Association between Thyroid Function and Respiratory Distress Syndrome in Preterm Infants
Abstract
1. Introduction
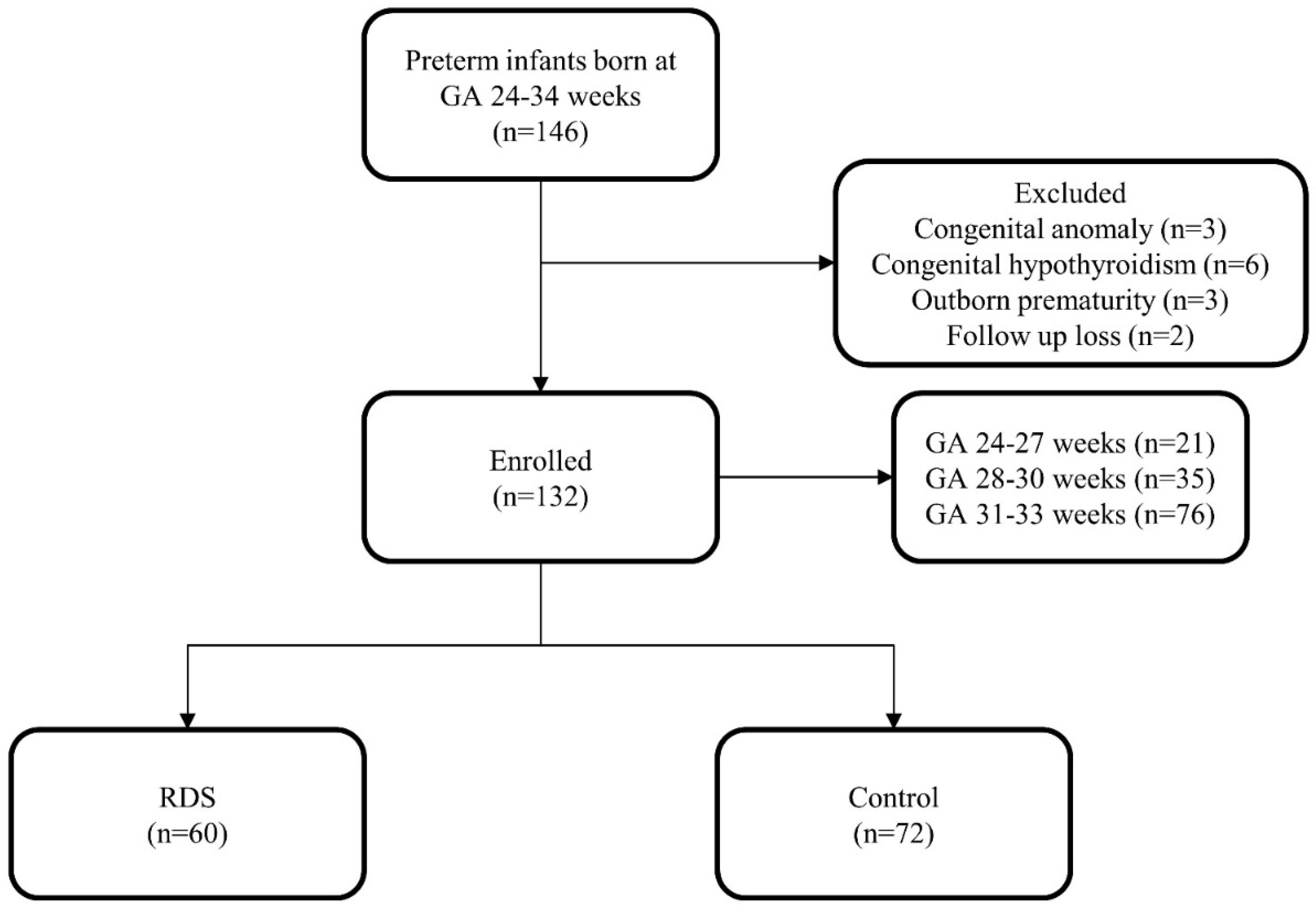
2. Materials and Methods
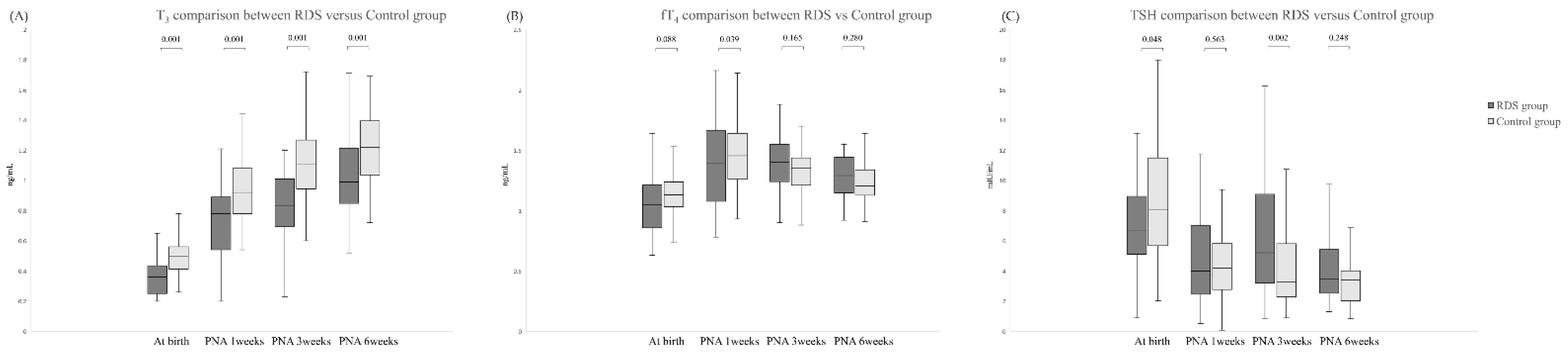
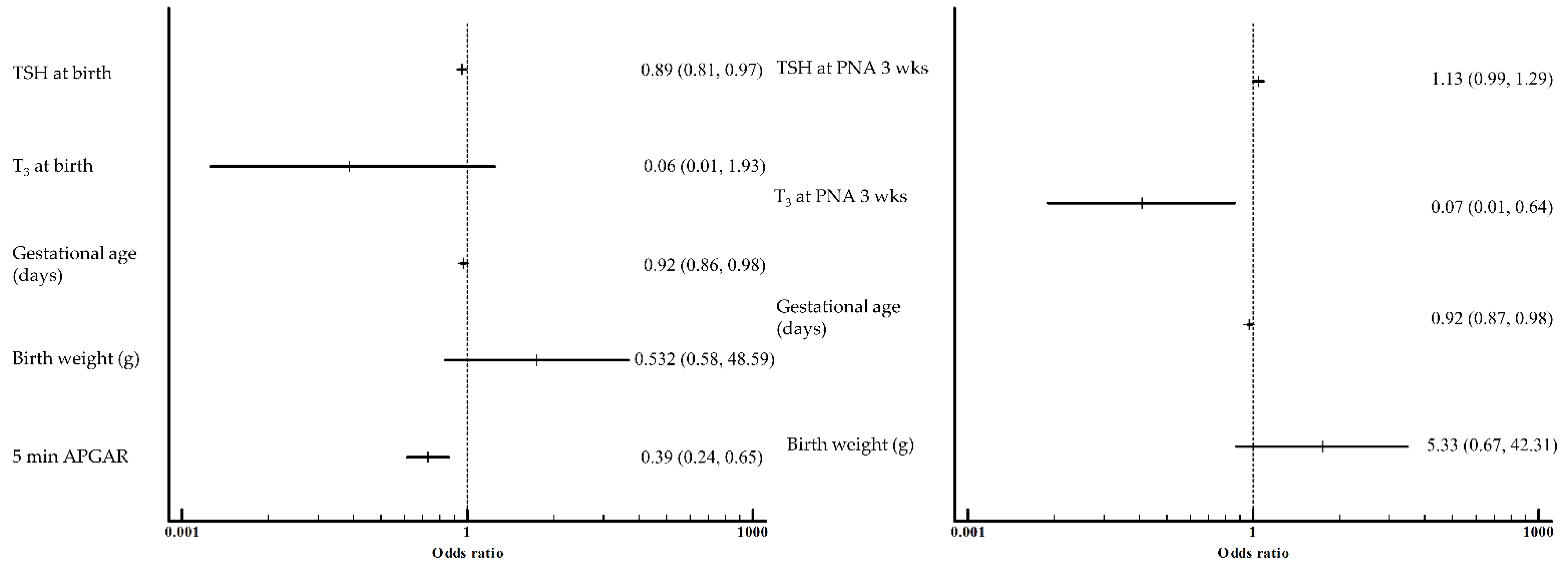
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carnielli, V.P.; Cogo, P.E. Surfactant metabolism in neonatal lung diseases. In Neonatology; Springer: Milano, Italy, 2012; pp. 433–440. [Google Scholar]
- Kowlessar, N.M.; Jiang, H.J.; Steiner, C. Hospital Stays for Newborns, 2011: Statistical Brief #163. In Healthcare Cost and Utilization Project (HCUP) Statistical Briefs; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2013. [Google Scholar]
- Altman, M.; Vanpée, M.; Cnattingius, S.; Norman, M. Risk factors for acute respiratory morbidity in moderately preterm infants. Paediatr. Perinat. Epidemiol. 2013, 27, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Ballard, P.L.; Hawgood, S.; Liley, H.; Wellenstein, G.; Gonzales, L.W.; Benson, B.; Cordell, B.; White, R.T. Regulation of pulmonary surfactant apoprotein SP 28–36 gene in fetal human lung. Proc. Natl. Acad. Sci. USA 1986, 83, 9527–9531. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, C.R.; Boggaram, V. Hormonal control of the surfactant system in fetal lung. Annu. Rev. Physiol. 1991, 53, 415–440. [Google Scholar] [CrossRef] [PubMed]
- Ballard, P.L.; Hovey, M.L.; Gonzales, L.K. Thyroid hormone stimulation of phosphatidylcholine synthesis in cultured fetal rabbit lung. J. Clin. Investig. 1984, 74, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Gunes, T.; Koklu, E.; Ozturk, M.A.; Koklu, S.; Cetin, N. Evaluation of serum cortisol levels in a relatively large and mature group of ventilated and nonventilated preterm infants with respiratory distress syndrome. Am. J. Perinatol. 2006, 23, 335–339. [Google Scholar] [CrossRef]
