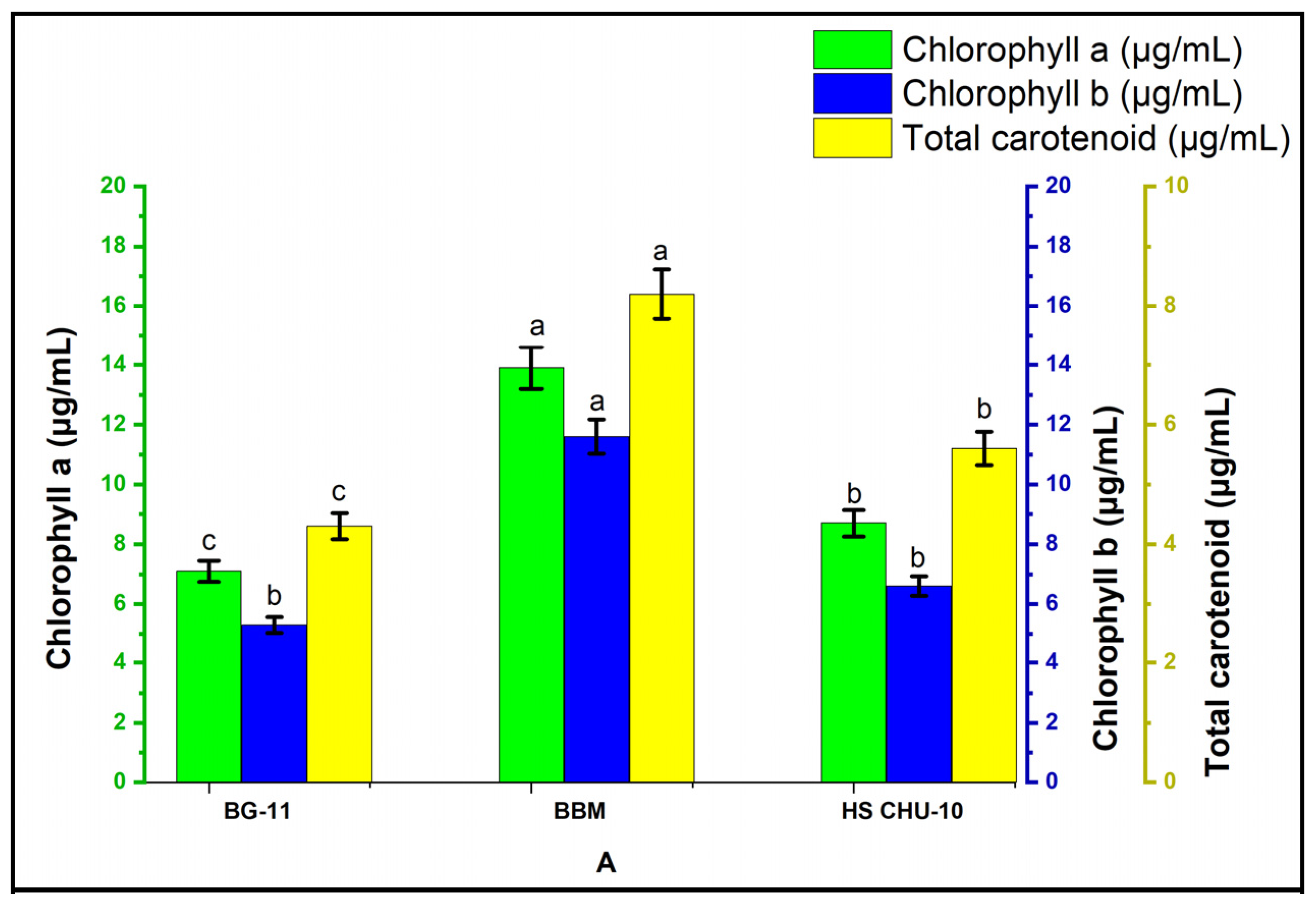
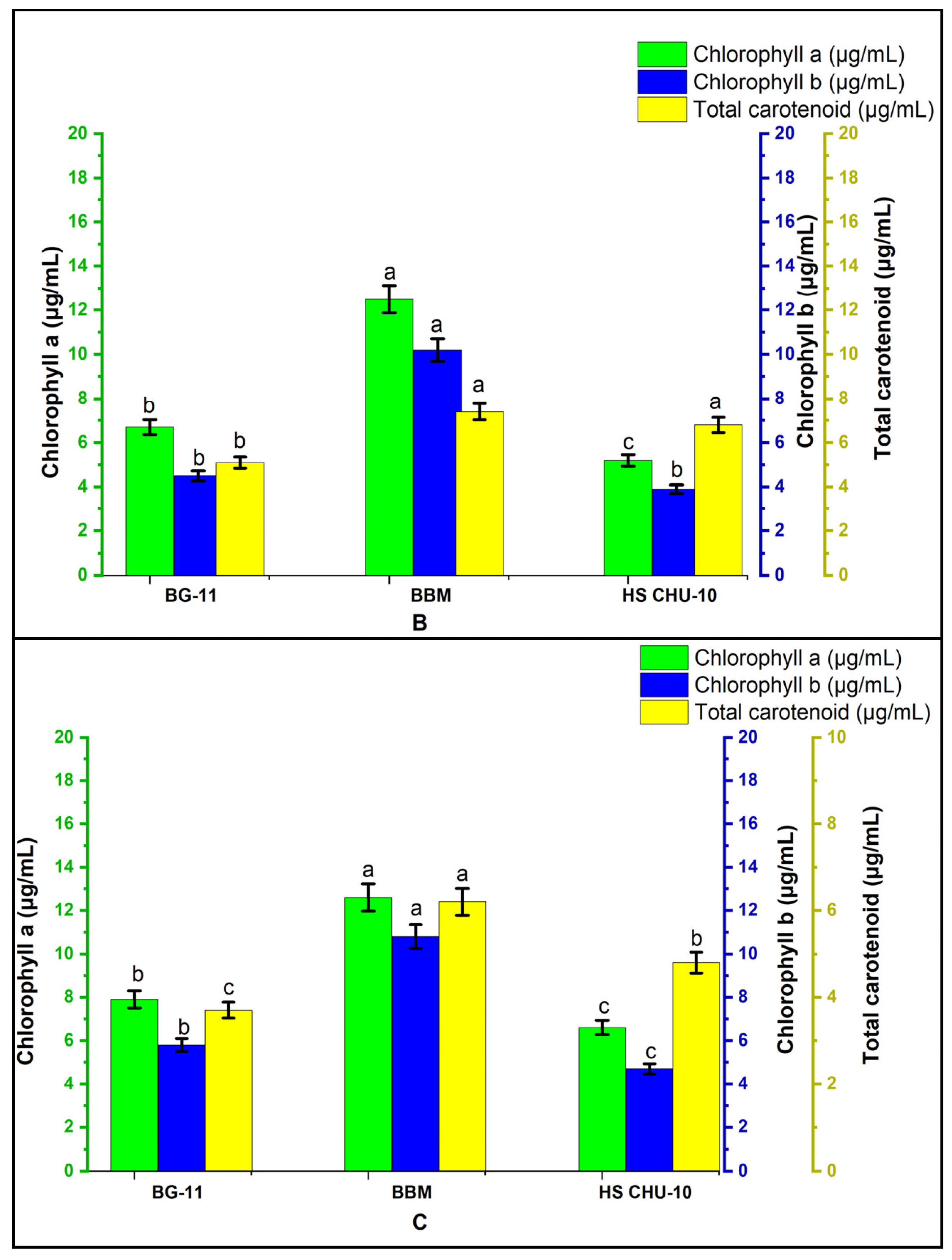
3.3. Pigment Analysis
3.4. Confocal Imaging for the Visualization of Lipophilic Compounds
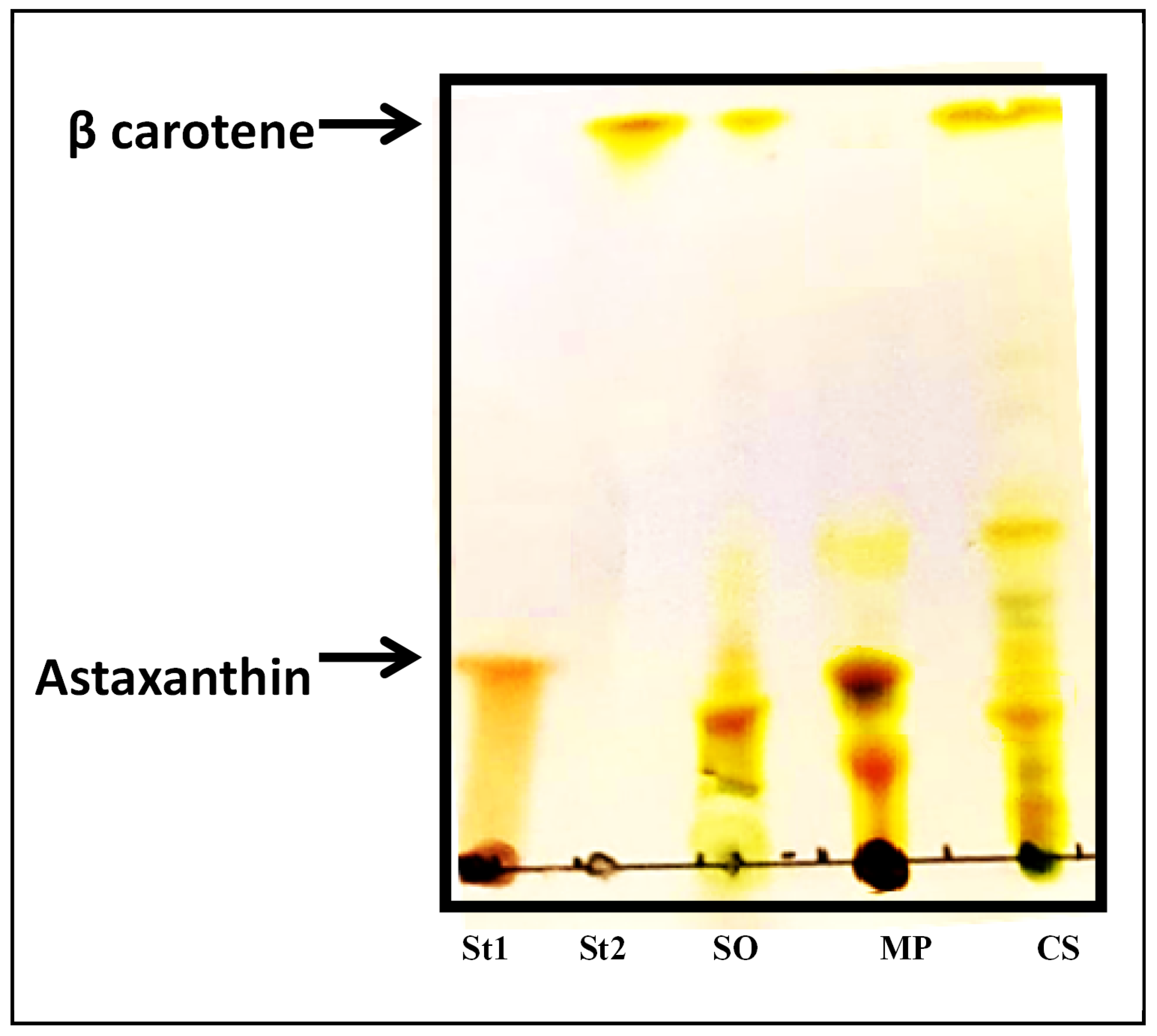
3.5. Carotenoid Chromatography
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Putri, R.D.; Pradana, Y.S.; Koerniawan, M.D.; Suwanti, L.T.; Siregar, U.J.; Budiman, A.; Suyono, E.A. Effect of distinct nitrate concentrations on pigment content of mixed culture of Chlorella vulgaris and Dunaliella sp. Asia-Pac. J. Mol. Biol. Biotechnol. 2022, 30, 15–23. [Google Scholar] [CrossRef]
- Singh, B.; Guldhe, A.; Singh, P.; Singh, A.; Rawat, I.; Bux, F. Sustainable production of biofuels from microalgae using a biorefinery approach. In Applied Environmental Biotechnology: Present Scenario and Future Trends; Springer: New Delhi, Inida, 2015; pp. 115–128. [Google Scholar]
- Sun, T.; Rao, S.; Zhou, X.; Li, L. Plant carotenoids: Recent advances and future perspectives. Mol. Hortic. 2022, 2, 3. [Google Scholar] [CrossRef]
- Watcharawipas, A.; Runguphan, W. Red yeasts and their carotenogenic enzymes for microbial carotenoid production. FEMS Yeast Res. 2023, foac063. [Google Scholar] [CrossRef] [PubMed]
- Siddiki, S.Y.A.; Mofijur, M.; Kumar, P.S.; Ahmed, S.F.; Inayat, A.; Kusumo, F.; Badruddin, I.A.; Khan, T.Y.; Nghiem, L.D.; Ong, H.C.; et al. Microalgae biomass as a sustainable source for biofuel, biochemical and biobased value-added products: An integrated biorefinery concept. Fuel 2022, 307, 121782. [Google Scholar] [CrossRef]
- Debnath, C.; Bandyopadhyay, T.K.; Bhunia, B.; Mishra, U.; Narayanasamy, S.; Muthuraj, M. Microalgae: Sustainable resource of carbohydrates in third-generation biofuel production. Renew. Sustain. Energy Rev. 2021, 150, 111464. [Google Scholar] [CrossRef]
