Life-Threatening Noninfectious Complications of Peritoneal Dialysis in an Infant with End-Stage Kidney Disease
Abstract
1. Introduction
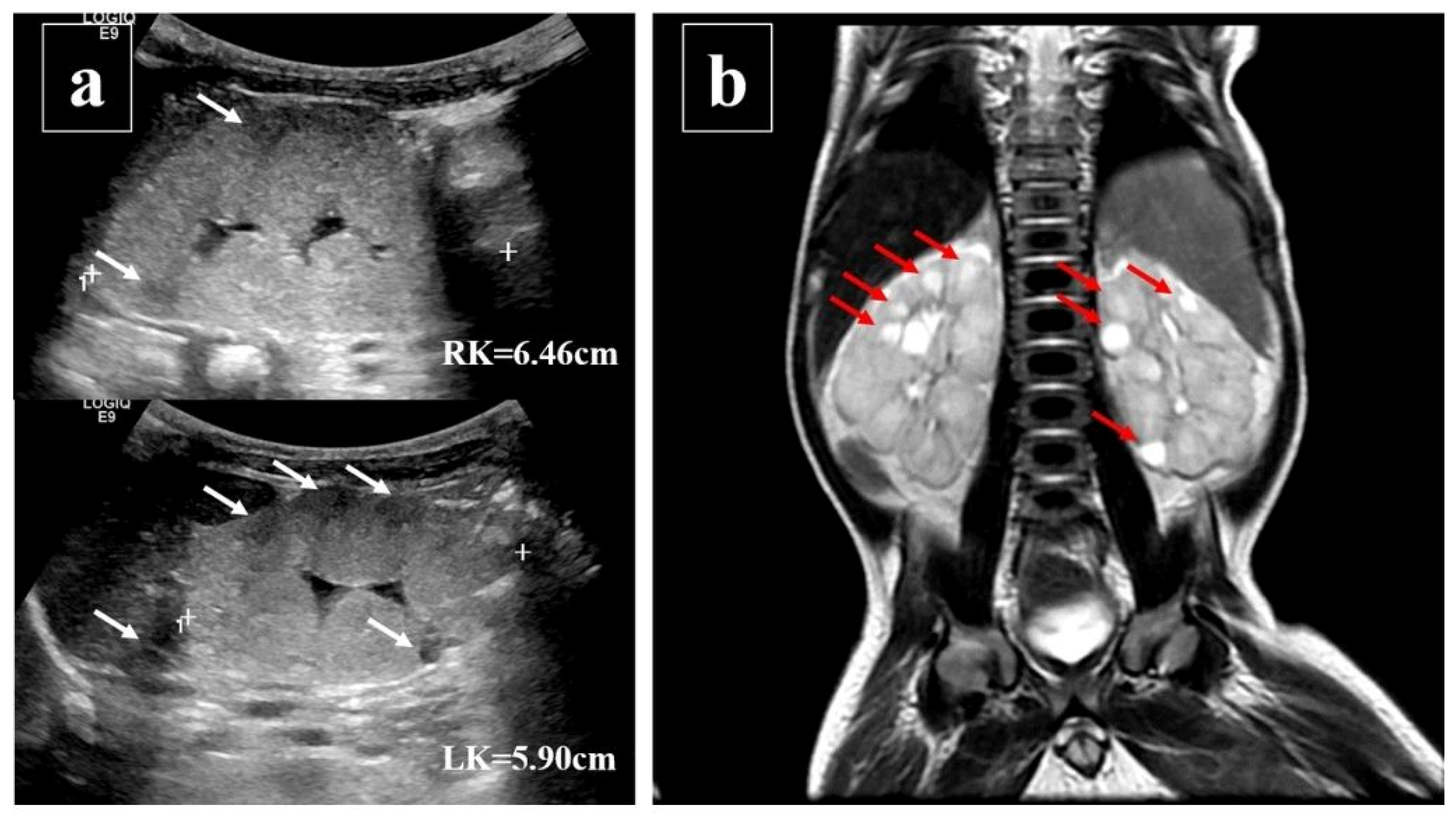
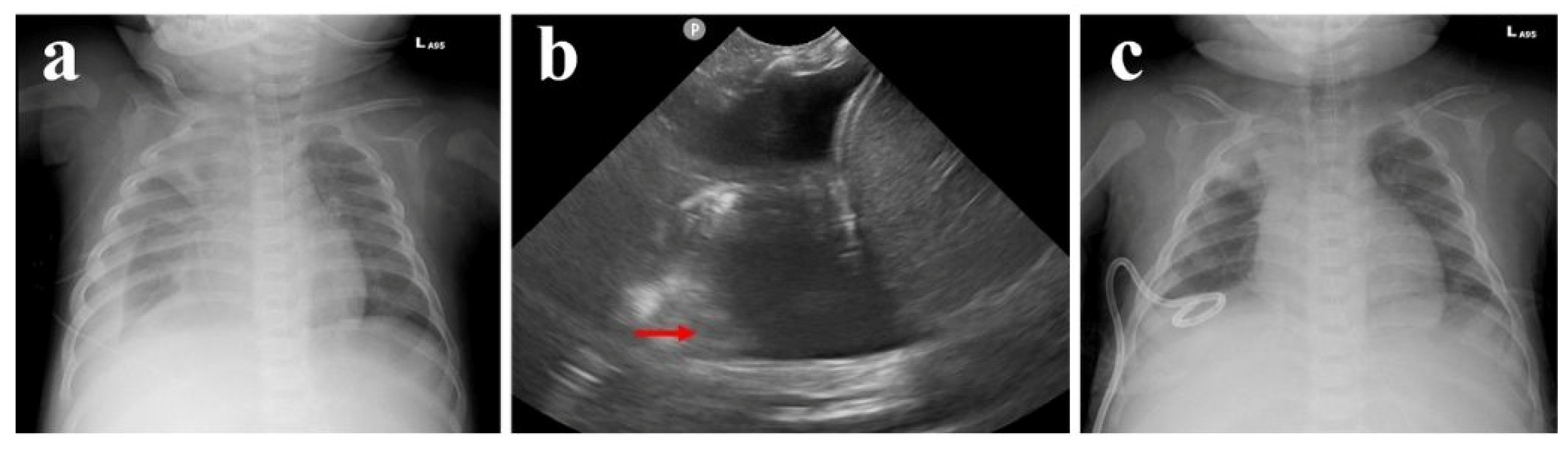
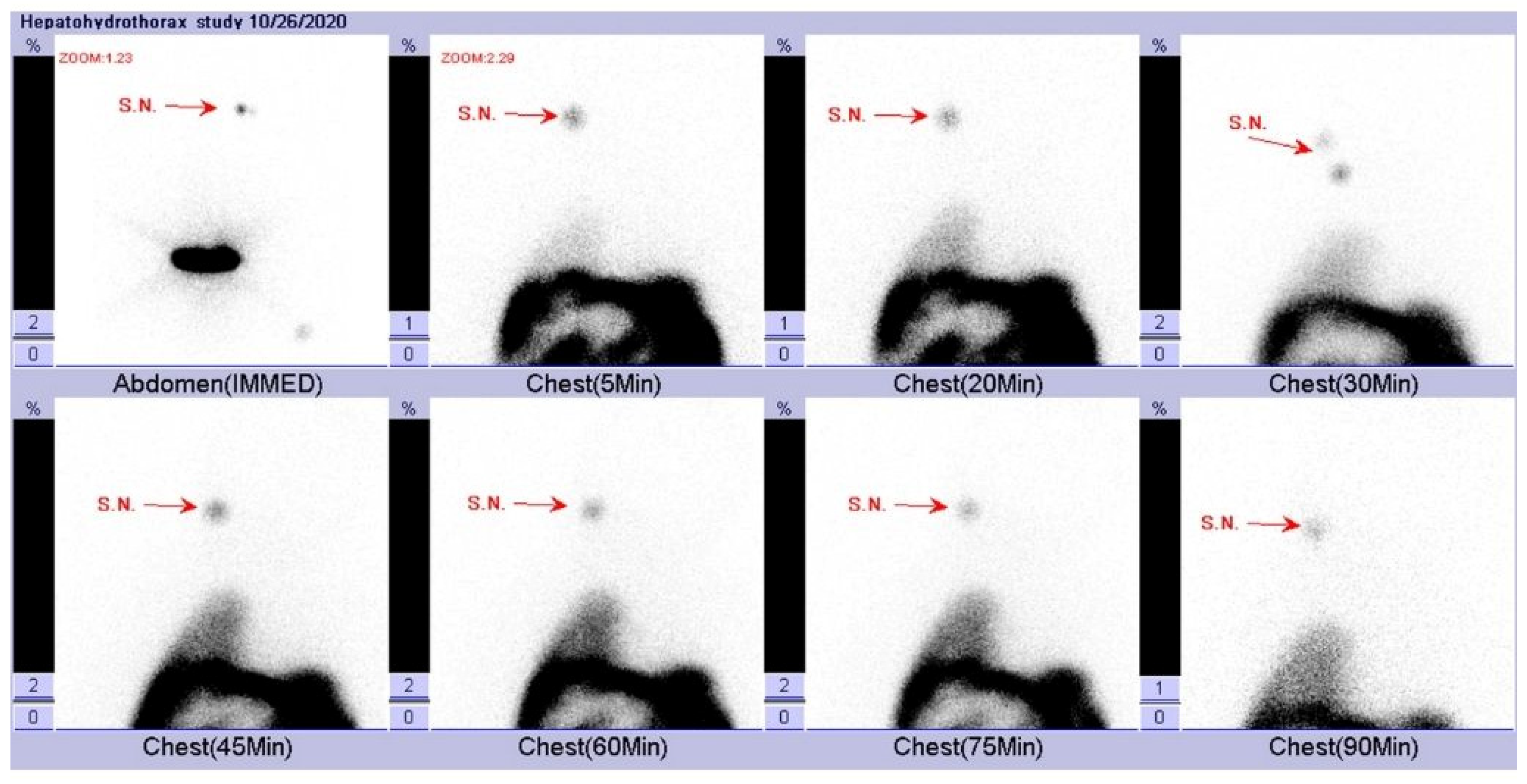
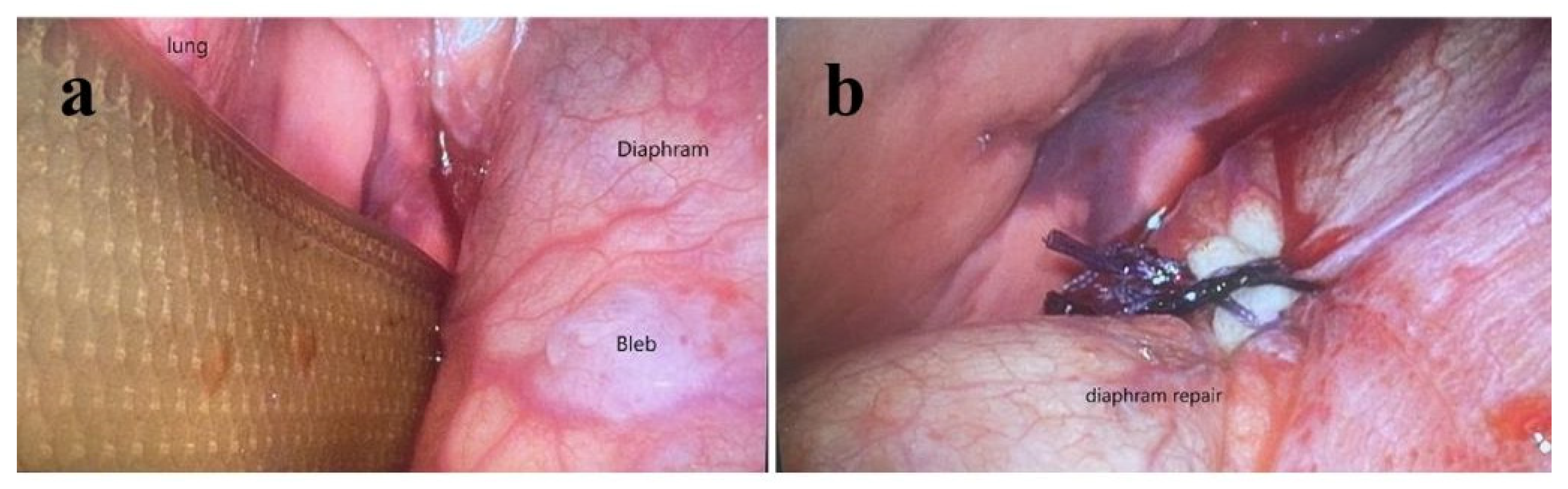
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Johansen, K.L.; Chertow, G.M.; Foley, R.N.; Gilbertson, D.T.; Herzog, C.A.; Ishani, A.; Israni, A.K.; Ku, E.; Tamura, M.K.; Li, S.; et al. US Renal Data System 2020 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 2021, 77 (Suppl. S1), A7–A8. [Google Scholar] [CrossRef]
- Vasudevan, A.; Phadke, K.; Yap, H.K. Peritoneal dialysis for the management of pediatric patients with acute kidney injury. Pediatr. Nephrol. 2017, 32, 1145–1156. [Google Scholar] [CrossRef] [PubMed]
- Nourse, P.; Cullis, B.; Finkelstein, F.; Numanoglu, A.; Warady, B.; Antwi, S.; McCulloch, M. ISPD guidelines for peritoneal dialysis in acute kidney injury: 2020 Update (paediatrics). Perit. Dial. Int. 2021, 41, 139–157. [Google Scholar] [CrossRef]
- Neu, A.M.; Warady, B.A.; Schaefer, F. Infectious Complications of Peritoneal Dialysis in Children. In Pediatric Dialysis; Springer: Cham, Switzerland, 2021; pp. 265–290. [Google Scholar]
- Borzych-Duzalka, D.; Aki, T.F.; Azocar, M.; White, C.; Harvey, E.; Mir, S.; Adragna, M.; Serdaroglu, E.; Sinha, R.; Samaille, C.; et al. Peritoneal Dialysis Access Revision in Children: Causes, Interventions, and Outcomes. Clin. J. Am. Soc. Nephrol. 2017, 12, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, G.K.; Ekim, M.; Bakkaloğlu, S.A.; Coşkun, S.; Delibaş, A.; Conkar, S.; Yılmaz, D.; Kara, A.; Saygılı, S.K.; Büyükkaragöz, B.; et al. Evaluation of non-infectious complications of peritoneal dialysis in children: A multicenter study. Pediatr. Nephrol. 2021, 36, 417–423. [Google Scholar] [CrossRef]