- Warburton, D.; Parton, L.; Buckley, S.; Cosico, L.; Enns, G.; Saluna, T. Combined effects of corticosteroid, thyroid hormones, and β-agonist on surfactant, pulmonary mechanics, and β-receptor binding in fetal lamb lung. Pediatr. Res. 1988, 24, 166–170. [Google Scholar] [CrossRef]
- Group, A.S. Australian collaborative trial of antenatal thyrotropin-releasing hormone (ACTOBAT) for prevention of neonatal respiratory disease. Lancet 1995, 345, 877–882. [Google Scholar] [CrossRef]
- Mohammad, T.; Fariba, G.; Susan, A.; Mohammad, A.; Shahla, A.; Zohreh, K.; Fatemeh, B.; Amin, S. Thyroid function test in pre-term neonates during the first five weeks of life. Int. J. Prev. Med. 2013, 4, 1271–1276. [Google Scholar]
- Ryckman, K.K.; Spracklen, C.N.; Dagle, J.M.; Murray, J.C. Maternal factors and complications of preterm birth associated with neonatal thyroid stimulating hormone. J. Pediatr. Endocrinol. Metab. 2014, 27, 929–938. [Google Scholar] [CrossRef]
- Tanaka, K.; Shimizu, T.; Hosaka, A.; Tokita, A.; Shiga, S.; Yamashiro, Y. Serum free T4 and thyroid stimulating hormone levels in preterm infants and relationship between these levels and respiratory distress syndrome. Pediatr. Int. 2007, 49, 447–451. [Google Scholar] [CrossRef]
- Jobe, A.H.; Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2001, 163, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.J.; Ternberg, J.L.; Feigin, R.D.; Keating, J.P.; Marshall, R.; Barton, L.; Brotherton, T. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann. Surg. 1978, 187, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.; Fagman, H. Development of the thyroid gland. Development 2017, 144, 2123–2140. [Google Scholar] [CrossRef] [PubMed]
