Abstract
The plant Ranunculus acris (L.) is widespread. It is used in folk medicine. The use of herbal medicines can have adverse effects on humans and requires studies of herbal raw materials for genotoxicity. The goals of the study were to examine the toxic and genotoxic effects of aqueous solutions of R. acris flowers at concentrations of 0.625%, 1.25%, 2.5%, 5% and 10% using the Allium test and to determine the numbers of flavonoids and polyphenols and the antioxidant activity of the extracts. It has been shown that aqueous solutions of buttercup flower extract have a depressing effect on mitosis in the cells of the root meristem of Allium cepa (L.) and cause chromosomal abnormalities (chromosomal stickiness, chromosome lagging, nuclear buds and bridges in anaphase).
1. Introduction
The Buttercup family (Ranunculaceae) is known for its use in both folk and traditional medicine [1]. The pharmaceutical importance of the buttercup family is reflected in the fact that a number of monographs describing various species of this family are a part of the pharmacopoeia. One well-known plant used in folk medicine is Ranunculus acris (L.) (Figure 1).

Figure 1.
Ranunculus acris (L.).
This plant is widely distributed in the territory of the Murmansk region, and every year it occupies new habitats, despite the severity of climatic factors and a short summer. However, scientific data on its phytochemical composition and biological activity of substances are scarce. The aerial parts of R. acris, and to a greater extent, the flowers, are known to contain high levels of ranunculin, a protoanemonin precursor. Protoanemonin can exhibit both positive and negative effects due to its chemical and ambivalent biological properties. Protoanemonin, γ-4-hydroxy-penta-2,4-dienoic acid lactone (γ-hydroxyvinylacrylic acid), known as a characteristic component produced by many members of the Ranunculaceae family, has been proposed as a taxonomic marker. The compound itself is not found in plant material but is released from its glycosidic precursor, ranunculin, upon drying by converting endogenous plant enzymes to protoanemonin. Protoanemonin is also known for its irritant properties [2]. Phytoderivatives for medical purposes undergo the necessary preclinical safety assessment for genotoxicity today. The need for such assessment is demonstrated by several examples of recent studies. [3]. A huge number of various test systems are used, including plant ones, when analysis is done. Plants most commonly used in such assays include beans (Vicia faba L.), onions (Allium cepa L.), corn (Zea mays L.), etc. For the present investigation, we selected the onion as a test plant, as it serves an excellent model due to the ease of working with the cells of its root meristem, which are large (both cells and chromosomes) [4]. The classic Allium test provides the detection of cytotoxicity and genotoxicity, which provides important information for assessing the mechanisms of action of chromosomal aberrations and disorders in the mitotic cycle [5]. Not enough data have been collected on the effect of R. acris extracts on test systems at present. The goal of the study was to examine the toxic and genotoxic effects of aqueous extracts of R. acris flowers using A. cepa.
2. Materials and Methods
2.1. Collection and Storage of Plant Material
Plant material was collected on the territory of the experimental site of the Polar-Alpine Botanical Garden-Institute of Kola Science Centre of the Russian Academy of Sciences, Kirovsk, Murmansk region (67°36′53″ N 33°40′21″ E). Altitude above sea level: 387 m. Plant collection date: 7 June 2022. Phenological phase: flowering. The plant material was identified by the employees of the botanical garden. The collection was carried out by the method of obtaining a composite sample. Aerial parts of plants were collected from a plot measuring 5 m × 5 m. After collection, the plant material was sorted into various parts: leaves, stems and flowers. Drying and storage of plant material was carried out in accordance with the rules of the International Pharmacopoeia [6]. To prepare the plant material for extraction, it was additionally crushed with an electric grinder and sifted through a sieve (d = 1 mm). Then, the material was further dried at 45 °C until it reached constant weight. After drying, the plant material was stored for no more than 7 days before the extraction process.
2.2. Extraction
Extraction was carried out using an ultrasonic bath, VILITEK VBS-3D (Moscow, Russia, 2020), at a temperature of 45 °C for 60 min. The ultrasound power was 120 W; ultrasound frequency was 40 kHz. Millipore quality deionized water was used as the solvent. Weighed portions of plant material were mixed with the solvent in a ratio of 1:10 (m/v). The obtained mixture after extraction was centrifuged for 10 min at 1500 rpm in an ELMI Multi CM-6 centrifuge (Riga, Latvia, 2005). Then, the obtained extract was diluted by the binary dilution method at concentrations of 10%, 5%, 2.5%, 1.25%, 0.6125% for Allium test.