- McCormick, B.B.; Bargman, J.M. Noninfectious complications of peritoneal dialysis: Implications for patient and technique survival. J. Am. Soc. Nephrol. 2007, 18, 3023–3055. [Google Scholar] [CrossRef]
- Vidal, E.; van Stralen, K.J.; Chesnaye, N.C.; Bonthuis, M.; Holmberg, C.; Zurowska, A.; Trivelli, A.; Da Silva, J.E.E.; Herthelius, M.; Adams, B.; et al. Infants Requiring Maintenance Dialysis: Outcomes of Hemodialysis and Peritoneal Dialysis. Am. J. Kidney Dis. 2017, 69, 617–625. [Google Scholar] [CrossRef]
- Parolin, M.; Ceschia, G.; Bertazza Partigiani, N.; La Porta, E.; Verrina, E.; Vidal, E. Non-infectious complications of peritoneal dialysis in children. Pediatr. Dial. 2012, 40, 257–271. [Google Scholar] [CrossRef]
- Bargman, J.M. Noninfectious complications of peritoneal dialysis. In Nolph and Gokal’s Textbook of Peritoneal Dialysis; Springer: Berlin/Heidelberg, Germany, 2009; pp. 571–609. [Google Scholar]
- Imani, P.D.; Carpenter, J.L.; Bell, C.S.; Brandt, M.L.; Braun, M.C.; Swartz, S.J. Peritoneal dialysis catheter outcomes in infants initiating peritoneal dialysis for end-stage renal disease. BMC Nephrol. 2018, 19, 231. [Google Scholar] [CrossRef]
- LaPlant, M.B.; Saltzman, D.A.; Segura, B.J.; Acton, R.D.; Feltis, B.A.; Hess, D.J. Peritoneal dialysis catheter placement, outcomes and complications. Pediatr. Surg. Int. 2018, 34, 1239–1244. [Google Scholar] [CrossRef]
- Fraser, N.; Hussain, F.K.; Connell, R.; Shenoy, M.U. Chronic peritoneal dialysis in children. Int. J. Nephrol. Renov. Dis. 2015, 8, 125–137. [Google Scholar] [CrossRef]