- Fisher, D.A.; Polk, D.H. Development of the thyroid. Baillière’s Clin. Endocrinol. Metab. 1989, 3, 627–657. [Google Scholar] [CrossRef]
- Kim, H.-R.; Jung, Y.H.; Choi, C.W.; Chung, H.R.; Kang, M.-J.; Kim, B.I. Thyroid dysfunction in preterm infants born before 32 gestational weeks. BMC Pediatr. 2019, 19, 391. [Google Scholar] [CrossRef]
- LaFranchi, S.H. Thyroid function in preterm/low birth weight infants: Impact on diagnosis and management of thyroid dysfunction. Front. Endocrinol. 2021, 12, 371. [Google Scholar] [CrossRef]
- Armanian, A.-M.; Hashemipour, M.; Esnaashari, A.; Kelishadi, R.; Farajzadegan, Z. Influence of perinatal factors on thyroid stimulating hormone level in cord blood. Adv. Biomed. Res. 2013, 2, 48. [Google Scholar]
- Poyekar, S.; Pratinidhi, S.; Prasad, S.S.; Sardar, Z.S.; Kankariya, B.; Bhole, O. Cord blood thyroid stimulating hormone level-and the influence of perinatal and other factors on it. Dysphagia 2019, 16, 79–82. [Google Scholar]
- Post, M.; Batenburg, J.; Van Golde, L. Effects of cortisol and thyroxine on phosphatidylcholine and phosphatidylglycerol synthesis by adult rat lung alveolar type II cells in primary culture. Biochim. Biophys. Acta 1980, 618, 308–317. [Google Scholar] [CrossRef]
- Gross, I.; Wilson, C.M.; Ingleson, L.D.; Brehier, A.; Rooney, S.A. Fetal lung in organ culture. III. Comparison of dexamethasone, thyroxine, and methylxanthines. J. Appl. Physiol. 1980, 48, 872–877. [Google Scholar] [CrossRef]
- Wu, B.; Kikkawa, Y.; Orzalesi, M.; Motoyama, E.; Kaibara, M.; Zigas, C.; Cook, C. The effect of thyroxine on the maturation of fetal rabbit lungs. Neonatology 1973, 22, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, M.A.; El Gendy, F.M.; Zidan, A.A. Free thyroxine and thyroid-stimulating hormone in preterm neonates with respiratory distress syndrome. Menoufia. Med. J. 2020, 33, 878. [Google Scholar]
