Abstract
Currently, crop physiological responses to waterlogging are considered only in a few crop models and in a limited way. Here, we examine the process bases of seven contemporary models developed to model crop growth in waterlogged conditions. The representation of plant recovery in these models is over-simplified, while plant adaptation or phenotypic plasticity due to waterlogging is often not considered. Aeration stress conceptualisation varies from the use of simple multipliers in equations describing transpiration and biomass to complex linkages of aeration-deficit factors with root growth, transpiration and nitrogen fixation. We recommend further studies investigating more holistic impacts and multiple stresses caused by plant behaviours driven by soils and climate. A sensitivity analysis using one model (a developer version of APSIM) with default parameters showed that waterlogging has the greatest impact on photosynthesis, followed by phenology and leaf expansion, suggesting a need for improved equations linking waterlogging to carbon assimilation. Future studies should compare the ability of multiple models to simulate real and in situ effects of waterlogging stress on crop growth using consistent experimental data for initialisation, calibration and validation. We conclude that future experimental and modelling studies must focus on improving the extent to which soil porosity, texture, organic carbon and nitrogen and plant-available water affect waterlogging stress, physiological plasticity and the ensuing temporal impacts on phenology, growth and yield.
Keywords:
anoxia; hypoxia; aeration stress; saturation; soil depth; plant-available water; water table; abiotic stress 1. Introduction
Globally, crop models are implemented for diverse purposes, including the assessment of crop responses to changes in climate. While the modelling of impacts of climate extremes such as drought and superfluous heat on crop growth has had extensive attention in many applications, modelling of crop responses to soil waterlogging has been considered in much fewer studies [1,2,3,4,5,6]. This may partly be because few crop models have the capability to simulate soil waterlogging, which is a complicated and complex process per se and accurately simulating crop behavior under waterlogging becomes even more complex [7]. Rötter et al. [4] found that only about 2% of the studies in the literature on impacts of climate extremes on crops focused on waterlogging. Thus, the scientific basis underpinning the modelling of waterlogging impacts has not advanced at the same pace as the level of the knowledge gained from field and laboratory experiments, which make up the majority of waterlogging studies. Very little of the considerable amount of knowledge obtained from these experiments is incorporated in crop models [4,5]. Similar observations could be applied to many other biotic and abiotic processes such as carbon and nitrogen cycling, greenhouse gas emissions and defoliation in agricultural systems models (e.g., [8,9,10,11,12,13], perhaps because the prevailing focus of the international scientific community has first been towards improving first principles of plant growth, such as photosynthesis, phenology, tiller development and yield formation.
Aspects of modelling waterlogging impacts on crop growth identified as important for further research include phenology of stress onset; root distribution, porosity, senescence and hydraulic conductance; adventitious roots; root cortical aerenchyma (RCA); nutrient dynamics and availability to plants; crop ion tolerance; oxidative stress damage, crop recovery, and hormone manipulation to enhance waterlogging tolerance [5,14,15,16,17,18,19,20]. To drill down into these research gaps, well-designed field and laboratory experiments are required with prescribed and quantified levels of waterlogging depth, duration, timing, frequency and crop stage, and crop recovery assessments for each level. Such experimental data can then be used to develop new and improved algorithms to be incorporated into crop models while ensuring interactions and feedbacks between soil, water, climatic variables and crop physiology as a whole. Experimental data is valuable not only in model improvement but also in identifying critical/sensitive growth stages and waterlogging conditions for decision making. Rigorous model testing and evaluation of enhanced algorithms using independent datasets should cover a range of crop species and cultivars, soil types, climates, and waterlogging and management conditions. In this way, the models can be used to extrapolate limited field or laboratory data to new environments, management and/or genotypes, while the field data can be used for model parameterization and validation (viz. [21]).
In agricultural fields, soil waterlogging can occur for many reasons. These may include excessive rainfall or irrigation, poor soil drainage, rising or perched water tables, as well as lateral surface or subsurface flows [5,22]. This may lead to reduced oxygen within soil pores, causing reduced growth and, sometimes, crop death. The low oxygen level (hypoxia) affects processes such as root growth [23,24], uptake of water and nutrients [14,23], nitrate concentrations in the soil [25], photosynthesis [15,26], leaf nitrogen (N) concentrations and senescence, plant hormones interaction and radiation-use efficiency [14]; grain quality and root aerenchyma [20], all of which may contribute to yield loss. The factors that determine the magnitude of these effects include timing and duration of the waterlogging event, crop sensitivity [18], soil water content, crop stage, crop genotype [27], the rate of crop and soil microbial respiration [28], water table depth, waterlogging frequency, temperature and ability to recover after waterlogging, for example through adaptations such as development of aerenchyma and adventitious roots [15,29]. Manik et al. [20] showed that in waterlogged conditions, the performance of barley genotypes with high RCA was much better than those with low RCA and that introgressed quantitative trait locus (QTL) for formation of RCA may alleviate yield losses due to waterlogging. Many of the interactions between these processes and factors observed in the field have not been comprehensively integrated into crop models [4,18], and in many cases are completely absent from contemporary models. It is worth noting that the science underpinning hydraulic and hydrological modelling aimed at simulating complex spatial and temporal ground water flows is well advanced on this area for decades e.g., [30,31]. In contrast, there are very few models that explicitly link hydrological spatial models with crop physiological processes. Indeed, crop models predominantly have a high level of precision temporally, but only simulate growth at a single point in space. It is however worth noting that recent work by [32] is an exception to this.
It is evident from field observations that crop production can be limited by waterlogging, but this very much depends on site-specific genotype x environment x management interactions [33,34]. It is thus important that future crop models have the ability to satisfactorily predict yields first under non-waterlogged conditions and second under a range of waterlogging conditions such as duration, timing, depth and recovery duration. Some processes in models are treated as though they are independent, though in practice they tend to be interlinked. For example, stresses related to sub- or supra-optimal water, nitrogen, phosphorus, temperature and oxygen can act together to impact growth even though some models only account for the most limiting stress. Crop responses to combined stresses have been shown to be different from those due to a single stress [35,36].
The challenge for researchers is thus to understand and represent in finer detail the processes that lead to reduced productivity, including interactions and feedback. To this end, the aim of this paper is thus to: (i) describe and compare the extent to which waterlogging is calculated in crop models designed for arable rainfed agricultural environments, (ii) identify sensitive crop growth processes in these models that are directly influenced by waterlogging, highlighting the main differences between them, and (iii) elucidate sensitive parameters influencing the interaction between crop growth and waterlogging.
2. Waterlogging and Crop growth in Crop Models
Models reviewed were selected based on the model’s capability to simulate crop growth and phenology under waterlogging and availability of adequate documentation such as technical or reference manuals, scientific journal papers and websites to enable a comparison of waterlogging-related equations. The models selected were those designed for simulating arable rainfed agricultural production and include APSIM [37,38], AquaCrop [39], DRAINMOD [40], DSSAT [41], EPIC [42], SWAP [36] and WOFOST [43]. The equations that define waterlogging and those that link waterlogging stresses to crop growth processes are provided, together with a discussion on inherent limitations.
Models reviewed are presented in Table 1, while crop growth processes directly impacted by waterlogging in the models are given in Table 2 (also see Supplementary Materials). Comprehensive reference manuals were available for AquaCrop, DRAINMOD, DSSAT, EPIC, SWAP and WOFOST while information for APSIM was obtained from published journal papers and the APSIM website. The main differences between these models are evident in the derivation and application of excess water stress factors. These factors are mainly derived using soil water content and critical thresholds based on soil, crop types and phenology, among other considerations. The processes directly impacted include at least one of the following: phenology, biomass, leaf area, photosynthesis, water and nitrogen (N) uptake, N-fixation, root growth, transpiration and yield. Phenology, crop specific factors and waterlogging duration are considered in some of these models while active plant recovery and adaptation mechanisms under waterlogging conditions are not considered in any of the models.

Table 1.
Aerations stress descriptions in crop models, and processes and variables directly impacted.

Table 2.
Crop growth variables and processes explicitly impacted by waterlogging in the crop models reviewed here.
2.1. Aeration Stress
In many models, waterlogging is described using air-filled pore space or excess water factors defined as a function of actual soil water content (θ), soil water content at saturation (θsat) and drained upper limit (θdul) or proximity to a water table and waterlogging duration (Table 1). Soil saturation occurs in the presence of a high water table (perched or regional groundwater table) which could result from excessive rainfall or irrigation and poor soil drainage. The water table in these models is represented in various ways that range from a prescribed water table (AquaCrop) to a water table simulated based on the bottom boundary condition and soil hydraulic properties (APSIM, DRAINMOD, DSSAT, SWAP) or based on other variables that are not directly linked to soil water processes (EPIC). The excess water stress factor is described using various terminologies such as ‘aeration stress factor’, ‘oxygen deficit stress factor’, ‘growth limiting factor’, ‘waterlogging coefficient’, ‘water stress factor’, ‘oxygen shortage reduction factor’ and ‘stress day index’. Some of these terminologies are intuitive e.g., ‘aeration’ stress while others such as ‘growth limiting factor’ and ‘stress day index’ are not. Therefore, hereafter, we use the term ‘aeration stress’ to represent all these terminologies except when describing specific waterlogging equations in a model.
All the models use soil water content to define aeration stress, except DRAINMOD which uses a ‘stress day index’ (SDI) based on a crop susceptibility factor for wet conditions, proximity to a water table and SWAP which uses pressure heads. APSIM, AquaCrop and SWAP use comparable forms of aeration stress equations where APSIM’s anaerobiosis point is defined as the drained upper limit. Equations describing aeration stress can be bilinear, making use of critical thresholds in soil water content (APSIM, AquaCrop,) or take more complex forms that consider other variables such as crop types, species and phenology (APSIM, EPIC). In AquaCrop, the aeration stress factor is applied directly on transpiration while a product of multiple stresses which include aeration stress, is used as a multiplier on yield (DRAINMOD), root water flux (SWAP) and transpiration (WOFOST). More complex applications of aeration stress factors on specific growth process such as root growth dynamics and transpiration are present in APSIM, SWAP and WOFOST.
Aeration stress factors are derived from soil water content measurements such as field capacity, wilting and saturation points and therefore the accuracy of these measurements is critical. Such stress factors are a simple extension of the existing pathways with which water stress is used to impact on crops and pastures [1,2,52]. The application of these stress factors in plant growth models requires knowledge of critical thresholds in soil air content for different crops and soils. In APSIM, vertical root growth in soybean is inhibited when volumetric soil moisture approaches 3% below saturation [17,32]. AquaCrop considers these thresholds as a percentage below soil saturation based on a crop’s tolerance to waterlogging. The values range from about 3% for tolerant crops to 15% for sensitive crops. The SWAP model provides critical pressure head values of root water extraction for various crops while DSSAT uses a minimum pore space value necessary for supplying oxygen to roots which also varies with crop type. The EPIC model sets the critical aeration factor, a fraction of soil porosity, at 0.85 (15% air-filled porosity) for many crops. A critical air-filled porosity of 0.1 to 0.15 m3m−3 is often assumed to be crucial for plant growth [14,53,54]. However, when held constant, this threshold can result in erroneous estimates of transpiration and plant growth [49,54,55]. Mohammadi et al. [56] derived critical air content based on root depth and the rate of oxygen consumption and showed that it varied linearly with soil depth. Bartholomeus et al. [49] found that a value of 0.1 can be too high for certain crop, soil and environmental conditions. Meskini-Vishkaee et al. [54] reported differences in critical aeration porosity, matric heads at field capacity (FC) and plant available water (PAW) between different soils and crops. Different methods such as those based on drainage flux and matric heads may also create differences in FC values, thus influencing aeration stress factors derived using FC. Certain soil and crop characteristics influence the critical values of aeration stress and thus should be considered simultaneously to accurately determine the impact of oxygen stress [36].
