Abstract
Biochemical and mineral peculiarities of plants inhabiting desert and semi-desert areas may provide important information about the mechanism of their adaptability and reveal the prospects of their utilization. Rheum tataricum L., known for its high tolerance to drought, salinity, and nutritional deficiency, is the least studied species of wild rhubarb. Using biochemical and ICP-MS analysis, the antioxidant status and mineral composition of R. tataricum were determined. Extremely high levels of antioxidant activity (148–155 mg GAE g−1 d.w.), polyphenols (24.6–25.1 mg GAE g−1 d.w.) and carotenoids (1.94 mg-eq β-carotene g−1 d.w.) were revealed in roots, proline in leaves (71.1 ± 6.2 mg kg−1 d.w.) and malic acid in stems (3.40 ± 0.50 mg g−1 d.w.). Compared to garden rhubarb, R. tataricum demonstrated significant root–leaves translocation of Li, Se, Si, and Mo, known to participate in plant antioxidant defense. Under high levels of Ca, Na, Mg, Fe, Cr and Si in soil, R. tataricum demonstrated the ability to significantly increase the accumulation of these elements in roots, showing a hyperaccumulation ability for Sr. The first broad picture of R. tataricum biochemical and mineral characteristics in semi-desert habitat and its nutritional value indicate the prospects of R. tataricum utilization in plant breeding, medicine, and nutrition.
Keywords:
rhubarb; salinity; antioxidants; carotenoids; organic acids; proline; minerals distribution 1. Introduction
Wild relatives of cultivated crops produce enormous opportunities in selection technology, revealing new prospects to improve crop quality and adaptability to environmental stress [1]. There are about 60 species of the genus Rheum L plants in nature, all of which are considered valuable in traditional medicine, demonstrating astringent, anti-inflammatory, laxative, wound healing, and fever relieve properties. High levels of food fiber provide a protection against heart diseases and vitamin K is valuable in osteoporosis prevention [2]. Rhubarb plants demonstrate antitumor properties [3], regulation of gastrointestinal flora [4], protection of the intestinal mucosal barrier [5,6], anti-inflammatory activity [7], inhibition of fibrosis [8] and heart protection [9]. The most common rhubarb species in Europe and the southwestern area of China are R. tanguticum Maxim., R. officinale Baill., R. palmatum L., R. acuminatum Hook. f. and Thomson., R. australe D. Don. Several species (R. tanguticum Maxim., R. officinale Baill. and R. palmatum L.) are officially included into the Chinese, Korean and Japanese Pharmacopoeia.
Among wild rhubarb species, R. tataricum is the least studied due to the restricted area of its habitat, occupying a narrow area of dry steppe and deserts of middle Asia, from the northeastern part of the Astrakhan region in Russia to lake Balkhash in Kazakhstan, as well as its extremely short vegetation period lasting from March to June and deformed small stems, one of the most valuable plant parts of garden rhubarb (Figure 1). Similar to desert rhubarb Rheum palaestinum, it has broad wrinkled leaves which presumably allow it to achieve rainfall collection, transport, and self-irrigation [10].

Figure 1.
Rheum tataricum of Bogdinsko-Baskunchak Nature Reserve.
Inhabiting semi-desert and desert areas, R. tataricum is highly tolerant to salinity, drought, and nutrient deficiency, which may become the basis for intensive selection aimed to improve plant quality and increase adaptability to environmental stress. Lack of information about the accumulation of biologically active compounds and minerals in this plant slows our knowledge of rhubarb adaptability and utilization possibilities.
Bogdinsko-Baskunchak Nature Reserve, which is situated on the peripheral part of the Caspian lowland on the border with Kazakhstan, occupies a territory of 18,478 ha and in 2021 was classified by UNESCO as a World Heritage Site. The climate of the Reserve is continental with low precipitation (the mean annual precipitation is 270 mm), differing from 150 to 400 mm in different years and the highest temperature range between −40 °C in winter and +40 °C in summer (the mean values are −8.1 °C and +24.8 °C, respectively). Soils are predominantly alkaline, light chestnut with a low amount of humus. Sodium chloride concentration in lake Baskunchak reaches 300 g L−1, with a surface salt deposit thickness of 10–18 m [11]. Gypsum outcrops and intense winds bringing dust and salt are additional factors which induce significant oxidative stress at the territory of the Reserve. In such extreme conditions there exist only 507 plant species [12].
The aim of the present work was to evaluate the biochemical and mineral composition of R. tataricum grown in the semi-desert territory of Bogdinsko-Baskunchak Nature Reserve, and the comparison of the results with the data obtained for European garden rhubarb grown in experimental fields of the Federal Scientific Vegetable Center. Due to lack of intensive environmental stresses, garden rhubarb is characterized by different leaf morphology, but to date, no comparison has been achieved between the biochemical characteristics and mineral content of R. tataricum and garden rhubarb.
2. Materials and Methods
2.1. Place of Sampling
Five plants of R. tataricum and soil samples under these plants were sampled each year, with three replicates, in May (1–5), 2021 and 2022 at the Eastern shore of the Baskunchak lake (48°13′18″2 N, 46°58′34″8 E). To perform a comparison, samples of 5 garden rhubarb plants, cv. Zaryanka, were sampled each year, with three replicates, grown on sod-podzolic clay-loam soil (pH 6.8, 2.1% organic matter, 1.1 g·kg−1 N, 0.045 g·kg−1 P2O5, 0.357 g·kg−1 K2O), at the Federal Scientific Center of Vegetable Production (Moscow region, 55°39.51′ N, 37°12.23′ E). In the semi-desert conditions of Bogdinsko-Baskunchak Nature Reserve, the beginning of May is the only time when it is possible to sample all plant tissues of R. tataricum, including not only roots, also leaves, stems and florets. By the end of May, the plants shed seeds and vegetation period ends. On the contrary, the garden rhubarb vegetation phase lasts until the end of autumn and, to make an appropriate comparison, rhubarb sampling at the experimental field of the Federal Scientific Vegetable Center was achieved at the end of May 2021–2022. A systematic uniform random sampling at fixed intervals along equally spaced three parallel transects was used [13], which allowed us to obtain five plant samples of R. tataricum, with three replicates each year by sampling the site according to its natural distribution within the research area in Bogdinsko-Baskunchak Nature Reserve. Mixed samples of all leaves of 5 plants from garden rhubarb, with three replicates, were used in this study.
Roots, stems, leaves and florets were separated. Roots were washed with fresh water to remove soil particles and root peel and pulp were separated and dried in an oven at 70 °C to constant weight. Stems, leaves and florets were cut into small slices, dried at 25–30 °C and homogenized. The same operations were performed with leaves of R. tataricum, but due to their large size a mixed sample of one leaf per plant from 5 plants was prepared. Soil samples were also dried, ground in a mortar, and shifted through a sieve.