- Ataoglu, E.; Cebeci, B.; Oguz, D.; Kurnaz, D.; Buyukkayhan, D. The assessment of thyroid hormone levels in term and preterm infants diagnosed with transient tachypnea of the newborn: A cross-sectional study. Med. Bull. Haseki. 2021, 59, 216–220. [Google Scholar] [CrossRef]
- Crowther, C.A.; Hiller, J.E.; Haslam, R.R.; Robinson, J.S.; Group, A.S. Australian collaborative trial of antenatal thyrotropin-releasing hormone: Adverse effects at 12-month follow-up. Pediatrics 1997, 99, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Braems, G. Fetal hypoxemia on a molecular level: Adaptive changes in the hypothalamic–pituitary–adrenal (HPA) axis and the lungs. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 110, S63–S69. [Google Scholar] [CrossRef]
- Chu, A.; Rooney, S. Estrogen stimulation of surfactant synthesis. Pediatr. Pulmonol. 1985, 1 (Suppl. 3), S110–S114. [Google Scholar]
- Engle, M.J.; Langan, S.M.; Sanders, R.L. The effects of insulin and hyperglycemia on surfactant phospholipid synthesis in organotypic cultures of type II pneumocytes. Biochim. Biophys. Acta 1983, 753, 6–13. [Google Scholar] [CrossRef]
| Variables | Total (n = 132) | RDS (n = 60) | Control (n = 72) | p Value |
|---|---|---|---|---|
| Gestational age (weeks) | 30.4 ± 2.5 | 28.9 ± 2.6 | 31.6 ± 1.6 | <0.05 |
| Birth weight (gram) | 1527.2 ± 450.5 | 1328.3 ± 503.1 | 1692.9 ± 320.8 | <0.05 |
| C-section delivery (%) | 102 (77.3) | 49 (81.7) | 53 (73.6) | 0.303 |
| Labor pain (%) | 93 (73.8) | 43 (71.7) | 54 (75) | 0.696 |
| Male (%) | 72 (54.5) | 33 (55.0) | 39 (54.2) | 1 |
| Multiple gestation (%) | 31 (23) | 11 (18.3) | 20 (27.8) | 0.055 |
| Small for gestational age (%) | 4 (3) | 1 (1.7) | 3 (4.2) | 0.63 |