- Timotius, V.; Suyono, E.A.; Suwanti, L.T.; Koerniawan, M.D.; Budiman, A.; Siregar, U.J. The content of lipid, chlorophyll, and carotenoid of Euglena sp. under various salinities. Asia-Pac. J. Mol. Biol. Biotechnol. 2022, 30, 114–122. [Google Scholar] [CrossRef]
- de Carvalho, J.C.; Sydney, E.B.; Tessari, L.F.; Soccol, C.R. Culture media for mass production of microalgae. In Biofuels from Algae; Elsevier: Amsterdam, The Netherlands, 2019; pp. 33–50. [Google Scholar]
- Zhang, L.; Pei, H.; Chen, S.; Jiang, L.; Hou, Q.; Yang, Z.; Yu, Z. Salinity-induced cellular cross-talk in carbon partitioning reveals starch-to-lipid biosynthesis switching in low-starch freshwater algae. Bioresour. Technol. 2018, 250, 449–456. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Yadav, K.; Kumar, S.; Nikalje, G.C.; Rai, M.P. Combinatorial Effect of Multiple Variables on Up-Regulation of Carotenoids and Lipids in Monoraphidium sp. for Pharmacological and Nutraceutical Applications. Appl. Sci. 2023, 13, 6107. [Google Scholar] [CrossRef]
- Agarwal, A.; Jeevanandham, S.; Kar, C.; Rai, M.P.; Kumar, V.; Bohidar, H.B.; Biswas, S.; Mukherjee, M. Crystalline Domains Nested on Two-Dimensional Nanosheets as Heterogeneous Nanomachineries for the Sustainable Production of Bioactive Compounds from Chlorella sorokiniana. ACS Sustain. Chem. Eng. 2022, 10, 9732–9748. [Google Scholar] [CrossRef]
- Zohir, W.F.; Kapase, V.U.; Kumar, S. Identification and Characterization of a New Microalga Dysmorphococcusglobosus-HI from the Himalayan Region as a Potential Source of Natural Astaxanthin. Biology 2022, 11, 884. [Google Scholar] [CrossRef] [PubMed]