- Hughes, C.R.; Angotti, D.M.; Jubelirer, R.A. Laparoscopic repositioning of a continuous ambulatory peritoneal dialysis (CAPD) catheter. Surg. Endosc. 1994, 8, 1108–1109. [Google Scholar] [CrossRef]
- Madenci, A.L.; Thiagarajan, R.R.; Stoffan, A.P.; Emani, S.M.; Rajagopal, S.K.; Weldon, C.B. Characterizing peritoneal dialysis catheter use in pediatric patients after cardiac surgery. J. Thorac. Cardiovasc. Surg. 2013, 146, 334–338. [Google Scholar] [CrossRef]
- Steinau, G.; Kaussen, T.; Bolten, B.; Schachtrupp, A.; Neumann, U.P.; Conze, J.; Boehm, G. Abdominal compartment syndrome in childhood: Diagnostics, therapy and survival rate. Pediatr. Surg. Int. 2011, 27, 399–405. [Google Scholar] [CrossRef]
- di Natale, A.; Moehrlen, U.; Neeser, H.R.; Zweifel, N.; Meuli, M.; Mauracher, A.A.; Brotschi, B.; Tharakan, S.J. Abdominal compartment syndrome and decompressive laparotomy in children: A 9-year single-center experience. Pediatr. Surg. Int. 2020, 36, 513–521. [Google Scholar] [CrossRef]
- Aksoy, O.Y.; Gulaldi, N.C.; Bayrakci, U.S. Hydrothorax in a pediatric patient on peritoneal dialysis: Answers. Pediatr. Nephrol. 2020, 35, 1421–1423. [Google Scholar] [CrossRef]
- Yang, S.-F.; Liu, C.-J.; Yang, W.-C.; Chang, C.-F.; Yang, C.-Y.; Li, S.-Y.; Lin, C.-C. The risk factors and the impact of hernia development on technique survival in peritoneal dialysis patients: A population-based cohort study. Perit. Dial. Int. 2015, 35, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Nomoto, Y.; Suga, T.; Nakajima, K.; Sakai, H.; Osawa, G.; Ota, K.; Kawaguchi, Y.; Sakai, T.; Sakai, S.; Shibat, M.; et al. Acute hydrothorax in continuous ambulatory peritoneal dialysis—A collaborative study of 161 centers. Am. J. Nephrol. 