Unilateral Transient Enhanced SEP during Integrated Multiparameter Neurophysiological Monitoring in a Newborn with Symptomatic Seizure
Abstract
:1. Introduction
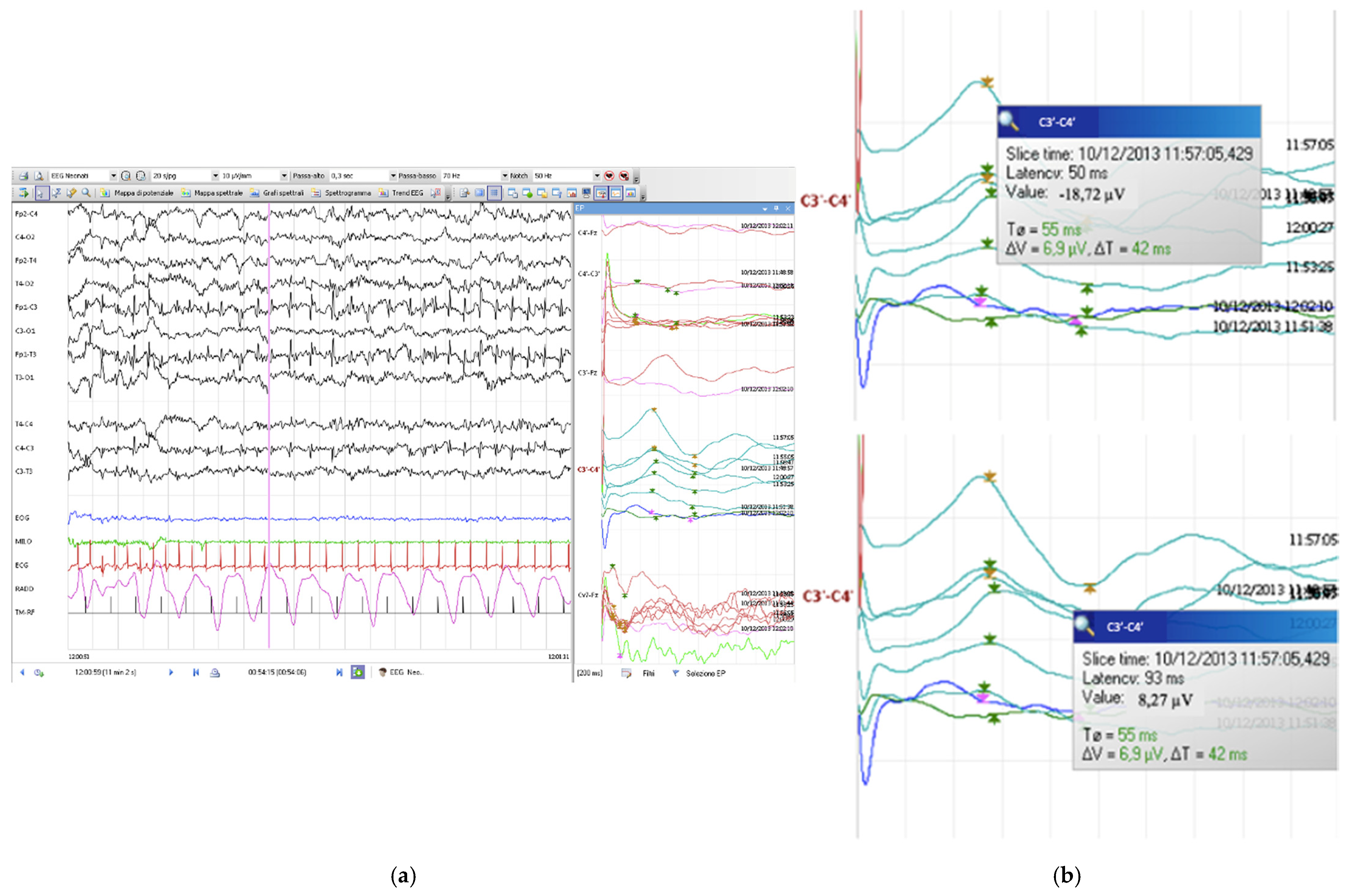
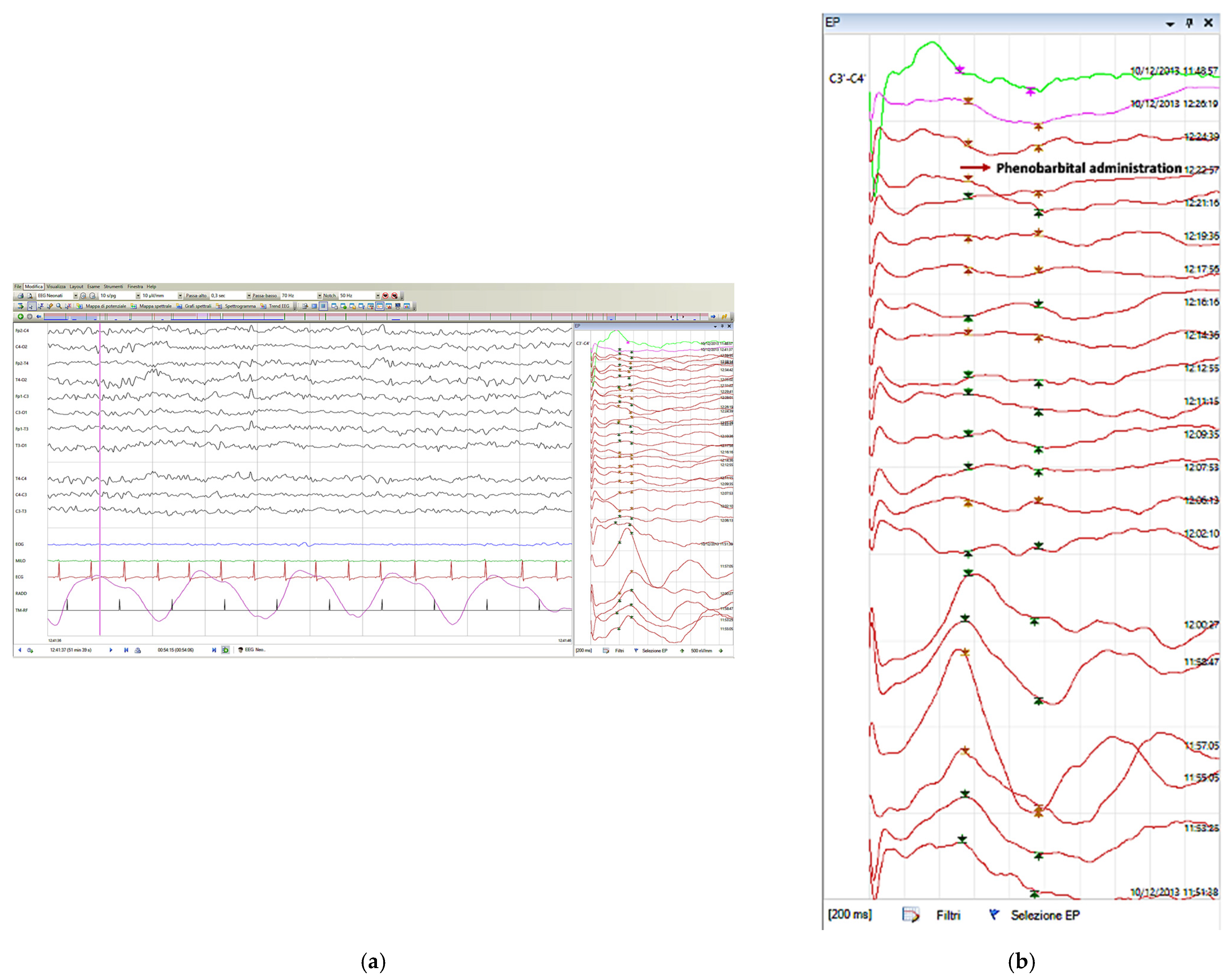
2. Case Description
Neurophysiological Assessments
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lori, S.; Gabbanini, S.; Bastianelli, M.; Bertini, G.; Corsini, I.; Dani, C. Multimodal neurophysiological monitoring in healthy infants born at term: Normative continuous somatosensory evoked potentials data. Dev. Med. Child Neurol. 2017, 59, 959–964. [Google Scholar] [CrossRef] [Green Version]
- Lori, S.; Bertini, G.; Molesti, E.; Gualandi, D.; Gabbanini, S.; Bastianelli, M.E.; Pinto, F.; Dani, C. The prognostic role of evoked potentials in neonatal hypoxic-ischemic insult. J. Matern. Fatal Neonatal. Med. 2011, 24 (Suppl. S1), 69–71. [Google Scholar] [CrossRef]
- Trollmann, R.; Nüsken, E.; Wenzel, D. Neonatal € somatosensory evoked potentials: Maturational aspects and prognostic value. Pediatr. Neurol. 2010, 42, 427–433. [Google Scholar] [CrossRef]
- Garfinkle, J.; Sant’Anna, G.M.; Rosenblatt, B.; Majnemer, A.; Wintermark, P.; Shevell, M.I. Somatosensory evoked potentials in neonates with hypoxic-ischemic encephalopathy treated with hypothermia. Eur. J. Paediatr. Neurol. 2015, 19, 423–428. [Google Scholar] [CrossRef]
- Muzyka, I.M.; Estephan, B. Somatosensory evoked potentials. Handb. Clin. Neurol. 2019, 160, 523–540. [Google Scholar] [CrossRef]
- George, S.R.; Taylor, M.J. Somatosensory evoked potentials in neonates and infants: Developmental and normative data. Electroencephalogr. Clin. Neurophysiol. Evoked Potentials Sect. 1991, 80, 94–102. [Google Scholar] [CrossRef]
