Abstract
In this study, twelve Combretum spp. were investigated for their phytochemical content, antioxidant potential, and their proximate and trace elements/minerals composition. The qualitative phytochemical composition of the leaves, stems, and ashes of Combretum plants analysed revealed the presence of saponins, tannins, terpenoids, steroids, cardiac glycosides, and flavonoids. The following phytoconstituents were lost in the ashes; tannins apart from C. mkuzense and C. padoides; cardiac glycosides; and flavonoids. The quantitative phytochemical analyses revealed that both the leaves, stems, and some ashes such as C. apiculatum and C. vendae contained levels of phenolic compounds, tannins, and flavonoids. DPPH screening method indicated great scavenging activity with the 70% acetone leaf extracts of C. kraussii, C. zeyheriim, and C. mkuzense. There was a significant decrease in the antioxidant activity in the ashes compared to the leaves and the stems. AOAC and ICPE protocols performed the proximate and nutritional analysis of the 70% acetone extracts. The extracts had substantial amounts of ash, moisture, protein, and energy. The leaves and ashes of C. adenogonium and C. apiculatum could provide a good source of calcium in the diet. This study presents valuable information on the phytochemical composition, nutritional composition, and antioxidant properties of some Combretum species.
1. Introduction
Plants are a good source of food, chemicals, and herbal medicines. Plant and fruits are the most important, safest, and most compatible sources of natural bioactives. Natural antioxidants are effectively used to prevent the destructive processes caused by oxidative stress [1]. Substantial evidence has played key roles in reactive oxygen species (ROS), free radicals, and other oxidants in causing numerous disorders and diseases such as cancer, cardiovascular diseases, Alzheimer’s, as well as neurodegenerative disorder [2]. Scientists are now focusing on natural antioxidants for the prevention and treatment of diseases and maintenance of human health [3]. Antioxidants often protect and neutralize your cells against free radicals [4]. Recently, interest in naturally occurring antioxidants has considerably increased for use in food, cosmetic and pharmaceutical products, because they possess the ability to neutralize free radicals, they can lower odds of some diseases [4,5]. Synthetic antioxidants such as butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) have been in use for several years as food additives, but now their use is restricted in dietary items due to their implications in chronic diseases such as cancers and cardiovascular disorders [6,7]. So, there is a need to explore natural antioxidants, which have few or no side effects and can be used in medicines in place of synthetic antioxidants [8]. Many plant materials have been identified and documented as promising sources of natural antioxidants [9,10]. Various studies have highlighted the evidence regarding variation in proximate composition, phenolics, and antioxidant activity with respect to different species of a plant [11,12]. The present study sought to investigate the differences in leaves from twelve Combretum plants.
Combretum is the biggest genus of the Combretaceae family with about 370 species [13]. Combretum plants are mainly used in traditional medicine against various diseases such mental problems, heart, and worm remedies to fever and microbial infections [14]. All parts of the Combretum species, in some cases even the fruits, are used for medicinal purposes [15]; some of the Combretum plants have been used in many African countries as food additives. Many species from the Combretaceae family have been reported to contain antioxidant activities. Research studies have shown that many of these antioxidant compounds possess anti-inflammatory, anti-atherosclerotic, antitumor, antimutagenic, anti-carcinogenic, antibacterial [16,17]. Studies by Masoko and Eloff [18] and Masoko et al. [14] have also reported antioxidant potential of 24 African combretum species. Combretum plants also possess nutritional components for energy metabolism and vital nutrients to maintain a state of optimal nutrition. The leaves of many Combretum plants are harvested from wild-growing populations and used as popular traditional herbal tea in several tropical West African countries [14,15]. It is very important to investigate plants’ nutritional properties, especially those that are easily accessed in all seasons.
2. Materials and Methods
2.1. Collection of Leaves
Fresh leaves of Combretum caffrum, C. vendae, C. erythrophyllum, C. elaegnoides, C. apiculatum, C. imberbe, C. adenogdium, C. padoides, C. bractesum, C. kraussi, C. mkuzense, and C. zeyheri were collected at Nelspruit, National Botanical Gardens, Mpumalanga, South Africa. Voucher specimens and tree labels were used to verify the identity of the plants. The voucher specimens were deposited at the Larry Leach Herbarium (UNIN) for confirmation. The leaves were air-dried for 30 days. When dry, the plant material was ground to a fine powder and stored in paper bags at room temperature.
2.2. Screening of Phytoconstituents
Screening of phytoconstituents included the following: terpenoids flavonoids, cardiac glycosides phlobatannin, steroids, saponins, alkaloids, and tannins [19,20].
- Saponins
The persistent frothing test was used to test for saponins by weighing 1 g of plant-powdered leaves and stems and mixed with 30 mL of tap water. The mixture was vigorously shaken and heated at 100 °C. The sample was observed for the formation of persistent froth [21].
- Tannins
The presence of tannins was tested by boiling 0.5 g of powdered leaves and stems in 5 mL of purified water in a test tube, then cooled and filtered. A few or three drops of 0.1% ferric chloride was added to 1 mL of the solution in a test tube and observed for brownish green or a blue-black colouration [20].
- Phlobotannins
Phlobotannins were tested by weighing 0.2 g of powdered leaves and stems of R. communis into 10 mL of purified water and filtered. The filtrate was boiled with 2% hydrochloric acid solution. The sample was observed for the formation of red colour of precipitate [21].
- Terpenes/terpenoids
The Salkowski’s test was used, and 5 mg of the leaves and powders were mixed in 2 mL of chloroform and 3 mL concentrated sulphuric acid (H2SO4) was carefully added to form a layer. The appearance of a reddish-brown colour indicates the presence of terpenes [21].
- Steroids
About 2 mL of acetic anhydride was added to 0.5 g of the powdered leaves and stems, followed by an addition of 2 mL of H2SO4. Blue colour was observed to draw an inference, indicating the presence of steroids [21].
- Cardiac glycosides
The Keller–Killani’s test was used. About 5 mL of the leaves and stem powders of the plant parts studied were treated with 2 mL of glacial acetic acid, containing one drop of ferric chloride solution. This was followed by an addition of 1 mL concentrated H2SO4. The colour changes (brown interface, violet ring below, and greenish ring at the lowest) were observed to draw inference, indicating the presence of cardiac glycosides [22].
- Flavonoids
About 5 mL of diluted ammonia solution was added to a portion of the filtrate of each plant extract, followed by the addition of concentrated H2SO4. Yellow colour change was observed to draw an inference, indicating the presence of flavonoids [23].
- Alkaloids
Drangendorff’s reagent was used to test for alkaloids by weighing 0.2 g of ground powdered leaves and stems with 95% ethanol using Soxhlet extractor. The extracting solvent was evaporated to dryness using a vacuum evaporator at 45 °C. The plant residues were dissolved in 5 mL of 1% hydrochloric acid and 5 drops of Drangendorff’s reagent was added. Reddish-brown colour change was observed to draw an inference [23,24].
2.2.1. Quantification of Phytochemicals
- Total phenolic content
The quantity of phenolics present in leaves was determined by using the Folin–Ciocaltleu reagent method [21] with minor modifications.
- Total tannin and total flavonoid content
The Folin–Ciocalteu and aluminium chloride colorimetric assay method described by [22] was used to determine the tannin and flavonoid content in the leaves, stems, and ashes.
2.2.2. Quantitative Antioxidant Activity Assay
Free radical scavenging activity of the plants was quantified and compared using the 2,2-Diphenyl-1-picrylhydrazyl (DPPH) (Sigma, Johannesburg, South Africa) method reported by [25] with modifications.
2.3. Proximate Analysis
Proximate analysis of the leaves was performed according to AOAC International [26].
2.3.1. Estimation of Moisture Content
Two grams of the dried sample was put the moisture analyser. The moisture analyser (Fisher Scientific, Johannesburg, South Africa) used the basic “loss-on-drying” technique to simultaneously weigh and heat the sample. Moisture was recorded as percentage moisture.
2.3.2. Estimation of Ash Content
Two grams of the dried sample was weighed into a dry porcelain dish and then heated in the maffle furnace at 600 °C for 6 h. It was cooled in desiccators and weighed. The percentage ash content was calculated by using the following formula:
2.3.3. Protein Determination
Crude protein was determined following the Dumas AOAC method 992.23 using LECO Corp, Truspec (Johannesburg, South Africa). Two grams of the sample was combusted at high temperature in an oxygen atmosphere. Via subsequent oxidation and reduction tubes, nitrogen is quantitatively converted to N2. A thermal conductivity detector measured the nitrogen content of the plant samples and was subsequently multiplied by 6.25 to get the protein content. A factor of 6.25 was used because most protein contains approximately 16% nitrogen.
2.3.4. Determination of Energy
The gross energy was determined for individual samples using the Isoperibol bomb calorimeter AC500 (LECO, Johannesburg, South Africa). About 1g of the sample was weighed in a crucible and placed inside a stainless-steel container (the “decomposition vessel”) filled with oxygen. Next, the sample was ignited through a cotton thread connected to an ignition wire inside the decomposition vessel and burned (combusted). The measurement result was presented in kilojoules.
2.4. Mineral or Trace Elements Analysis
- Digestion of the Dried Leaves and Stems
Four hundred milligrams of the sample were weighed into the digestion vessels. Five millilitres of HNO3 and 3 mL of H2O2 was added and the mixture was shaken. A waiting period of 10 min was observed before closing the vessel. The microwave heated following the program in the Table 1.

Table 1.
Microwave digester conditions.
2.5. Statistical Analysis
Statistical analysis of results was performed using Statistix 10 data analysis software; a completely randomised test and the Welch’s Test was used to compare any significant differences between the means. Statistical analysis was performed to determine variation between the plants in terms of proximate, nutritional, phytoconstituents, and antioxidant properties. Values were considered significantly different when p ˂ 0.05.
3. Results
3.1. Phytochemicals
Phytochemicals are compounds of plants known to exhibit diverse pharmacological and biochemical effects on living organisms [27]. The qualitative phytochemical composition of the leaves Combretum plants analysed in this study revealed the presence of saponins, tannins, terpenoids, steroids, cardiac glycosides, and flavonoids (Table 2).

Table 2.
Qualitative test for phytochemicals in the leaves.
The ashes of the plants contain only saponins, terpenoids, and steroids. However, tannins were also detected in the ashes of C. mkuzense and C. padoides—cardiac glycosides and flavonoids (Table 3).

Table 3.
Qualitative test for phytochemicals in the ashes of twelve Combretum species.
The ashes of the plants contain only saponins, terpenoids, and steroids. However, tannins were also detected in the ashes of C. mkuzense and C. padoides—cardiac glycosides and flavonoids (Table 4).

Table 4.
Qualitative test for phytochemicals in the ashes of twelve Combretum species.