2.3. Determination of the Total Content of Flavonoids
Total flavonoid content (TFC) was determined by reaction with 2% aluminum chloride (AlCl3). The technique is described in detail in previous work. [7]. The extracts were preliminarily diluted 100 times. Then, the optical density (A) of the solution was measured at a wavelength of 420 nm. Calibration was carried out using rutin solutions in the concentration range of 100–1000 µg/mL; calibration equation: y = 4.363x + 0.4755 (R2 = 0.9914). The total content of flavonoids in the obtained extract is expressed as mg of rutin equivalent (RE) per 1 g of plant material.
2.4. The Total Content of Phenolic Components
The total phenolic content (TPC) was determined by reaction with the Folin–Ciocalteu reagent [8]. The extracts were preliminarily diluted 100 times. Then, the optical density was measured at a wavelength of 765 nm. Calibration was carried out using gallic acid in the concentration range of 10–200 µg/mL; calibration equation: y = 4.630x + 45.505 (R2 = 0.9979). The total content of phenolic components was expressed in mg of gallic acid equivalent (GAE) per gram of plant material.
2.5. General Antioxidant Activity
The total antioxidant activity (TAC) was determined using the phosphomolybdenum reduction assay [9]. The optical density of solutions was measured at a wavelength of 805 nm. Calibration was carried out using ascorbic acid in the concentration range of 100–1000 µg/mL; calibration equation: y = 0.154x − 0.0865 (R2 = 0.9965). The total content of antioxidant substances in the extract was expressed as mg of ascorbic acid equivalent (AAE) per gram of plant material.
2.6. Free Radical Scavenging
Free radical scavenging (FRS) was determined by reaction with the free radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) [10]. The extract was diluted 50 times before analysis. Optical density was measured at 517 nm. The degree of inhibition was calculated by the formula:
where and are the optical densities at a wavelength of 517 nm of the measured sample and blank one, respectively.
All optical density measurements were carried out with a KFK-3-01 photocolorimeter (ZOMZ, Sergiyev Posad, Russia, 2010) in a 5.024 mm cuvette.
2.7. Allium Test
Onion bulbs of the Stuttgarter Riesen variety (Allium cepa L., 2n = 16) were kept in a cool dark place (+4–5 °C) for 14 days before the experiment in order to synchronize their biological functions and mitosis. Then, the bulbs were cleaned of dry scales and visually inspected. Five bulbs were taken for each concentration and control. Distilled water was used as a control. The experiment lasted 5 days, including preliminary germination of the bulbs in distilled water for 24 h. The roots were cut and fixed in Clark’s fixative for 24 h after 5 days, then washed three times in 80% alcohol (each time for one hour) and placed in sealed test tubes for long-term storage. To obtain preparations, the roots were simultaneously hydrolyzed and stained in ceramic crucibles in a 2% acetoorcein solution (PANECO, Moscow, Russia, 2019), and the solution was boiled in the flame of an alcohol lamp [11]. The roots were left in the dye for 24 h at 4 °C after cooling. To obtain preparations, the root tip was crushed in the presence of one drop of 45% acetic acid. The number of dividing cells was determined in 1000 examined cells per field of view and phase, and chromosome aberrations were noted at ×400 and ×1000 magnification (in immersion oil) using a Micromed 1, v.1-20” (MICROMED, Moscow, Russia, 2019). The shooting was carried out with a Toupcam 2.0 digital Cmos camera (TOUPTEC, Hangzhou, China, 2019) equipped with ToupView software (version 4.10.17614.20200822) for a 1/2.7″ sensor with a resolution of 1920 × 1080 pixels. Over 30,000 cells were counted in total. The mitotic index (MI) was calculated as the portion of dividing cells among the total number of cells in the preparation.
2.8. Statistical Analysis
Statistical analysis was performed in MS Excel 2010. Statistically significant differences were compared at p ≤ 0.05 using ANOVA and Tukey’s HSD (honestly significant difference) tests. All measurements were performed three times for each analysis. The results are expressed as mean ± standard deviation (SD).