- Dawson, G.D. Investigations on a patient subject to myoclonic seizures after sensory stimulation. J. Neurol. Neurosurg. Psychiatry 1947, 10, 141–162. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, B.; Thun-Hohenstein, L.; Molinari, L.; Superti-Furga, A.; Boltshauser, E. Somatosensory evoked potentials with high cortical amplitudes: Clinical data in 31 children. Neuropediatrics 1994, 25, 78–84. [Google Scholar] [CrossRef]
- Shibasaki, H.; Yamashita, Y.; Neshige, R.; Tobimatsu, S.; Fukui, R. Pathogenesis of giant somatosensory evoked potentials in progressive myoclonic epilepsy. Brain 1985, 108 Pt 1, 225–240. [Google Scholar] [CrossRef]
- Ebner, A.; Deuschl, G. Frontal and parietal components of enhanced somatosensory evoked potentials: A comparison between pathological and pharmacologically induced conditions. Electroencephalogr. Clin. Neurophysiol. Evoked Potentials Sect. 1988, 71, 170–179. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Kaga, M.; Suzuki, H.; Sakuragawa, N.; Arima, M. Giant somatosensory evoked potentials in the Rett syndrome. Brain Dev. 1991, 13, 36–39. [Google Scholar] [CrossRef]
- Farnarier, G.; Regis, H.; Roger, J. Potentiels evoques somesthesiques et myoclonus d’action. Rev. D’electroencéphalographie Neurophysiol. Clin. 1985, 15, 37–43. [Google Scholar] [CrossRef]
- Mauguière, F.; Allison, T.; Babiloni, C.; Buchner, H.; Eisen, A.A.; Goodin, D.S.; Jones, S.J.; Kakigi, R.; Matsuoka, S.; Nuwer, M.; et al. Somatosensory evoked potentials. The International Federation of Clinical Neurophysiology. Electroencephalogr. Clin. Neurophysiol. Suppl. 1999, 52, 79–90. [Google Scholar]
- Dawson, G.D. The relation between the electroencephalogram and muscle action potentials in certain convulsive states. J. Neurol. Neurosurg. Psychiatry 1946, 9, 5–22. [Google Scholar] [CrossRef] [Green Version]
- Halliday, A.M. The electrophysiological study of myoclonus in man. Brain 1967, 90, 241–284. [Google Scholar] [CrossRef]