- Quantitative Phytochemical Composition of the Combretum Plants
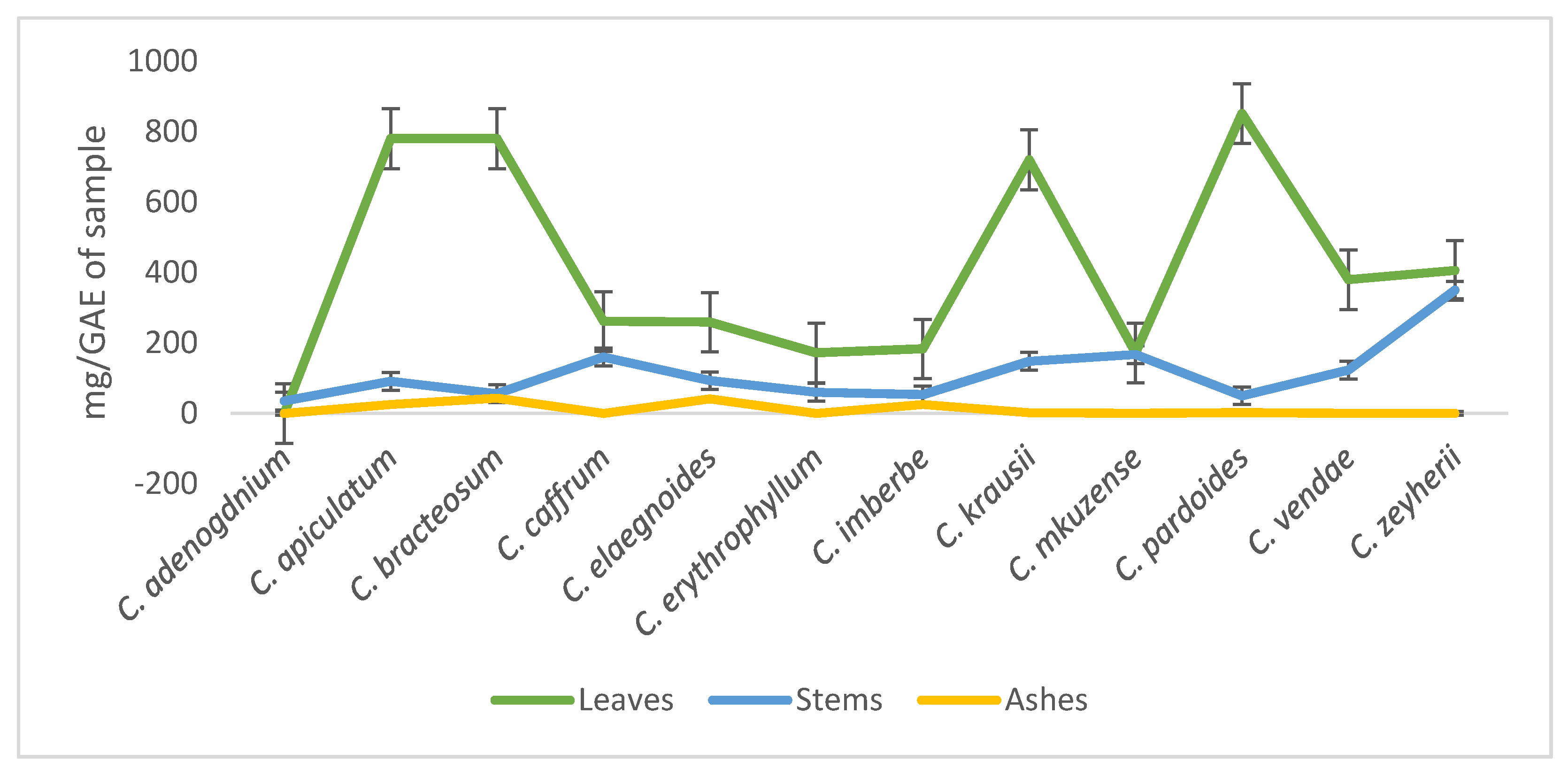
The leaves showed the presence of high amounts of phenolic compounds when compared to the stems with C. pardoides, C. apiculatum, and C. bracteosum having the highest concentration of phenols. The phenolic compounds of the leaves, stems, and ashes of the plants ranged between 172–851, 35–350, and 0–43 mg of GAE/g of sample, respectively. (Figure 1).
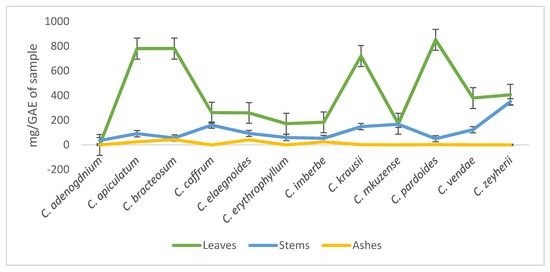
Figure 1.
Evaluation of the phenolic compounds in the leaves, stems, and ashes of twelve Combretum species.
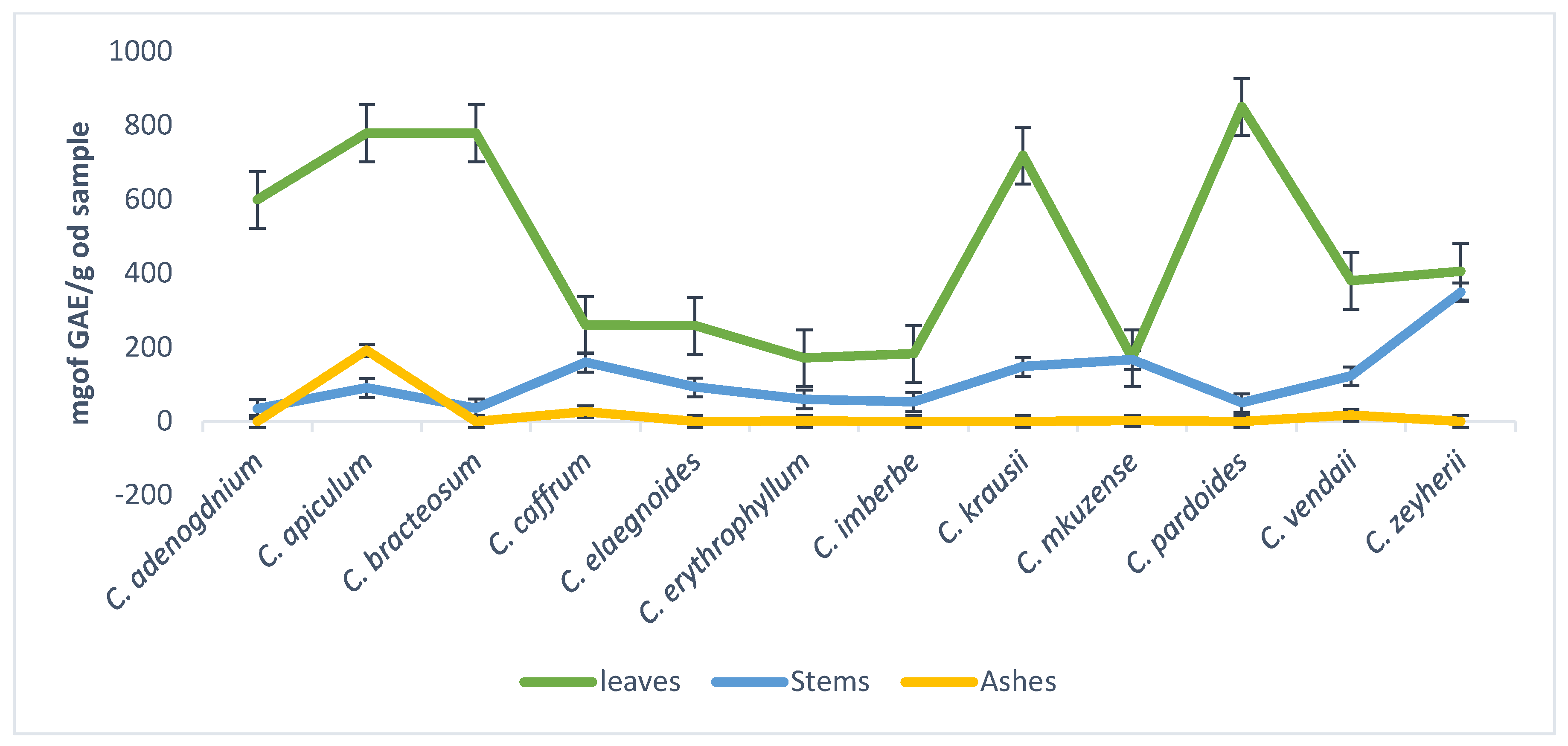
The concentration of tannins in leaves, stems, and ashes of the plants ranged from 172–851, 93.3–508.3, and 0–50 mg of GAE/g of sample, respectively. The ashes of C. apiculatum had the highest concentration of tannins when compared to the stems (Figure 2).
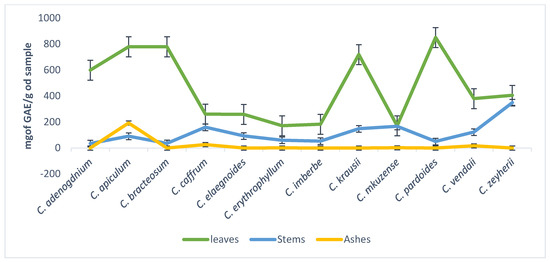
Figure 2.
Total tannins content concentrations of the 70% aqueous acetone extracts of twelve Combretum species.
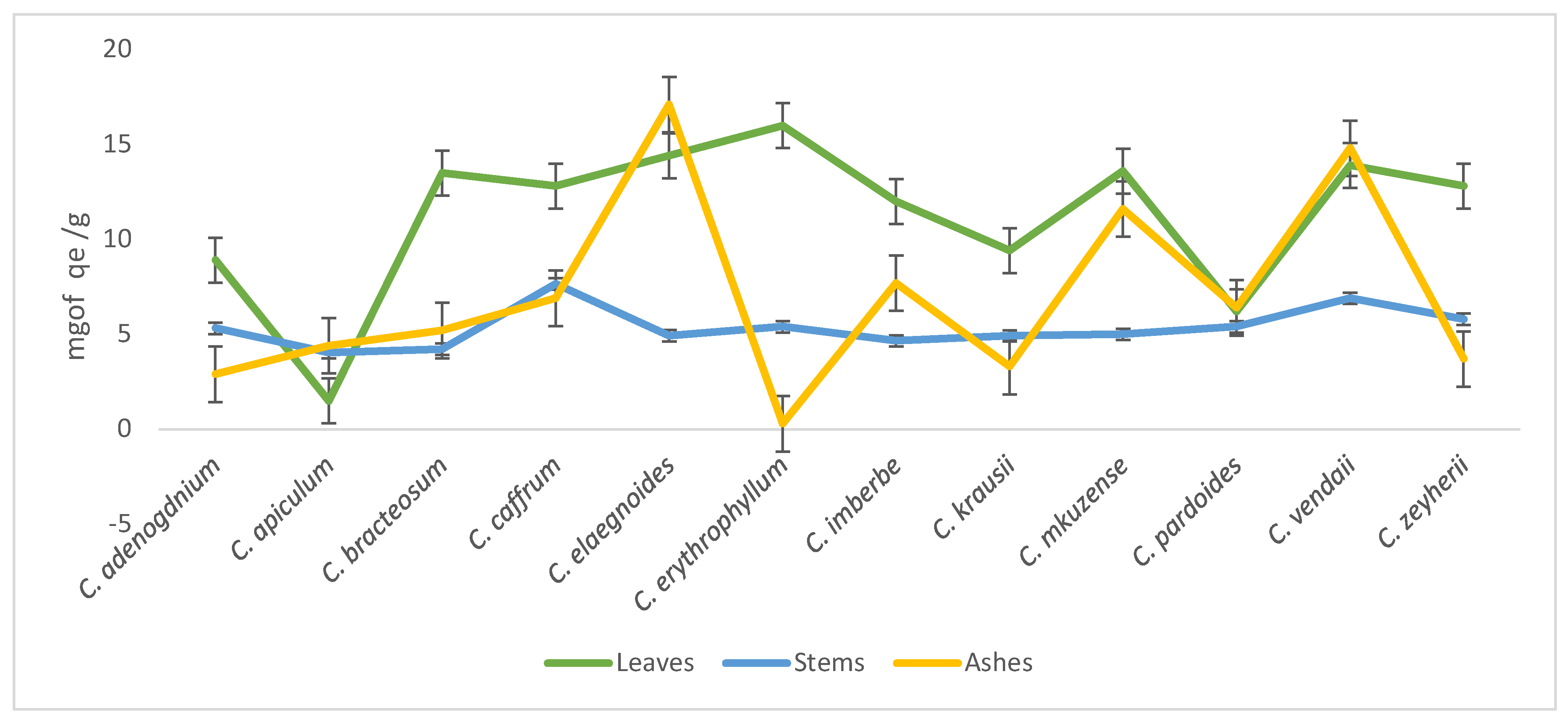
Most of the leaves had the highest flavonoids concentration when compared to the stems apart from C. kraussii, C. apiculatum, C. pardoides, and C. adenogdnium. Interestingly, ashes of C. apiculatum, C. elaegnoides, and C. vendae had the highest concentrations of the flavonoids when compared with other species of Combretum (Figure 3).