2.2. Mineral Composition
Al, As, B, Ca, Cd, Co, Cr, Cu, Fe, Hg, I, K, Li, Mg, Mn, Mo, Na, Ni, P, Pb, Se, Si, Sn, Sr, V, and Zn contents in dried homogenized rhubarb roots and leaves were assessed using ICP-MS on quadruple mass-spectrometer Nexion 300D (Perkin Elmer Inc., Shelton, CT, USA), equipped with the seven-port FAST valve and ESI SC DX4 autosampler (Elemental Scientific Inc., Omaha, NE, USA) at the Biotic Medicine Center (Moscow, Russia). Rhodium 103 Rh was used as an internal standard to eliminate instability during measurements. Quantitation was performed using external standard (Merck IV, multi-element standard solution); Perkin–Elmer standard solutions for P, Si, and V, and all the standard curves were obtained at five different concentrations. For quality control purposes, internal controls and reference materials were tested together with the samples daily. Microwave digestion of samples was carried out with sub-boiled HNO3 diluted 1:150 with distilled deionized water (Fluka No. 02, 650 Sigma–Aldrich, Co., Saint Louis, MO, USA) in the Berghof SW-4 DAP-40 microwave system (Berghof Products + Instruments Gmb H, 72, 800 Eningen, Germany). The instrument conditions and acquisition parameters were: plasma power and argon flow, 1500 and 18 L min−1, respectively; aux argon flow, 1.6 L min−1; nebulizer argon flow, 0.98 L min−1; sample introduction system, ESI ST PFA concentric nebulizer and ESI PFA cyclonic spray chamber (Elemental Scientific Inc., Omaha, NE, USA); sampler and slimmer cone material, platinum; injector, ESI Quartz 2.0 mm I.D.; sample flow, 637 L min−1; internal standard flow, 84 L min−1; dwell time and acquisition mode, 10–100 ms and peak hopping for all analytes; sweeps per reading, 1; reading per replicate, 10; replicate number, 3; DRC mode, 0.55 mL min−1 ammonia (294993-Aldrich Sigma–Aldrich, Co., St. Louis, MO 63103, USA) for Ca, K, Na, Fe, Cr, V, optimized individually for RPa and RPq; STD mode, for the rest of analytes at RPa = 0 and RPq = 0.25. Trace levels of Hg in samples were not taken into account and, accordingly, they were not included in the tables.
Total content of Fe, Mn, Zn, Cu, Cd, Fe, Cr and Sr in soil samples was analyzed on AAS spectrophotometer (Hitachi 7001, Japan) using 3% HNO3. Mobile forms of elements were determined in ammonium-acetate buffer according to [14].
2.3. Total Polyphenols (TP)
Total polyphenols in rhubarb tissues (roots, stems and leaves) were determined in 70% ethanol and water extracts using the Folin–Ciocalteu colorimetric method as previously described [15]. One gram of dry homogenates was extracted with 20 mL of 70% ethanol/water at 80 °C for 1 h. The mixture was cooled down and quantitatively transferred to a volumetric flask, and the volume was adjusted to 25 mL. The mixture was filtered through filter paper, and 1 mL of the resulting solution was transferred to a 25 mL volumetric flask, to which 2.5 mL of saturated Na2CO3 solution and 0.25 mL of diluted (1:1) Folin–Ciocalteu reagent were added. The volume was brought to 25 mL with distilled water. One hour later the solutions were analyzed through a spectrophotometer (Unico 2804 UV, Suite E Dayton, NJ, USA), and the concentration of polyphenols was calculated according to the absorption of the reaction mixture at 730 nm. As an external standard, 0.02% gallic acid was used. The results were expressed as mg of Gallic acid equivalent per g of dry weight (mg GAE g−1 d.w).
2.4. Antioxidant Activity (AOA)
The antioxidant activity of samples (roots, stems and leaves) was assessed using a redox titration method according to [15] via titration of 0.01 N KMnO4 solution with ethanolic/water extracts of dry samples, produced as described in Section 2.4. The reduction of KMnO4 to colorless Mn+2 in this process reflects the quantity of antioxidants dissolvable in 70% ethanol/water. The values were expressed in mg Gallic acid equivalents (mg GAE g−1 d.w.).
2.5. Carotenoids
Carotenoids content in rhubarb roots powder was determined using hexane extract of samples, quantitative thin layer chromatography (TLC) on chromatographic paper Watman 3A and spectrophotometric analysis by the spectrophotometer Unico 2804 UV (Suite E Dayton, Newark, NJ, USA) [15].
2.6. Proline
Proline concentration was determined according to [16] with slight modification. About 50 mg of dry homogenized rhubarb roots were ground with 10 mL of 3% sulfur salicylic acid in a mortar. The mixture was filtered and 1 mL of the resulting filtrate, 2 mL of ninhydrin reagent and 2 mL of acetic acid were heated at 95 °C during 1 h. Proline concentration was evaluated using absorption value of the reaction mixture at 505 nm (Unico 2804 UV spectrophotometer, Suite E Dayton, Newark, NJ, USA) and a calibration curve with 5 different proline (Merck) concentrations.
2.7. Organic Acids
Rhubarb stems were determined using HPLC (Agilent 1100): column Zorbax Bonus-RP C18, 4.6 × 250 ID mm, 5 µM; current speed—1.0 mL min−1; wavelength—210 nm. Mobile phase—isocratic elution with phosphate buffer, pH 2.5 [17].
2.8. Statistical Analysis
The data were processed by analysis of variance and mean separations were performed through the Duncan’s multiple range test, with reference to 0.05 probability level, using SPSS software version 21 (Armonk, NY, USA). Data expressed as percentages were subjected to angular transformation before processing.
3. Results and Discussion
R. tataricum belongs to a group of rhubarb species with high adaptability to semi- desert and desert conditions, where both morphological changes (large area of leaves, deep roots) and biochemical peculiarities ensure plant survival. Despite intensive morphological investigations of these plants [1,10], nothing is known about the biochemical characteristics and mineral composition of R. tataricum, which is directly connected with a restricted habitat and short vegetation period. On the other hand, garden rhubarb’s biochemical characteristics are still rather fragmentary [18,19], suggesting the necessity of intensive investigations.
3.1. Antioxidant Status of Plants
The analysis of total antioxidant activity (AOA) and total phenolic content (TP) revealed an unusual distribution of antioxidants in rhubarb, and the highest levels of antioxidants were recorded in roots and the lowest in florets (Table 1). No differences in phenolics content were found between rhubarb plant parts, with mean values of 24.0 ± 0.8 mg GAE g−1 d.w.

Table 1.
Total antioxidant activity and polyphenols accumulation by R. tataricum and garden rhubarb, cultivar Zaryanka, using ethanolic extracts of tissues.