2.9. Statistical Analysis of Allium Test
The accumulated data were analyzed using the R programming language in the RStudio software environment. A Shapiro–Wilk test was performed to establish the normality of sampling distribution, and a Bartlett test was performed to determine variance homogeneity.
The optimal sampling size was estimated considering the test power of 80% at p < 0.05. A Tukey test was carried out for multiple comparisons. The differences of total aberrations in the cells of the A. cepa root meristem were calculated using the Kruskal–Wallis statistic and Dunn’s multiple comparison post hoc test.
3. Results
The data of chemical analyzes showed that the determined parameters for different parts of the plant differ significantly at p < 0.05. Based on these results, all aerial parts of the R. acris plant had low free radical scavenging activity. In addition, low levels of polyphenolic components and flavonoids were observed in all above-ground parts. The data obtained in the course of chemical analyzes indicated that the leaves had the highest antioxidant activity (TAC) (Figure 2), and the highest values in terms of TFC, TPC and DPPH (FRS) were observed in the leaves (Figure 3, Figure 4 and Figure 5). Similar patterns of change, depending on the plant organ, were observed for the TPC, TFC and DPPH parameters. To study genotoxicity, flowers were chosen because they are the main source of protoanemonin which has a toxic effect [3].
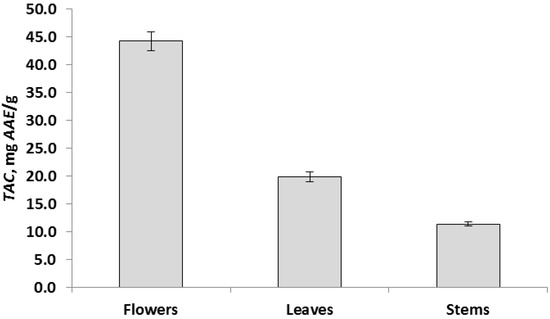
Figure 2.
Total antioxidant activity, TAC, expressed as mean ± SD (in error bar).
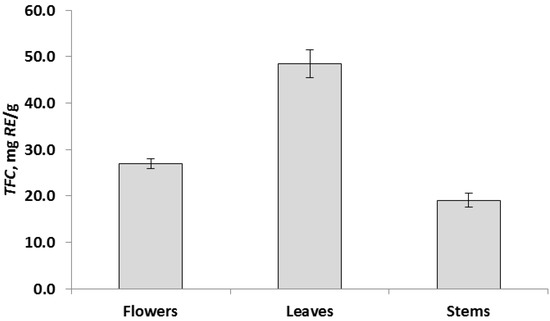
Figure 3.
Total flavonoid content, TFC, expressed as mean ± SD (in error bar).
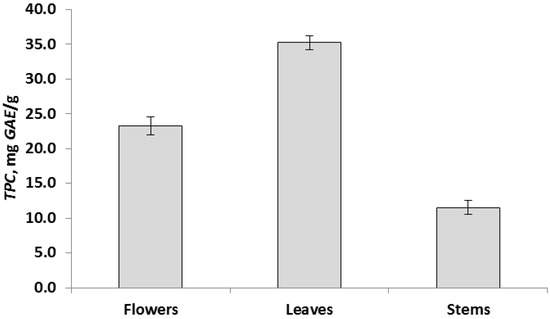
Figure 4.
Total phenolic content, TPC, expressed as mean ± SD (in error bar).
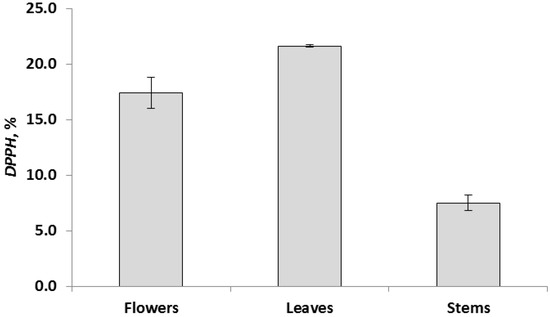
Figure 5.
Free radical scavenging, FRS, expressed as mean ± SD (in error bar).