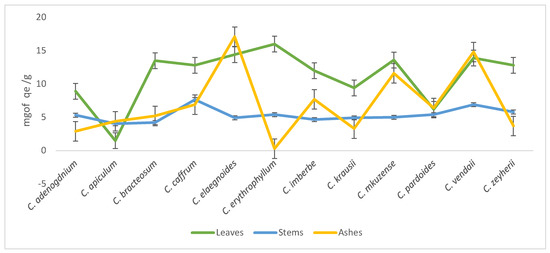
Figure 3.
Total flavonoids content concentrations of the 70% aqueous acetone extracts of twelve Combretum species.
3.2. Antioxidants
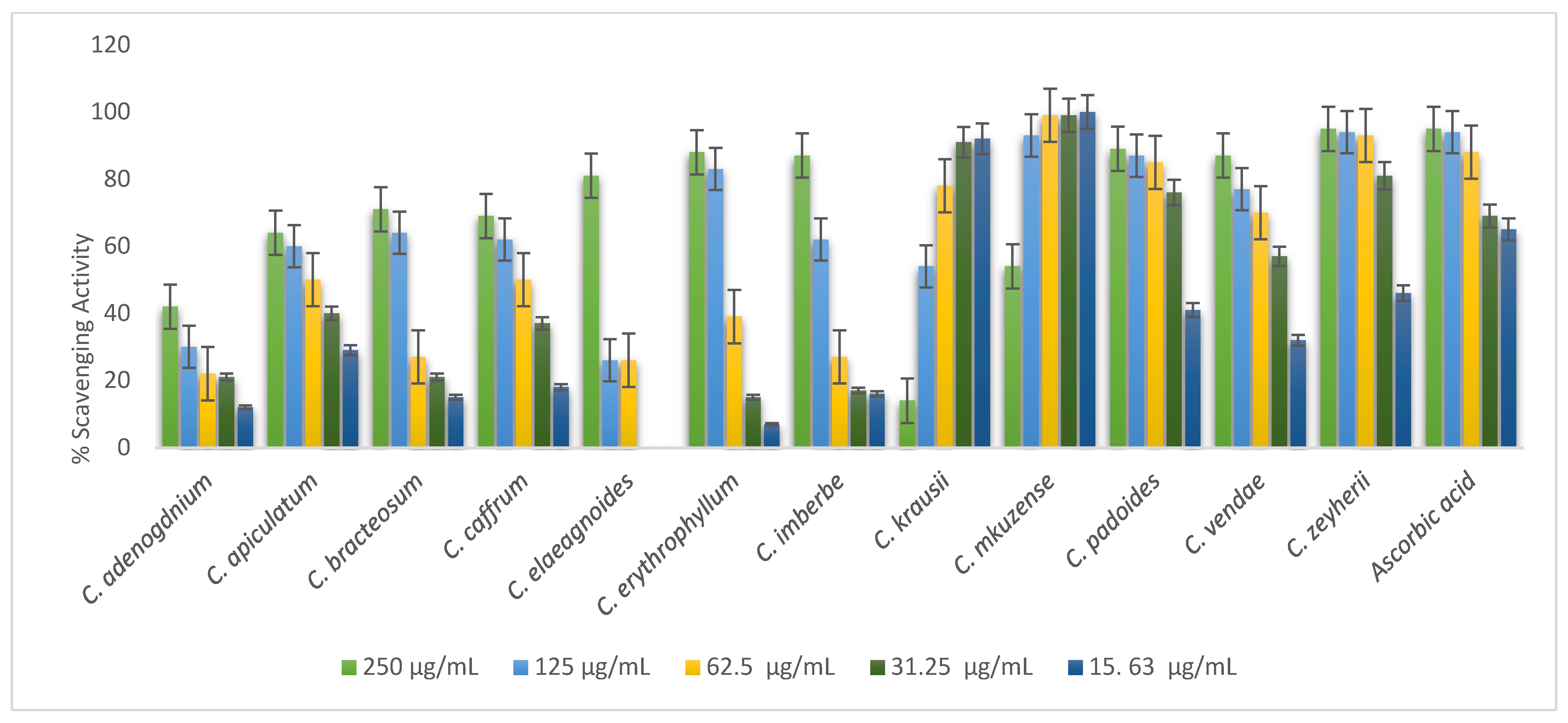
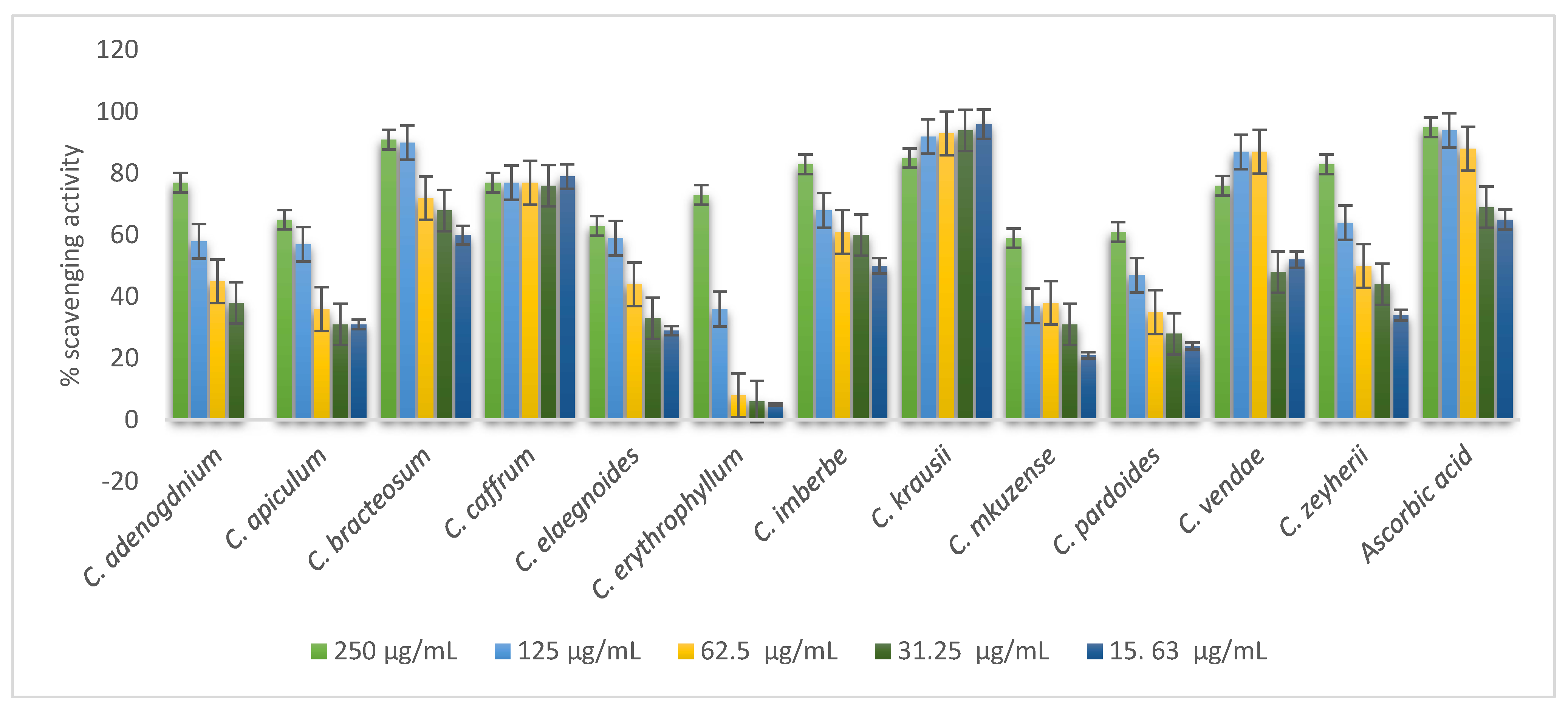
Free radical scavenging properties of 70% of leaf extracts are presented in Figure 4. Most of the leaves tested exhibited a dose-dependent manner of antioxidant activity. The following leaf extracts showed highest scavenging activity: C mkuzense, C. zeyherii, C. kraussii, and C. padoides. Statistically, there was no significant difference (p = 0.01) in the scavenging activity of the following plants: C. mkuzense (0.0625; 0.125; 0.25; and 0.5 mg/mL); C. zeyherii (0.25 and 0.5 mg/mL); C. kraussii (25 and 0.5 mg/mL), and C. padoides (1 mg/mL). C. elaegnoides, C. erythrophyllum, and C. bracteosum had the lowest radical scavenging potential compared to other leaves in the study, as depicted in Figure 4.
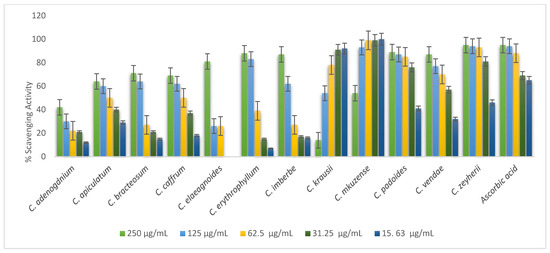
Figure 4.
Evaluation of the antioxidant activity of the 70% acetone leaf extracts of the twelve Combretum species.
Figure 5 shows the concentration-dependent activity of the leaf extracts of the plants on DPPH radical scavenging by stems of Combretum spp. except for C. kraussii. As sample concentration increased, the percentage inhibition of DPPH radical also increased. In the plants that acted in a concentration-dependent manner, the following stems: C. zeyherii, C. bracteosum, C. imberbe, and C. adenogonium, showed the greatest percentage scavenging activity (Figure 4).