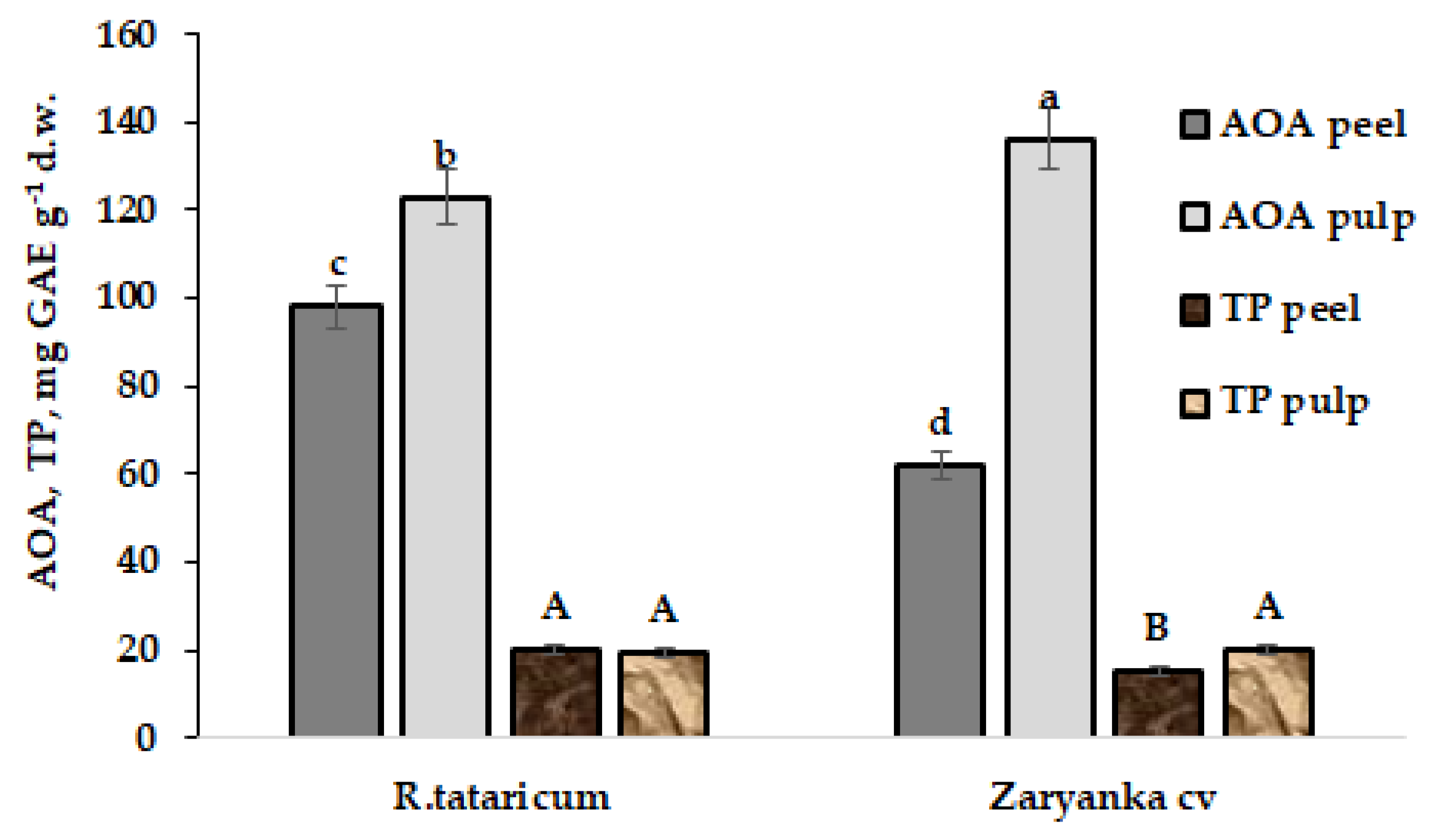
On the contrary, water extracts demonstrated significantly higher levels of AOA and TP in root peel of R. tataricum compared to garden rhubarb, cv. Zaryanka (Figure 2).
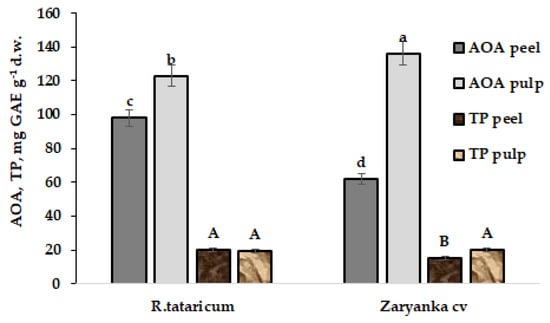
Figure 2.
Total antioxidant activity (AOA) and polyphenol content (TP) in water extracts of R. tataricum roots peel/pulp and garden rhubarb cv. Zaryanka. The values with the same letters do not differ statistically according to Duncan’s test at p < 0.05.
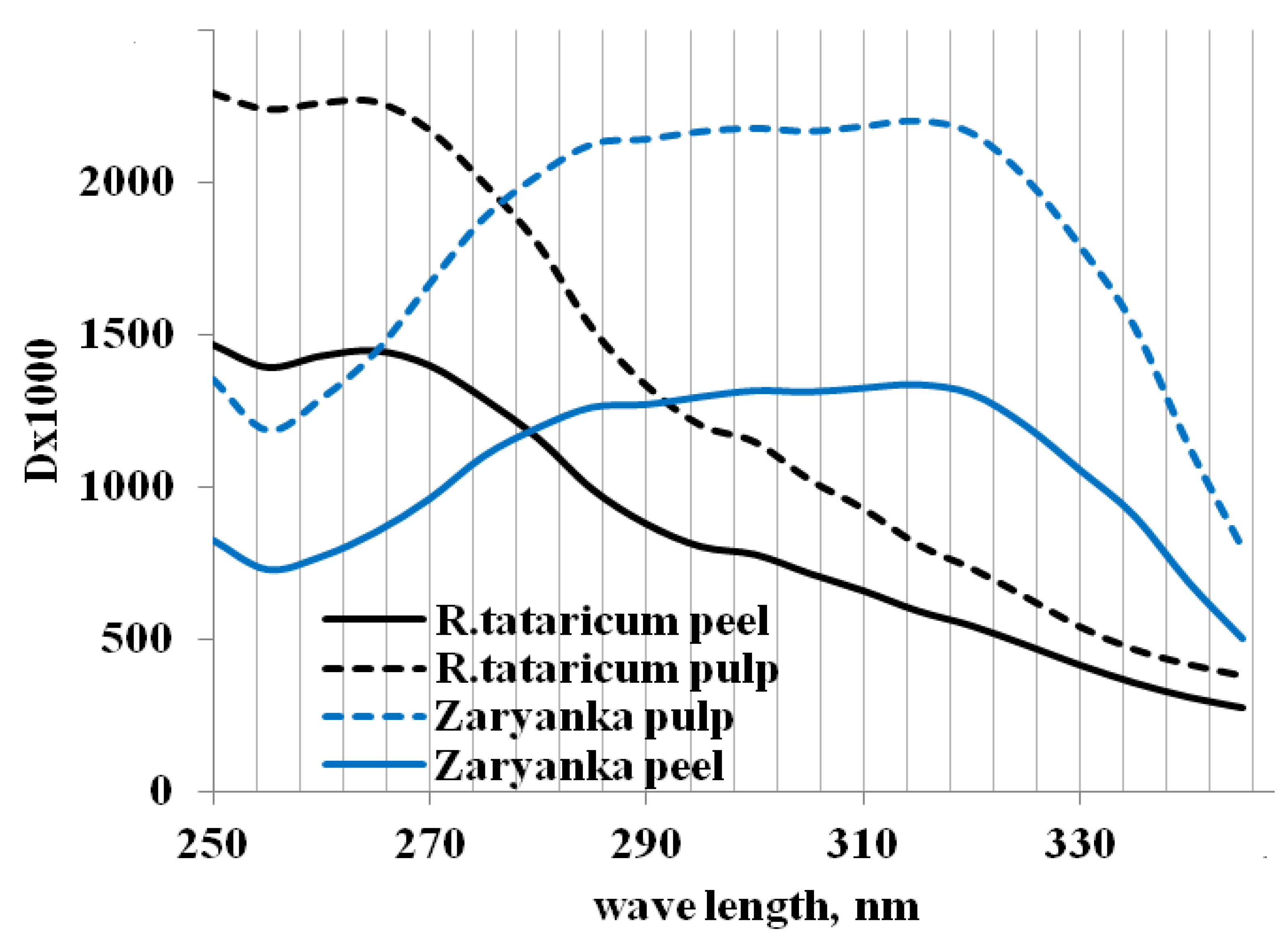
The UV spectrum of R. tataricum root water extracts (Figure 3) indicates different characteristics of the extracts’ absorption compared to garden rhubarb extracts, which proved significant differences in chemical composition of water-soluble compounds in R. tataricum and Zaryanka roots. According to the literature reports, rhubarb roots accumulate different forms of polyphenols, such as anthraquinones, anthrones, naphthalenes, chromones, phenylbutanones, stilbenes and tannins which absorb light in a range of 280–400 nm [20,21,22,23] and phenolic content and composition are greatly affected both by the genetic and environmental factors [24]. Consequently, the detected differences in the UV-spectra (Figure 3) may refer to both factors.