In addition to aeration stress, there are other stresses related to nutrients, temperature, salinity, pests and soil compaction that impact crop growth. These stresses are treated independently in most models but in reality, they occur simultaneously and plants can develop adaptation mechanisms, producing a unique response which can be considered as a new emergent stress [57,58,59]. This unique response to concurrent stresses is rarely captured in models which use simplistic approaches to account for this phenomenon. For example, the most limiting stress (Law of the Minimum), which may exclude aeration stress, is often used as a multiplier on variables such as biomass (APSIM and EPIC) and N-fixation (DSSAT) while other models such as AquaCrop, SWAP and WOFOST use a multiplicative approach. The models that use the most limiting stress approach assume that the contribution of all other stresses is insignificant, even though plant responses to combined stresses can differ markedly from those due to a single stress [35,58,60,61]. On the other hand, a multiplicative model of the stresses can be inadequate and could lead to unreliable yield predictions [62]. Impacts of a single stress on various crops have been investigated more than those of combined stresses. Further, combined stresses involving aeration stress have not had as much attention as other stresses. Studies on combined stresses that include aeration stress are aeration and salt stress in tomatoes [63], waterlogging and pathogen (Fusarium poae) stress on wheat and barley [64], and temperature and waterlogging stress on rice [65]. Given that concurrent stresses on a crop can produce a synergistic effect, the multiplicative and the most limiting stress approaches in models may be unrealistic and further research is needed to understand the various stress response pathways for combined stresses. Decline in oxygen is accompanied by a decline in redox potential which in turn causes the crop to deteriorate. The redox status of the soil is a good indicator of waterlogging intensity but is not captured in these crop models.
2.2. Waterlogging Duration
The duration of waterlogging affects crop growth and the models that consider this are APSIM, AquaCrop, DRAINMOD and WOFOST. In APSIM, three days with aeration stress below a given threshold are required for impact on root depth while WOFOST requires four days of waterlogging for a maximum impact on transpiration. For WOFOST, if there is oxygen deficit on the fifth day, the reduction in transpiration remains similar to that on the fourth day. In AquaCrop, the number of days when waterlogging stress fully affects transpiration is specified, in addition to setting a threshold for the aeration coefficient. DRAINMOD considers waterlogging duration using the SEW30 method (see equation S18 in Supplementary Materials) as an indicator of waterlogging severity and is a scalar applied to the final grain yield rather than incrementally affecting crop growth processes. SEW30 is “Sum of Excess Water” in the top 30 cm of soil, calculated from depth of the water table beneath the ground surface. Waterlogging duration impacts also depend on growth stage. Generally, the longer the waterlogging duration the more adverse are the effects on crop growth [15,66]. Marti et al. [66] found that wheat yields were affected according to the length of waterlogging during stem elongation. Ren et al. [67] reported variable results from various studies on sensitivity of waterlogging duration for maize at the three and six-leaf stages. This variability in results is more likely due to the piecemeal nature of published information regarding the design of field experiments, varying field conditions etc. which hinders a systematic comparison across the studies. Due to the interaction of different waterlogging durations, crop types and varieties, the duration of waterlogging before the plant is affected should not be fixed in the model to allow further testing using field observations. More research is needed to investigate how the number of consecutive days of waterlogging affects different crops, and how this interacts with waterlogging frequency.
2.3. Root Growth and Transpiration
Root growth in waterlogging conditions is simulated in APSIM, DSSAT and SWAP and depends on the extent of soil saturation, although the fraction of roots affected is determined differently in these models. The differences are due to consideration of other variables such as the aeration stress threshold and saturation duration (APSIM), soil water deficit factor (DSSAT) and a minimum gas filled porosity at which aeration stress occurs based on plant physiological and soil physical processes (SWAP). Impacts of waterlogging on transpiration/water-uptake are simulated in AquaCrop and WOFOST. In AquaCrop the waterlogging coefficient (the air-filled pore space in the root zone) is used to adjust transpiration while specifying the number of days of saturation to account for crop resistance to transient periods of waterlogging. WOFOST reduces transpiration when the soil water content exceeds a critical value for aeration while also taking into consideration the number of consecutive days with oxygen stress. The SWAP model calculates a reduction factor using the multiplicative approach of multiple stresses, including an aeration stress factor, to calculate root water uptake. Root growth and uptake of water and nutrients are affected by waterlogging. In certain conditions, root tissues can adapt to low oxygen levels. For example, wheat roots can survive anoxic conditions longer when exposed first to relatively reduced oxygen levels compared to a sudden change from aerated to anoxic conditions [68]. This phenomenon is not captured in the models reported here.
2.4. Leaf Growth
The models APSIM, EPIC and WOFOST directly incorporate aeration stress on leaf growth. APSIM uses the proportion of the affected root system due to aeration stress to adjust leaf area. EPIC calculates leaf area index using the most limiting of a number of stresses, including aeration stress. The leaf area index is a function of the crop development stage, the minimum of all crop stress factors and a heat unit factor. The WOFOST model uses the product of water and oxygen shortage reduction factors, including a crop-specific maximum death rate of leaves, to determine the death rate of leaves due to water stress.
2.5. Phenology and Crop-Specific Factors
The models APSIM, WOFOST and SWAP/WOFOST incorporating improvements by [49] have some capability in simulating waterlogging impacts taking into consideration important variables such as phenology, waterlogging duration and crop-specific factors for waterlogging. Growth stage is considered in APSIM and WOFOST while crop-specific waterlogging stress factors are incorporated in APSIM, DRAINMOD, EPIC and SWAP. In an approach implemented in GLAM (General Large Area Model) based on the WOFOST/CGMS (Crop Growth Monitoring System) models, the critical air content is modified using a crop specific parameter to define the lower limit of soil water content where a plant suffers waterlogging stress [27]. A limitation of most crop specific factors is that they are fitting parameters with no physical meaning, rendering them untransferable across models due to the diverse formulations of aeration stress factors in the models.
Waterlogging can cause delays in phenological events such as shoot development, ear emergence and maturity [34,69]. Additionally, some crop varieties have some tolerance to waterlogging conditions and show reduced sensitivity in yield hence the need for variety-specific waterlogging stress factors. Sensitivities of different growth stages and crop species are important in evaluating impacts of waterlogging in crop models. For instance, a crop might show greater sensitivity to waterlogging when it occurs early in the growth cycle compared to that at a later period as shown in the case of barley and rapeseed in [70], while a crop such as field pea is adversely affected in both early and late waterlogging. Wang et al. [71] observed larger reductions in yield when cotton was waterlogged during flowering than at the boll-opening stage.
2.6. Nitrogen Cycling Processes Impacted by Waterlogging
A reduction in nitrate concentrations in soil can occur due to waterlogging [29,72] due to effects of superfluous soil water on denitrification, leaching, N uptake, runoff, gaseous losses etc. [8,9,73]. None of the models reported here have explicitly incorporated aeration stress impacts on nitrogen losses and uptake except for DSSAT, which calculates N-fixation rate based on excess water stress.
2.7. Crop Recovery after Waterlogging (Phenotypic Plasticity)
Crop recovery, adaptation and plasticity after waterlogging have not been accounted for in any of the crop models presented here. The assumption in some models is that the crop recovers when waterlogging ceases e.g., recovery from waterlogging in APSIM AgPasture happens every day when soil water is below saturation and is proportional to the water free porosity. However, for some crops, short waterlogging durations can have longer-term adverse effects. For instance, three days of waterlogging severely retarded development in wheat and barley even after drainage [74]. Colmer et al. [68] showed that seminal roots of wheat stopped growing soon after waterlogging and the capacity for re-growth after drainage was diminished. On the other hand, adventitious roots can grow in waterlogged soils and can even grow longer when waterlogging ceases [68]. Sometimes waterlogging can lead to complete crop failure depending on crop stage and duration of waterlogging [75]. Therefore, the basic assumption in some models of crop recovery when waterlogging ceases needs further investigation. Crop growth during the recovery period is key in estimating yield losses due to waterlogging [76]. Zhang et al. [77] found that the spraying of cotton stem leaves with sodium nitroprusside (SNP) during waterlogging increased nitric oxide even after waterlogging stress which led to enhanced photosynthetic recovery and increased yield compared to the non-SNP waterlogged treatment. The re-aeration impact on crops that occurs after waterlogging can cause stress but is fundamentally disregarded in crop models. This re-aeration stress has been discussed in few studies [78,79,80]. Rapid re-aeration after hypoxia causes damage mainly attributed to formation of excessive reactive oxygen species (ROS), by-products of aerobic metabolism [78]. An imbalance between ROS and antioxidant systems activated by re-aeration can occur due to environmental stresses, creating oxidative stress. Re-aeration can also induce photosynthetic stress in crops due to an abrupt increase in light intensity when floods recede. Therefore, plant adaptation mechanisms under waterlogging conditions and transition between hypoxic/anoxic conditions and re-aeration deserve further research.
2.8. Crop Yields
Numerous experimental studies have shown that waterlogging generally reduces crop yield, but this varies depending on other factors discussed previously. In most dynamic models, yield is an outcome of climate-soil-crop process interactions. However, in some large-scale models where processes such as crop growth are less detailed compared to those in field scale models, a yield gap parameter is used to adjust crop yield. The yield gap parameter lumps processes or conditions such as sub-optimal management and biotic stresses not captured in the model. For example, GLAM [81] does not explicitly incorporate factors that impact yield such as waterlogging. Rather, GLAM uses a yield gap parameter that can be calibrated to match simulated yields with observed yields. The limitation with this approach is obvious in that it is difficult to attribute a simulated yield reduction to a particular factor unless it is the only factor impacting growth and the rest have been well controlled. DRAINMOD uses a different approach to the aeration stress factor method: the model calculates SDI which adjusts yield for a given stress type (see Supplementary Materials). SDI is a function of water table depth (up to 30 cm from the surface), a crop susceptibility factor for wet conditions [46] which depends on crop species and growth stage, and potential yield. Although this index uses the water table depth, it does not directly influence growth variables and processes such as transpiration, root and leaf growth among others.
2.9. Soil Water Dynamics
The discussion presented above linking crop growth to soil water content and aeration stress implies that the success in simulating crop growth under waterlogged conditions is contingent on adequate mathematical formulations of soil water dynamics, among other processes [82,83]. Soil saturation can be caused by water ponding on the surface due excessive rainfall or irrigation, perching water tables above a layer of low permeability in the subsoil, rising of the groundwater table into the root zone and lateral surface or subsurface flows. Waterlogging is simulated in crop models via soil water balance modules that vary in their structure. Some crop models also contain a water table that may cause sub-surface waterlogging. The two main approaches for simulating soil water balance are based on Richard’s equation and the cascading bucket model. The Richard’s equation is used in APSIM (SWIM3 module) and SWAP to simulate soil water flow while the cascading bucket model is used in APSIM (SoilWAT module), AquaCrop, WOFOST and DSSAT, where drainage is calculated as a fraction of the soil water content above the drained upper limit (θdul). Hysteresis in soil is not accounted for in AquaCrop, APSIM, DSSAT and WOFOST, which could lead to uncertainties in simulated soil water related processes such as infiltration and lateral flow. Capillary rise is important especially where the water table is close to the surface. APSIM-SWIM and SWAP use the Richard’s equation and simulate capillary rise and the water table depth is updated. Capillary rise in AquaCrop and DSSAT is simulated based on soil hydraulic properties, water table depth and soil water. The depth and variation in time of the water table in AquaCrop is specified as an input to the model and is thus not updated by the model while DSSAT can simulate perched water tables. It would be a worthwhile contribution to waterlogging impact studies to investigate the influence of capillary rise and hysteresis on waterlogging and crop growth. It is necessary that in environments where shallow water tables exist, and capillary flow or bypass flows are presumed dominant that the adopted crop model adequately predict soil water dynamics, including surface water ponding.