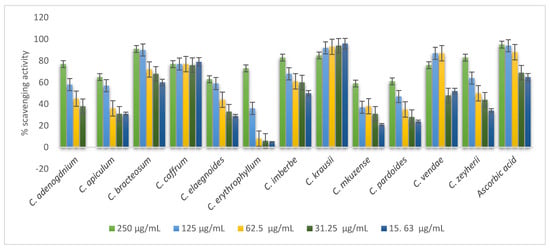
Figure 5.
Evaluation of the antioxidant activity of the stems of the twelve Combretum species.
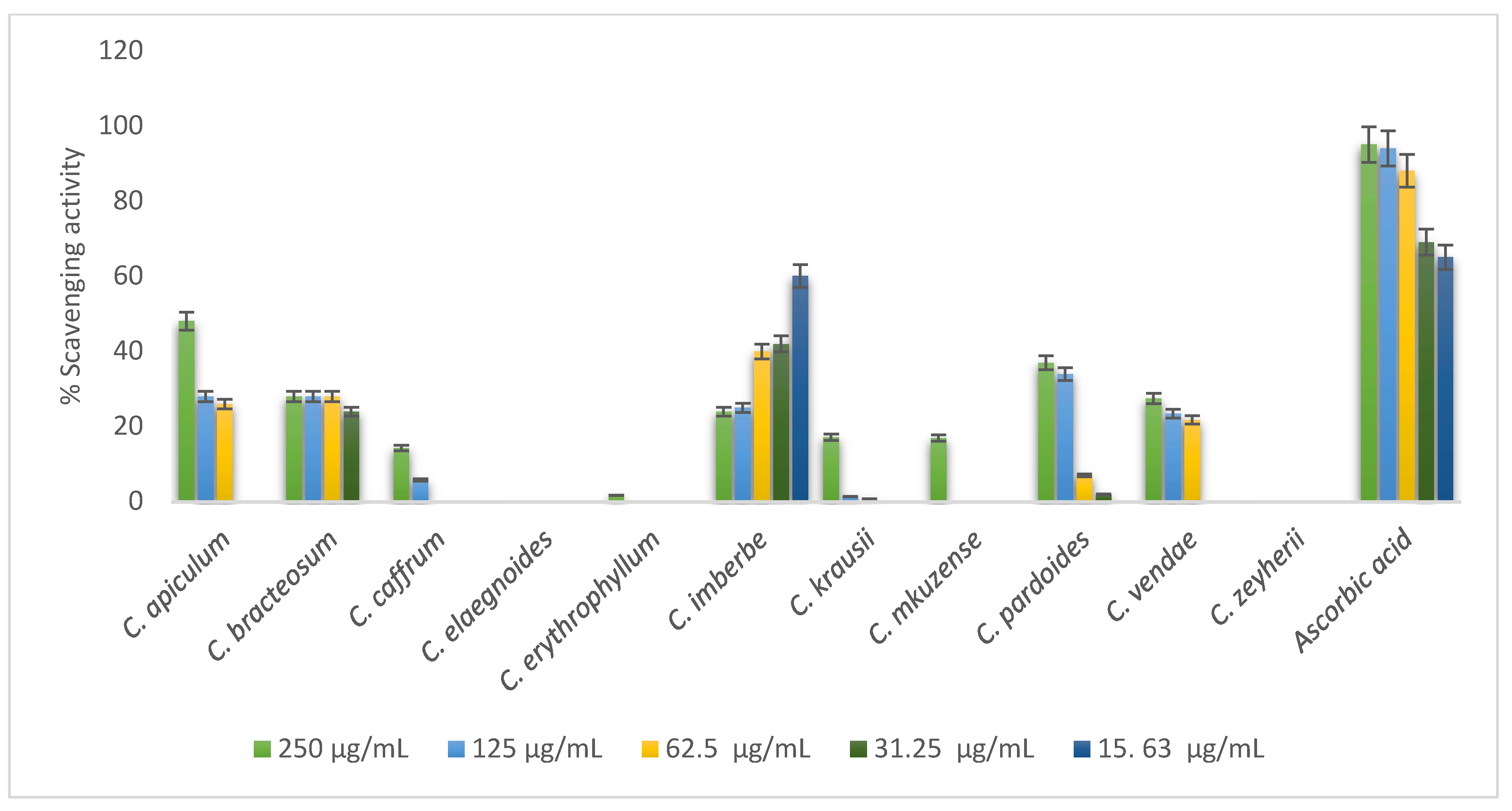
The majority of the Combretum ash extracts such as C. elaegnoides and C. zeyherii did not possess any antioxidant activity. Ashes from C. apiculatum, C. caffrum, C. padoides, and C. vendae showed some antioxidant activity in a concentration-dependent manner while the activity of C. imberbe was observed to be opposite, as depicted in Figure 6.
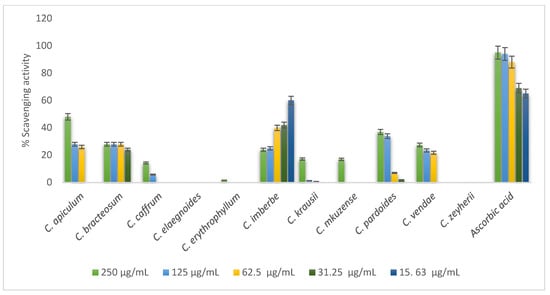
Figure 6.
DPPH radical scavenging activity of 70% acetone ash extracts of twelve Combretum species.
3.3. Proximate Analysis
3.3.1. Ash
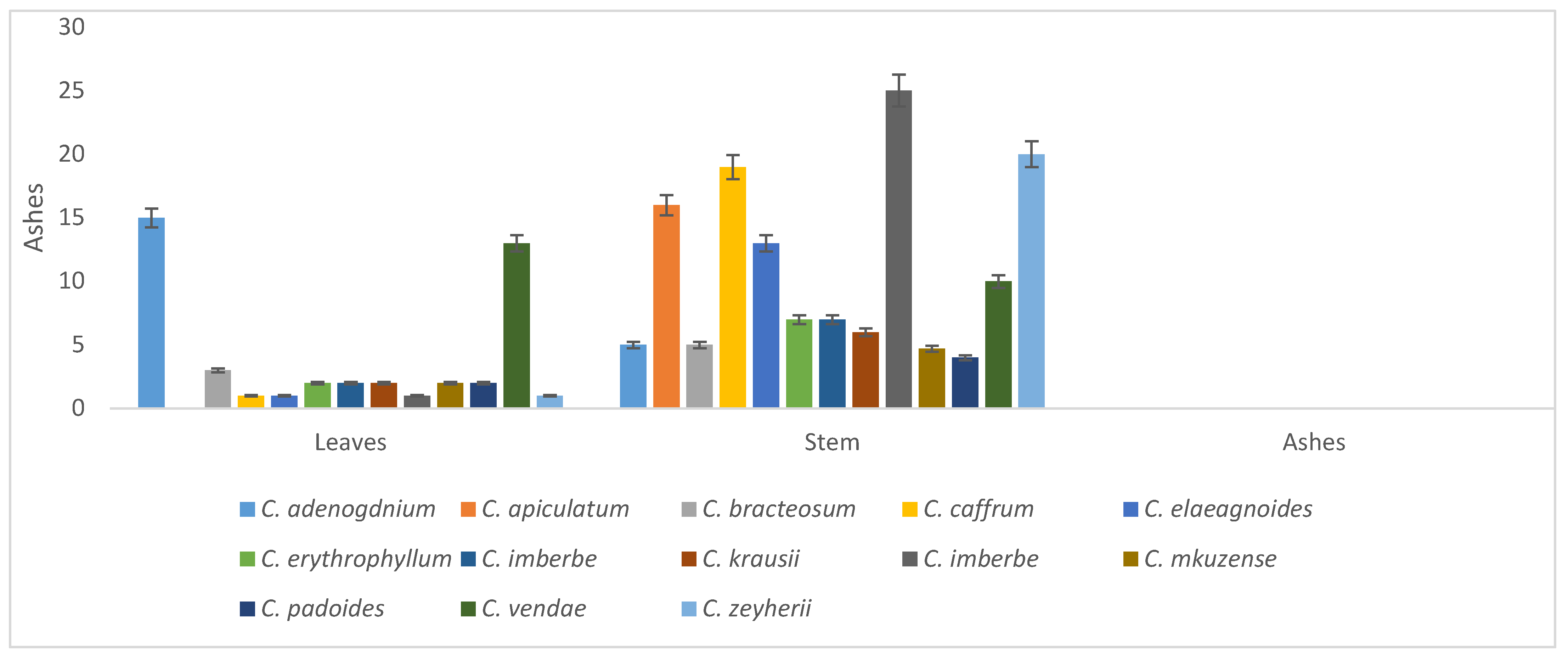
Ash percentage ranged from 1–15 in the leaves and 4–35 in the stems. The leaves of C. vendae and C. adenogdnium had the highest ash levels. Generally, the stems (83%) had the highest ash percentage levels when compared to the leaves (17%), as shown in Figure 7.
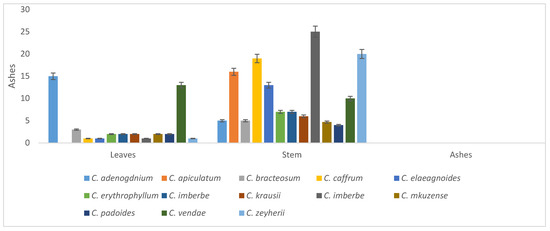
Figure 7.
Ash percentage of the leaves and stems of the Combretum species.