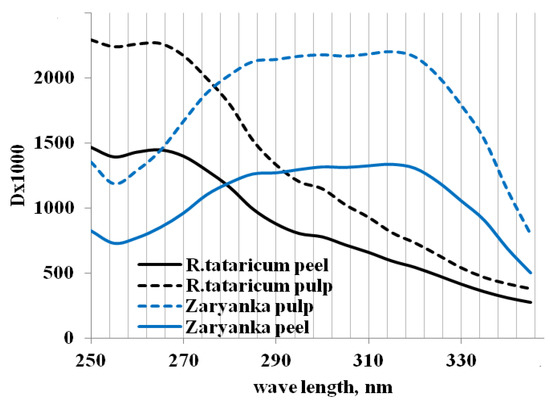
Figure 3.
UV spectrum of rhubarb root peel and pulp water extracts.
Furthermore, taking into consideration a significant role of antioxidants in plants protection against oxidative stress (salinity, drought and high temperature in particular) [25], the results demonstrate the importance of high root peel antioxidant status in R. tataricum grown in stress conditions.
3.2. Proline
Salt stress is usually accompanied by ionic, osmotic, and oxidative stress due to the overproduction of reactive oxygen species (ROS) in plants, causing the oxidation of protein, membrane lipids and nucleic acids, and inhibiting plant growth and development [26]. Additionally, under salt stress, plants must extensively adjust various physiological and biochemical processes, including ion and osmotic homeostasis, as well as stress damage control and repair [27].
Proline plays a highly beneficial role in plants exposed to various stress conditions [28]. Besides acting as an excellent osmolyte, proline plays three major roles during stress, i.e., as a metal chelator, an antioxidative defense molecule and a signaling molecule.
Apart from acting as osmolyte for osmotic adjustment, proline contributes to stabilizing sub-cellular structures (e.g., membranes and proteins), scavenging free radicals and buffering cellular redox potential under stress conditions [29]. In many plant species, proline accumulation under salt stress has been correlated with stress tolerance, and its concentration has been shown to be generally higher in salt tolerant than in salt sensitive plants [30,31]. Despite the genetic differences between R. tataricum and garden rhubarb, and significant differences in environmental stress values between the semi-desert conditions of Bogdinsko-Baskunchak Nature Reserve and the experimental fields of the Federal Scientific Vegetable Center, the obtained data indicated significant peculiarities in rhubarb biochemistry under stress condition.
Data presented in Table 2 indicate that R. tataricum accumulates levels of proline 1.3 (root pulp), 1.4 (leaves) and 2.08 (root peel) times higher compared to the correspondent values in garden rhubarb, indicating root peel as the most important plant part in anti-stress tolerance. On the contrary, cv. Zaryanka accumulated thrice higher levels of proline in stems, the most developed plant part of garden rhubarb, while R. tataricum contained an extremely low amount in stems.

Table 2.
Proline content in rhubarb root peel and pulp (mg kg−1 d.w.).
3.3. Carotenoids
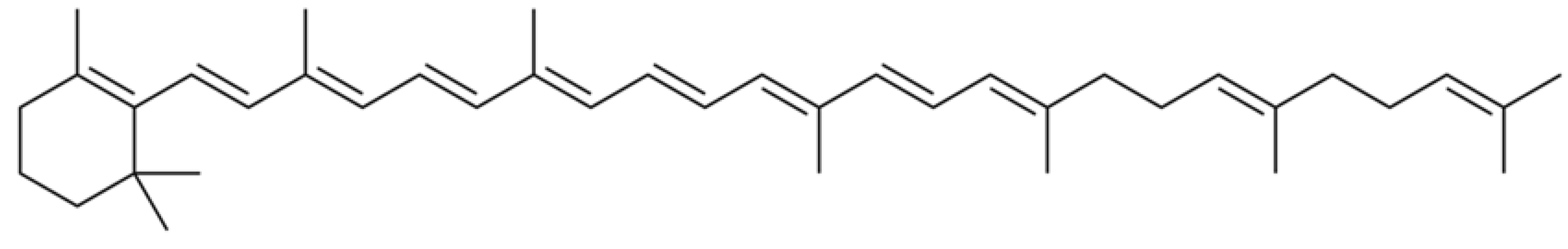
The protective role of carotenoids in plants grown in stress conditions is connected with their antioxidant properties and participation in phytohormone biosynthesis (especially abscisic acid, known to protect plants against heat stress) [32]. Furthermore, salinity is known to alter carotenoid biosynthesis, capable of increasing the accumulation of these compounds [33]. In this respect, the remarkable increase in β-zeacarotene (Figure 4) in rhubarb roots grown in conditions of high salinity may be connected with Baskunchak Reserve salt pollution. UV-spectra of hexane extracts of R. tataricum and garden rhubarb cv. Zaryanka indicated an almost 10 times increase in β -zeacarotene accumulation in roots of R. tataricum, compared to garden rhubarb roots which reached the concentration of 1.94 mg-eq β-carotene g−1 d.w. versus 0.13 mg-eq β-carotene g−1 d.w in roots of cv. Zaryanka. Notably, β -zeacarotene showed maximum values at 410, 430 and 450 nm in hexane [34], which had been seldom found in plants and never described in rhubarb species. Thin layer chromatography (TLC) data revealed the presence of only one spot (Rf 0.91), indicating the peculiarity of the R. tataricum and garden rhubarb carotenoid profile.
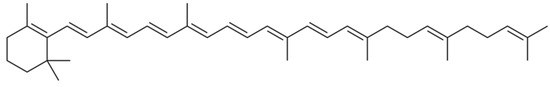
Figure 4.
Chemical formula of β-zeacarotene.