R. acris flower extract had a concentration-dependent mitodepressive effect on A. cepa root meristem cell division (Figure 6).
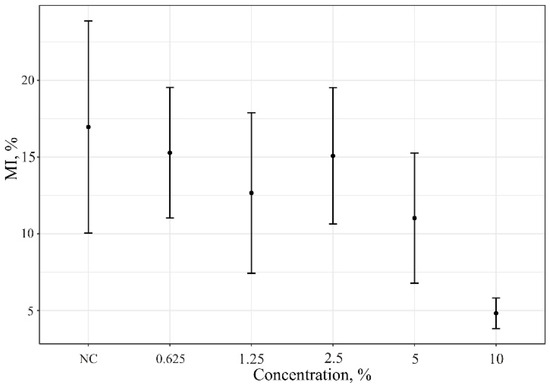
Figure 6.
Mitotic index data of A. cepa root meristem cells after treatment with of R. acris flower extract. NC—control (distiller water); MIs (%)—mitotic index.
Chromosomal aberrations in A. cepa root meristematic cells are shown in Table 1 and Figure 7. The noted absence of aberrations in the 10% R. acris flower extract may indicate the arrest of cell division and the antimitotic effect of substances in the solution. The cessation of root growth after 96 h of exposure and coloration in the color of the solution due to the pigments contained in it were also noted at this concentration.

Table 1.
Aberrations in A. cepa root meristem cells after treatment with R. acris flower extract.
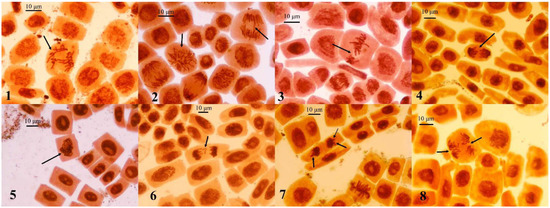
Figure 7.
Chromosomal aberrations and other disturbances in mitosis of A. cepa root meristem cells after treatment with of R. acris flower extract. (1) Disruption of spindle in metaphase; (2) multipolar mitosis and bridge in anaphase; (3) disturbance in metaphase; (4) stickiness of chromosomes in anaphase; (5) stickiness of chromosomes in metaphase; (6) fragments of chromosomes in telophase; (7) lagging chromosomes and the appearance of nuclear buds in telophase; (8) disruption of spindle in anaphase. Staining with acetoorcein, ×400 magnification.
4. Discussion
Plants are valuable sources of useful substances for medical needs. According to some data, about 30% of the pharmaceutical market and 11% of essential medicines are herbal products [12,13]. Plant extracts are composed of complex mixtures of phytochemicals that can act synergistically, additively or antagonistically. This can affect living organisms [14]. Therefore, the need to study plant extracts for genotoxicity and cytotoxicity using a variety of test systems is growing. The Allium test was used because of its sensitivity and wide distribution as a system for evaluating the effects of various factors in one study [15]. The effect of plant extracts on mitosis in the meristematic cells of onion roots is described by a number of changes: an increase or decrease in the cell division index (mitotic index) relative to the control, the appearance of various chromosomal disorders both at the stages of division and in interphase cells. The mitotic index is a measure of cytotoxicity. A decrease in the rate of cell division as the concentration of the R. acris extract in solution increased indicates a cytotoxic effect of the substances in the solution. This effect has already been described in the literature. It is called the dose-dependent mitodepressive effect. It may occur due to inhibition of DNA synthesis or blockage of the G2 phase of the cell cycle. The decrease in the mitotic index and the observed chromosomal aberrations can also be explained by impaired nucleoprotein synthesis and a decrease in the level of ATP, which provides energy for spindle elongation, microtubule dynamics and chromosome movement [16,17].
The noted absence of aberrations in an aqueous solution of the extract at a 10% concentration, along with a low mitotic index compared to the control, may indicate the antimitotic effect of the substances from the extract. Antimitotics are considered as blockers of mitosis and inducers of cell death. Antimitotic agents widely used in chemotherapy target exclusively proliferating cells and usually cause prolonged arrest of mitosis [18]. The antimitotic effect of protoanemonin realized from Ranunculus spp. was described in 1966 when inhibition of root growth and blocking of mitosis were observed in an experiment on the root meristems of corn.
