Extremely Premature Infant and Digestive Malformations: Case Report of Atypical Postoperative Journeys
Abstract
1. Introduction and Clinical Significance
2. Case Presentation
2.1. Prenatal History and Delivery
- Oligohydramnios (likely secondary to prolonged rupture of membranes).
- Maternal SARS-CoV-2 infection.
- Maternal hypothyroidism.
- Positive Escherichia coli culture.
- Membranes ruptured > 48 h before delivery.
2.2. Initial Assessment and Resuscitation
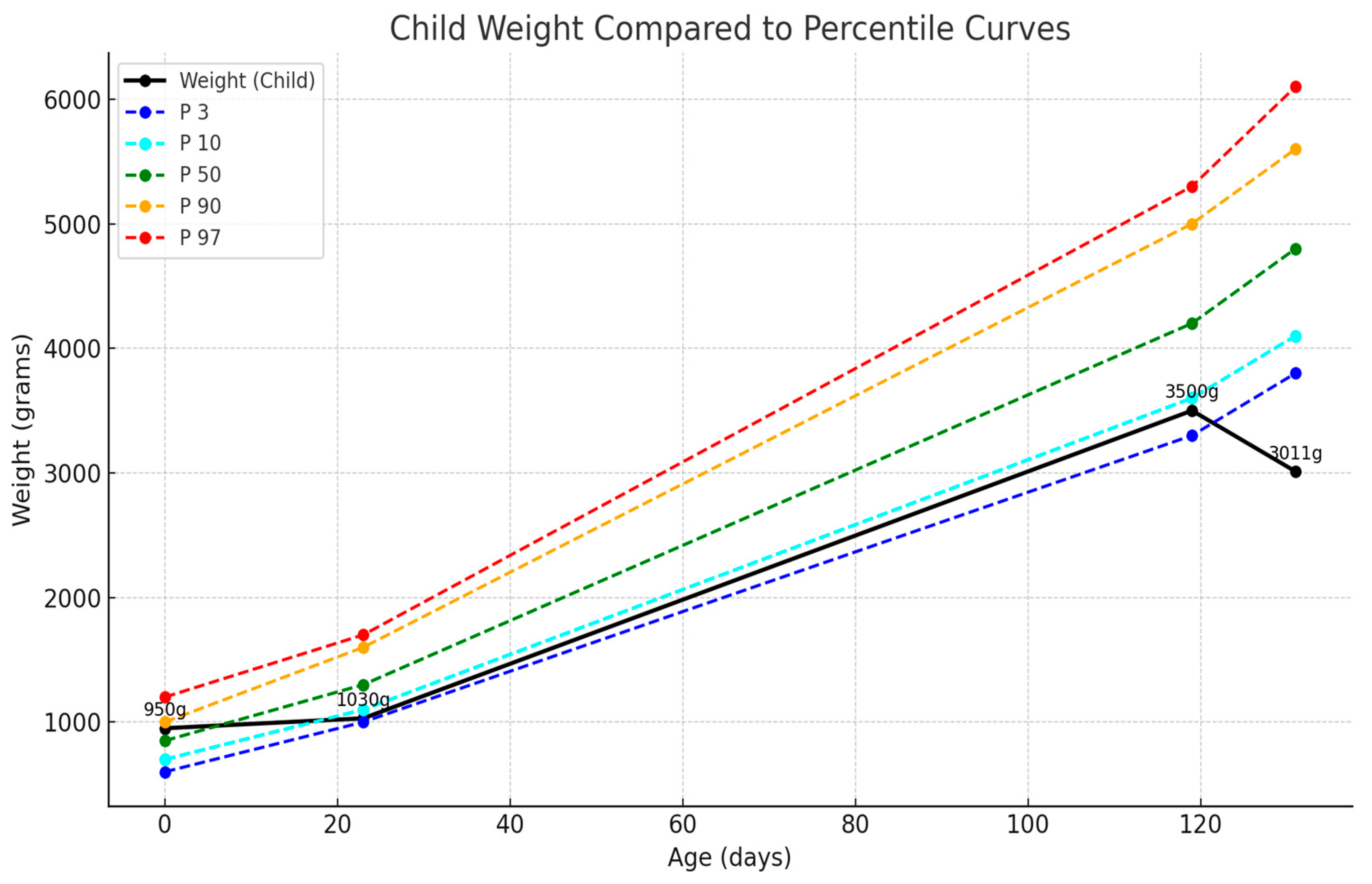
2.3. NICU Course—First Two Weeks
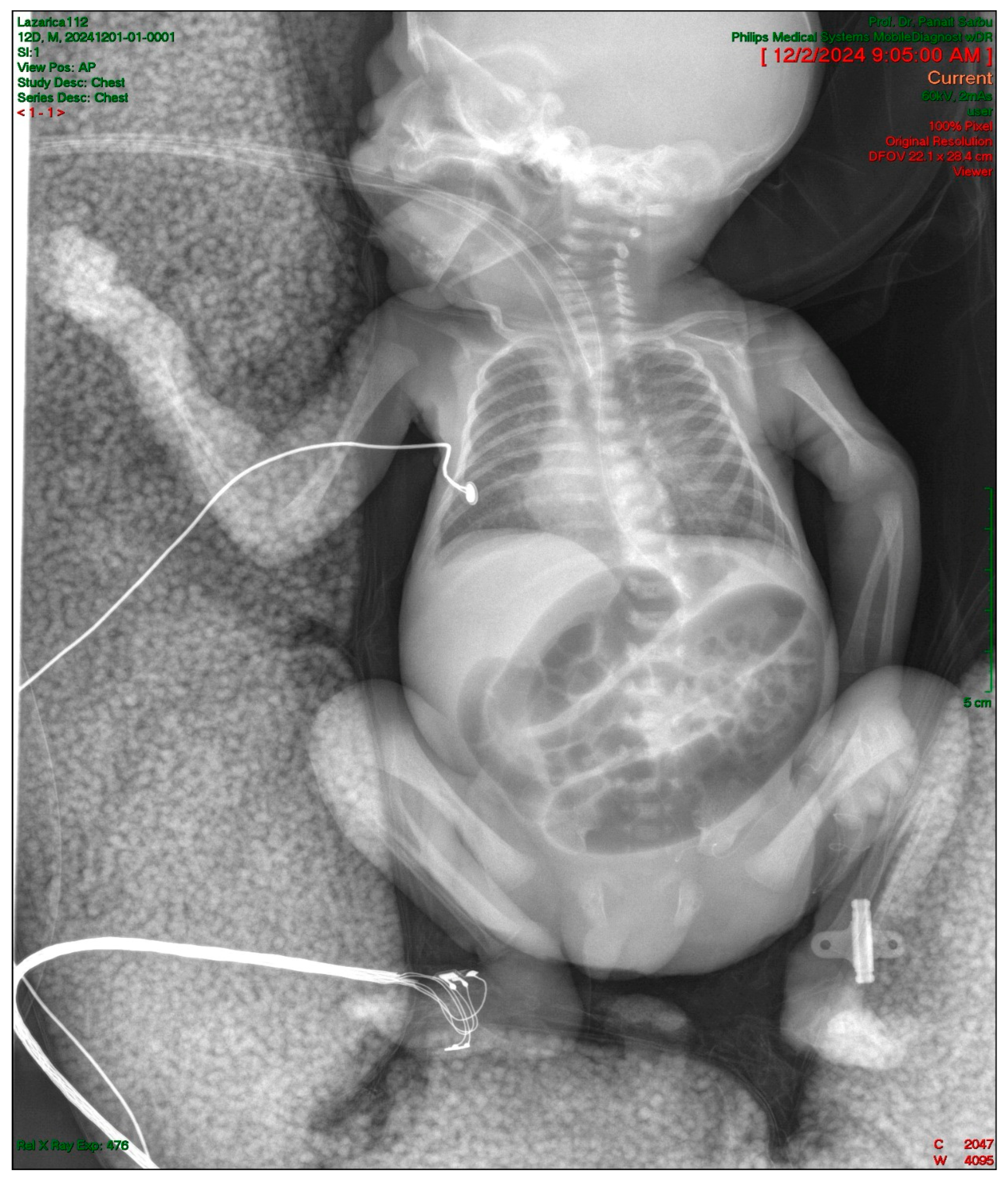
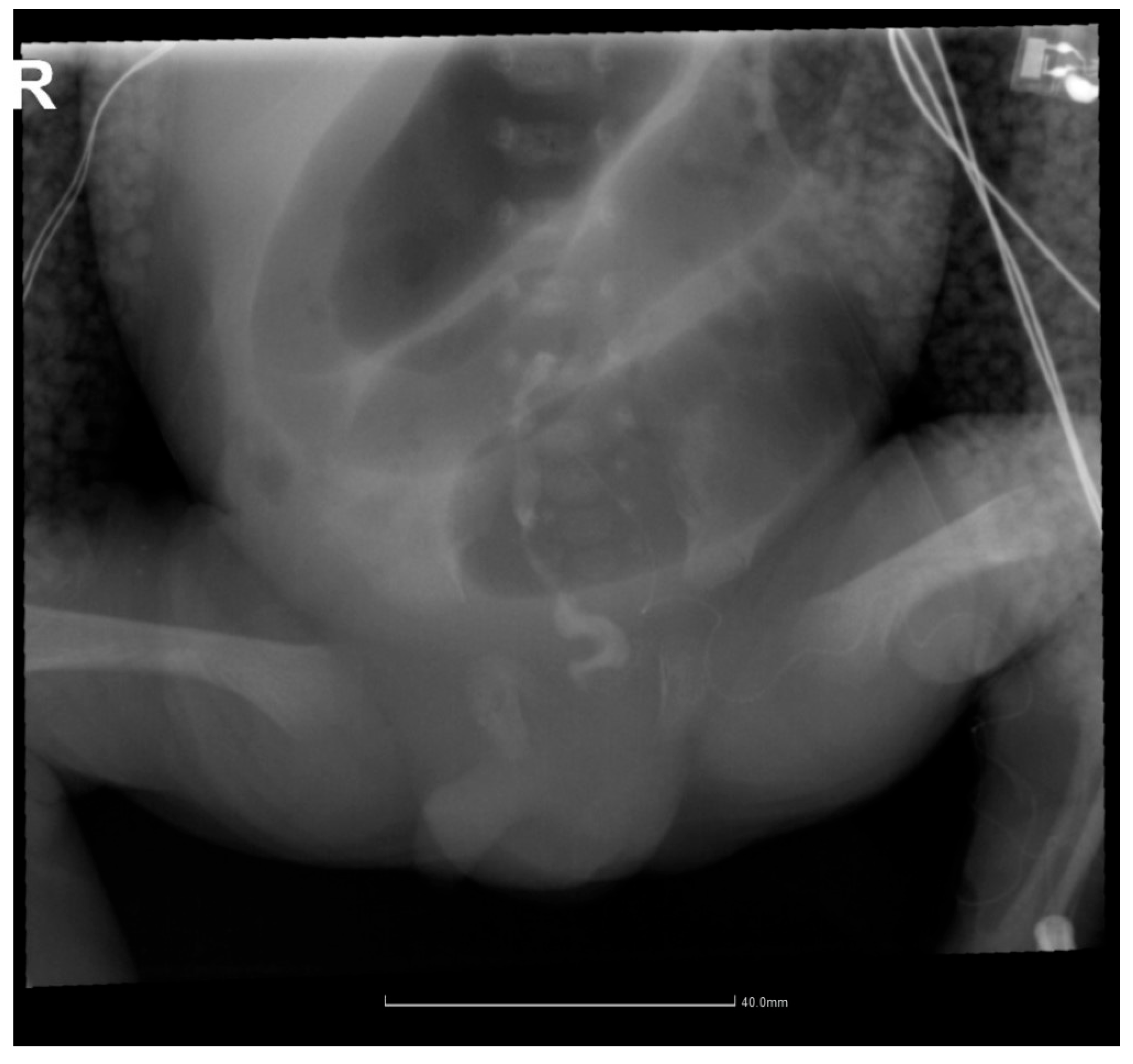
2.4. Gastrointestinal Complications and Diagnosis