- Radzun, K.A.; Wolf, J.; Jakob, G.; Zhang, E.; Stephens, E.; Ross, I.; Hankamer, B. Automated nutrient screening system enables high-throughput optimisation of microalgae production conditions. Biotechnol. Biofuel. 2015, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Chankhong, K.; Chotigeat, W.; Iewkittayakorn, J. Effects of culture medium on growth kinetics and fatty acid composition of Chlorella sp. T12. Songklanakarin J. Sci. Technol. 2018, 40, 1098–1104. [Google Scholar]
- George, B.; Pancha, I.; Desai, C.; Chokshi, K.; Paliwal, C.; Ghosh, T.; Mishra, S. Effects of different media composition, light intensity and photoperiod on morphology and physiology of freshwater microalgae Ankistrodesmusfalcatus–A potential strain for bio-fuel production. Bioresour. Technol. 2014, 171, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Sangapillai, K.; Marimuthu, T. Isolation and selection of growth medium for freshwater microalgae Asterarcys quadricellulare for maximum biomass production. Water Sci. Technol. 2019, 80, 2027–2036. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.S.; Gondi, R.; Vincent, G.S.; JohnSamuel, G.C.; Jeyakumar, R.B. Microalgae Biomass and Lipids as Feedstock for Biofuels: Sustainable Biotechnology Strategies. Sustainability 2022, 14, 15070. [Google Scholar] [CrossRef]
- Yee, W. Microalgae from the Selenastraceae as emerging candidates for biodiesel production: A mini review. World J. Microbiol. Biotechnol. 2016, 32, 64. [Google Scholar] [CrossRef]
- Kirrolia, A.; Bishnoi, N.R.; Singh, R. Effect of shaking, incubation temperature, salinity and media composition on growth traits of green microalgae Chlorococcum sp. J. Algal Biomass Util. 2012, 3, 46–53. [Google Scholar]