The main chromosomal abnormalities noted in the study were nuclear buds, stickiness of chromosomes, anaphase bridges, chromosome fragments and disruption of spindle fibers. Chromosomal aberrations in treated onion root cells were caused by chemicals in the aqueous extracts of R. acris flowers, as such aberrations were not observed in controls. Stickiness of chromosomes was a common disorder of mitosis in a variety of onion studies. Several degrees of stickiness of chromosomes in anaphase and telophase (mild, moderate and severe) exist. In its classical sense, this term indicates severity when the chromosomes form an amorphous mass (cluster) due to defects in non-histone proteins responsible for chromosome organization during mitosis and proper chromatid segregation [19,20]. Chromosomal bridges can also be caused by stickiness of chromosomes because the process of condensing chromatin into separate chromatids and chromosomes is disrupted. Stickiness of chromosomes makes their separation incomplete; therefore, they remain connected by bridges. Nuclear buds originate from the nuclear membrane in situ in certain areas of the nuclei during the cell interphase stage. This may be the result of excessive production of nucleic acids and proteins induced by cytotoxicants [21]. Wandering and lagging chromosomes and fragments are indicators that the work of the cell division spindles is disrupted [22]. All these disorders in A. cepa meristematic cells are usually irreversible. Their presence in the study indicates the toxic properties of the tested aqueous solutions of the R. acris flower extract.
5. Conclusions
The paper presents data in the content of various biologically active substances and the features of the antioxidant activity and free radical scavenging of individual parts of the Ranunculus acris plant. It was shown that the highest values of the content of flavonoids and polyphenolic components and the degree of inhibition of free radicals are found in plant leaves, and the antioxidant activity is higher in flowers. It was established for the first time that extracts of R. acris have mitodepressive and cytotoxic effects. The obtained data can be useful for the use of parts of the Ranunculus acris plant as raw materials for the pharmaceutical production of dietary supplements and drugs. Additional evaluation of various parts of the plant for genotoxicity and cytotoxicity is required.
Author Contributions
Conceptualization, M.V.S., A.A.K. and N.S.T.; methodology, M.V.S., A.A.K. and N.S.T.; software, M.V.S., A.A.K. and N.S.T.; data curation, M.V.S., A.A.K. and N.S.T.; writing—original draft preparation, M.V.S., A.A.K. and N.S.T.; writing—review and editing, M.V.S., A.A.K. and N.S.T.; visualization, M.V.S., A.A.K. and N.S.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been carried out in the framework of Scientific Research Contract No. FMEZ-2023-0012 and financially supported by Scholarship of President of Russian Federation (SP-1326.2022.4).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Müller, M.B.; Bertrams, J.; Stintzing, F.C. Stability of protoanemonin in plant extracts from Helleborus niger L. and Pulsatilla vulgaris mill. J. Pharm. Biomed. Anal. 2020, 188, 113370. [Google Scholar] [CrossRef] [PubMed]
- Schink, M.; Garcia-Käufer, M.; Bertrams, J.; Duckstein, S.M.; Müller, M.B.; Huber, R.; Stintzing, F.C.; Gründemann, C. Differential cytotoxic properties of Helleborus niger L. on tumour and immunocompetent cells. J. Ethnopharmacol. 2015, 159, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Durnev, A.D.; Lapickaya, A.S. Genotoxicology of compounds of plant origin. Ekol. Genet. 2012, 10, 41–52. (In Russian) [Google Scholar] [CrossRef]