| APGAR at 5 min | 7.8 ± 1.6 | 6.9 ± 1.6 | 8.5 ± 1.1 | <0.05 |
| Maternal age (years) | 32.7 ± 5.7 | 32.6 ± 6.7 | 32.8 ± 4.8 | 0.789 |
| GDM (%) | 19 (14.4) | 7 (11.7) | 12 (16.7) | 0.464 |
| Preeclampsia (%) | 29 (22) | 16 (26.7) | 13 (18.1) | 0.292 |
| Pathological chorioamnionitis (%) | 53 (44.9) | 20 (37) | 33 (51.6) | 0.139 |
| Maternal thyroid disease (%) | 6 (4.5) | 4 (6.7) | 2 (2.8) | 0.41 |
| Antenatal steroids (%) | 69 (52.7) | 33 (55.5) | 36 (50.7) | 0.296 |
| Variables | RDS (n = 60) | Control (n = 72) | p Value |
|---|---|---|---|
| BPD | 28 (50.0) | 6 (8.6) | <0.001 |
| PDA (Treated) | 15 (26.8) | 2 (2.9) | <0.001 |
| Pulmonary hypertension | 5 (8.9) | 1 (1.4) | 0.061 |
| NEC | 9 (16.1) | 1 (1.4) | 0.030 |
| PVL | 1 (1.8) | 1 (1.4) | 0.693 |
| IVH | 0 (0.0) | 1 (1.4) | 0.556 |
| ROP with PRP | 5 (8.9) | 1 (1.4) | 0.061 |
| Death | 2 (3.6) | 0 (0.0) | 0.196 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Kim, Y.; Chang, M.; Lee, B. Association between Thyroid Function and Respiratory Distress Syndrome in Preterm Infants. Pediatr. Rep. 2022, 14, 497-504. https://doi.org/10.3390/pediatric14040058
Kim Y, Kim Y, Chang M, Lee B. Association between Thyroid Function and Respiratory Distress Syndrome in Preterm Infants. Pediatric Reports. 2022; 14(4):497-504. https://doi.org/10.3390/pediatric14040058
Chicago/Turabian StyleKim, Yonghyuk, Youngjin Kim, Meayoung Chang, and Byoungkook Lee. 2022. "Association between Thyroid Function and Respiratory Distress Syndrome in Preterm Infants" Pediatric Reports 14, no. 4: 497-504. https://doi.org/10.3390/pediatric14040058
APA StyleKim, Y., Kim, Y., Chang, M., & Lee, B. (2022). Association between Thyroid Function and Respiratory Distress Syndrome in Preterm Infants. Pediatric Reports, 14(4), 497-504. https://doi.org/10.3390/pediatric14040058