- Yadav, K.; Vasistha, S.; Nawkarkar, P.; Kumar, S.; Rai, M.P. Algal biorefinery culminating multiple value-added products: Recent advances, emerging trends, opportunities, and challenges. 3 Biotech 2022, 12, 244. [Google Scholar] [CrossRef]
- Mapelli-Brahm, P.; Gómez-Villegas, P.; Gonda, M.L.; León-Vaz, A.; León, R.; Mildenberger, J.; Rebours, C.; Saravia, V.; Vero, S.; Vila, E.; et al. Microalgae, Seaweeds and Aquatic Bacteria, Archaea, and Yeasts: Sources of Carotenoids with Potential Antioxidant and Anti-Inflammatory Health-Promoting Actions in the Sustainability Era. Mar. Drugs 2023, 21, 340. [Google Scholar] [CrossRef]
- Japar, A.S.; Takriff, M.S.; Yasin, N.H. Microalgae acclimatization in industrial wastewater and its effect on growth and primary metabolite composition. Algal Res. 2021, 53, 102163. [Google Scholar] [CrossRef]
- Rajput, A.; Singh, D.P.; Khattar, J.S.; Swatch, G.K.; Singh, Y. Evaluation of growth and carotenoid production by a green microalga Scenedesmus quadricauda PUMCC 4.1. 40. under optimized culture conditions. J. Basic Microbiol. 2022, 62, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Kaha, M.; Iwamoto, K.; Yahya, N.A.; Suhaimi, N.; Sugiura, N.; Hara, H.; Othman, N.A.; Zakaria, Z.; Suzuki, K. Enhancement of astaxanthin accumulation using black light in Coelastrum and Monoraphidium isolated from Malaysia. Sci. Rep. 2021, 11, 11708. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.S.; Cokus, S.J.; Gallaher, S.D.; Walter, A.; Lopez, D.; Erickson, E.; Endelman, B.; Westcott, D.; Larabell, C.A.; Merchant, S.S.; et al. Chromosome-level genome assembly and transcriptome of the green alga Chromochloriszofingiensis illuminates astaxanthin production. Proc. Natl. Acad. Sci. USA 2017, 114, E4296–E4305. [Google Scholar] [CrossRef]