- Chandel, S.; Kaur, S.; Issa, M.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Exposure to mobile phone radiations at 2350 MHz incites cyto- and genotoxic effects in root meristems of Allium cepa. J. Environ. Health Sci. Eng. 2019, 17, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, I.A. Allium Test in Enviromental Monitoring and Health; Novas Edições Acadêmicas; OmniScriptum Publishing: Riga, Latvia, 2018; p. 94. [Google Scholar]
- Russian Federation. State Pharmacopoeia of the Russian Federation, 14th ed.; Russian Federation: Moscow, Russia, 2018; Volume 2, pp. 1962–1967. (In Russian)
- Korovkina, A.V.; Tsvetov, N.S.; Nikolaev, V.G. Flavonoid content and antioxidant activity of extracts of Polygonum weyrichii fr. Schmidt. IOP Conf. Ser. Earth Environ. Sci. 2020, 42, 52044. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Medvedeva, M.Y.; Bolsunovsky, A.Y.; Zotina, T.A. Cytogenetic abnormalities in aquatic plant Elodea canadensis in anthropogenic contamination zone of Yenisei River. Contemp. Probl. Ecol. 2014, 7, 422–432. (In Russian) [Google Scholar] [CrossRef]
- Sousa, S.G.; Oliveira, L.A.; de Aguiar, M.D.; de Brito, T.V.; Batista, J.A.; Pereira, C.M.C.; de Souza Costa, M.; Mazulo, J.C.R.; de Carvalho, F.M.; Vasconselos, D.F.P.; et al. Chemical structure and anti-inflammatory effect of polysaccharide extracted from Morinda citrifolia Linn (Noni). Carbohydr. Polym. 2018, 197, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Nadaf, M.; Joharchi, M.R.; Amiri, M.S. Ethnomedicinal uses of plants for the treatment of nervous disorders at the herbal markets of Bojnord, North Khorasan Province, Iran. Avicenna J. Phytomed. 2019, 9, 153–163. [Google Scholar] [PubMed]
- Prajitha, V.; Thoppil, J.E. Genotoxic and antigenotoxic potential of the aqueous leaf extracts of Amaranthus spinosus Linn. using Allium cepa assay. S. Afr. J. Bot. 2016, 102, 18–25. [Google Scholar] [CrossRef]
- Bonciu, E.; Firbas, P.; Fontanetti, C.S.; Wusheng, J.; Karaismailoglu, M.C.; Liu, D.; Menicucci, F.; Pesnya, D.S.; Popescu, A.; Romanovsky, A.V.; et al. An evaluation for the standardization of the Allium cepa test as cytotoxicity and genotoxicity assay. Caryologia 2018, 71, 191–209. [Google Scholar] [CrossRef]
- Frescura, V.D.-S.; Kuhn, A.W.; Laughinghouse, H.D., IV; Nicoloso, F.T.; Lopes, S.J.; Tedesco, S.B. Evaluation of the allelopathic, genotoxic, and antiproliferative effect of the medicinal species Psychotria brachypoda and Psychotria birotula (Rubiaceae) on the germination and cell division of Eruca sativa (Brassicaceae). Caryologia 2013, 66, 138–144. [Google Scholar] [CrossRef]
- Madić, V.; Stojanović-Radić, Z.; Jušković, M.; Dragana, J.; Žabar Popović, A.; Vasiljević, P. Genotoxic and antigenotoxic potential of herbal mixture and five medicinal plants used in ethnopharmacology. S. Afr. J. Bot. 2019, 125, 290–297. [Google Scholar] [CrossRef]
- Antu, M.J.; Vaishnavi, A.; Aswathy, A. Anti-mitotic activity of aqueous leaf extracts of Azadirachta indica A. Juss. and Simarouba glauca DC. on Allium cepa L. root tips. J. Pharmacogn. Phytochem. 2020, 9, 485–489. [Google Scholar]
- Çavuşoğlu, D.; Tabur, S.; Çavuşoğlu, K. The effects of Aloe vera L. leaf extract on some physiological and cytogenetical parameters in Allium cepa L. seeds germinated under salt stress. Cytologia 2016, 81, 103–110. [Google Scholar] [CrossRef]
- Farizan, A.; Norfatimah, M.Y.; Aili, Z.N.; Lyena, W.Z.A.; Indah, M.A. Use of cytological and molecular biological method for water pollution monitoring. IOP Conf. Ser. Earth Environ. Sci. 2021, 674, 012108. [Google Scholar] [CrossRef]
- Nisha, K.K. Cytotoxic Effect of Crotalaria laburnifolia L. leaf extract on Allium cepa root tip cells. J. Adv. Biol. Sci. 2017, 4, 72–75. [Google Scholar]
- Redli, P.M.; Gasic, I.; Meraldi, P.; Nigg, E.A.; Santamaria, A. The Ska complex promotes Aurora B activity to ensure chromosome biorientation. J. Cell Biol. 2016, 215, 77–93. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).