3.3.2. Moisture
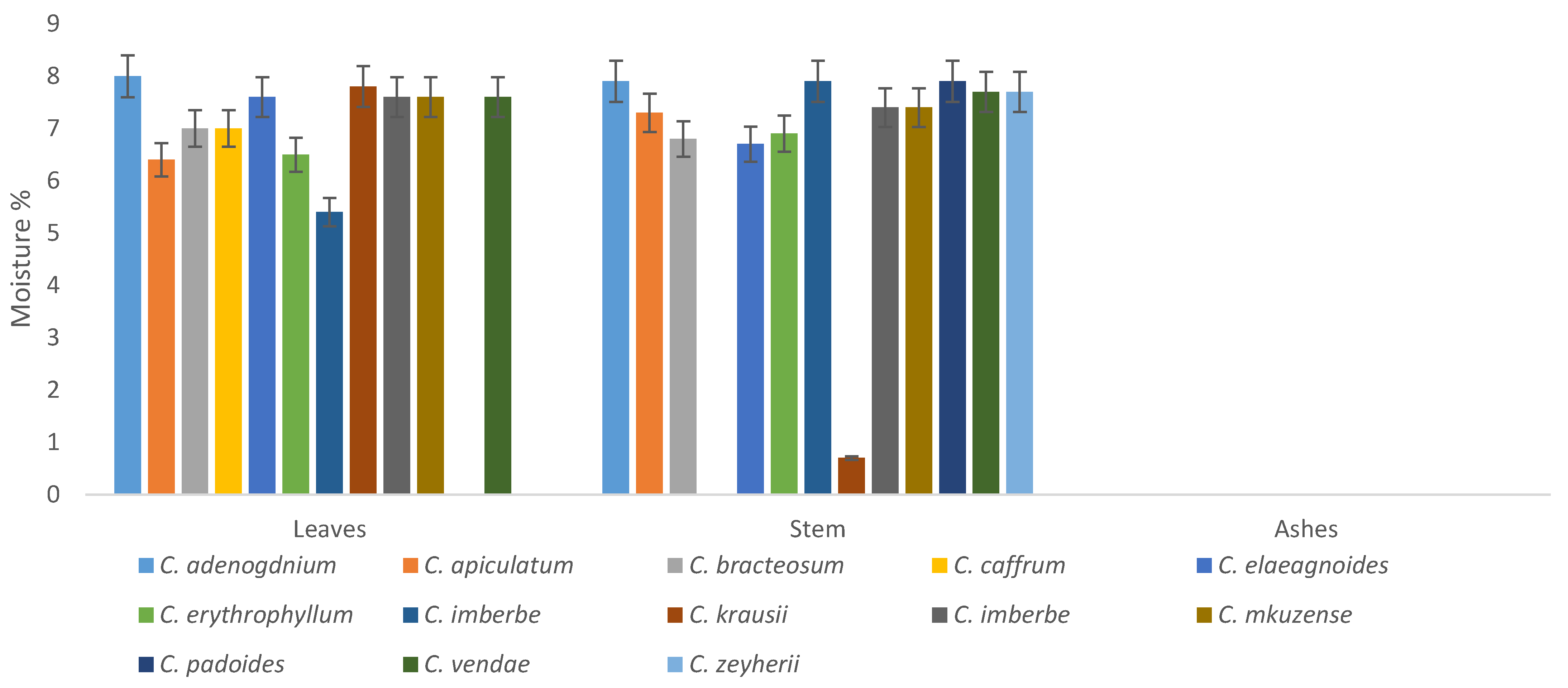
Moisture content of the plants ranged from 0–9% in both the ash, leaves, and stems. The leaves of C. vendae and C. adenogonium had no moisture; it was only detected in the stems. The leaves of C. caffrum had the highest moisture content, however; the stems had 0% of moisture content, as depicted in Figure 8.
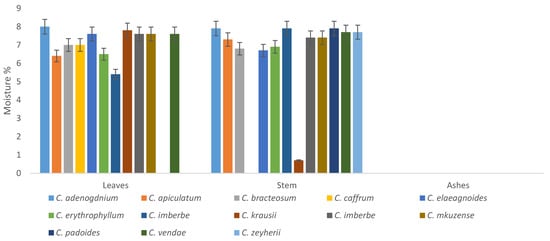
Figure 8.
Moisture content (%) of the leaves and stems of the Combretum plants.
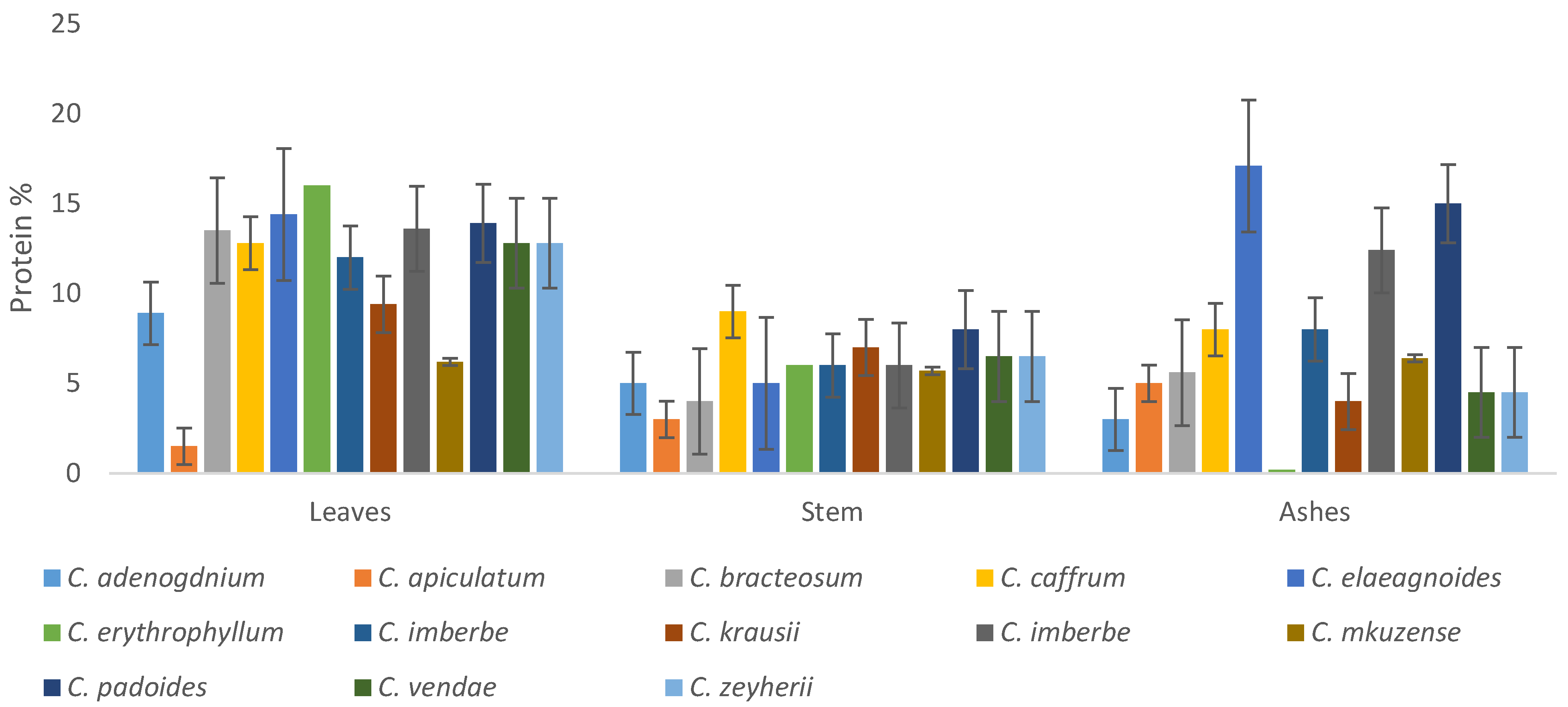
3.3.3. Protein Percentage
The protein content in the leaves ranged between, 2 and 14.4%, stems 4.04 and 7.66%, while the ashes contain protein in the range of 0.29–17.1%, as depicted in Figure 9. Generally, the leaves had higher protein content compared to the stems and ashes of other species. However, the protein contents of the C. elaegnoides and C. padoides were interestingly high.
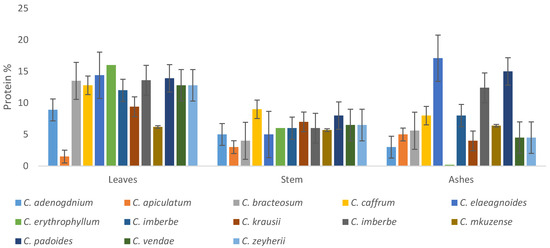
Figure 9.
Protein percentage of the leaves, stems, and ashes of the Combretum species.
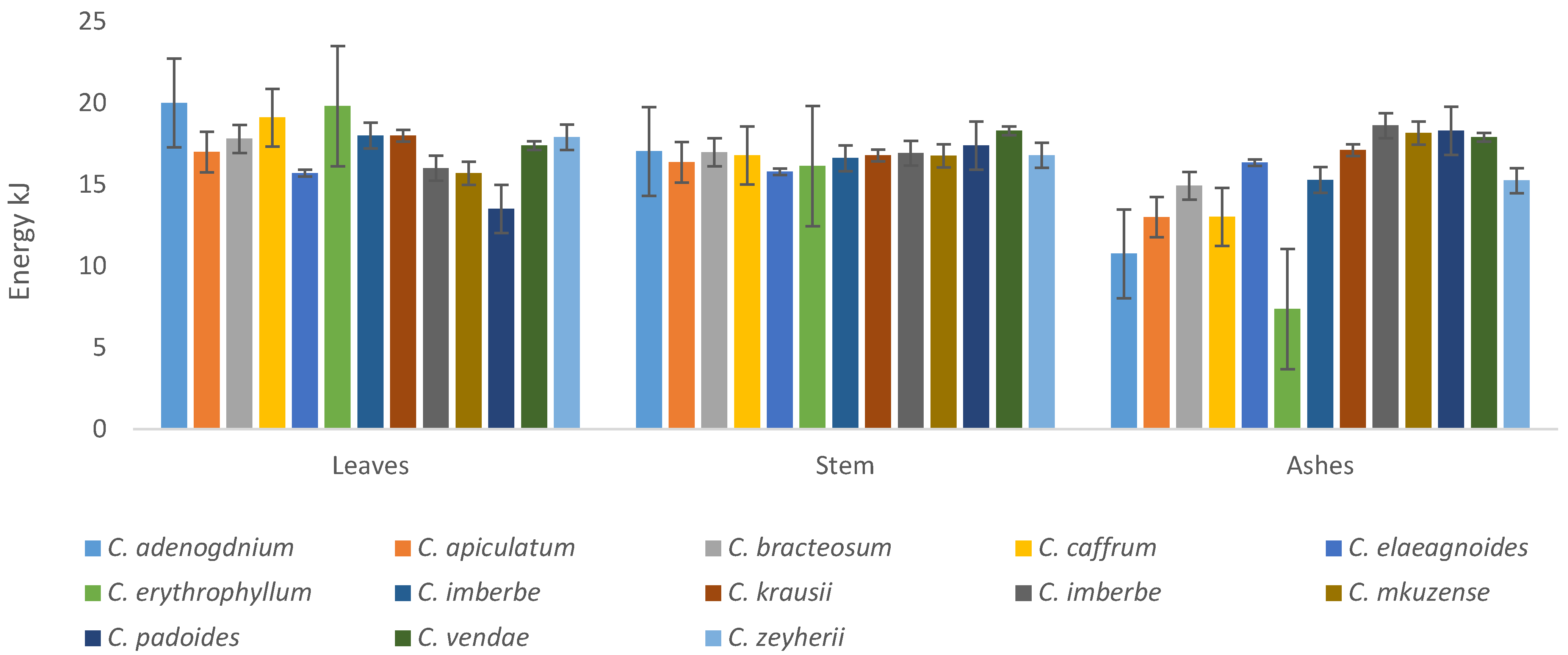
All the plants had appreciable amounts of energy, with C. adenogdnium exhibiting the highest concentrations for both leaves and stems, as illustrated in Figure 10. The ashes C. erythrophyllum had the lowest energy overall.
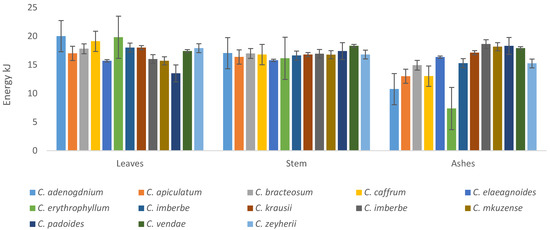
Figure 10.
Energy content of the leaves, stems, and ashes of the Combretum plants tested in the study.