| BG-11 | g/L | BBM | g/L | HS-CHU10 | g/L |
|---|---|---|---|---|---|
| NaNO3 | 1.5 | NaNO3 | 0.25 | Ca(NO3)2.4H2O | 2 |
| K2HPO4 | 0.04 | K2HPO4 | 0.075 | K2HPO4 | 0.25 |
| MgSO4.7H2O | 0.075 | MgSO4.7H2O | 0.075 | MgSO4.7H2O | 1.25 |
| CaCl2.2H2O | 0.036 | CaCl2.2H2O | 0.025 | Na2CO3 | 1 |
| Na2CO3 | 0.02 | KH2PO4 | 0.175 | Na2SiO3 | 1.25 |
| Na2EDTA | 0.001 | NaCl | 0.025 | FeCl3 | 0.04 |
| Ferric ammonium citrate | 0.006 | H3BO3 | 0.011 | ||
| Citric acid | 0.006 | FeSO4.7H2O | 0.004 | ||
| Na2 EDTA | 0.05 | ||||
| KOH | 0.031 | ||||
| H2SO4 conc. | 1 µL | ||||
| Micronutrients | |||||
| g/L | mg/L | mg/L | |||
| H3BO3 | 2.86 | ZnSO4.7H2O | 8.82 | H3BO3 | 0.24 |
| MnCl2.4H2O | 1.81 | MnCl2.4H2O | 0.44 | MnSO4.H2O | 0.147 |
| ZnSO4.7H2O | 0.22 | MoO3 | 0.71 | ZnSO4.7H2O | 0.023 |
| Na2MoO4.2H2O | 0.039 | CuSO4.5H2O | 1.57 | CuSO4.5H2O | 0.010 |
| CuSO4.5H2O | 0.079 | Co(NO3)2.6H2O | 0.49 | (NH4)6Mo7O24.4H2O | 0.007 |
| Co(NO3)2.6H2O | 0.049 | Vit B1 | 10 µg | Co(NO3)2.6H2O | 0.014 |
| Vit B12 | 10 µg | Vit B1 | 5 | ||
| Vit B7 | 2.5 | ||||
| Vit B12 | 2.5 |
| Species | Pigment Compositions | BG-11 | BBM | HS CHU10 |
|---|---|---|---|---|
| Scenedesmus obliquus | Chlorophyll-a (µg/mL) | 12.8 ± 0.64 | 13.9 ± 0.69 | 13.2 ± 0.66 |
| Chlorophyll-b (µg/mL) | 10.9 ± 0.54 | 11.6 ± 0.58 | 11.3 ± 0.56 | |
| Chlorophyll a + b (µg/mL) | 23.7 ± 1.18 | 25.5 ± 1.27 | 24.5 ± 1.22 | |
| Chlorophyll a/b | 1.17 ± 0.05 | 1.19 ± 0.05 | 1.16 ± 0.05 | |
| Carotenoid (µg/mL) | 7.6 ± 0.07 | 8.2 ± 0.08 | 7.9 ± 0.07 | |
| Monoraphidium sp. | Chlorophyll-a (µg/mL) | 10.7 ± 0.53 | 12.5 ± 0.62 | 11.2 ± 0.56 |
| Chlorophyll-b (µg/mL) | 9.5 ± 0.47 | 10.2 ± 0.51 | 9.9 ± 0.49 | |