Due to the central role that soil water content plays in defining waterlogging, it is imperative that soil hydraulic properties be properly quantified and soil water dynamics adequately represented in the model. Parameters such as soil field capacity and porosity are often assumed constant but can be variable [1,2,84,85] and this may bias the values of excess water stress factors calculated using these variables. In waterlogged conditions, the simulation of soil water content depends on the movement of the water table in and out of the soil profile or rootzone, which is represented in a rudimentary fashion in many crop models. The simplistic coupling of the soil profile and the water table in these models has led researchers to link groundwater models to crop models [86,87] to calculate the water table more accurately and improve soil water simulation. A question for future research is whether accuracy would be more improved by better simulating crop growth processes or better simulating fluctuating water tables, or both.
2.10. Evaluation of Waterlogging—Crop Growth Algorithms in Models
Many crop models have been adequately tested in their ability to simulate crop growth under varying climate and management conditions such as limited water availability, and irrigation and fertilizer applications. While some of these models are reported to have some capability in modelling crop growth in waterlogged conditions (Table 1), very little work has been published on their testing and application in conditions of excess soil water. To effectively simulate impacts of waterlogging on crops, algorithms that can satisfactorily capture the multifaceted nature of crop responses to excess soil water are required. From the discussion presented above, the evaluation of these algorithms should have at its core an interaction matrix of waterlogging duration, depth (and/or critical aeration factors), frequency, phenology, crop type and genotype and crop recovery respectively. Further, the new and improved waterlogging-crop growth algorithms should ensure that crop-specific variables such as aeration stress thresholds and waterlogging duration are not hard-coded in the models to provide opportunities for model sensitivity testing and evaluation. The diverse waterlogging crop algorithms calls for their evaluation in a given crop model. For example, Bartholomeus et al. [49] compared two aeration stress algorithms using the SWAP model and showed that in their approach (shown in Table 1) actual oxygen stress started at slightly drier conditions than when using the Feddes function. Given the wide-ranging crop model structures, systematic intra- and inter-model comparisons of the waterlogging-crop algorithms are vital.
The accuracy and sufficiency of data (model input and calibration data) such as soil hydraulic properties, crop parameters, yield etc. are crucial and should be considered when evaluating the waterlogging-crop growth schemes. For example, soil porosity can be variable in shrink-swell soils and may also be affected by compaction, but it is often assumed to be constant which can lead to errors in soil water balance [84]. Further, the definition of field capacity measurements used to quantify plant available water has been debated over time [54,85,88] and this has implications also for aeration stress factors based on this measurement. Mohammadi et al. [56] observed large differences between the field capacity derived in their study using –10kPa suction, soil and plant characteristics and those derived using −33 kPa suction and hydraulic conductivity. These differences can lead to errors in estimates of plant available water, soil water balance and consequently crop growth. They reported that water uptake by crops under waterlogged conditions continued until almost saturation in some soils, whereas in other soils this threshold was a lower water content. de Jong van Lier [88] showed that up to 50% of water above field capacity can be available for use by crops. This means that in a model where plant available water is defined by field capacity, soil water above field capacity would be routed into drainage, whereas it could actually be transpired by the plant. This could lead to inaccurate estimates of transpiration and soil water balance not to mention the impact on other growth processes. Consequently, crop sensitivity to waterlogging would also not be reliably simulated in models that use field capacity to calculate aeration stress. Root water uptake can occur even when soil water is above field capacity and that field capacity determined in a laboratory should not be regarded as the upper limit of plant extractable water [85]. A recent study by [89] developed a method to calculate the least limiting water range (LLWR) for plants using Genuchten’s water retention curve parameters [90] which included the minimum air-filled porosity and field capacity. They found that changes in soil structure and clay content influenced the thresholds that determine LLWR. These studies among others, demonstrate the need to reassess the determination of soil properties such as soil porosity, field capacity and plant available water, not just for waterlogging modelling studies but for improving biophysical models as a whole.
Errors in data or an inadequate model structure can mask the effects of waterlogging and therefore a robust uncertainty analysis, which may include intra- and inter-model comparisons. Advances in crop model enhancements are hampered by challenges such as lack of open-source model code and data sharing protocols across research bodies, not to mention incomplete model documentation. Initiatives such as AgMIP (Agricultural Model Intercomparison and Improvement Project), Big Data Driven Agriculture etc. are a step in the right direction toward overcoming these challenges. These initiatives can provide data useful for improving and testing crop models and enable a robust uncertainty analysis as an integral part of model enhancement.
3. Case Study: Sensitivity of Processes Impacted by Waterlogging Using the APSIM Model
3.1. Sensitivity Analysis of Waterlogging Parameters Using APSIM
A study by [5] comparing the structure of several models with experimental findings found that APSIM-Soybean simulations under waterlogging conditions most closely agreed with the measured data. Following this pioneering work, APSIM-Soybean [17] was adopted for case study as the most developed point-based dynamic model capable of simulating transient waterlogging conditions. As such, a derivative of this model (in APSIM version 7.9) was used to evaluate the sensitivity of the waterlogging functions on plant growth.
3.2. Overview of APSIM and Approach Used to Model Waterlogging
APSIM is a field-scale crop model used worldwide to simulate agricultural systems [37]. The model contains modules for soil water dynamics, soil temperature, soil carbon and N routines and several crop models. There are two modules in APSIM to simulate soil water dynamics, namely SoilWat, a ‘bucket’ type model and SWIM3 which uses Richard’s equation [91,92]. In this study, the SoilWat module was used to simulate the water table by configuring the bottom soil layer to restrict drainage from the soil profile thus forming a layer of low permeability via parameters for controlling movement of water to the soil layer below (MWCON), soil water conductivity (SWCON) and saturated hydraulic conductivity (KS). Depending on settings of these parameters, water can saturate the bottom layers, moving upward to create a perched water table or ponding on the surface controlled by a parameter max_pond. Any excess goes to runoff. Details about other modules can be found on the APSIM website (www.apsim.info; accessed on 24 May 2022). APSIM simulates the effect of waterlogging on crops using an aeration stress factor (AeSF) derived based on crop sensitivity to aeration stress which varies by growth stage. AeSF reduces root depth if it less than a given threshold (0.6), after three days. Photosynthesis, leaf area development and tillering are reduced according to the proportion of the affected roots. AeSF also adjusts biomass accumulation depending on the most limiting of temperature, nitrogen, phosphorus and aeration stress factors.
3.3. Sensitivity Analysis
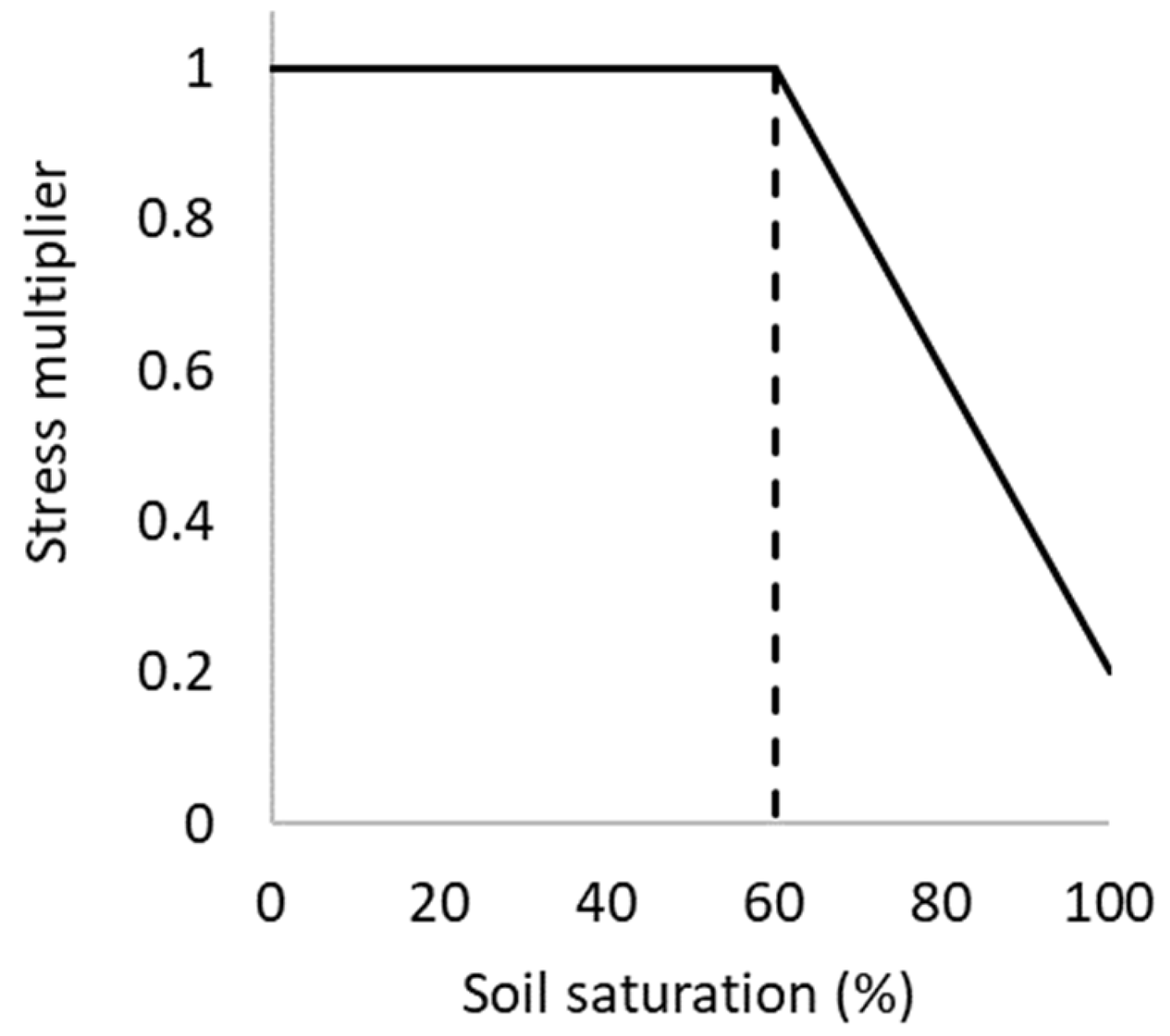
A sensitivity analysis was conducted to evaluate the impact of soil moisture states of varying duration (long or short) and application periods (early or late) on crop yield. As detailed in [18], several algorithms are incorporated into the model to simulate excessive moisture impacts on photosynthesis, phenology, leaf expansion and N fixation rates when soil water exceeds field capacity. In the case of photosynthesis, the developed algorithm modifies radiation use efficiency as a function of excess moisture stress due to flooding, whereas the phenology algorithm delays crop development. The basic shape of all functions is of the form shown in Figure 1, consisting of a threshold soil moisture at which the plant is affected, and the waterlogging stress index at full saturation. There is capacity to specify these parameters according to growth stage, but this option was not tested here due to the added complexity necessary to test and report all model configuration permutations.