3.3.4. Mineral and Trace Metals Composition of the Leaves
The minerals detected in the leaves ranged from 4.86–283 (Ca); 0.114–166 (Co); 0.15–0.661 (Cu); 0.209–3.19 (Fe); 2.98–65.5 (K); 2.03–51.2 (Mg) 0.0319–3.98 (Mn); 1.8–14 (Na); 0.0139–3.5 (Ni); and 0.006–1.04 (Zn). C. elaegnoides had the lowest concentrations of minerals apart from Mg and Zn. C. adenogonium had the highest Ca, Cu, and Fe concentration while C. apiculatum had the highest concentration of Co, K, Mg, and Mn (Table 5).

Table 5.
Concentration (mg/mL) of trace minerals in the leaves of some Combretum species.
C. krausii had the lowest concentration of the following minerals: Ca, Co, Cu, K, and Mn while C. erythrophyllum had the highest concentration of Cu and Zn. The mineral that was detected in high concentrations was Ca whereas Zn is lowest as shown in Table 6.

Table 6.
Concentration (mg/mL) of trace minerals in the stem of some Combretum species.
The mineral compositions in percentage in the ashes ranged from 38.9–980 (Ca); 0–1.73 (Co), 0–1.07 (Cu) 0–25.1(Fe); 0–196 (K); 0–57.9 (Mg); 0–2.71 (Mn); 0–24.4 (Na); 0–2.22 (Ni); 0–18.7 (Zn). Overall C. zeyherii had the highest concentration of all the minerals, as highlighted in Table 7.

Table 7.
Concentration (mg/mL) of trace minerals in the ashes of some Combretum species.
4. Discussion
All the plants investigated are traditionally used for medicinal purposes in South Africa and other African countries. Most of the plants are indigenous to South Africa.
Phytochemicals: Phytochemicals are substances produced by plants known to possess diverse pharmacological and biochemical effects on living organisms [28]. The qualitative phytochemical composition of both the leaves and stems of some Combretum species analysed in this study revealed the presence of saponins, tannins, terpenoids, steroids, cardiac glycosides, and flavonoids. These phytoconstituents were not detected in the ashes of the plants. However, tannins were present in C. mkuzense, while cardiac glycosides and flavonoids were contained in C. padoides. These phytoconstituents, which were not detected in the ashes, may have been destroyed by heat. The quantitative phytochemical analyses revealed that both the leaves, stems, and some ashes such as C. apiculatum and C. vendae contained appreciable levels of phenolic compounds, tannins, and flavonoids. As detected in this study, these phytoconstituents have been associated with antimicrobial activities and numerous physiological activities in mammalian cells in various studies [23,24,25,26]. Generally, the leaves showed to have higher concentrations of the phytoconstituents when compared to the stems. The phenolic compounds contained in the plants’ leaves were found to vary in the following order: C. padoides > C. apiculatum ≥ C. bracteosum > C. krausii > C. zeyheri > C. vendae > C. caffrum > C. adenogdnium > C. elaeagnoides > C. imberbe > C. mkuzense > C. erythrophyllum. These indicate that the leaves of C. padoides, C. bracteosum, and C. apiculatum are a good source of phenolic compounds when compared to other plant leaves in the study. These results support results presented by Masoko and Eloff [18], who investigated the qualitative antioxidant activity and phytochemical properties of 30 members of the Combretaceae. The phenolic compounds of the stems were found to be in the following order C. mkuzense > C. caffrum > C. krausii > C. vendae > C. apiculum = C. elaeagnoides > C. bracteosum > C. imberbe > C. padoides > C. zeyheri > C. adenogdnium > C. erythrophyllum. Although there was a significant decrease (p = 0.01) in the concentration of phenolic compounds in the stems when compared to the leaves, they still possessed substantial amounts and could still be used during seasons when leaves are scarce. Phenolic compounds exhibit various physiological properties, such as anti-allergenic, anti-inflammatory, anti-microbial, antioxidant, anti-thrombotic, cardio-protective, and vasodilatory effects [29,30,31]. Phenolic compounds have been associated with the health benefits of consuming high fruits and vegetables [32,33]. The antioxidant activities found in phenols causes them to have great health benefits [34]. In the ashes, notable amounts of phenolic compounds and tannins were only observed in C. apiculatum, C. bracteosum, and C. caffrum. Flavonoids play various biological activities in living organisms [35]. The study revealed that the all the Combretum plants tested possess substantial concentrations of flavonoids, with their leaves having higher concentrations than the stem. It was interesting to observe that the ashes of C. apiculatum, C. mkuzense, and C. vendae were significantly higher than those of the leaves and stems (p = 0.01). Currently, few or no studies report the presence of phytoconstituents in the ashes of these plants. The flavonoids in the leaves were found to be in the following order: C. elaeagnoides > C. vendae > C. padoides > C. imberbe > C. apiculatum > C. bracteosum > C. erythrophyllum > C. caffrum > C. zeyheri > C. krausii > C. mkuzense. Several studies [36,37,38] have reported the presence of flavonoids in C. apiculatum, C. caffrum, C. kraussii, and C. erythrophyllum, supporting the findings of this study. Several reports have revealed that other Combretum plants that were not included such as C. hereroense [39], C. nigricans, C. leprosum [40], and C. micranthum [41,42,43] contain flavonoids. These reports, together with these study findings, indicate that Combretum plants are a good source of flavonoids. Flavonoids have a capacity to act as antioxidants because of polyphenolic compounds. The functional groups mediate antioxidants by scavenging free radicals or by chelating metals ions [44,45,46]. As a dietary component, flavonoids are thought to have health-promoting properties due to their high antioxidant capacity in both in vivo and in vitro systems [47,48]. Saponins are used to treat the following medical conditions: hypercholesterolemia, hyperglycaemia, antioxidant, anticancer, anti-inflammatory, and weight loss. Reports have shown that they also possess antifungal properties [49]. Saponins have great cytotoxic effect and growth inhibition against different cell lines, making them potential anticancer agents [50]. Terpenes are a crucial group of organic compounds that have been reported as potent drugs used to treat a wide range of ailments. Terpenes are the most rapidly acting anti-malarial Artemisinin and its derivate [51]. Phenols, also found in plant sources, are a major group of compounds acting as primary antioxidants or free radical scavengers [52].
Antioxidants: The antioxidant activities of many plants are of great interest in the food, cosmetics, and pharmaceutical industries since their possible use as natural additives emerged from a growing tendency to replace synthetic preservatives with natural ones. DPPH assay is widely used to evaluate antioxidant activity of biological samples. DPPH is a stable free radical with characteristic absorption at 520 nm, and antioxidants react with DPPH radical and convert it to diamagnetic 2,2-diphenyl-1-picrylhydrazine molecule. The discolouration degree indicates the antioxidant extract’s scavenging potential, which is due to the hydrogen-donating ability [53,54]. In this study, as the sample concentration increased, the percentage inhibition of DPPH radical also increased. However, in the case of C. kraussii and C. mkuzense, the opposite was observed, i.e., at the lowest concentration, the scavenging activity was the highest. This means that, out of all the leaves tested, C. kraussii and C. mkuzense are good sources of antioxidants. Concentration-dependently, C. zeyherii leaves showed the overall highest scavenging activity when compared to the other leaves. It was observed to have scavenging activity, which was tested at concentrations of 250 μg/mL and 125 μg/mL. The methanol and acetone extracts of the leaves of C. zeyherii were found to possess antioxidant activity [55]. The stems of the plants had a relatively good antioxidant activity, which was found to be concentration-dependent, except for C. kraussii (Figure 6). There was a significant decrease in the antioxidant activity in the ashes (p = 0.01), compared to both the leaves and the stems. This may be due to the loss of some phytoconstituents, (tannins, cardiac glycosides, and flavonoids). Flavonoids and tannins have been shown to act as a secondary antioxidant defence system in plant tissues exposed to different abiotic and biotic stresses [56]. Ashes from C. imberbe, C. apiculatum, and C. padoides still possessed antioxidant activity after the burning process. Combretum plants are a good source of phytochemicals that possess an antioxidant capacity.
Proximate analysis: The study of the proximate content—for instance, the moisture content presented in the different parts of the plants—could reflect the plant’s ability to resist harsh environmental conditions such as drought. Reports have shown that most Combretum spp, such as C. erythrophyllum and C. zeyherii, are drought-resistant [57]. Moisture content is measuring the amount of water contained in a material. Water is an essential compound of many food products. About 20% of the total water is consumed through food [58].
The percentage ash content of the plants could be important reflection of the nutritional mineral contents [59]. Ash refers to the inorganic residue remaining after either ignition or complete oxidation of organic matter in a foodstuff. Ash contains inorganic material of the plant, which includes oxides and salts containing anions [60]. The ash content is a measure of the total amount of minerals present within a food product. Generally, the leaves had the lowest ash percentage when compared to the stems, apart from C. adenogdium and C. vendae. C. kraussii had the highest ash percentage, followed by C. zeyherii. This means that these plants should possess the highest concentration of mineral content. High ash content indicates the presence of heavy amounts of inorganic nutrients in plant material [61]. Proteins are chains of amino acids which are involved in nearly every process in the body. These macromolecules are involved in metabolic reactions such DNA replication, transcription, and act as enzymes as well as signalling proteins [62,63]. The protein contents in the plants were observed in the following order: leaves > stem > ashes for the following plants (C. adenogdium, C. caffrum, C. erythrophyllum, C. krausii, and C. zeyherii); ashes > leaves > stem (C. elaegnoides, C. apiculatum, and C. vendae); leaves > ashes > stem (C. bracteosum, C. mkuzense, and C. padoides). Overall, the leaves had the highest protein content apart from C. elaegnoides at 17.1%. Foods that provide more than 12% of their calorific value from proteins are considered a good source of proteins [64]. Since many of the plants tested in the study had appreciable levels of protein content, they can be considered for use in the food industry. These plants showed appreciable levels of energy content within them. Plant-based proteins are regarded as functional ingredients in food formulations, including thickening and gelling agents, stabilisers of emulsions and foams, and binding agents for fat and water. Moreover, some proteins have biological activities such as antioxidant or antimicrobial properties [65].
Minerals: Trace elements are dietary minerals essential for proper growth, development, and maintaining and recovering the organism’s health [66]. Some trace elements control important biological processes through such actions as catalysts in enzymes systems and oxidation-reduction in metabolism. In this study, the leaves contain the highest calcium concentration, while zinc has the lowest concentration. C. bracteosum had the lowest levels of minerals such as calcium, copper, nickel, and zinc when compared to leaves of other Combretum species used in this study. C. adenogonium had the highest levels of copper and iron, while C. caffrum had the highest levels of magnesium and manganese. C. elaegnoides had the lowest concentrations of the following minerals: Ca, Co, Cu, Fe, K, Mg, Mn, and Ni.