2.5. Postoperative Course and Complications
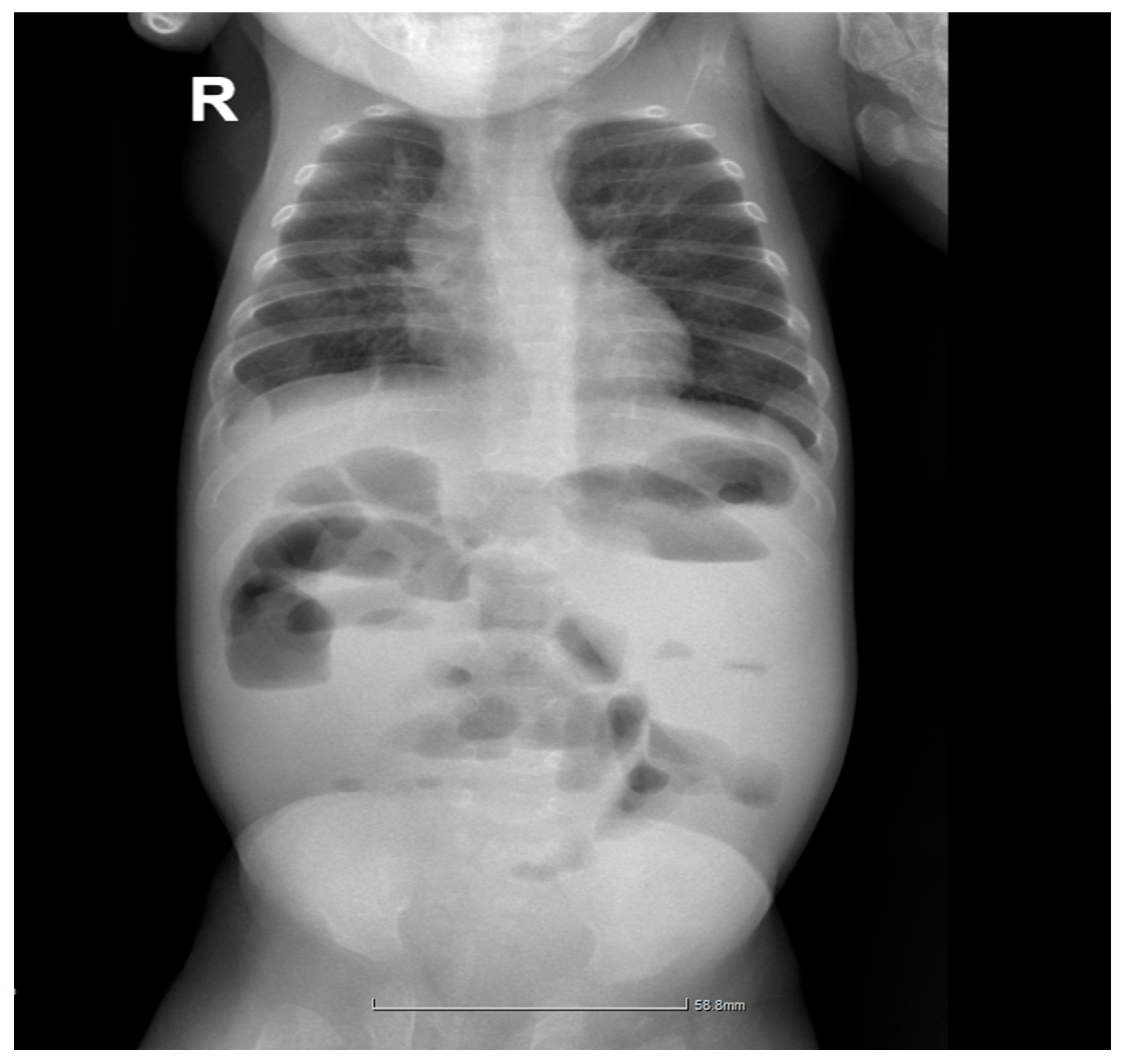
2.6. Long-Term Follow-Up and Complications
2.7. Current Status and Long-Term Outcomes
3. Discussion
3.1. Unique Aspects of This Case
3.2. Literature Review
3.3. Nutritional Considerations
3.4. Long-Term Follow-Up and Neurodevelopmental Outcomes
3.5. Study Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bamat, N.; Eichenwald, E.C. Approach to Mechanical Ventilation in Very Preterm Neonates. Available online: https://www.uptodate.com/contents/approach-to-mechanical-ventilation-in-very-preterm-neonates (accessed on 4 January 2025).