3.4. Nitrates and Total Dissolved Solids
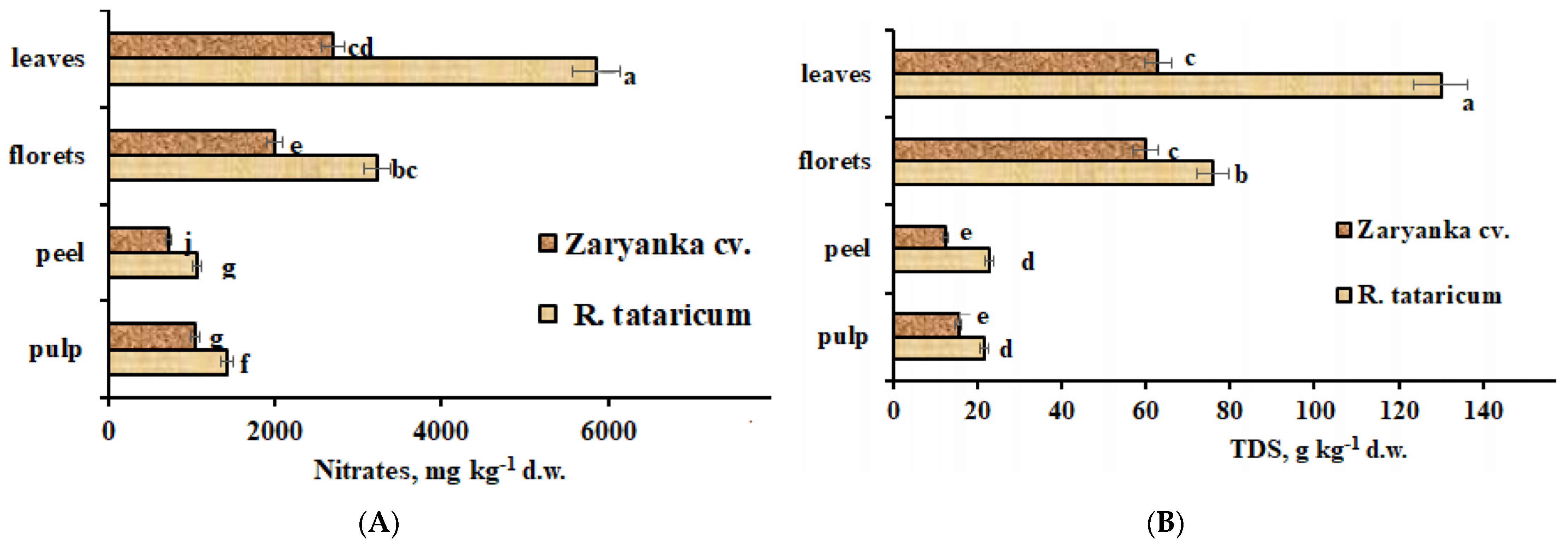
High environmental stress, especially drought, salinity, and high temperature, is known to cause nitrates accumulation in plants [35]. The present results indicate significantly higher levels of nitrates in R. tataricum leaves, florets, and roots, compared to correspondent parameters of cv. Zaryanka. The exception is represented by stems, where the nitrates levels are significantly higher in garden rhubarb (Figure 5).
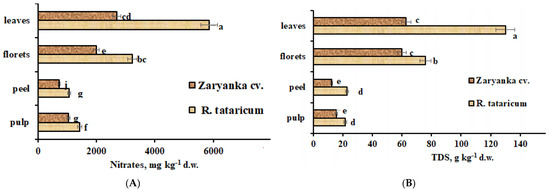
Figure 5.
Nitrates (A) and total dissolved solids (TDS) (B) accumulation in R. tataricum and garden rhubarb cv. Zaryanka. Values with the same letters do not differ statistically according to Duncan’s test at p < 0.05.
The highest differences were recorded in leaves where the differences reached more than 2. While N is an essential macronutrient for plant growth and development, it is also closely associated with plant adaptations to various abiotic stressors. As nitrogen is considered the most important nutrient for plant growth from a quantitative perspective, plants have evolved efficient strategies to manage N levels in response to various complex stressors [36].
On the one hand, salt stress in soils impairs the plant’s ability to take up water from the rhizosphere, leading to water limitation and growth inhibition.
Total dissolved solids (TDS) accumulation to a large extent showed the same phenomenon of nitrates distribution in plant parts, though this parameter reflects the content of all soluble substances including nitrates, salts, organic acids, and sugar.
3.5. Organic Acids
Organic acids are a core component inside cellular metabolism. Many plant stress responses involve the exudation of organic acids at the root–soil interface, which can improve soil mineral acquisition and toxic metal tolerance [37]. Thus, oxalate in soils may enhance phosphate availability, promote mineral dissolution, and increase the mobility of aluminum and heavy metal cations by complexation. Oxalate might stimulate microbiological growth and phosphate mobilization in the rhizosphere [38]. The mitochondria are the prime site for the intracellular biosynthesis of organic acids such as citrate, malate, and oxalate. Citrate, malate, and oxalate are implicated mainly for their roles in Al detoxification at the root–soil interface. The chelating property of organic acids is well expressed by citric and oxalic ones. After synthesis, organic acids are circulated among various plant parts, via various transporters, for functions such as the xylem loading of minerals, cell pH and redox equilibrium maintenance, drought tolerance, and tolerance towards fungal pathogens. Sometimes these organic acids are secreted by the roots into the soil, where they can mobilize minerals that are fixed due to various chemicals and microbial activities in the soil. They can also bind to toxic cationic species, such as Al and Mn+2, to inhibit their binding to the root tips. Secretion of organic acids into the soil also helps soil carbon sequestration, which is an important phenomenon for underground carbon fixation. Organic acids can also attract various microbes towards the root for helpful symbiotic associations and malate can also help in Mn detoxification internally by chelating it in both photosynthetic and non-photosynthetic tissues [39]. Moreover, citrate and malate can chelate other heavy metals that affect plants adversely, such as Cu, Ni, and Cd. The transportation of organic acids is not confined to the root–soil interface. In a few cases, the apoplastic secretion of organic acids plays a pivotal role in plant stress tolerance. Recently, it was established that organic acids also participate in regulating primary root growth under P deficiency. Furthermore, organic acids are known to protect plants against drought and other environmental stresses [37].
Compared to garden rhubarb, R. tataricum is characterized by a lower content of oxalic and succinic acids (1.7 and 3.5 times, respectively) and significantly higher content of malic and tartaric acids (12.6 and 4.2 times, respectively) (Table 3). The investigations of Sun et al. [40] revealed a significant role of malic enzyme (regulating malic acid metabolism) in antioxidant defense of plants via reduction in oxidative damage caused by reactive oxygen species including salinity and H2O2 production.

Table 3.
Organic acids content in dry Rhubarb stems (mg g₋1 d.w.).
3.6. Mineral Composition
The mineral composition of plants reflects both genetic peculiarities of a species and elements’ bioavailability in soil. Despite great differences in soil characteristics, salinity levels and climate parameters of the semi-desert territory of Bogdinsko-Baskunchak Nature Reserve and the experimental fields of the Federal Scientific Vegetable Center in Moscow region, comparison of the mineral distribution of leaves/roots in R. tataricum and garden rhubarb provides an indirect evaluation of the role of macro and trace elements in plant adaptability. Indeed, mineral distribution between different plant tissues is highly valuable in plant physiology and molecular biology [41]. In the conditions of salt stress of the Baskunchak environment, which is reflected in an Na content 63 times higher in rhubarb roots and 288 times higher in leaves compared to the garden rhubarb, grown in non-stressed condition, significant changes in Ca and Mg content were also recorded (Table 4).

Table 4.
Root and leaf mineral composition of R. tataricum and rhubarb cv. Zaryanka (mg kg−1 d.w.).
Salinity is known to cause ion and osmotic stresses resulting in raising Ca2+ via activation of Ca2+ channels [42]. Comparison of macroelements content in R. tataricum and garden rhubarb root revealed twice higher levels of Ca content and 2.4 times higher levels of Mg in R. tataricum roots. These facts may both be connected to the Ca protection of plants against salt loading and the existence of Ca/Mg excess in the environment. A high content of oxalic acid in rhubarb results in Ca precipitation. Thu et al. [43] indicated that salt-tolerant rice genotypes accumulated several times higher levels of Na in roots that non-tolerant genotypes, preventing the penetration of this element into leaves. Tester et al. [44] reported that the most significant plant adaptation to salinity is the ability to restrict the transportation and accumulation of Na in leaves. High root Mg in salt-tolerant varieties may increase the osmotic pressure in the roots, thereby allowing them to absorb water from saline solutions [45].