- Hallett, M.; Chadwick, D.; Marsden, C.D. Cortical reflex myoclonus. Neurology 1979, 29, 1107–1125. [Google Scholar] [CrossRef]
- Kofler, M.; Müller, J.; Reggiani, L.; Wenning, G.K. Somatosensory evoked potentials in progressive supranuclear palsy. J. Neurol. Sci. 2000, 179, 85–91. [Google Scholar] [CrossRef]
- Miwa, H.; Mizuno, Y. Enlargements of somatosensory-evoked potentials in progressive supranuclear palsy. Acta Neurol. Scand. 2002, 106, 209–212. [Google Scholar] [CrossRef]
- Kochs, E.; Treede, R.D.; Schulte Amesch, J. Vergrösserung somatosensorisch evozierter Potentiale während Narkoseeinleitung mit Etomidat [Increase in somatosensory evoked potentials during anesthesia induction with etomidate]. Anaesthesist 1986, 35, 359–364. (In German) [Google Scholar] [CrossRef]
- MacDonald, D.B.; Dong, C.; Quatrale, R.; Sala, F.; Skinner, S.; Soto, F.; Szelényi, A. Recommendations of the International Society of Intraoperative Neurophysiology for intraoperative somatosensory evoked potentials. Clin. Neurophysiol. 2019, 130, 161–179. [Google Scholar] [CrossRef]
- Schorl, M. Giant somatosensory evoked potentials as indicator of nonconvulsive status epilepticus. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2008, 119, 726–728. [Google Scholar] [CrossRef]
- Pacifici, G.M. Clinical Pharmacology of Phenobarbital in Neonates: Effects, Metabolism and Pharmacokinetics. Curr. Pediatr. Rev. 2016, 12, 48–54. [Google Scholar] [CrossRef]
- Gehin, P.; Huttin, B.; Brichet, B.; Weber, M. Signification des potentiels évoqués somesthésiques (PES) d’amplitude anormalement élevée [Significance of abnormally high somatosensory evoked potentials (SEV)]. Rev. D’électroencéphalographie Neurophysiol. Clin. 1985, 15, 155–161. (In French) [Google Scholar] [CrossRef]