- Suciu, L.M.; Puscasiu, L.; Szabo, B.; Cucerea, M.; Ognean, M.L.; Oprea, I.; Bell, E.F. Mortality and Morbidity of very Preterm Infants in Romania: How Are We Doing? Pediatr. Int. 2014, 56, 200–206. [Google Scholar] [CrossRef]
- Taylor, G.L.; O’Shea, T.M. Extreme Prematurity: Risk and Resiliency. Curr. Probl. Pediatr. Adolesc. Health Care 2022, 52, 101132. [Google Scholar] [CrossRef]
- Villar, J.; Cavoretto, P.I.; Barros, F.C.; Romero, R.; Papageorghiou, A.T.; Kennedy, S.H. Etiologically Based Functional Taxonomy of the Preterm Birth Syndrome. Clin. Perinatol. 2024, 51, 475–495. [Google Scholar] [CrossRef]
- Boni, L.; Gradellini, C.; Miari, M.; Cocconi, P.; Montorsi, A.; Capone, R.; Costi, S.; Di Leo, S.; Puglisi, C.; Ghirotto, L. How Parents and Health Professionals Experience Prematurity in an Italian Neonatal Intensive Care: A Grounded Theory Study. J. Pediatr. Nurs. 2022, 67, e172–e179. [Google Scholar] [CrossRef]
- Ferretti, E.; Daboval, T.; Rouvinez-Bouali, N.; Lawrence, S.L.; Lemyre, B. Extremely Low Gestational Age Infants: Developing a Multidisciplinary Care Bundle. Paediatr. Child. Health 2021, 26, e240–e245. [Google Scholar] [CrossRef]
- Merenstein, G.B.; Gardner, S.L. Handbook of Neonatal Intensive Care, 6th ed.; Mosby: St. Louis, MO, USA, 2006. [Google Scholar]
- Mandy, G.T. Preterm Birth: Definitions of Prematurity, Epidemiology, and Risk Factors for Infant Mortality. Available online: https://www.uptodate.com/contents/preterm-birth-definitions-of-prematurity-epidemiology-and-risk-factors-for-infant-mortality/print (accessed on 13 July 2025).
- Fenton, T.R.; Elmrayed, S.; Alshaikh, B.N. Fenton Third–Generation Growth Charts of Preterm Infants Without Abnormal Fetal Growth: A Systematic Review and Meta–Analysis. Paediatr. Perinat. Epidemiol. 2025, 39, 543–555. [Google Scholar] [CrossRef]
- Amodio, J.; Berdon, W.; Abramson, S.; Stolar, C. Microcolon of Prematurity: A Form of Functional Obstruction. Am. J. Roentgenol. 1986, 146, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Emil, S.; Nguyen, T.; Sills, J.; Padilla, G. Meconium Obstruction in Extremely Low-Birth-Weight Neonates: Guidelines for Diagnosis and Management. J. Pediatr. Surg. 2004, 39, 731–737. [Google Scholar] [CrossRef]
- Solaz-García, A.J.; Segovia-Navarro, L.; Rodríguez de Dios-Benlloch, J.L.; Benavent-Taengua, L.; Castilla-Rodríguez, D.Y.; Company-Morenza, M.A. Prevención de La Obstrucción Meconial En Recién Nacidos Prematuros de Muy Bajo Peso. Enferm. Intensiv. 2019, 30, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, C.; Barone, G.; Capitanucci, M.L.; Zaccara, A.M.; Fusaro, F.; Iacobelli, B.D.; Scorletti, F.; Conforti, A.; De Angelis, P.; Diamanti, A.; et al. Megacystis–Microcolon–Intestinal Hypoperistalsis Syndrome: Don’t Forget the Bladder. Pediatr. Surg. Int. 2024, 40, 124. [Google Scholar] [CrossRef] [PubMed]
- Al-Alaiyan, S.; Nazer, H. Megacystis-Microcolon-Intestmal Hypoperistalsis Syndrome. Ann. Saudi Med. 1996, 16, 353–355. [Google Scholar] [CrossRef] [PubMed]