The variation of elemental content from plant to plant was mainly attributed to the differences between the botanical structure and the mineral composition of the soil in which plants are cultivated. Other factors responsible for the variation include absorbability of the plants, the use of fertilizers, irrigation water, and climatic conditions [64]. Compared to other foods, they are good sources of minerals. The analysed leaves were found to have higher concentrations of minerals when compared to consumable vegetables such as Allium sativum and Allium tuberosum [67]. In addition, they were found to have higher concentrations than some wild plants that are used as spices such as F. xanthoxyloïdes, H. gabonii (bark and fruit), M. myristica, M. whitei, P. brazzeana, P. guineense, P. umbellatum, S. melongena, S. striatinux, S. zenkeri (fruit), S. zenkeri (bark), T. tetraptera, and X. aethiopica [65]. Regarding stems, the mineral content was found to have decreased significantly compared to the leaves. Apart from calcium, there was a further decrease in mineral content regarding the ashes. It was interesting to observe that the calcium concentration in all the ashes increased significantly, approximately four times more than the leaves and stems. Based on these findings, it can be concluded that the leaves and ashes of C. adenogonium and C. apiculatum could provide a good source of calcium. Calcium is also important for blood coagulation and the normal functioning of the cardiac muscles [68]. It is also noteworthy to highlight that C. zeyherii has high mineral content. These minerals are necessary for the maintenance of good health in both animals and humans. Furthermore, the mineral elements affect biochemical processes and play crucial roles in living organisms, specifically the biological, metabolic, and enzymatic reactions leading to the development of active organic components [69]. Sodium and potassium maintain the human body’s ionic balance and tissue excitability. Sodium plays an important role in the transport of metabolites [70]. The ratio of potassium/sodium in any food is an important factor associated with hypertension and arteriosclerosis. Sodium enhances and potassium depresses blood pressure [71]. In this study, the leaves and ashes of C. adenogonium, C. bracteosum, and C. apiculatum proved to be a good source of sodium. Iron is essential in oxygen binding to haemoglobin and acts as a catalyst for many enzymes such as cytochrome oxidase [72]. In the current study, the leaves of C. adenogonium, C. bracteosum, and ashes of C. zeyherii possessed high levels of concentrations of iron. Thus, these plants can be recommended against anaemia. Magnesium helps to support muscle and nerve function and energy production and prevent impaired spermatogenesis and bleeding disorders [73]. The leaves of C. bracteosum, stems of C. apiculatum, and ashes of C. caffrum and C. bracteosum possessed appreciable amounts compared to other plants in the study. The recommended dietary allowance (RDA) for calcium (1000 mg/day), magnesium (400 mg/day), and iron (8 mg/day) suggest that these plants contribute substantially to improving the diet in terms of mineral requirement. The manganese concentration ranged between 0.00 and 3.9 mg/mg, which is higher than the values obtained from wild edible plants such as Aegle marmelos (L.) Corrêa, Argyreia speciosa (L. f.) Sweet, Butea monosperma (Lam Taub) reported by [74] Zn concentration ranged between 0.006 and 1.04 mg/mL, which is like the levels reported in some wild and leafy vegetables in India [74,75,76]. According to Shirwaikar et al. [38], minerals such as copper, manganese, and zinc are well-known antioxidants. The presence of these minerals might be attributed to the increase in the antioxidant activity of certain plants.
5. Conclusions
The pharmacological effect of the phytochemical constituents such as alkaloids, glycoside, tannins, and flavonoids as well as the antioxidant activity of the plants and ashes in the study explains the rationale for using these plants in traditional medicine and their use as food additives. The outcome of this study suggests that the selected plants and ashes could probably be a cheaper alternative to conventional drugs and preservatives since the plants are easily obtainable and can be cultivated on a sustainable basis. The plants and ashes contained appreciable amounts of nutrients such as protein, energy, and mineral elements that could enhance the nutrition of humans and livestock. Furthermore, the results suggest that these plants and their ashes could serve as a feed supplement to improve health and growth performance in humans and livestock. It can be ascertained that the Combretum extracts, together with the ashes, can be exploited as a source of natural nutrients and minerals, especially C. adenogonium, C. bracteosum, and C. apiculatum. Therefore, Combretum species could be potential nutraceuticals as nutritional supplements. This indicates that the Combretum plants will offer their users both nutritional and medicinal benefits.
Author Contributions
Conceptualization and editing, P.M.; investigation, formal analysis, and writing original draft, M.M.M. Co-supervision of the project, M.S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to acknowledge the staff in the Department of Biochemistry, Microbiology, and Biotechnology at the University of Limpopo for their outstanding support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zengin, G.; Cakmak, Y.S.; Guler, G.O.; Aktumsek, A. Antioxidant properties of methanolic extract and fatty acid composition of Centaurea urvillei DC. Subspecies hayekiana Wagenitz. Rec. Nat. Prod. 2011, 5, 123–132. [Google Scholar]
- Adom, K.K.; Liu, R.H. Antioxidant activity of grains. J. Agric. Food Chem. 2002, 50, 6182–6187. [Google Scholar] [CrossRef] [PubMed]
- Adom, K.K.; Sorrells, M.E.; Liu, R.H. Phytochemical profiles and antioxidant activity of wheat varieties. J. Agric. Food Chem. 2003, 51, 7825–7834. [Google Scholar] [CrossRef] [PubMed]
- Nunes, P.X.; Silva, S.F.; Guedes, R.J.; Almeida, S. Biological Oxidations and Antioxidant Activity of Natural Products, Phytochemicals as Nutraceuticals-Global Approaches to Their Role in Nutrition and Health; Intech Open: London, UK, 2012. [Google Scholar] [CrossRef]
- Djeridane, A.; Yousfi, M.; Nadjemi, B.; Boutassouna, D.; Stocker, P.; Vidal, N. Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006, 97, 654–660. [Google Scholar] [CrossRef]
- Wannes, W.A.; Mhamdi, B.; Sriti, J.; Jemia, M.B.; Ouchikh, O.; Hamdaoui, G.; Kchouk, M.E.; Marzouk, B. Antioxidant activities of the essential oil and methanol extracts from myrtle (Myrtus communis var. italica L.) leaf, stem and flower. Food Chem. Toxicol. 2010, 48, 1362–1370. [Google Scholar] [CrossRef] [PubMed]
- Anagnostopoulou, M.A.; Kefalas, P.; Papageorgiou, V.P.; Assimopoulou, A.N.; Boskou, D. Radical scavenging activity of various extracts and fractions of sweet orange peel (Citrus sinensis). Food Chem. 2006, 94, 19–25. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Wahid, A.; Siddique, K.H.M. Drought Stress in Plants: An Overview. In Plant Responses to Drought Stress; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- Kulisica, T.; Radonicb, A.; Katalinicc, V.; Milosa, M. Use of different methods for testing antioxidative activity of Oregano essential oil. Food Chem. 2004, 85, 633–640. [Google Scholar] [CrossRef]
- Wada, L.; Ou, B. Antioxidant activity and phenoliccontent of Oregon cane berries. J. Agric. Food Chem. 2002, 50, 3495–3500. [Google Scholar] [CrossRef] [PubMed]
- Lachman, J.; Orsák, M.; Pivec, V. Antioxidant contents and composition in some fruits and their role in human nutrition. Hortic. Sci. 2000, 27, 103–117. [Google Scholar]
- Zia-ul-haq, M.; Iqbal, S.; Ahmad, S.; Bhanger, M.I.; Wiczkowski, W.; Amarowicz, R. Antioxidant potential of Desi chickpea varieties commonly consumed in Pakistan. J. Food Lipids. 2009, 15, 326–342. [Google Scholar] [CrossRef]
- Perez-Lamela, C.; Garcia-Falcon, M.S.; Simal-Gandara, J.; Orriols-Fernandez, I. Influence of grape variety, vine system and enological treatments on the colour stability of young red wines. Food Chem. 2007, 101, 601–606. [Google Scholar] [CrossRef]
- McGaw, L.J.; Rabe, T.; Sparg, S.G.; Jäger, A.K.; Eloff, J.N.; Van Staden, J. An investigation of the biological activity of Combretum species. J. Ethnopharmacol. 2001, 75, 45–50. [Google Scholar] [CrossRef]
- Atindehou, K.K.; Schmid, C.; Brun, R.; Koné, M.W.; Traore, D. Antitrypanosomal anti-plasmodial activity of medicinal plants from Côte d‘Ivoire. J. Ethnopharmacol. 2009, 90, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Owen, R.W.; Giacosa, A.; Hull, W.E.; Haubner, R.; Spiegelhalder, B.; Bartsch, H. The antioxidant/anticancer potential of phenolic compounds isolated from olive oil. Eur. J. Cancer 2000, 36, 1235–1247. [Google Scholar] [CrossRef]
- Sala, A.; Recio, M.D.; Giner, R.M.; Manez, S.; Tournier, H.; Schinella, G.; Rios, J.L. Anti-inflammatory, and antioxidant properties of Helichrysum italicum. J. Pharm. Pharmacol. 2002, 54, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Masoko, P.; Eloff, J.N. Screening of twenty-four South African Combretum and six Terminalia species (Combretaceae) for antioxidant activities. Afr. J. Tradit. Complement. Altern. Med. 2007, 4, 231–239. [Google Scholar]
- Rogers, C.B.; Verotta, L. Chemistry and biological properties of the African Combretaceae. In Proceedings of the First International IOCD-Symposium, Victoria Falls, Zimbabwe, 25–28 February 1996; UZ Publications: Harare, Zimbabwe, 1996; pp. 121–141. [Google Scholar]
- Eloff, J.N.; Katerere, D.R.; McGaw, L.J. The biological activity and chemistry of the southern African Combretaceae J. Ethnopharmacol. 2008, 119, 686–699. [Google Scholar] [CrossRef]
- Muthu, C.; Ayyanar, M.; Raja, N.; Ignacimuthu, S. Medicinal plants used by traditional healers in Kancheepuram District of Tamil Nadu, India. J. Ethnobiol. Ethnomed. 2006, 2, 43. [Google Scholar] [CrossRef] [PubMed]
- Borokini, T.I.; Omotayo, F.O. Phytochemical and ethnobotanical study of some selected medicinal plants from Nigeria. J. Med. Plant Res. 2012, 6, 1106–1118. [Google Scholar]