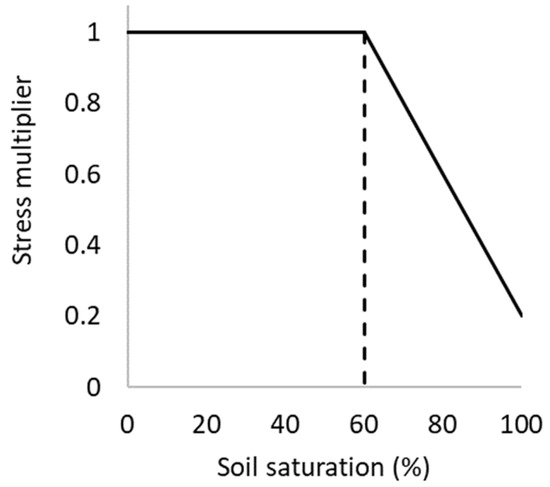
Figure 1.
APSIM waterlogging function with an inflection point (threshold value) of 60%.
The design of the sensitivity analysis was to evaluate the impact of varying soil moisture states of varying duration and application periods on crop yield. In this analysis the crop was wheat, and the climate, soil and management were based on field trials conducted at the Hamilton Smart Farm in western Victoria, Australia [93]. Each simulation was based on weather data recorded at the location from 2010 to 2020 inclusive. Irrigation was applied at a rate of 10 mm per application either throughout the growing season or at specific growth stages. The frequency of irrigation varied from 1 to 5 days per week. In each simulation the threshold value of a specific waterlogging function was altered, while all other parameters remained fixed. The threshold value was bounded by values ranging from 0.2 to 0.8. For a given waterlogging function sensitivity test, all other waterlogging functions were turned off. The N fixation function was not included in the sensitivity analysis because wheat is not a legume.
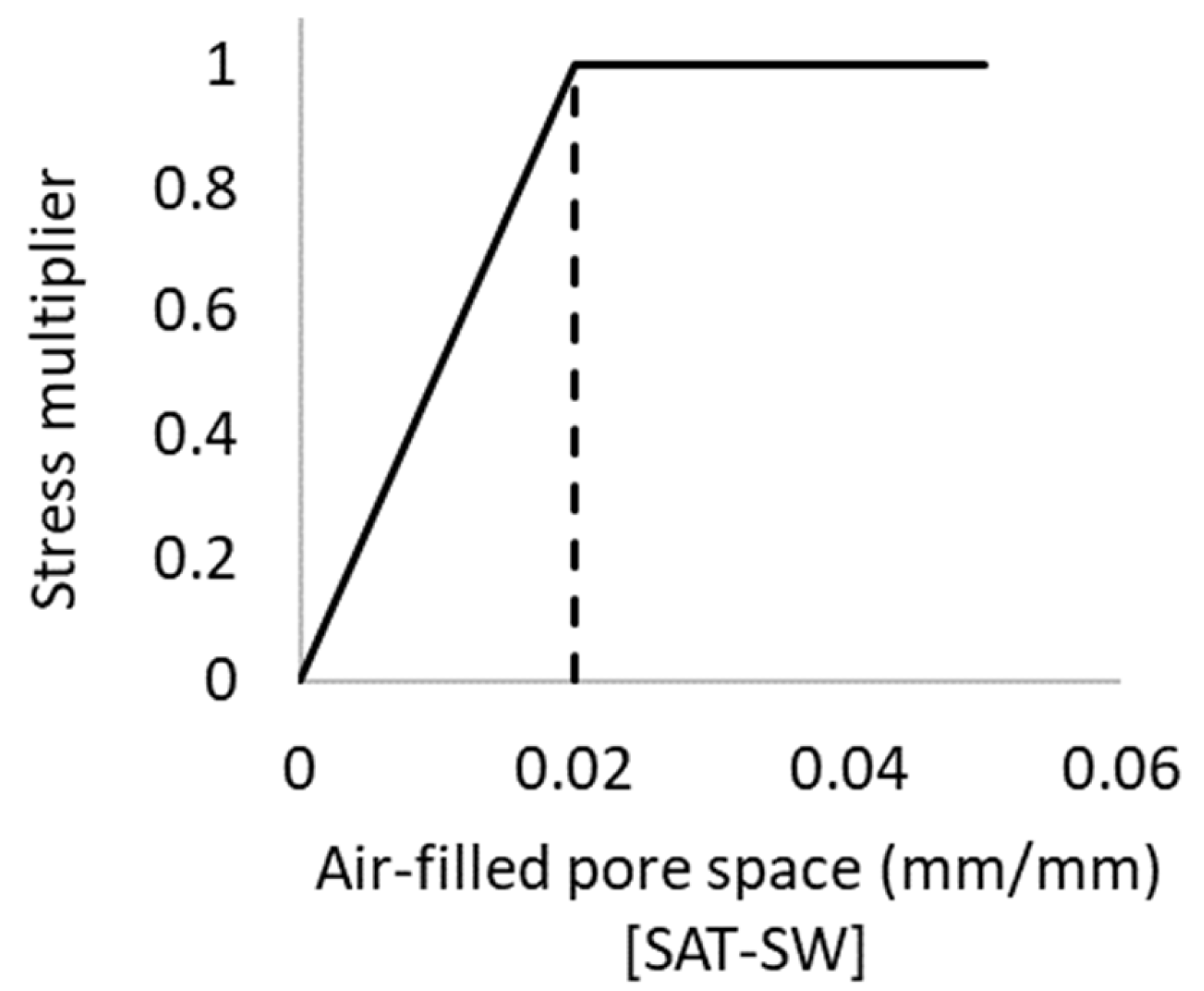
The threshold value for the aeration stress factor used to inhibit root growth was set at 0.02, nominated based on a range of reported observations [94,95,96] and simulations in APSIM [38]. The shape of the function defining the effects of excessive moisture on the rate of root growth is shown in Figure 2. As the air-filled pore space decreases below 0.02 with increasing soil saturation, root development linearly declines due a lack of oxygen until cessation when the soil is fully saturated.