- Bello, T.; Fadiora, S. Micro-Colon Associated with Multiple Ileal Atresia in a Newborn Infant—Case Report and Literature Review. West. Afr. J. Med. 2004, 22, 350–351. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mohammed, D.; Abubakar, M.; Haruna, G.I. Radiological Diagnosis of Microcolon in a 5-Day Old Neonate: A Case Report. World J. Adv. Res. Rev. 2020, 8, 238–241. [Google Scholar] [CrossRef]
- Cabrera Valerio, C.; Díaz, Z.; Alcántara, E.; Castillo, R. Colonic Atresia: A Rare Entity in the Newborn. A Six-Case Report and a Bibliographic Review. Cir. Pediatr. 2021, 34, 74–78. [Google Scholar] [PubMed]
- Juang, D.; Snyder, C.L. Neonatal Bowel Obstruction. Surg. Clin. N. Am. 2012, 92, 685–711. [Google Scholar] [CrossRef]
- Rausch, L.A.; Hanna, D.N.; Patel, A.; Blakely, M.L. Review of Necrotizing Enterocolitis and Spontaneous Intestinal Perforation Clinical Presentation, Treatment, and Outcomes. Clin. Perinatol. 2022, 49, 955–964. [Google Scholar] [CrossRef]
- Feng, W.; Zhang, H.; Yan, H.; Yang, Z.-B.; Zhao, J.-L.; Zhang, L.-B. Gastrointestinal Perforation in Extremely Low Birth Weight Infants: A Single Center Retrospective Study in China. Pediatr. Neonatol. 2024, 65, 111–116. [Google Scholar] [CrossRef]
- Okello, I.; Kakembo, N.; Kisa, P.; Nimanya, S.; Stephens, C.Q.; Yap, A.; Wesonga, A.S.; Naluyimbazi, R.; Sekabira, J. Epidemiologic Factors Associated with Neonatal Bowel Perforations in Uganda: Experience from a Single Tertiary Referral Hospital. Ann. Pediatr. Surg. 2023, 19, 43. [Google Scholar] [CrossRef]
- Jańczewska, I.; Wierzba, J.; Jańczewska, A.; Szczurek-Gierczak, M.; Domżalska-Popadiuk, I. Prematurity and Low Birth Weight and Their Impact on Childhood Growth Patterns and the Risk of Long-Term Cardiovascular Sequelae. Children 2023, 10, 1599. [Google Scholar] [CrossRef]
- Morniroli, D.; Tiraferri, V.; Maiocco, G.; De Rose, D.U.; Cresi, F.; Coscia, A.; Mosca, F.; Giannì, M.L. Beyond Survival: The Lasting Effects of Premature Birth. Front. Pediatr. 2023, 11, 1213243. [Google Scholar] [CrossRef]
- de Jesus, L.E.; Lund, T.C.; Regadas, C.T.; de Oliveira, A.P.P.; Bruno, R.R.; de Moraes, A.C.G.; Dekermacher, S. Meconium Ileal Obstruction and Functional Immaturity: Review. J. Pediatr. Surg. 2024, 59, 161935. [Google Scholar] [CrossRef] [PubMed]
- Devavarapu, P.K.V.; Uppaluri, K.R.; Nikhade, V.A.; Palasamudram, K.; Sri Manjari, K. Exploring the Complexities of Megacystis-Microcolon-Intestinal Hypoperistalsis Syndrome: Insights from Genetic Studies. Clin. J. Gastroenterol. 2024, 17, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Kiran, Z.; Sheikh, A.; Humayun, K.N.; Islam, N. Neonatal Outcomes and Congenital Anomalies in Pregnancies Affected by Hypothyroidism. Ann. Med. 2021, 53, 1560–1568. [Google Scholar] [CrossRef] [PubMed]
- Allegaert, K.; Vanhole, C.; Van Helvoirt, M.; Vanhaesebrouck, S.; Gewillig, M.; Debeer, A.; Devlieger, H.; de Zegher, F. Association of Patent Ductus Arteriosus and Severe Hypothyroidism in Term Newborns: Coincidence or Causal? J. Matern. Fetal Neonatal Med. 2004, 16, 339–341. [Google Scholar] [CrossRef]