- Odebiyi, O.O.; Sofowora, E.A. Phytochemical screening of Nigerian medicinal plants II. Lloydia 1977, 41, 234–246. [Google Scholar]
- Humadi, S.S.; Istudor, V. Quantitative analysis of bioactive compound Hibiscus sabadariffa L. extracts note 1: Quantitative analysis of flavonoids. Farmácia 2008, 6, 699–707. [Google Scholar]
- Tambe, V.D.; Bhambar, R.S. Estimation of total phenol, tannin, alkaloid and flavonoid in Hibiscus Tiliaceus Linn. wood extracts. Res. Rev. 2014, 2, 2321–6182. [Google Scholar]
- Chigayo, K.; Mojapelo, P.E.L.; Moleele, S.M. Phytochemical and antioxidant properties of different solvent extracts of Kirkia wilmsii tubers. Asian Pac. J. Trop. Biomed. 2016, 6, 1037–1043. [Google Scholar] [CrossRef]
- AOAC. Official methods of analysis of the Association of Official Analytical Chemists; AOAC International: Rockville, MD, USA, 1994. [Google Scholar]
- Zhang, X.R.; Kaunda, J.S.; Zhu, H.T.; Wang, D.; Yang, C.R.; Zhang, Y.J. The Genus Terminalia (Combretaceae): An Ethnopharmacological, Phytochemical and Pharmacological Review, Natural Products, and Bioprospecting; Springer: Singapore, 2019. [Google Scholar]
- Trease, G.E.; Evans, W.C. Pharmacognosy, 14th ed.; W.B. Sanders: London, UK, 1989; p. 288. [Google Scholar]
- Sofowora, L.A. Medicinal Plants and Traditional Medicine in Africa; Spectrum Books Ltd.: Ibaban, Philippines, 1993; pp. 55–71. [Google Scholar]
- Abo, K.A.; Ogunleye, V.O.; Ashidi, J.S. Antimicrobial Potential of Spondiasmonbin, croton zambesicus and zygotritonia crocea. Phytother. Res. 1999, 13, 494–497. [Google Scholar] [CrossRef]
- Nweze, E.I.; Okafor, J.I.; Njoku, O. Methabolic Extracts of Treme guineenes (Schumm and thorn) and Morinda lucida Benth used in Nigeria Herbal Medicinal practice. Biol. Res. 2004, 2, 39–48. [Google Scholar]
- Mishra, A.K.; Kehri, H.K.; Sharma, B.; Pandey, A.K. Inhibitory activity of Indian spice plant Cinnamomum zeylanicum extracts against Alternaria solani and Curvularia lunata, the pathogenic dematiaceous moulds. Ann. Clin. Microbiol. Antimicrob. 2009, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E.J. Effect of plant flavonoids on immune and inflammatory cell function. Adv. Exp. Med. Biol. 1998, 439, 175–182. [Google Scholar] [PubMed]
- Puupponen-Pimia¨, R.; Nohynek, L.; Meier, C.; Ka¨hko¨nen, M.; Heinonen, M.; Hopia, A. Antimicrobial properties of phenolic compounds from berries. J. Appl. Microbiol. 2001, 9, 494–507. [Google Scholar] [CrossRef]
- Hertog, M.G.L.; Hollman, P.C.H.; Katan, M.B.; Kromhout, D. Intake of potentially anticarcinogenic flavonoids and their determinants in adults in the Netherlands. Nutr. Cancer 1993, 20, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Parr, A.J.; Bolwell, G.P. Phenols in the plant and in man. The potential for possible nutritional enhancement of the diet by modifying the phenols content or profile. J. Sci. Food Agric. 2000, 80, 985–1012. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure–activity relationships. J. Nutri. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Antioxidant, lipo-protective and antibacterial activities of phytoconstituents present in Solanum xanthocarpum root. Int. Rev. Biophys. Chem. 2012, 3, 42–47. [Google Scholar]
- Pettit, G.R.; Smith, C.R.; Singh, S.B. Recent advances in the chemistry of plant antineoplastic constituents. In Biologically Active Natural Products, Proceedings of the Phytochemical Society of Europe; Hostettmann, K., Lea, P.J., Eds.; Oxford Science Publications: Oxford, UK, 1987. [Google Scholar]
- Schwikkard, S.; Zhou, B.N.; Glass, T.E.; Sharp, J.L.; Mattern, M.R.; Johnson, R.K.; Kingston, D.G.I. Bioactive compounds from Combretum erythrophyllum. J. Nat. Prod. 2000, 63, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Letcher, R.M.; Nhamo, L.R.M. Chemical constituents of the Combretaceae. Part IV. Phenanthrene derivatives from the heartwood of Combretum hereroense. Perkin Trans. 1973, 1, 1179–1181. [Google Scholar] [CrossRef]
- Facundo, V.A.; Andrade, C.H.S.; Silveira, E.R.; Braz-Filho RHufford, C.D. Triterpenes and flavonoids from Combretum leprosum. Phytochem. Lett. 1993, 32, 411–415. [Google Scholar] [CrossRef]
- Masoko, P.; Picard, J.; Eloff, J.N. The antifungal activity of twenty-four southern African Combretum species (Combretaceae). S. Afr. J. Bot. 2007, 73, 173–183. [Google Scholar] [CrossRef]
- Masoko, P.; Picard, J.; Eloff, J.N. Antifungal activities of six South African Terminalia species (Combretaceae). J. Ethnopharmacol. 2005, 99, 301–308. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Cook, N.C.; Samman, S. Review: Flavonoids-chemistry, metabolism, cardio protective effects and dietary sources. J. Nutr. Biochem. 1996, 7, 66–76. [Google Scholar] [CrossRef]
- Tijjani, M.A.; Abdurahman, F.I.; Buba, S.W.; Mala, G.I.; Akan, J.C.; Aji, B.M. Abdullahi. A.S. Chemical and proximate contents of methanolic leaf extract of Piliostigma thonningii schum (Camel Foot). J. Chem. Pharm. Res. 2012, 4, 2409–2414. [Google Scholar]
- Iniaghe, O.M.; Malomo, S.O.; Adebayo, J.O. Proximate composition and phytochemical constituents of leaves of some Acalypha species. Pak. J. Nutr. 2009, 8, 256–258. [Google Scholar] [CrossRef]
- Adesuyi, A.O.; Awosanya, O.A.; Adaramola, F.B.; Omeonu, A.I. Nutritional and phytochemical screening of Aloe barbadensis. Curr. Res. J. Biol. 2011, 4, 4–9. [Google Scholar]
- Von Gadow, A.; Joubert, E.; Hansmann, C.F. Comparison of the antioxidant activity of aspalathin with that of other plant phenols of Rooibos tea (Aspalathus linearis), α-tocopherol, BHT, and BHA. J. Agric. Food Chem. 1997, 45, 632–638. [Google Scholar] [CrossRef]
- Jaitak, V.; Sharma, K.; Kalia, K.; Kumar, N.; Singh, H.P.; Kaul, V.K.; Singh, B. Antioxidant activity of Potentilla fulgens: An alpine plant of western Himalaya. J. Food Compos. Anal. 2010, 23, 142–147. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Lima De Morais, G.R.; De Sales, I.R.P.; Filho, M.R.D.C.; De Jesus, N.Z.T.; De Sousa Falcao, H.; Barbosa-Filho, J.M.; Cabral, A.G.S.; Souto, A.L.; Tavares, J.F.; Batista, L.M. Bioactivities of the genus Combretum (Combretaceae): A review. Molecules 2012, 17, 9142–9206. [Google Scholar] [CrossRef]
- Srivastava, P.; Prasad, S.G.M.; Mohd Nayeem, A.; Prasad, M. Analysis of antioxidant activity of herbal yoghurt prepared from different milk. J. Pharm. 2015, 4, 18–20. [Google Scholar]
- FNB. Food and Nutrition Board Dietary Reference Intakes for Water Potassium, Sodium, Chloride and Sulphate; Institute of Medicine, National Academies, National Academies Press: Washington, DC, USA, 2005. [Google Scholar]
- Raman, R.C.; Ipper, G.B.; Subhash, J.D. Preliminary phytochemical investigation of extract of leaves of Pergularia daemia Linn. Int. J. Pharm. Sci. 2010, 1, 111–116. [Google Scholar]
- Gopalan, C.; Ramasastri, B.V.; Balasubramanian, S.C.; Narsinagarao, B.S.; Deosthale, Y.G.; Pant, K.C. Nutritive Value of Indian Foods; India National Institute of Nutrition: Hyderabad, India, 1999. [Google Scholar]
- Odhav, B.; Beekrum, S.; Akula, U.; Baijnath, H. Preliminary assessment of nutritional value of traditional leafy vegetables in KwaZulu-Natal, South Africa. J. Food Compos. Anal. 2007, 20, 430–435. [Google Scholar] [CrossRef]
- Perrett, D. From ‘protein’ to the beginnings of clinical proteomics. Proteom.—Clin. Appl. 2007, 1, 720–738. [Google Scholar] [CrossRef] [PubMed]
- Keskin, O.; Tuncbag, N.; Gursoy, A. Characterization, and prediction of protein interfaces to infer protein-protein interaction networks. Curr. Pharm. Biotechnol. 2007, 9, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Ali, A. Proximate and mineral composition of the marchubeh (Asparagus officinalis). World J. Dairy Food Sci. 2009, 4, 142–149. [Google Scholar]
- Jafarirad, S.; Mehrabi, M.; Divband, B.; Kosari-Nasab, M. Biofabrication of zinc oxide nanoparticles using fruit extract of Rosa canina and their toxic potential against bacteria: A mechanistic approach. Mater. Sci. Eng. 2016, 59, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Aiasgharpour, M.; Farzami, M. Trace Elements in Human Nutrition: A Review. Int. J. Med. 2013, 2, 115–128. [Google Scholar]
- Chaturvedi, V.C.; Shrivastava, R.; Upreti, R.K. Viral infections and trace elements: A complex trace element. Curr. Sci. 2004, 87, 1536–1554. [Google Scholar]
- Khalid, N.; Ahmed, I.; Latif, M.S.Z.; Rafique, T.; Fawad, S.A. Comparison of antimicrobial activity, phytochemical profile and minerals composition of garlic Allium sativum and Allium tuberosum. J. Appl. Biol. Chem. 2014, 57, 311–317. [Google Scholar] [CrossRef]
- Bouba, A.A.; Njintang, N.Y.; Foyet, H.S.; Scher, J.; Montet, D.; Mbofung, C.M.F. Agricultural systems, a literature review and annotated bibliography. Int. J. Food Sci. Nutr. 2012, 1, 213–224. [Google Scholar]
- Sundriyal, M.; Sundriyal, R.C. Wild edible plants of the Sikkim Himalaya: Nutritive values of selected species. Econ. Bot. 2004, 58, 286–299. [Google Scholar] [CrossRef]
- Serfor-Armah, Y.; Nyarko, B.J.B.; Akaho, E.H.K.; Kyere, A.W.K.; Osae, S.; Oppong-Boachie, K. Multielemental analysis of some traditional plant medicines used in Ghana. J. Trace Microprobe Technol. 2002, 20, 419–427. [Google Scholar] [CrossRef]
- Sinha, B.K.; Bhattacharjee, S.; Tapan, S. Nutritional composition, mineral content, antioxidant activity and quantitative estimation of water-soluble vitamins and phenolics by RP-HPLC in some lesser-used wild edible plants. Heliyon 2019, 5, e01431. [Google Scholar]
- Saupi, N.; Zakaria, M.H.; Bujang, J.S. Analytic chemical composition, and mineral content of yellow velvet leaf (Limnocharis flava L. Buchenau)’s edible parts. J. Appl. Sci. 2009, 9, 2969–2974. [Google Scholar] [CrossRef]
- Geissler, C.A.; Powers, H.J. Human Nutrition, 11th ed.; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Brookes, K.B.; Doudoukina, O.V.; Katsoulis, L.C.; Veale, D.J.H. Uteroactive constituents from Combretum kraussii. S. Afr. J. Chem. 1999, 52, 127–132. [Google Scholar]
- Seal, T.; Chaudhuri, K. Nutritional analysis of some selected wild edible plants consumed by the tribal people of Meghalaya state in India. Int. J. Food Sci. Nutr. 2016, 1, 39–43. [Google Scholar]
- Saikia, P.; Deka, D.C. Mineral content of some wild green leafy vegetables of North-East India. J. Chem. Pharm. 2013, 5, 117–121. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).