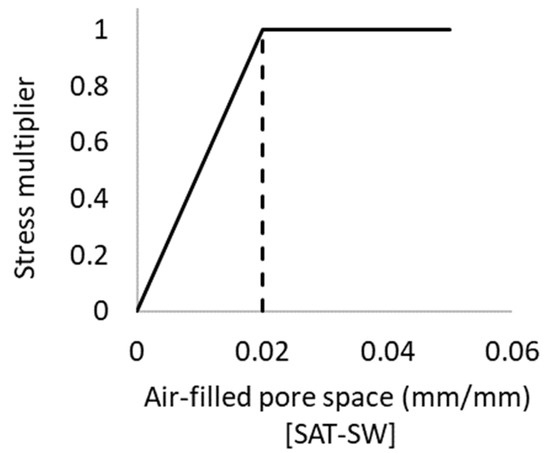
Figure 2.
Function describing excessive moisture effects on the rate of root growth with an inflection point (threshold value) of 0.02.
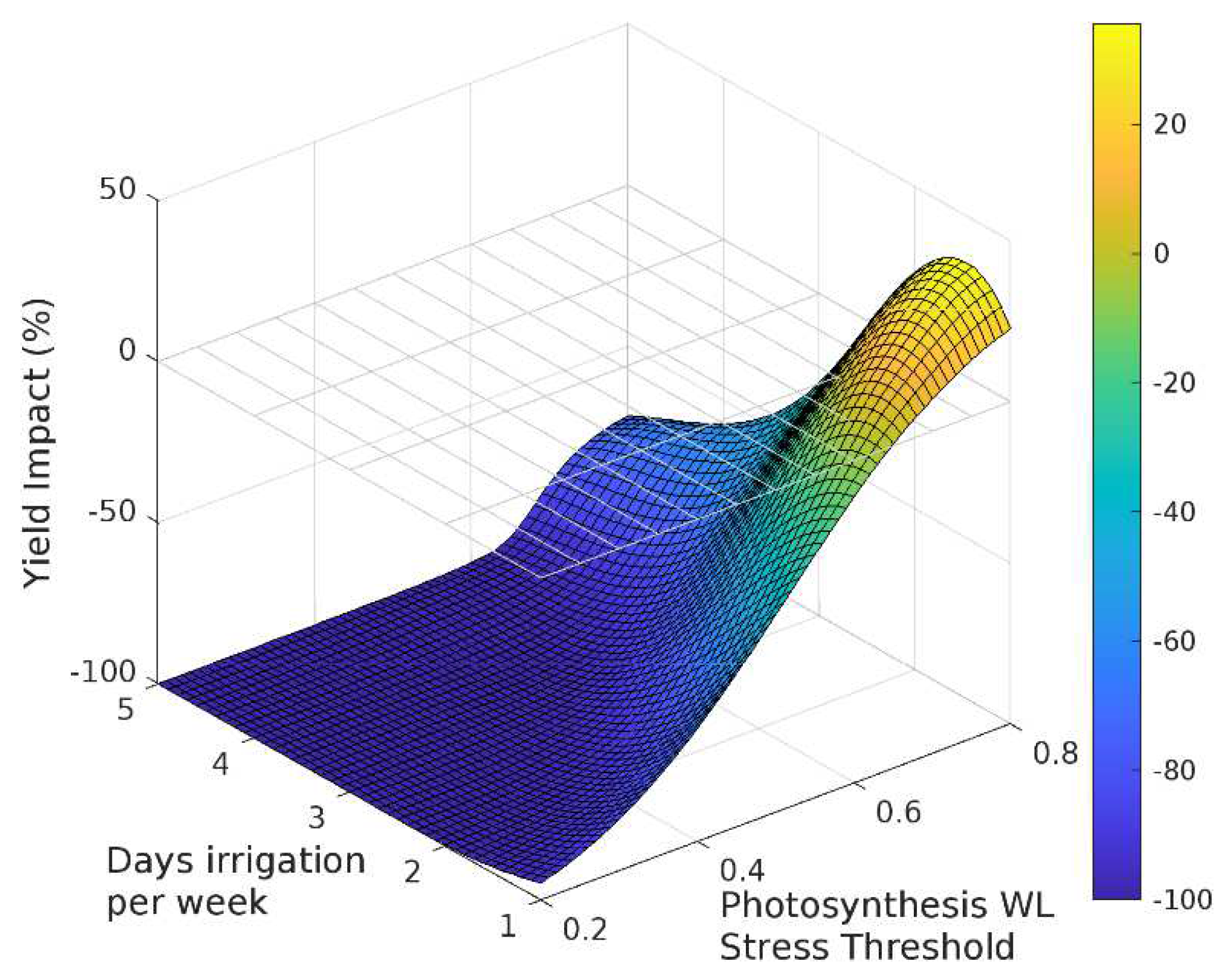
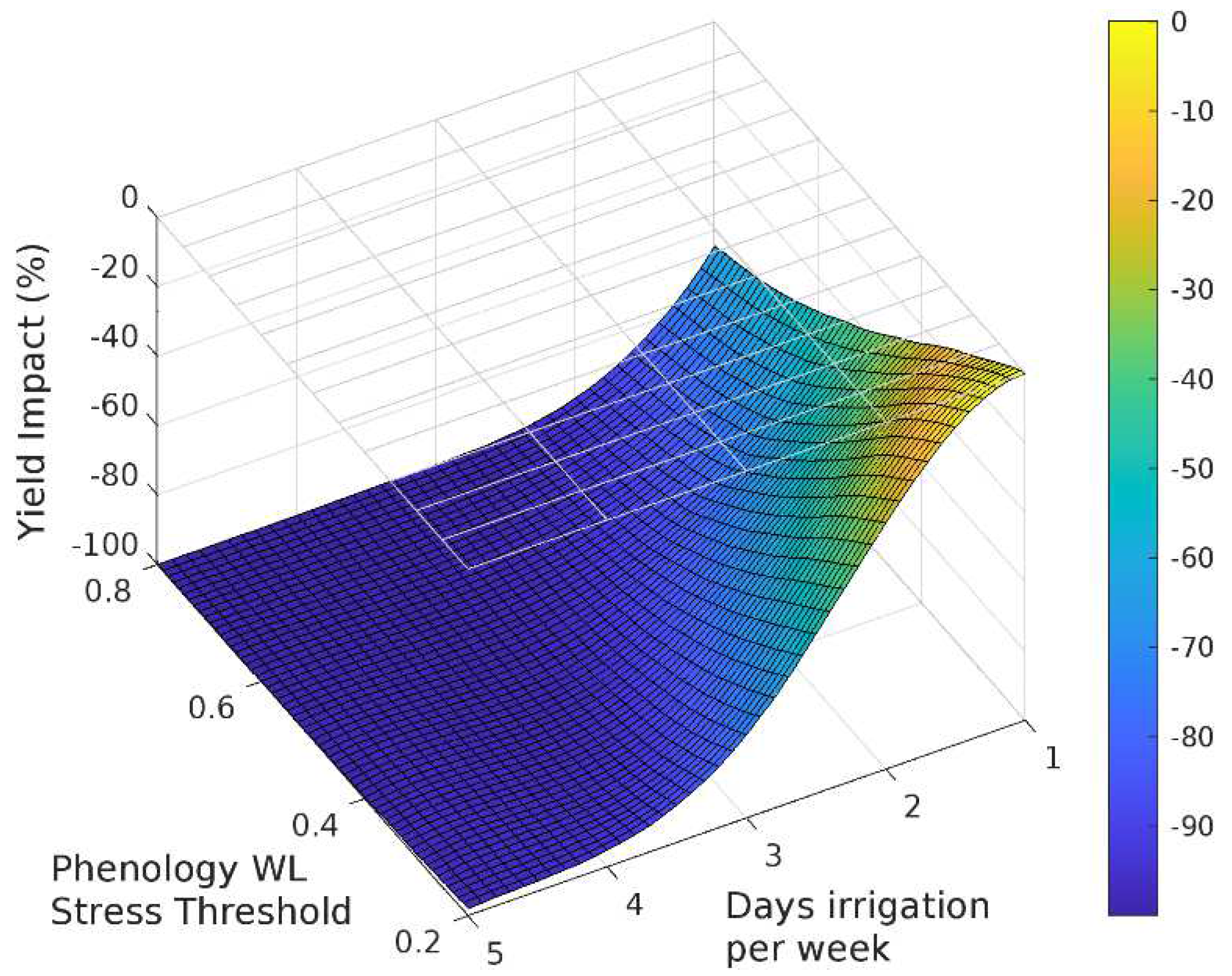
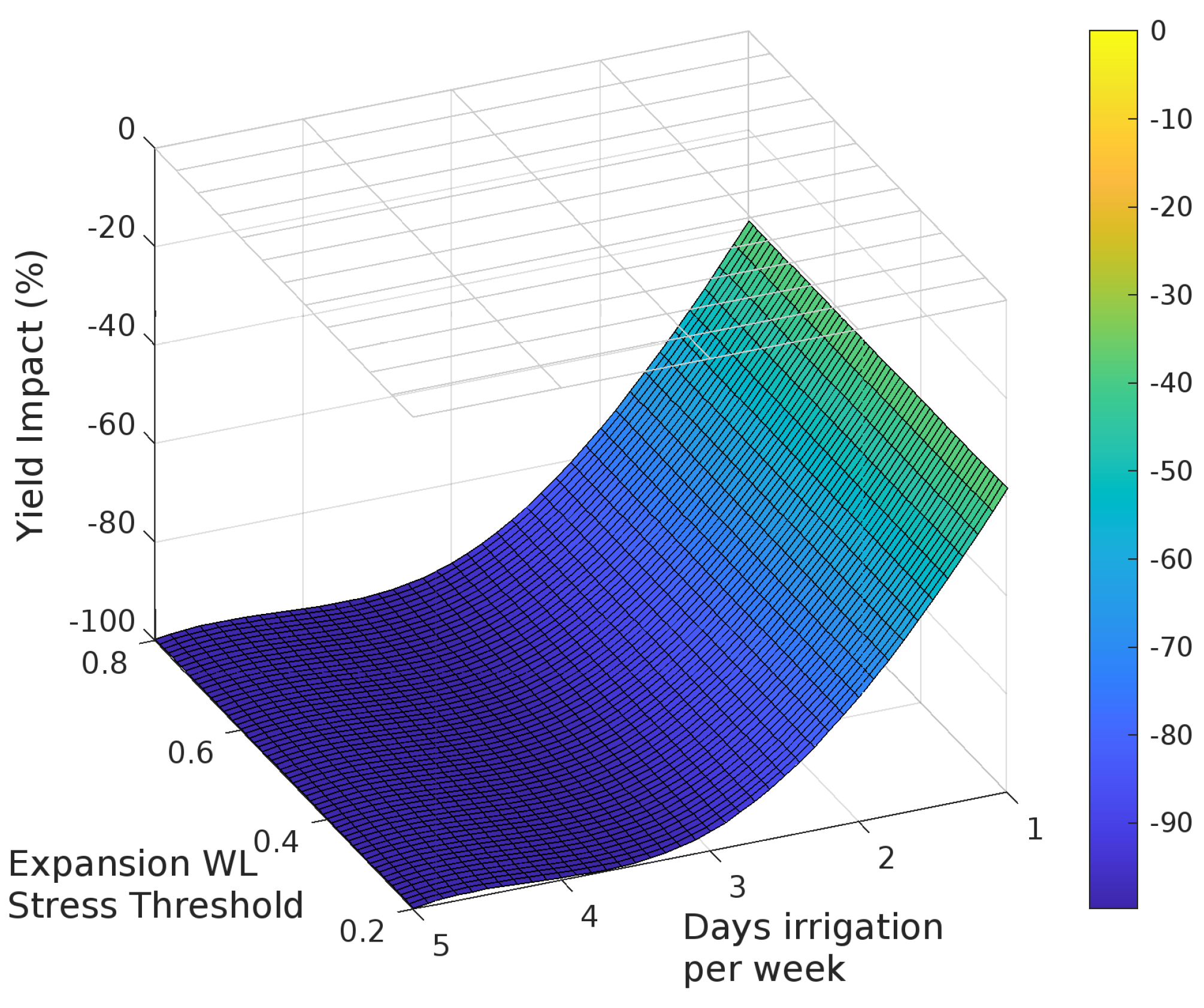
The sensitivity analysis with APSIM showed that for irrigation treatments applied for the entire season, grain yield decreased as the irrigated days per week increased (Figure 3, Figure 4 and Figure 5). Yield was most sensitive to the stress threshold for photosynthesis, followed by the phenology threshold, and virtually unaffected by the leaf expansion threshold. Figure 6 summarizes the yield response relative to a non-waterlogged control for various irrigation frequencies applied at either discrete growth stages. The average root zone saturation is depth weighted and defined by:
where l is the number of soil layers in which roots exist. The number of days the root zone soil moisture exceeds the drained upper limit defines those days in which any root zone soil layer is saturated.
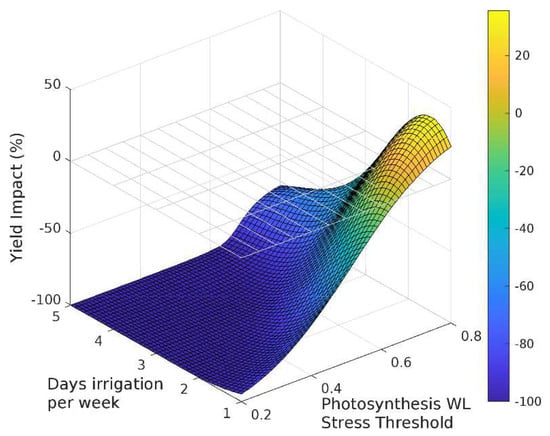
Figure 3.
Sensitivity of the response function associated with photosynthesis on grain yield.
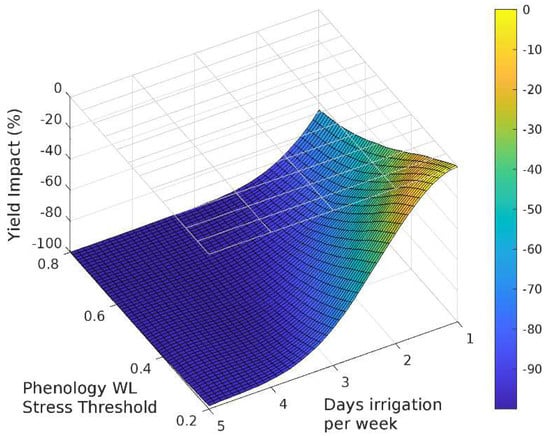
Figure 4.
Sensitivity of the response function associated with phenology on grain yield.
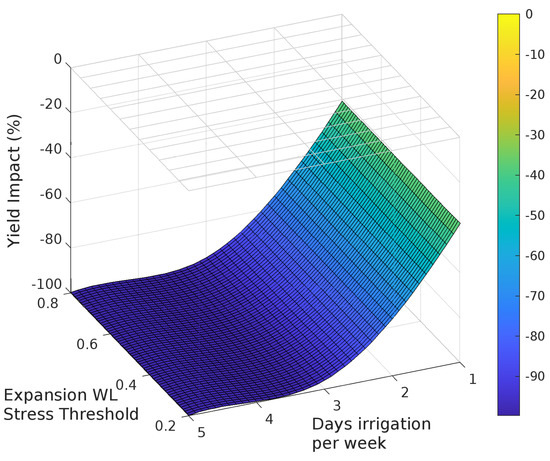
Figure 5.
Sensitivity of yield to waterlogging associated with leaf expansion on grain yield.