- Năstase, L.; Cristea, O.; Diaconu, A.; Stoicescu, S.-M.; Mohora, R.; Pascu, B.M.; Tala, S.T.; Roșca, I. Two Cases of Congenital Hypothyroidism Revealing Thyroid Agenesis. Medicina 2023, 59, 1887. [Google Scholar] [CrossRef]
- Gurol-Urganci, I.; Jardine, J.E.; Carroll, F.; Draycott, T.; Dunn, G.; Fremeaux, A.; Harris, T.; Hawdon, J.; Morris, E.; Muller, P.; et al. Maternal and Perinatal Outcomes of Pregnant Women with SARS-CoV-2 Infection at the Time of Birth in England: National Cohort Study. Am. J. Obstet. Gynecol. 2021, 225, 522.e1–522.e11. [Google Scholar] [CrossRef]
- Allotey, J.; Fernandez, S.; Bonet, M.; Stallings, E.; Yap, M.; Kew, T.; Zhou, D.; Coomar, D.; Sheikh, J.; Lawson, H.; et al. Clinical Manifestations, Risk Factors, and Maternal and Perinatal Outcomes of Coronavirus Disease 2019 in Pregnancy: Living Systematic Review and Meta-Analysis. BMJ 2020, 370, m3320. [Google Scholar] [CrossRef]
- Woodworth, K.R.; Olsen, E.O.; Neelam, V.; Lewis, E.L.; Galang, R.R.; Oduyebo, T.; Aveni, K.; Yazdy, M.M.; Harvey, E.; Longcore, N.D.; et al. Birth and Infant Outcomes Following Laboratory-Confirmed SARS-CoV-2 Infection in Pregnancy—SET-NET, 16 Jurisdictions, March 29–October 14, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1635–1640. [Google Scholar] [CrossRef]
- Patberg, E.T.; Adams, T.; Rekawek, P.; Vahanian, S.A.; Akerman, M.; Hernandez, A.; Rapkiewicz, A.V.; Ragolia, L.; Sicuranza, G.; Chavez, M.R.; et al. Coronavirus Disease 2019 Infection and Placental Histopathology in Women Delivering at Term. Am. J. Obstet. Gynecol. 2021, 224, 382.e1–382.e18. [Google Scholar] [CrossRef]
- Popescu, D.E.; Roșca, I.; Jura, A.M.C.; Cioca, A.; Pop, O.; Lungu, N.; Popa, Z.-L.; Rațiu, A.; Boia, M. Prompt Placental Histopathological and Immunohistochemical Assessment after SARS-CoV-2 Infection during Pregnancy—Our Perspective of a Small Group. Int. J. Mol. Sci. 2024, 25, 1836. [Google Scholar] [CrossRef]
- Popescu, D.-E.; Cerbu, S.; Rosca, I.; Lungu, N.; Trușculescu, A.A.; Belengeanu, V.; Manea, A.M.; Dima, M.A.; Gorun, F.; Popa, Z.L.; et al. Comparative Analysis of Hematological and Biochemical Changes in Neonates among Women with and without COVID-19 Infection during Pregnancy. Children 2023, 10, 1370. [Google Scholar] [CrossRef]
- Lungu, N.; Popescu, D.-E.; Jura, A.M.C.; Zaharie, M.; Jura, M.-A.; Roșca, I.; Boia, M. Enhancing Early Detection of Sepsis in Neonates through Multimodal Biosignal Integration: A Study of Pulse Oximetry, Near-Infrared Spectroscopy (NIRS), and Skin Temperature Monitoring. Bioengineering 2024, 11, 681. [Google Scholar] [CrossRef] [PubMed]
- Preda, M.; Chivu, R.D.; Ditu, L.M.; Popescu, O.; Manolescu, L.S.C. Pathogenesis, Prophylaxis, and Treatment of Candida Auris. Biomedicines 2024, 12, 561. [Google Scholar] [CrossRef] [PubMed]
- Gomella, T.L.; Eyal, F.G.; Zenk, K.E. Neonatology, Management, Procedures, On-Call Problems, Diseases, and Drugs, 6th ed.; McGraw-Hill Professional: Columbus, OH, USA, 2009. [Google Scholar]
- Mandy, G.T. Overview of the Long-Term Complications of Preterm Birth. Available online: https://www.uptodate.com/contents/overview-of-the-long-term-complications-of-preterm-birth (accessed on 13 July 2025).