| Chlorophyll a + b (µg/mL) | 20.2 ± 1.01 | 22.7 ± 1.13 | 21.1 ± 1.05 | |
| Chlorophyll a/b | 1.12 ± 0.05 | 1.22 ± 0.06 | 1.13 ± 0.05 | |
| Carotenoid (µg/mL) | 6.8 ± 0.34 | 7.4 ± 0.37 | 8.9 ± 0.44 | |
| Chlorella sorokiniana | Chlorophyll-a (µg/mL) | 11.6 ± 0.58 | 12.6 ± 0.63 | 12.3 ± 0.61 |
| Chlorophyll-b (µg/mL) | 9.7 ± 0.48 | 10.8 ± 0.54 | 10.2 ± 0.51 | |
| Chlorophyll a + b (µg/mL) | 21.3 ± 1.06 | 23.4 ± 1.17 | 22.5 ± 1.12 | |
| Chlorophyll a/b | 1.19 ± 0.05 | 1.16 ± 0.05 | 1.2 ± 0.06 | |
| Carotenoid (µg/mL) | 5.7 ± 0.28 | 6.2 ± 0.31 | 7.36 ± 0.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, K.; Nikalje, G.C.; Pramanik, D.; Suprasanna, P.; Rai, M.P. Screening of the Most Effective Media for Bioprospecting Three Indigenous Freshwater Microalgae Species. Int. J. Plant Biol. 2023, 14, 558-570. https://doi.org/10.3390/ijpb14030044
Yadav K, Nikalje GC, Pramanik D, Suprasanna P, Rai MP. Screening of the Most Effective Media for Bioprospecting Three Indigenous Freshwater Microalgae Species. International Journal of Plant Biology. 2023; 14(3):558-570. https://doi.org/10.3390/ijpb14030044
Chicago/Turabian StyleYadav, Kushi, Ganesh Chandrakant Nikalje, Dibyajyoti Pramanik, Penna Suprasanna, and Monika Prakash Rai. 2023. "Screening of the Most Effective Media for Bioprospecting Three Indigenous Freshwater Microalgae Species" International Journal of Plant Biology 14, no. 3: 558-570. https://doi.org/10.3390/ijpb14030044
APA StyleYadav, K., Nikalje, G. C., Pramanik, D., Suprasanna, P., & Rai, M. P. (2023). Screening of the Most Effective Media for Bioprospecting Three Indigenous Freshwater Microalgae Species. International Journal of Plant Biology, 14(3), 558-570. https://doi.org/10.3390/ijpb14030044