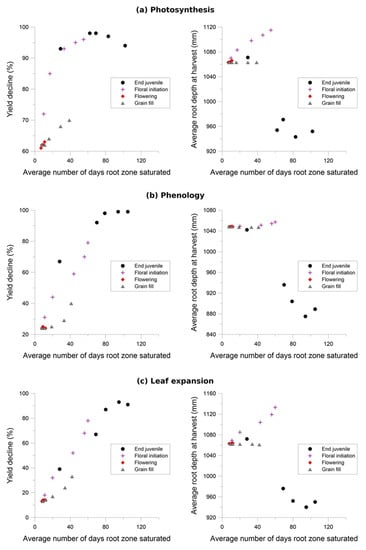
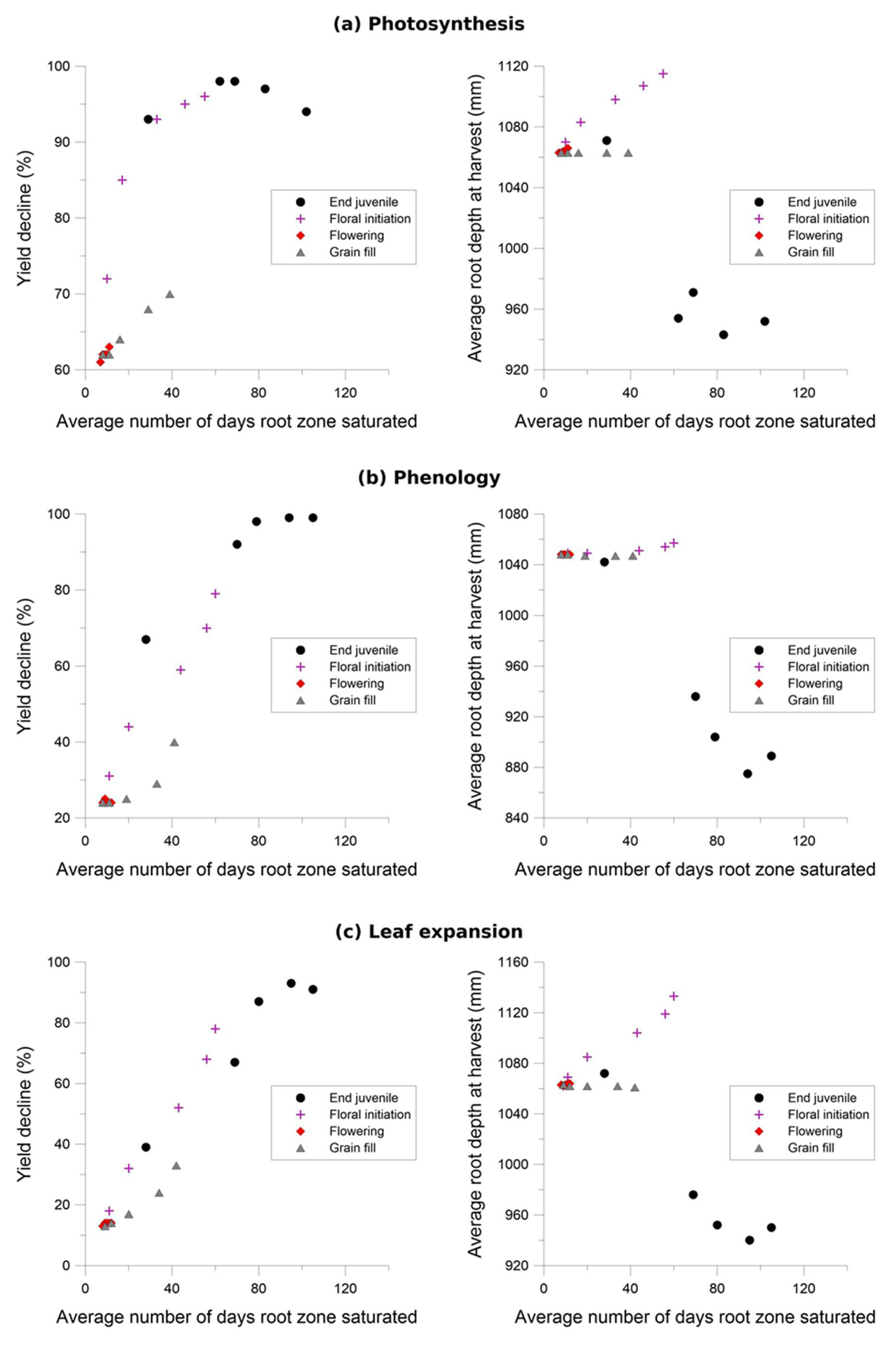
Figure 6.
The impact of three waterlogging functions: (a) photosynthesis, (b) phenology and (c) leaf expansion, on yield and root depth.
Grain yield was highly sensitive to the photosynthesis waterlogging function, with even the mildest of waterlogging treatments causing yield to decline to 39% of the non-waterlogged control, and the most intense treatment of waterlogging throughout the crop life caused complete yield failure (Figure 6a). Yield was also affected by the phenology waterlogging function, but not as strongly. Waterlogging at germination or during flowering caused yields to decline to 74–76% of the non-waterlogged control, but again waterlogging throughout the crop life caused complete yield failure (Figure 6b). The leaf expansion waterlogging function was the least sensitive, causing a decline to only 86–87% of the non-waterlogged control when waterlogging was imposed in the germination or flowering periods, and only the most intense waterlogging treatment caused yield failure (Figure 6c). Across each of the tests the most sensitive phenological stage was the juvenile phase, followed in declining order by the floral initiation phase, grain-fill, germination and lastly flowering.
Rooting depth was also shown to reduce with increasing days of root zone soil saturation, with an estimated maximum variation of 38%, 78% and 62% due to changes in the photosynthesis, phenology and expansion thresholds respectively (Figure 6). The effect of the three waterlogging functions did not have a significant effect on root depth for the flowering and grain filling stages. In all cases, the onset of significant changes in root development occurs when the average growing season root zone saturation exceeds approximately 30%.
As expected, growing season evapotranspiration is highly correlated to root depth which is in itself correlated with root zone saturation (R2 of 0.90, 0.96 and 0.94 for photosynthesis function, phenology function and expansion function respectively).
The sensitivity analysis indicates that the waterlogging impact functions caused reduced root extension and lower crop yields. Evapotranspiration was increased with a longer duration of waterlogging if irrigation was applied to all growth stages. However, if only a single growth stage was waterlogged, this led to a decrease in total crop evapotranspiration, presumably because the smaller canopy was less able to extract water from the soil and evaporate it during subsequent growth stages.
4. Conclusions
Waterlogging stress and its effect on crop growth in models have received comparatively less attention than impacts of drought and heat on crop productivity. Current models incorporate waterlogging impacts without considering detailed interactions and feedback mechanisms in crop growth. To advance the modelling of waterlogging impacts on crop growth, there is need for experiments aimed at detailed investigations into the factors (crop type/genotype, adaptation, growth stage, duration, depth and frequency of waterlogging, and recovery) that affect the crop during and after waterlogging and unravel how these factors interact.
Crop-specific variables such as aeration stress thresholds and waterlogging duration should not be fixed in any model, because crop types tend to behave differently under different waterlogging conditions. Further redevelopment of the definition of soil properties such as field capacity and porosity used in the derivation of aeration stress factors and plant available water is also necessary.
Multiple stress interactions, including aeration stress, and their effect on crop growth require further research to quantify adequately the impact of aeration stress on crops. Concurrent stresses on a crop can produce a unique response and therefore, alternative approaches to the multiplicative and “Law of the Minimum” stress approaches in models may be required to capture the unique response phenomena.
Further studies enabling comparison of the ability of multiple models such as those reported here to simulate real effects of waterlogging stress on crop growth using the same calibration and validation data are needed. Satisfactory evaluation of the existing, improved or new waterlogging-crop growth algorithms will require intra- and inter-model comparisons which are possible through joint initiatives across multiple organizations and stakeholders.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijpb13030017/s1, Equations linking waterlogging stress to crop growth processes (root growth and water uptake, transpiration, leaf growth, N-fixation and yield) in crop models.
Author Contributions
Preparation of initial manuscript, discussion and reviewing/editing of the manuscript, F.G.; sensitivity analysis section of the paper, C.B. and M.A.; discussion and reviewing/editing of the manuscript and project management, M.M.; provision of the APSIM version for the sensitivity analysis, conceptual design and structure of the paper, assistance with discussion and reviewing/editing of the manuscript, K.L. and M.T.H. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Grains Research and Development Corporation (project VGIP2C) and Agriculture Victoria Research. We acknowledge finance provided by the Grains Research and Development Corporation (GRDC project UOT1906-002RTX) to support the time of Matthew Harrison and Ke Liu in contributing to this work.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge the role of Fiona Robertson in leading the project through its initial stages.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Harrison, M.T.; Evans, J.R.; Moore, A.D. Using a Mathematical Framework to Examine Physiological Changes in Winter Wheat after Livestock Grazing: 1. Model Derivation and Coefficient Calibration. Field Crops Res. 2012, 136, 116–126. [Google Scholar] [CrossRef]
- Harrison, M.; Evans, J.; Moore, A. Using a Mathematical Framework to Examine Physiological Changes in Winter Wheat after Livestock Grazing: 2. Model Validation and Effects of Grazing Management. Field Crops Res. 2012, 136, 127–137. [Google Scholar] [CrossRef]
- Chang-Fung-Martel, J.; Harrison, M.T.; Rawnsley, R.; Smith, A.P.; Meinke, H. The Impact of Extreme Climatic Events on Pasture-Based Dairy Systems: A Review. Crop Pasture Sci. 2017, 68, 1158–1169. [Google Scholar] [CrossRef]
- Rötter, R.P.; Appiah, M.; Fichtler, E.; Kersebaum, K.C.; Trnka, M.; Hoffmann, M.P. Linking Modelling and Experimentation to Better Capture Crop Impacts of Agroclimatic Extremes—A Review. Field Crops Res. 2018, 221, 142–156. [Google Scholar] [CrossRef]
- Liu, K.; Harrison, M.T.; Shabala, S.; Meinke, H.; Ahmed, I.; Zhang, Y.; Tian, X.; Zhou, M. The State of the Art in Modeling Waterlogging Impacts on Plants: What Do We Know and What Do We Need to Know. Earth Future 2020, 8, e2020EF001801. [Google Scholar] [CrossRef]
- Yan, H.; Harrison, M.T.; Liu, K.; Wang, B.; Feng, P.; Fahad, S.; Meinke, H.; Yang, R.; Liu, D.L.; Archontoulis, S.; et al. Crop Traits Enabling Yield Gains under More Freq.Uent Extreme Climatic Events. Sci. Total Environ. 2022, 808, 152170. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Smith, C.J. Modelling the Growth and Water Uptake Function of Plant Root Systems: A Review. Aust. J. Agric. Res. 2004, 55, 501–523. [Google Scholar] [CrossRef]
- Christie, K.M.; Smith, A.P.; Rawnsley, R.P.; Harrison, M.T.; Eckard, R.J. Simulated Seasonal Responses of Grazed Dairy Pastures to Nitrogen Fertilizer in SE Australia: N Loss and Recovery. Agric. Syst. 2020, 182, 102847. [Google Scholar] [CrossRef]
- Christie, K.M.; Smith, A.P.; Rawnsley, R.P.; Harrison, M.T.; Eckard, R.J. Simulated Seasonal Responses of Grazed Dairy Pastures to Nitrogen Fertilizer in SE Australia: Pasture Production. Agric. Syst. 2018, 166, 36–47. [Google Scholar] [CrossRef]
- Harrison, M.T.; Christie, K.M.; Rawnsley, R.P.; Eckard, R.J. Modelling Pasture Management and Livestock Genotype Interventions to Improve Whole-Farm Productivity and Reduce Greenhouse Gas Emissions Intensities. Anim. Prod. Sci. 2014, 54, 2018–2028. [Google Scholar] [CrossRef]
- Harrison, M.T.; Cullen, B.R.; Tomkins, N.W.; McSweeney, C.; Cohn, P.; Eckard, R.J. The Concordance between Greenhouse Gas Emissions, Livestock Production and Profitability of Extensive Beef Farming Systems. Anim. Prod. Sci. 2016, 56, 370–384. [Google Scholar] [CrossRef]