- Scharf, R.J.; Stroustrup, A.; Conaway, M.R.; DeBoer, M.D. Growth and Development in Children Born Very Low Birthweight. Arch. Dis. Child. Fetal Neonatal Ed. 2016, 101, F433–F438. [Google Scholar] [CrossRef] [PubMed]
- Haiden, N.; Luque, V.; Domellöf, M.; Hill, S.; Kivelä, L.; de Koning, B.; Köglmeier, J.; Moltu, S.J.; Norsa, L.; De Pipaon, M.S.; et al. Assessment of Growth Status and Nutritional Management of Prematurely Born Infants after Hospital Discharge: A Position Paper of the ESPGHAN Nutrition Committee. J. Pediatr. Gastroenterol. Nutr. 2025, 81, 421–441. [Google Scholar] [CrossRef]
- Rosca, I.; Turenschi, A.; Nicolescu, A.; Constantin, A.T.; Canciu, A.M.; Dica, A.D.; Bratila, E.; Coroleuca, C.A.; Nastase, L. Endocrine Disorders in a Newborn with Heterozygous Galactosemia, Down Syndrome and Complex Cardiac Malformation: Case Report. Medicina 2023, 59, 856. [Google Scholar] [CrossRef]
- Khan, T.F.; Romeu, A.M.; Franco-Liñán, M.; Torres, A.R. Guide to Neurological Follow-up in Premature Newborns. Medicina 2024, 84 (Suppl. S3), 26–31. [Google Scholar]
| Timeline | Complication/Intervention | Management |
|---|---|---|
| Day 1 | Respiratory distress, intubation | HFO ventilation, surfactant |
| Day 3 | Patent ductus arteriosus (PDA) | Fluid restriction, IV paracetamol |
| Day 5 | Ureaplasma suspected | Added clarithromycin |
| Day 10 | Leukocytosis | Changed to imipenem/cilastatin, colistin |
| Day 12 | Intestinal obstruction suspected | Surgical consultation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matran, E.R.; Dinulescu, A.; Prejmereanu, A.; Peta, O.-A.; Tiron, R.-I.; Pavelescu, M.L. Extremely Premature Infant and Digestive Malformations: Case Report of Atypical Postoperative Journeys. Pediatr. Rep. 2025, 17, 101. https://doi.org/10.3390/pediatric17050101
Matran ER, Dinulescu A, Prejmereanu A, Peta O-A, Tiron R-I, Pavelescu ML. Extremely Premature Infant and Digestive Malformations: Case Report of Atypical Postoperative Journeys. Pediatric Reports. 2025; 17(5):101. https://doi.org/10.3390/pediatric17050101
Chicago/Turabian StyleMatran, Elena Roxana, Alexandru Dinulescu, Ana Prejmereanu, Oana-Alexandra Peta, Radu-Ioan Tiron, and Mirela Luminița Pavelescu. 2025. "Extremely Premature Infant and Digestive Malformations: Case Report of Atypical Postoperative Journeys" Pediatric Reports 17, no. 5: 101. https://doi.org/10.3390/pediatric17050101
APA StyleMatran, E. R., Dinulescu, A., Prejmereanu, A., Peta, O.-A., Tiron, R.-I., & Pavelescu, M. L. (2025). Extremely Premature Infant and Digestive Malformations: Case Report of Atypical Postoperative Journeys. Pediatric Reports, 17(5), 101. https://doi.org/10.3390/pediatric17050101