High levels of Na, Ca, Mg, Fe in R. tataricum indicate typical characteristics of the Reserve soil enriched with NaCl, and gypsum [12]. Increased levels of Cr and Sr reflect the peculiarities of the Reserve environment and the ability of plants to hyperaccumulate Sr in roots.
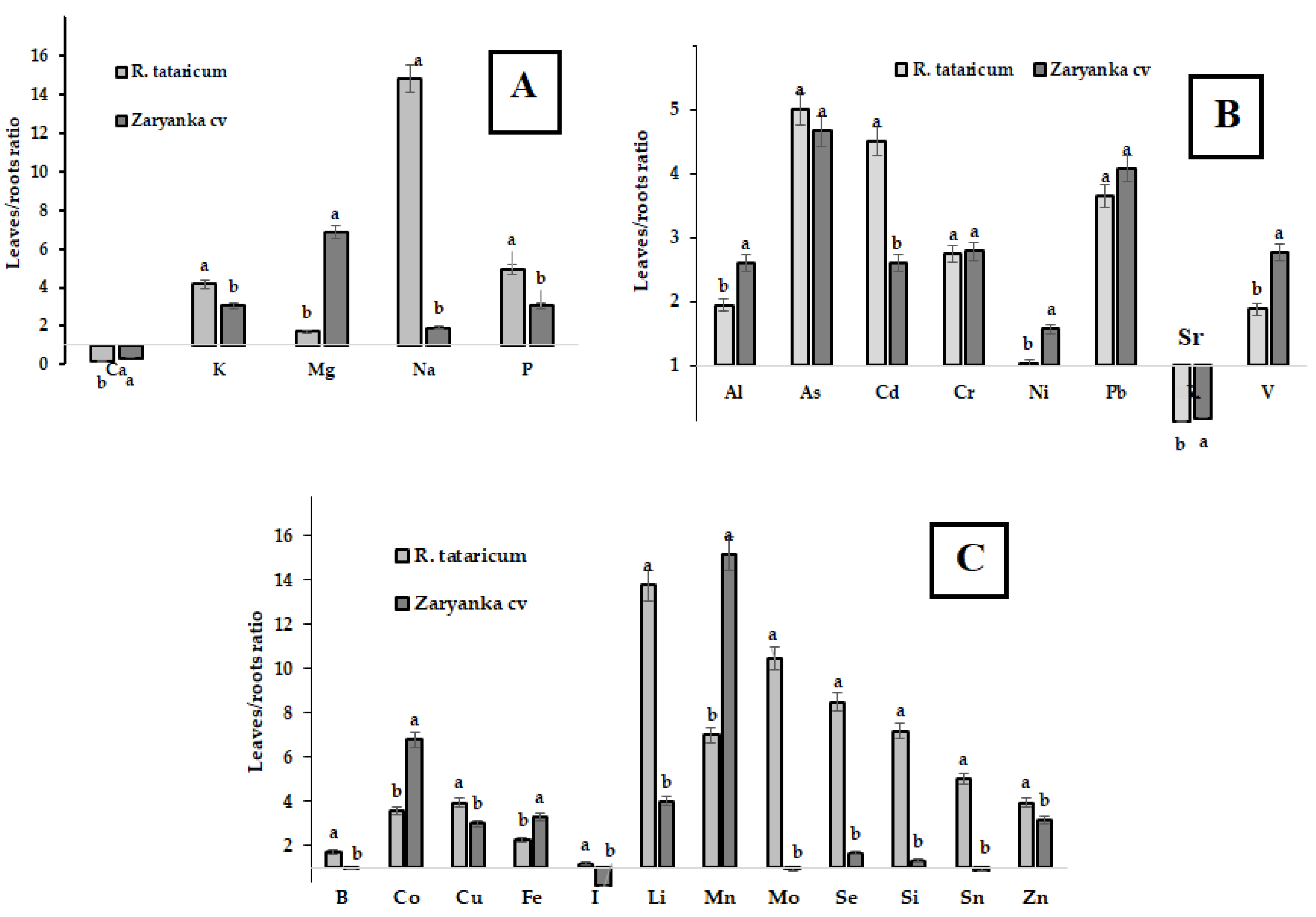
Despite the well-known restriction of Na accumulation in soil and the translocation of this toxic ion to leaves in many plant species tolerant to salinity and drought, Na leaves/roots distribution in R. tataricum indicates enormous levels of Na concentration in leaves. The data in Figure 6 indicate that intensive translocation to leaves in R. tataricum is also typical for such elements as Li, Mo, Se, Si, Sn and Cd. Among them, Mo, Se, and Si are known to enhance plants tolerance to draught and salinity. Li is the analog of Na, and its behavior repeats the behavior of Na. Known to be toxic for plants at high concentrations, Li may stimulate plant growth and the absorption of water by macromolecules at low concentrations [46]. Thus, intensive translocation of Li to leaves in R. tataricum may be beneficial in plant tolerance to high salinity.
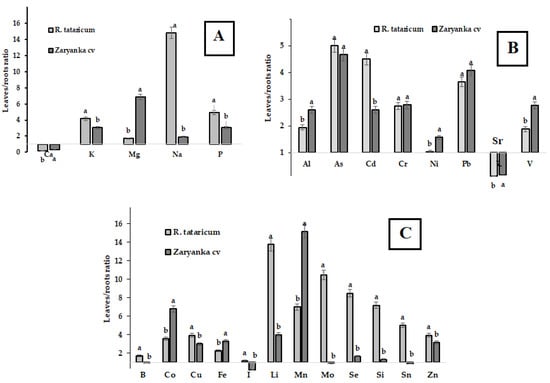
Figure 6.
Leaves/roots distribution of macro elements (A), Al, As and heavy metals (B) and microelements (C) in R. tataricum and rhubarb cv. Zaryanka. For each element values with similar letters do not differ statistically according to Duncan’s test at p < 0.05.
Molybdenum involved in ABA synthesis is known to play an important role in transpiration and water absorption, especially in stress-related responses including salinity and drought [47]. On the other hand, the protection role of Si against drought and salinity has also been documented [48]. The beneficial effects of silicon include regulation of Na+ uptake, transport, and distribution, improvement of antioxidant defense, root water uptake [49] and modulation of various genes expression. Despite low Si content, its significant translocation from roots to leaves in R. tataricum seems to be directly connected with increased proline accumulation in leaves [50]. Furthermore, studies proved that Si and Se demonstrate synergism in alleviating the toxic effects of salt stress via improvement of plants antioxidant status and accumulation of osmoprotectants such as proline and soluble sugar [51]. Selenium may also affect nitrogen accumulation [52] which is in accordance with the obtained data on elevated levels of nitrates and Se in R. tataricum leaves (Figure 6, Table 4). It worth mentioning that Se, a powerful natural antioxidant, may provide significant antioxidant protection to R. tataricum grown in semi-desert conditions and increased salinity levels.
In general, compared to garden rhubarb, R. tataricum accumulated significantly higher levels of Mn, Fe, Co, B, Li, V, Sr, Pb, Cr and Al. Soil mineral composition revealed low levels of Pb and Cd and high contents of Fe, Sr, and Cr at the territory of Baskunchak Nature Reserve. According to the data in Table 5, R. tataricum recorded a Sr hyperaccumulation phenomenon reflected in high coefficient of biological accumulation. reaching 16.