- Alcock, D.J.; Harrison, M.T.; Rawnsley, R.P.; Eckard, R.J. Can Animal Genetics and Flock Management Be Used to Reduce Greenhouse Gas Emissions but Also Maintain Productivity of Wool-Producing Enterprises? Agric. Syst. 2015, 132, 25–34. [Google Scholar] [CrossRef]
- Sándor, R.; Ehrhardt, F.; Grace, P.; Recous, S.; Smith, P.; Snow, V.; Soussana, J.-F.; Basso, B.; Bhatia, A.; Brilli, L.; et al. Ensemble Modelling of Carbon Fluxes in Grasslands and Croplands. Field Crops Res. 2020, 252, 107791. [Google Scholar] [CrossRef]
- Najeeb, U.; Bange, M.P.; Tan, D.K.Y.; Atwell, B.J. Consequences of Waterlogging in Cotton and Opportunities for Mitigation of Yield Losses. AoB Plants 2015, 7, plv080. [Google Scholar] [CrossRef] [PubMed]
- Herzog, M.; Striker, G.G.; Colmer, T.D.; Pedersen, O. Mechanisms of Waterlogging Tolerance in Wheat—A Review of Root and Shoot Physiology. Plant Cell Environ. 2016, 39, 1068–1086. [Google Scholar] [CrossRef]
- Manik, S.M.N.; Pengilley, G.; Dean, G.; Field, B.; Shabala, S.; Zhou, M. Soil and Crop Management Practices to Minimize the Impact of Waterlogging on Crop Productivity. Front. Plant Sci. 2019, 10, 140. [Google Scholar] [CrossRef]
- Ebrahimi-Mollabashi, E.; Huth, N.I.; Holzwoth, D.P.; Ordóñez, R.A.; Hatfield, J.L.; Huber, I.; Castellano, M.J.; Archontoulis, S. V Enhancing APSIM to Simulate Excessive Moisture Effects on Root Growth. Field Crops Res. 2019, 236, 58–67. [Google Scholar] [CrossRef]
- Pasley, H.R.; Huber, I.; Castellano, M.J.; Archontoulis, S.V. Modeling Flood-Induced Stress in Soybeans. Front. Plant Sci. 2020, 11, 62. [Google Scholar] [CrossRef]
- Liu, K.; Harrison, M.T.; Archontoulis, S.V.; Huth, N.; Yang, R.; Liu, D.L.; Yan, H.; Meinke, H.; Huber, I.; Feng, P.; et al. Climate Change Shifts Forward Flowering and Reduces Crop Waterlogging Stress. Environ. Res. Lett. 2021, 16, 094017. [Google Scholar] [CrossRef]
- Manik, S.M.N.; Quamruzzaman, M.; Livermore, M.; Zhao, C.; Johnson, P.; Hunt, I.; Shabala, S.; Zhou, M. Impacts of Barley Root Cortical Aerenchyma on Growth, Physiology, Yield Components, and Grain Quality under Field Waterlogging Conditions. Field Crops Res. 2022, 279, 108461. [Google Scholar] [CrossRef]
- Harrison, M.T.; Jackson, T.; Cullen, B.R.; Rawnsley, R.P.; Ho, C.; Cummins, L.; Eckard, R.J. Increasing Ewe Genetic Fecundity Improves Whole-Farm Production and Reduces Greenhouse Gas Emissions Intensities: 1. Sheep Production and Emissions Intensities. Agric. Syst. 2014, 131, 23–33. [Google Scholar] [CrossRef]
- Cox, J.W.; McFarlane, D.J. The Causes of Waterlogging in Shallow Soils and Their Drainage in Southwestern Australia. J. Hydrol. 1995, 167, 175–194. [Google Scholar] [CrossRef]
- Elzenga, J.T.M.; van Veen, H. Waterlogging and Plant Nutrient Uptake. In Waterlogging Signalling and Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2010; pp. 23–35. [Google Scholar]
- Zhou, W.; Chen, F.; Meng, Y.; Chandrasekaran, U.; Luo, X.; Yang, W.; Shu, K. Plant Waterlogging/Flooding Stress Responses: From Seed Germination to Maturation. Plant Physiol. Biochem. 2020, 148, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Thorburn, P.J.; Biggs, J.S.; Collins, K.; Probert, M.E. Using the APSIM Model to Estimate Nitrous Oxide Emissions from Diverse Australian Sugarcane Production Systems. Agric. Ecosyst. Environ. 2010, 136, 343–350. [Google Scholar] [CrossRef]
- Harrison, M.T.; Kelman, W.M.; Moore, A.D.; Evans, J.R. Grazing Winter Wheat Relieves Plant Water Stress and Transiently Enhances Photosynthesis. Funct. Plant Biol. 2010, 37, 726–736. [Google Scholar] [CrossRef]
- Li, S.; Tompkins, A.M.; Lin, E.; Ju, H. Simulating the Impact of Flooding on Wheat Yield—Case Study in East China. Agric. For. Meteorol. 2016, 216, 221–231. [Google Scholar] [CrossRef]
- Grable, A.R.; Siemer, E.G. Effects of Bulk Density, Aggregate Size, and Soil Water Suction on Oxygen Diffusion, Redox Potentials, and Elongation of Corn Roots. Soil Sci. Soc. Am. J. 1968, 32, 180–186. [Google Scholar] [CrossRef]
- Kaur, G.; Singh, G.; Motavalli, P.P.; Nelson, K.A.; Orlowski, J.M.; Golden, B.R. Impacts and Management Strategies for Crop Production in Waterlogged or Flooded Soils: A Review. Agron. J. 2020, 112, 1475–1501. [Google Scholar] [CrossRef]
- Clark, M.P.; Bierkens, M.F.P.; Samaniego, L.; Woods, R.A.; Uijlenhoet, R.; Bennett, K.E.; Pauwels, V.R.N.; Cai, X.; Wood, A.W.; Peters-Lidard, C.D. The Evolution of Process-Based Hydrologic Models: Historical Challenges and the Collective Quest for Physical Realism. Hydrol. Earth Syst. Sci. 2017, 21, 3427–3440. [Google Scholar] [CrossRef]
- Singh, V.P. Hydrologic Modeling: Progress and Future Directions. Geosci. Lett. 2018, 5, 15. [Google Scholar] [CrossRef]
- Deng, C.; Zhang, Y.; Bailey, R.T. Evaluating Crop-Soil-Water Dynamics in Waterlogged Areas Using a Coupled Groundwater-Agronomic Model. Environ. Model. Softw. 2021, 143, 105130. [Google Scholar] [CrossRef]
- Ibrahim, A.; Harrison, M.T.; Meinke, H.; Zhou, M. Examining the Yield Potential of Barley Near-Isogenic Lines Using a Genotype by Environment by Management Analysis. Eur. J. Agron. 2019, 105, 41–51. [Google Scholar] [CrossRef]
- Liu, K.; Harrison, M.T.; Ibrahim, A.; Manik, S.M.N.; Johnson, P.; Tian, X.; Meinke, H.; Zhou, M. Genetic Factors Increasing Barley Grain Yields under Soil Waterlogging. Food Energy Secur. 2020, 9, e238. [Google Scholar] [CrossRef]
- Zhang, H.; Sonnewald, U. Differences and Commonalities of Plant Responses to Single and Combined Stresses. Plant J. 2017, 90, 839–855. [Google Scholar] [CrossRef] [PubMed]
- Kroes, J.G.; van Dam, J.C.; Bartholomeus, R.P.; Groenendijk, P.; Heinen, M.; Hendriks, R.F.A.; Mulder, H.M.; Supit, I.; van Walsum, P.E.V. SWAP Version 4; Wageningen University & Research: Wageningen, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Holzworth, D.P.; Huth, N.I.; deVoil, P.G.; Zurcher, E.J.; Herrmann, N.I.; McLean, G.; Chenu, K.; van Oosterom, E.J.; Snow, V.; Murphy, C.; et al. APSIM—Evolution towards a New Generation of Agricultural Systems Simulation. Environ. Model. Softw. 2014, 62, 327–350. [Google Scholar] [CrossRef]
- Asseng, S.; Keating, B.A.; Huth, N.; Eastham, J. Simulation of Perched Water Tables in Duplex Soils. In Proceedings of the MODSIM 97: International Congress on Modelling and Simulation, Hobert, Australia, 8–11 December 1997. [Google Scholar]
- Raes, D.; Steduto, P.; Hsiao, T.C.; Fereres, E. Chapter 1. AquaCrop—FAO Crop-Water Productivity Model to Simulate Yield Response to Water. In AquaCrop Version 6.0–6.1; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018. [Google Scholar]
- Skaggs, R.W. DRAINMOD Reference Report: Methods for Design and Evaluation of Drainage-Water Management Systems for Soils with High Water Tables; North Carolina State University: Raleigh, NC, USA, 1980. [Google Scholar]
- Jones, J.W.; Hoogenboom, G.; Porter, C.H.; Boote, K.J.; Batchelor, W.D.; Hunt, L.A.; Wilkens, P.W.; Singh, U.; Gijsman, A.J.; Ritchie, J.T. The DSSAT Cropping System Model. Eur. J. Agron. 2003, 18, 235–265. [Google Scholar] [CrossRef]
- Williams, J.R.; Jones, C.A.; Kiniry, J.R.; Spanel, D.A. EPIC Crop Growth Model. Trans. Am. Soc. Agric. Eng. 1989, 32, 497–511. [Google Scholar] [CrossRef]
- De Wit, A.; Boogaard, H.; Fumagalli, D.; Janssen, S.; Knapen, R.; van Kraalingen, D.; Supit, I.; van der Wijngaart, R.; van Diepen, K. 25 Years of the WOFOST Cropping Systems Model. Agric. Syst. 2019, 168, 154–167. [Google Scholar] [CrossRef]
- Steduto, P.; Hsiao, T.C.; Raes, D.; Fereres, E. Aquacrop-the FAO Crop Model to Simulate Yield Response to Water: I. Concepts and Underlying Principles. Agron. J. 2009, 101, 426–437. [Google Scholar] [CrossRef]
- Raes, D.; Steduto, P.; Hsiao, T.C.; Fereres, E. Aquacrop-The FAO Crop Model to Simulate Yield Response to Water: II. Main Algorithms and Software Description. Agron. J. 2009, 101, 438–447. [Google Scholar] [CrossRef]
- Evans, R.O.; Skaggs, R.W. Stress Day Index Models to Predict Corn and Soybean Yield Response to Water Table Management. In Proceedings of the 15th International Congress on Irrigation and Drainage, The Hague, The Netherlands, 4–11 September 1993. [Google Scholar]
- Jones, J.W.; Hoogenboom, G.; Wilkens, P.; Porter, C.; Tsuji, G. Decision Support System for Agrotechnology Transfer (DSSAT) Version 4.5; University of Hawaii: Honolulu, HI, USA, 2010. [Google Scholar]
- Sharpley, A.N.; Williams, J.R. EPIC—Erosion/Productivity Impact Calculator: 1. Model Documentation; Technical Bulletin 1768; U.S. Department of Agriculture, Agricultural Research Service (ARS): Washington, DC, USA, 1990.