1989, 9, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Szeto, C.C.; Chow, K.M. Pathogenesis and management of hydrothorax complicating peritoneal dialysis. Curr. Opin. Pulm. Med. 2004, 10, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Chow, K.M.; Szeto, C.C.; Wong, T.Y.; Li, P.K. Hydrothorax complicating peritoneal dialysis: Diagnostic value of glucose concentration in pleural fluid aspirate. Perit. Dial. Int. 2002, 22, 525–528. [Google Scholar] [CrossRef]
- Mohamad Alahmad, M.A.; Kasmani, R. Sweet hydrothorax: A common presentation of a rare condition. Avicenna J. Med. 2019, 9, 111–114. [Google Scholar] [CrossRef]
- Momenin, N.; Colletti, P.M.; Kaptein, E.M. Low pleural fluid-to-serum glucose gradient indicates pleuroperitoneal communication in peritoneal dialysis patients: Presentation of two cases and a review of the literature. Nephrol. Dial. Transplant. 2012, 27, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Bohra, N.; Sullivan, A.; Chaudhary, H.; Demko, T. The Use of Pleural Fluid to Serum Glucose Ratio in Establishing the Diagnosis of a Not So Sweet PD-Related Hydrothorax: Case Report and Literature Review. Case Rep. Nephrol. 2020, 2020, 8811288. [Google Scholar] [CrossRef] [PubMed]
- Harry, L.; Nyakale, N.; Tinarwo, P. Scintigraphic peritoneography in the diagnosis of pleuroperitoneal leak complicating peritoneal dialysis: A comparison with conventional diagnostic methods. Medicine 2020, 99, e21029. [Google Scholar] [CrossRef]
- Lizaola, B.; Bonder, A.; Trivedi, H.D.; Tapper, E.B.; Cardenas, A. Review article: The diagnostic approach and current management of chylous ascites. Aliment. Pharmacol. Ther. 2017, 46, 816–824. [Google Scholar] [CrossRef]
- Wu, X.; Vega, M.; Swartz, S.J.; Michael, M. Milky appearance of peritoneal fluid in a neonate on peritoneal dialysis due to end-stage renal disease: Answers. Pediatr. Nephrol. 2018, 33, 73–76. [Google Scholar] [CrossRef]