Table 5.
R. tataricum coefficients of biological accumulation (CBA) in accordance with the correspondent soil data (mg kg−1 d.w.).
4. Conclusions
A comparison of the biochemical characteristics and mineral content between R. tataricum and European garden rhubarb revealed, for the first time, the factors participating in R. tataricum stress tolerance, with much higher levels of plant antioxidants in the semi-desert conditions of Bogdinsko-Baskunchak Nature Reserve. These included the accumulation of: polyphenols, carotenoids and proline in roots; malic acid in stems; more intensive nitrate; and Na, Li, Mo, Si and Se roots–leaves translocation in R. tataricum compared to garden rhubarb grown in conditions of low intensity of environmental stresses. Further investigations are needed to reveal the actual biologically active compounds of R. tataricum and evaluate the chances of its utilization in plant breeding, medicine, and the food industry.
Author Contributions
Experiment conceptualization: N.G., V.K. and G.C.; samples collection and preparation for the analysis: N.G., S.S. and N.P.; laboratory determinations: M.B., A.K., N.G. and O.K.; data statistical processing: N.G., V.K., S.S. and G.C.; data interpretation, and draft and final-version manuscript writing: N.G., M.B., O.K., A.K., N.P. and G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any grants from public, commercial, or not-for-profit agencies.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The work was carried out in accordance with an agreement on scientific cooperation between Bogdinsko-Baskunchak Nature Reserve, Federal Scientific Vegetable Center and Voronezh State University of Forestry and Technologies (No 23, 22 April 2021) which allowed the participants to work at the territory of the Reserve and extract legally protected species.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Mareri, L.; Parrotta, L.; Cai, G. Environmental Stress and Plants. Int. J. Mol. Sci. 2022, 23, 5416. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Zuo, J.; Guo, F.; Dong, D. What we already know about rhubarb: A comprehensive review. Chin. Med. 2020, 15, 88. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.S.; Huang, Y.; Cai, L.Q.; Zhu, J.; Duan, Q.; Duan, Y.; Imperato-McGinley, J. The Chinese medicinal herbal formula ZYD88 inhibits cell growth and promotes cell apoptosis in prostatic tumor cells. Oncol. Rep. 2003, 10, 1633–1639. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, L.; Chen, D.C. Effect of Rhubarb on Gastrointestinal Dysfunction in Critically Ill Patients: A Retrospective Study Based on Propensity Score Matching. Chin. Med. J. 2018, 131, 1142–1150. [Google Scholar] [CrossRef]
- Chen, D.; Wang, L. Mechanisms of therapeutic effects of rhubarb on gut origin sepsis. Chin. J. Traumatol. 2009, 12, 365–369. [Google Scholar]
- Chen, D.C.; Ma, L.Q.; Liu, S.Z. Effects of rhubarb on intestinal flora and bacterial translocation in rats with sepsis. Chin. Crit. Care Med. 2009, 21, 17–20. [Google Scholar]
- Kolodziejczyk-Czepas, J.; Czepas, J. Rhaponticin as an anti-inflammatory component of rhubarb: A mini review of the current state of the art and prospects for future research. Phytochem. Rev. 2019, 18, 1375–1386. [Google Scholar] [CrossRef]
- Tian, S.L.; Yang, Y.; Liu, X.L.; Xu, Q.B. Emodin Attenuates bleomycin-induced pulmonary fibrosis via anti-inflammatory and anti-oxidative activities in rats. Med. Sci. Monitor. 2018, 24, e937532. [Google Scholar] [CrossRef]
- Liudvytska, O.; Kolodziejczyk-Czepas, J. A Review on Rhubarb-Derived Substances as Modulators of Cardiovascular Risk Factors—A Special Emphasis on Anti-Obesity Action. Nutrients 2022, 14, 2053. [Google Scholar] [CrossRef]
- Lev-Yadun, S.; Katzir, G.; Ne’eman, G. Self-irrigation in the desert rhubarb Rheum palaestinum—A response to Khammash. Plant Ecol. Evol. 2017, 150, 109–111. [Google Scholar] [CrossRef]
- Chuikov, J.S. Bogdinsko-Baskunchack Nature Reserve Complex and Its Protection; Proceedings of the Bogdinsko-Baskunchak Nature Reserve: Akhtubinsk, Russia, 1998. (In Russian) [Google Scholar]
- Volobaeva, O.V. Bogdinsko-Baskunchak Nature Reserve Flora. Ph.D. Thesis, Bashkir State University, Ufa, Russia, 2021. (In Russian). [Google Scholar]
- Wulfsohn, D. Sampling Techniques for Plants and Soil; Landbauforschung Völkenrode, University of Copenhagen, Denmark, 2010, Special Issue 340, pp. 3–30.
- Kidin, V.V.; Derugin, I.P.; Kobzarenko, V.I. Workshop on Agrochemistry; Kolos: Moscow, Russia, 2007. (In Russian) [Google Scholar]
- Golubkina, N.A.; Kekina, H.G.; Molchanova, A.V.; Antoshkina, M.S.; Nadezhkin, S.M.; Soldatenko, A.V. Plants Antioxidants and Methods of Their Determination; Infra-M: Moscow, Russia, 2020. (In Russian) [Google Scholar]
- Quertani, R.N.; Abid, G.; Karmous, C.; Chikha, M.B.; Boudaya, O.; Mahmoudi, H.; Mejri, S.; Jansen, K.; Ghorbel, A. Evaluating the contribution of osmotic and oxidative stress components on barley growth under salt stress. AoB Plants 2021, 13, plab034. [Google Scholar] [CrossRef]
- Guidance on Methods of Quality Control and Safety of Biologically Active Food Supplements P 4.1.1672-03; Organic Acids Determination, 2004; Ministry of Health of Russia: Moscow, Russia, 2004; pp. 109–111. (In Russian)
- Kalisz, S.; Oszmiański, J.; Kolniak-Ostek, J.; Grobelna, A.; Kieliszek, M.; Cendrowski, A. Effect of a variety of polyphenols compounds and antioxidant properties of rhubarb (Rheum rhabarbarum). LWT-Food Sci. Technol. 2020, 118, 108775. [Google Scholar] [CrossRef]
- Kolodziejczyk-Czepas, J.; Liudvytska, O. Rheum rhaponticum and Rheum rhabarbarum: A review of phytochemistry, biological activities and therapeutic potential. Phytochem. Rev. 2021, 20, 589–607. [Google Scholar] [CrossRef]
- Golubkina, N.A.; Kharchenko, V.A.; Caruso, G. Selenium. Prospects of functional food production with high antioxidant activity. In Plant Antioxidants and Health; Reference Series in Phytochemistry; Ekiert, H.M., Ramawat, K.G., Arora, J., Eds.; Springer Nature: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Aichner, D.; Ganzera, M. Analysis of anthraquinones in rhubarb (Rheum palmatum and Rheum officinale) by supercritical fluid chromatography. Talanta 2015, 144, 1239–1244. [Google Scholar] [CrossRef] [PubMed]