- Bartholomeus, R.P.; Witte, J.P.M.; van Bodegom, P.M.; van Dam, J.C.; Aerts, R. Critical Soil Conditions for Oxygen Stress to Plant Roots: Substituting the Feddes-Function by a Process-Based Model. J. Hydrol. 2008, 360, 147–165. [Google Scholar] [CrossRef]
- Feddes, R.A.; Kowalik, P.J.; Zaradny, H. Simulation of Field Water Use and Crop Yield. Simulation Monographs; John Wiley & Sons: New York, NY, USA, 1978. [Google Scholar]
- De Wit, A.J.W.; Boogaard, H.L.; Supit, I.; van den Berg, M. System Description of the WOFOST 7.2 Cropping Systems Model; Wageningen Environmental Research: Wageningen, The Netherlands, 2020. [Google Scholar]
- Harrison, M.T.; Cullen, B.R.; Armstrong, D. Management Options for Dairy Farms under Climate Change: Effects of Intensification, Adaptation and Simplification on Pastures, Milk Production and Profitability. Agric. Syst. 2017, 155, 19–32. [Google Scholar] [CrossRef]
- Leão, T.P.; da Silva, A.P.; Macedo, M.C.M.; Imhoff, S.; Euclides, V.P.B. Least Limiting Water Range: A Potential Indicator of Changes in near-Surface Soil Physical Quality after the Conversion of Brazilian Savanna into Pasture. Soil Tillage Res. 2006, 88, 279–285. [Google Scholar] [CrossRef]
- Meskini-Vishkaee, F.; Mohammadi, M.H.; Neyshabouri, M.R. Revisiting the Wet and Dry Ends of Soil Integral Water Capacity Using Soil and Plant Properties. Soil Res. 2018, 56, 331–345. [Google Scholar] [CrossRef]
- Khataar, M.; Mohhamadi, M.H.; Shabani, F. Soil Salinity and Matric Potential Interaction on Water Use, Water Use Efficiency and Yield Response Factor of Bean and Wheat. Sci. Rep. 2018, 8, 2679. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.H.; Asadzadeh, F.; Vanclooster, M. Refining and Unifying the Upper Limits of the Least Limiting Water Range Using Soil and Plant Properties. Plant Soil 2010, 334, 221–234. [Google Scholar] [CrossRef]
- Mittler, R. Abiotic Stress, the Field Environment and Stress Combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef]
- Sewelam, N.; Oshima, Y.; Mitsuda, N.; Ohme-Takagi, M. A Step towards Understanding Plant Responses to Multiple Environmental Stresses: A Genome-Wide Study. Plant Cell Environ. 2014, 37, 2024–2035. [Google Scholar] [CrossRef]
- Nguyen, D.; Rieu, I.; Mariani, C.; van Dam, N.M. How Plants Handle Multiple Stresses: Hormonal Interactions Underlying Responses to Abiotic Stress and Insect Herbivory. Plant Mol. Biol. 2016, 91, 727–740. [Google Scholar] [CrossRef]
- Grigorova, B.; Vaseva, I.; Demirevska, K.; Feller, U. Combined Drought and Heat Stress in Wheat: Changes in Some Heat Shock Proteins. Biol. Plant. 2011, 55, 105–111. [Google Scholar] [CrossRef]
- Prasch, C.M.; Sonnewald, U. Signaling Events in Plants: Stress Factors in Combination Change the Picture. Environ. Exp. Bot. 2015, 114, 4–14. [Google Scholar] [CrossRef]
- Yin, X.; Struik, P.C. C3 and C4 Photosynthesis Models: An Overview from the Perspective of Crop Modelling. NJAS-Wagen. J. Life Sci. 2009, 57, 27–38. [Google Scholar] [CrossRef]
- Li, Y.; Niu, W.; Cao, X.; Wang, J.; Zhang, M.; Duan, X.; Zhang, Z. Effect of Soil Aeration on Root Morphology and Photosynthetic Characteristics of Potted Tomato Plants (Solanum Lycopersicum) at Different NaCl Salinity Levels. BMC Plant Biol. 2019, 19, 1–15. [Google Scholar] [CrossRef]
- Martínez, M.; Arata, A.F.; Lázaro, L.; Stenglein, S.A.; Dinolfo, M.I. Effects of Waterlogging Stress on Plant-Pathogen Interaction between Fusarium Poae and Wheat/ Barley. Acta Sci. Agron. 2019, 41, 1–9. [Google Scholar] [CrossRef]
- Zhen, B.; Li, H.; Niu, Q.; Qiu, H.; Tian, G.; Lu, H.; Zhou, X. Effects of Combined High Temperature and Waterlogging Stress at Booting Stage on Root Anatomy of Rice (Oryza sativa L.). Water 2020, 12, 2524. [Google Scholar] [CrossRef]
- Marti, J.; Savin, R.; Slafer, G.A. Wheat Yield as Affected by Length of Exposure to Waterlogging During Stem Elongation. J. Agron. Crop Sci. 2015, 201, 473–486. [Google Scholar] [CrossRef]
- Ren, B.; Zhang, J.; Li, X.; Fan, X.; Dong, S.; Liu, P.; Zhao, B. Effects of Waterlogging on the Yield and Growth of Summer Maize under Field Conditions. Can. J. Plant Sci. 2014, 94, 23–31. [Google Scholar] [CrossRef]
- Colmer, T.D.; Atwell, B.J.; Ismail, A.M.; Pedersen, O.; Shabala, S.; Sorrell, B.; Voesenek, L.A.C.J. Chapter 18: Waterlogging and Submergence. In Plants in Action. Available online: https://www.rseco.org/index.html (accessed on 30 June 2022).
- Dickin, E.; Wright, D. The Effects of Winter Waterlogging and Summer Drought on the Growth and Yield of Winter Wheat (Triticum aestivum L.). Eur. J. Agron. 2008, 28, 234–244. [Google Scholar] [CrossRef]
- Ploschuk, R.A.; Miralles, D.J.; Colmer, T.D.; Striker, G.G. Waterlogging Differentially Affects Yield and Its Components in Wheat, Barley, Rapeseed and Field Pea Depending on the Timing of Occurrence. J. Agron. Crop Sci. 2020, 206, 363–375. [Google Scholar] [CrossRef]
- Wang, X.; Deng, Z.; Zhang, W.; Meng, Z.; Chang, X.; Lv, M. Effect of Waterlogging Duration at Different Growth Stages on the Growth, Yield and Quality of Cotton. PLoS ONE 2017, 12, e0169029. [Google Scholar] [CrossRef]
- Zurweller, B.A.; Motavalli, P.P.; Nelson, K.A.; Dudenhoeffer, C.J. Short-Term Soil Nitrous Oxide Emissions as Affected by Enhanced Efficiency Nitrogen Fertilizers and Temporarily Waterlogged Conditions. J. Agric. Sci. 2015, 7, 1. [Google Scholar] [CrossRef]
- Rawnsley, R.P.; Smith, A.P.; Christie, K.M.; Harrison, M.T.; Eckard, R.J. Current and Future Direction of Nitrogen Fertiliser Use in Australian Grazing Systems. Crop Pasture Sci. 2019, 70, 1034–1043. [Google Scholar] [CrossRef]
- Malik, A.I.; Colmer, T.D.; Lambers, H.; Setter, T.L.; Schortemeyer, M. Short-Term Waterlogging Has Long-Term Effects on the Growth and Physiology of Wheat. New Phytol. 2002, 153, 225–236. [Google Scholar] [CrossRef]
- Kaur, G.; Zurweller, B.A.; Nelson, K.A.; Motavalli, P.P.; Dudenhoeffer, C.J. Soil Waterlogging and Nitrogen Fertilizer Management Effects on Corn and Soybean Yields. Agron. J. 2017, 109, 97–106. [Google Scholar] [CrossRef]
- Ciancio, N.; Miralles, D.J.; Striker, G.G.; Abeledo, L.G. Plant Growth Rate after, and Not during, Waterlogging Better Correlates to Yield Responses in Wheat and Barley. J. Agron. Crop Sci. 2021, 207, 304–316. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, G.; Xu, S.; Dai, J.; Li, W.; Li, Z.; Zhang, D.; Cui, Z.; Li, C.; Dong, H. Nitric Oxide Reduces the Yield Loss of Waterlogged Cotton by Enhancing Post-Stress Compensatory Growth. Field Crop. Res. 2022, 283, 108524. [Google Scholar] [CrossRef]
- Yeung, E.; Bailey-Serres, J.; Sasidharan, R. After The Deluge: Plant Revival Post-Flooding. Trends Plant Sci. 2019, 24, 443–454. [Google Scholar] [CrossRef]
- Jayawardhane, J.; Cochrane, D.W.; Vyas, P.; Bykova, N.V.; Vanlerberghe, G.C.; Igamberdiev, A.U. Roles for Plant Mitochondrial Alternative Oxidase Under Normoxia, Hypoxia, and Reoxygenation Conditions. Front. Plant Sci. 2020, 11, 566. [Google Scholar] [CrossRef]
- León, J.; Castillo, M.C.; Gayubas, B. The Hypoxia–Reoxygenation Stress in Plants. J. Exp. Bot. 2021, 72, 5841–5856. [Google Scholar] [CrossRef]
- Challinor, A.J.; Wheeler, T.R.; Craufurd, P.Q.; Slingo, J.M.; Grimes, D.I.F. Design and Optimisation of a Large-Area Process-Based Model for Annual Crops. Agric. Forest Meteorol. 2004, 124, 99–120. [Google Scholar] [CrossRef]
- Harrison, M.T.; Evans, J.R.; Dove, H.; Moore, A.D. Recovery Dynamics of Rainfed Winter Wheat after Livestock Grazing 2. Light Interception, Radiation-Use Efficiency and Dry-Matter Partitioning. Crop Pasture Sci. 2011, 62, 960–971. [Google Scholar] [CrossRef]
- Taylor, C.A.; Harrison, M.T.; Telfer, M.; Eckard, R. Modelled Greenhouse Gas Emissions from Beef Cattle Grazing Irrigated Leucaena in Northern Australia. Anim. Prod. Sci. 2016, 56, 594–604. [Google Scholar] [CrossRef]
- Whitmore, A.P.; Whalley, W.R. Physical Effects of Soil Drying on Roots and Crop Growth. J. Exp. Bot. 2009, 60, 2845–2857. [Google Scholar] [CrossRef] [PubMed]
- Logsdon, S. Should Upper Limit of Available Water Be Based on Field Capacity? Agrosyst. Geosci. Environ. 2019, 2, 1–6. [Google Scholar] [CrossRef]
- Xu, X.; Huang, G.; Zhan, H.; Qu, Z.; Huang, Q. Integration of SWAP and MODFLOW-2000 for Modeling Groundwater Dynamics in Shallow Water Table Areas. J. Hydrol. 2012, 412–413, 170–181. [Google Scholar] [CrossRef]
- Xiang, Z.; Bailey, R.T.; Nozari, S.; Husain, Z.; Kisekka, I.; Sharda, V.; Gowda, P. DSSAT-MODFLOW: A New Modeling Framework for Exploring Groundwater Conservation Strategies in Irrigated Areas. Agric. Water Manag. 2020, 232, 106033. [Google Scholar] [CrossRef]
- De Jong van Lier, Q. Field Capacity, a Valid Upper Limit of Crop Available Water? Agric. Water Manag. 2017, 193, 214–220. [Google Scholar] [CrossRef]
- De Lima, R.P.; Tormena, C.A.; Figueiredo, G.C.; da Silva, A.R.; Rolim, M.M. Least Limiting Water and Matric Potential Ranges of Agricultural Soils with Calculated Physical Restriction Thresholds. Agric. Water Manag. 2020, 240, 106299. [Google Scholar] [CrossRef]
- Van Genuchten, M.T. A Closed-Form Equation for Predicting the Hydraulic Conductivity of Unsaturated Soils. Soil Sci. Soc. Am. J. 1980, 44, 892–898. [Google Scholar] [CrossRef]
- Huth, N.I.; Bristow, K.L.; Verburg, K. SWIM3: Model Use, Calibration, And Validation. Trans. ASABE 2012, 55, 1303–1313. [Google Scholar] [CrossRef]
- Paydar, Z.; Huth, N.; Snow, V. Modelling Irrigated Eucalyptus for Salinity Control on Shallow Watertables. Aust. J. Soil Res. 2005, 43, 587–597. [Google Scholar] [CrossRef]
- Robertson, F.; Suraweera, D.; McCaskill, M.; Christy, B.; Armstrong, R.; Zollinger, R.; Byron, J.; Partington, D.; Clark, S. Waterlogging Effects on Soils and Wheat Crops in the High Rainfall Zone of Victoria. In Proceedings of the 19th Australian Agronomy Conference, Wagga Wagga, Australia, 25–29 August 2019. [Google Scholar]
- Huck, M.G. Variation in Taproot Elongation Rate as Influenced by Composition of the Soil Air. Agron. J. 1970, 62, 815–818. [Google Scholar] [CrossRef]
- Boru, G.; Vantoai, T.; Alves, J.; Hua, D.; Knee, M. Responses of Soybean to Oxygen Deficiency and Elevated Root-Zone Carbon Dioxide Concentration. Ann. Bot. 2003, 91, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Colmer, T.D.; Pedersen, O.; Nakazono, M. Regulation of Root Traits for Internal Aeration and Tolerance to Soil Waterlogging-Flooding Stress. Plant Physiol. 2018, 176, 1118–1130. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).