- Kumar, J.; Gordillo, R.; Del Rio, M.; Flynn, J.T. Recurrent chyloperitoneum: A rare complication of peritoneal dialysis. Pediatr. Nephrol. 2008, 23, 671–674. [Google Scholar] [CrossRef]
- Tsai, M.K.; Lai, C.H.; Chen, L.M.; Jong, G.P. Calcium Channel Blocker-Related Chylous Ascites: A Systematic Review and Meta-Analysis. J. Clin. Med. 2019, 8, 466. [Google Scholar] [CrossRef]
- Kim, S.; Yu, Y.M.; Kwon, J.; Yoo, H.; Jung, S.H.; Lee, E. Calcium Channel Blocker-Associated Chyloperitoneum in Patients Receiving Peritoneal Dialysis: A Systematic Review. Int. J. Environ. Res. Public Health 2019, 16, 1333. [Google Scholar] [CrossRef]
- Basualdo, J.E.; Rosado, I.A.; Morales, M.I.; Fernández-Ros, N.; Huerta, A.; Alegre, F.; Landecho, M.F.; Lucena, J.F. Lercanidipine-induced chylous ascites: Case report and literature review. J. Clin. Pharm. Ther. 2017, 42, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Park, S.J.; Oh, J.Y.; Kim, J.H.; Lee, J.S.; Kim, P.K.; Shin, J.I. Noninfectious Complications of Peritoneal Dialysis in Korean Children: A 26-Year Single-Center Study. Yonsei Med. J. 2015, 56, 1359–1364. [Google Scholar] [CrossRef] [PubMed]
- Bonthuis, M.; Harambat, J.; Jager, K.J.; Vidal, E. Growth in children on kidney replacement therapy: A review of data from patient registries. Pediatr. Nephrol. 2021, 36, 2563–2574. [Google Scholar] [CrossRef] [PubMed]
- Laakkonen, H.; Happonen, J.-M.; Marttinen, E.; Paganus, A.; Hölttä, T.; Holmberg, C.; Rönnholm, K. Normal growth and intravascular volume status with good metabolic control during peritoneal dialysis in infancy. Pediatr. Nephrol. 2010, 25, 1529–1538. [Google Scholar] [CrossRef][Green Version]



| Laboratory Data | Pleural Effusion | Dialysate Fluid | Serum |