- Kashiwada, Y.; Nanaka, G.I.; Nishioka, I. Tannins and related compounds. XLVIII. Rhubarb (7). Isolation and characterization of new dimeric and trimeric procyanidins. Chem. Pharmaceut. Bull. 1986, 34, 4089–4091. [Google Scholar] [CrossRef]
- Kashiwada, Y.; Nanaka, G.I.; Nishioka, I. Chromone glucosides from rhubarb. Phytochemistry 1990, 29, 1007–1009. [Google Scholar] [CrossRef]
- Yagi, A.; Koizumi, Y.; Nishioka, I. Studies on rhubarb (Rheim Rhizoma). I—Stilbene derivatives from “Dodaioo” (Chinese inferior rhubarb). Jap. J. Pharmacol. 1971, 25, 52–54. [Google Scholar]
- Ge, Y.; Sun, M.; Salomé Abarca, L.F.; Wang, M.; · Choi, Y.H. Investigation of species and environmental effects on rhubarb roots metabolome using 1 H NMR combined with high performance thin layer chromatography. Metabolomics 2018, 14, 137. [Google Scholar] [CrossRef]
- Ahmad, P.; Latef, A.; Hashem, A.; Hashem, A.; Allah, E.; Gucel, S.; Tran, L.-S. Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front. Plant Sci. 2016, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, Y. Unraveling salt stress signaling in plants. J. Integr. Plant Biol. 2018, 60, 796–804. [Google Scholar] [CrossRef]
- Hare, P.D.; Cress, W.A. Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul. 1997, 21, 79–102. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Petrusa, L.M.; Winicov, I. Proline status in salt tolerant and salt sensitive alfalfa cell lines and plants in response to NaCl. Plant Physiol. Biochem. 1997, 35, 303–310. [Google Scholar]
- Fougère, F.; Le Rudulier, D.; Streeter, J.G. Effects of salt stress on amino acid, organic acid, and carbohydrate composition of roots, bacteroids, and cytosol of alfalfa (Medicago sativa L.). Plant Physiol. 1991, 96, 1228–1236. [Google Scholar] [CrossRef]
- Larkindale, J.; Knight, M.R. Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol. 2002, 128, 682–695. [Google Scholar] [CrossRef]
- Borghesi, E.; González-Miret, M.L.; Escudero-Gilete, M.L.; Malorgio, F.; Heredia, F.J.; Meléndez-Martínez, A.J. Effects of Salinity Stress on Carotenoids, Anthocyanins, and Color of Diverse Tomato Genotypes. J. Agric. Food Chem. 2011, 59, 11676–11682. [Google Scholar] [CrossRef]
- Rodrigo, M.J.; Lado, J.; Alós, E.; Alquezar, B.; Dery, O.; Hirschberg, J.; Zacarias, L. A mutant allele of ζ-carotene isomerase (Z-ISO) is associated with the yellow pigmentation of the “Pinalate” sweet orange mutant and reveals new insights into its role in fruit carotenogenesis. BMC Plant Biol. 2019, 19, 465. [Google Scholar] [CrossRef]
- Ye, J.Y.; Tian, W.H.; Jin, C.W. Nitrogen in plants: From nutrition to the modulation of abiotic stress adaptation. Stress Biol. 2022, 2, 4. [Google Scholar] [CrossRef]
- Zhang, G.-B.; Meng, S.; Gong, J.-M. The Expected and Unexpected Roles of Nitrate Transporters in Plant Abiotic Stress Resistance and Their Regulation. Int. J. Mol. Sci. 2018, 19, 3535. [Google Scholar] [CrossRef]
- Panchal, P.; Miller, A.J.; Giri, J. Organic acids: Versatile stress-response roles in plants. J. Exp. Bot. 2021, 72, 4038–4052. [Google Scholar] [CrossRef]
- Strobel, B.W.; Kristensen, F.; Hansen, H.C.B. Oxalate distribution in soils under rhubarb (Rheum rhaponticum). Int. J. Environ. Anal. Chem. 2004, 84, 909–917. [Google Scholar] [CrossRef]
- Fernando, D.R.; Mizuno, T.; Woodrow, I.E.; Baker, A.J.; Collins, R.N. Characterization of foliar manganese (Mn) in Mn (hyper)accumulators using X-ray absorption spectroscopy. New Phytol. 2010, 188, 1014–1027. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Hana, G.; Meng, Z.; Lin, L.; Sui, N. Roles of malic enzymes in plant development and stress responses. Plant Signal. Behav. 2019, 14, e1644596. [Google Scholar] [CrossRef] [PubMed]
- Kopittke, P.M.; Lombi, E.; van der Ent, A.; Wang, P.; Laird, J.S.; Moore, K.L.; Persson, D.P.; Husted, S. Methods to Visualize Elements in Plants. Plant Physiol. 2020, 182, 1869–1882. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Jin, L.; Peng, R. Crosstalk between Ca2+ and Other Regulators Assists Plants in Responding to Abiotic Stress. Plants 2022, 11, 1351. [Google Scholar] [CrossRef]
- Phong Thu, T.T.; Yasui, H.; Yamakawa, T. Effects of salt stress on plant growth characteristics and mineral content in diverse rice genotypes. Soil Sci. Plant Nutr. 2017, 63, 264–273. [Google Scholar] [CrossRef]
- Tester, M.; Davenport, R. Na+ tolerance and Na+ transport in higher plants. Ann. Bot. 2003, 91, 503–527. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Z.; Cheng, J.; Lai, Y.; Wang, J.; Bao, Y.; Huang, J.; Zhang, H. QTL Analysis of Na+ and K+ Concentrations in Roots and Shoots under Different Levels of NaCl Stress in Rice (Oryza sativa L.). PLoS ONE 2012, 7, 51202. [Google Scholar] [CrossRef]
- Anderson, C.E. Lithium in Plants. In Lithium and Cell Physiology; Bach, R.O., Gallicchio, V.S., Eds.; Springer: New York, NY, USA, 1990. [Google Scholar] [CrossRef]
- Abou Seeda, M.A.; Yassen, A.A.; Abou El-Nour, E.A.A.; Zaghlou, S.M. Importance of Molybdenum and it Diverse Role in Plant Physiology: A Review. Middle East J. Appl. Sci. 2020, 10, 228–249. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Huynh, W.Q.; Kronzucker, H.J. The Role of Silicon in Higher Plants under Salinity and Drought Stress. Front. Plant Sci. 2016, 7, 1072. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Xu, X.B.; Hu, Y.H.; Han, W.H.; Yin, J.L.; Li, H.L.; Gong, H.J. Silicon improves salt tolerance by increasing root water uptake in Cucumis sativus L. Plant Cell Rep. 2015, 34, 1629–1646. [Google Scholar] [CrossRef] [PubMed]
- Sattar, A.; Cheema, M.A.; Abbas, T.; Sher, A.; Ijaz, M.; Hussain, M. Separate and combined effects of silicon and selenium on salt tolerance of wheat plants. Russ. J. Plant Physiol. 2017, 64, 341–348. [Google Scholar] [CrossRef]
- Xu, S.; Zhao, N.; Qin, D.; Liu, S.; Jiang, S.; Xu, L.; Sun, Z.; Yan, D.; Hu, A. The synergistic effects of silicon and selenium on enhancing salt tolerance of maize plants Environ. Exp. Bot. 2021, 187, 104482. [Google Scholar] [CrossRef]
- Elkelish, A.A.; Soliman, M.H.; Alhaithloul, H.A.; El-Esawi, M.A. Selenium protects wheat seedlings against salt stress-mediated oxidative damage by up-regulating antioxidants and osmolytes metabolism. Plant Physiol. Biochem. 2019, 137, 144–153. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).