|---|---|---|---|
| Protein (g/L) | 4 | 2 | 47 |
| Glucose (mmol/L) | 7.493 | 25.364 | 7.604 |
| Laboratory Data | Dialysate Fluid | Serum |
|---|---|---|
| Triglyceride (mmol/L) | 4.396 | 3.277 |
| Cholesterol (mmol/L) | 0.311 | 5.569 |
| Albumin (g/L) | 2 | 29 |
| Glucose (mmol/L) | 7.493 | 5.717 |
| Protein (g/L) | 6 | 52 |
| Lactate dehydrogenase (µkat/L) | 0.433 | 3.9 |
| WBC (109/L) | 0.002 | N/A |
| RBC (1012/L) | 0.000027 | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, C.-T.; Tang, Y.-H.; Wang, H.-H.; Lee, Y.-S.; Liu, C.-S.; Tsao, P.-C.; Guo, M.-C.; Chen, H.-L.; Lin, C.-H. Life-Threatening Noninfectious Complications of Peritoneal Dialysis in an Infant with End-Stage Kidney Disease. Pediatr. Rep. 2025, 17, 100. https://doi.org/10.3390/pediatric17050100
Teng C-T, Tang Y-H, Wang H-H, Lee Y-S, Liu C-S, Tsao P-C, Guo M-C, Chen H-L, Lin C-H. Life-Threatening Noninfectious Complications of Peritoneal Dialysis in an Infant with End-Stage Kidney Disease. Pediatric Reports. 2025; 17(5):100. https://doi.org/10.3390/pediatric17050100
Chicago/Turabian StyleTeng, Chao-Ting, Yi-Hsuan Tang, Hsin-Hui Wang, Yu-Sheng Lee, Chin-Su Liu, Pei-Chen Tsao, Meei-Chyi Guo, Hui-Lan Chen, and Chien-Hung Lin. 2025. "Life-Threatening Noninfectious Complications of Peritoneal Dialysis in an Infant with End-Stage Kidney Disease" Pediatric Reports 17, no. 5: 100. https://doi.org/10.3390/pediatric17050100
APA StyleTeng, C.-T., Tang, Y.-H., Wang, H.-H., Lee, Y.-S., Liu, C.-S., Tsao, P.-C., Guo, M.-C., Chen, H.-L., & Lin, C.-H. (2025). Life-Threatening Noninfectious Complications of Peritoneal Dialysis in an Infant with End-Stage Kidney Disease. Pediatric Reports, 17(5), 100. https://doi.org/10.3390/pediatric17050100