- Báez-Martín, M.M.; Morales-Chacón, L.; Gómez-Fernández, L.; Cabrera-Abreu, I.; Álvarez, L.; Araujo, F. Potenciales evocados gigantes [Giant evoked potentials]. Rev. Neurol. 2001, 33, 1120–1125. (In Spanish) [Google Scholar]
- Karhu, J.; Hari, R.; Paetau, R.; Kajola, M.; Mervaala, E. Cortical reactivity in progressive myoclonus epilepsy. Electroencephalogr. Clin. Neurophysiol. 1994, 90, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Visani, E.; Canafoglia, L.; Sebastiano, D.R.; Agazzi, P.; Panzica, F.; Scaioli, V.; Ciano, C.; Franceschetti, S. Giant SEPs and SEP-recovery function in Unverricht-Lundborg disease. Clin. Neurophysiol. 2013, 124, 1013–1018. [Google Scholar] [CrossRef]
- Valeriani, M.; Restuccia, D.; Di Lazzaro, V.; Le Pera, D.; Tonali, P. The pathophysiology of giant SEPs in cortical myoclonus: A scalp topography and dipolar source modelling study. Electroencephalogr. Clin. Neurophysiol. Evoked Potentials Sect. 1997, 104, 122–131. [Google Scholar] [CrossRef]
- Ikeda, A.; Shibasaki, H.; Nagamine, T.; Xu, X.; Terada, K.; Mima, T.; Kaji, R.; Kawai, I.; Tatsuoka, Y.; Kimura, J. Peri-rolandic and fronto-parietal components of scalp-recorded giant SEPs in cortical myoclonus. Electroencephalogr. Clin. Neurophysiol. Evoked Potentials Sect. 1995, 96, 300–309, Erratum in Electroencephalogr. Clin. Neurophysiol. 1995, 96, 484. [Google Scholar] [CrossRef]
- Desmedt, J.E.; Bourguet, M. Color imaging of parietal and frontal somatosensory potential fields evoked by stimulation of median or posterior tibial nerve in man. Electroencephalogr. Clin. Neurophysiol. Evoked Potentials Sect. 1985, 62, 1–17. [Google Scholar] [CrossRef]
- Ragazzoni, A.; Ferri, R.; Di Russo, F.; Del Gracco, S.; Barcaro, U.; Navona, C. Giant somatosensory evoked potentials in different clinical conditions: Scalp topography and dipole source analysis. Electroencephalogr. Clin. Neurophysiol. Suppl. 1999, 49, 81–89. [Google Scholar]
- Kwon, S.H.; Scheinost, D.; Lacadie, C.; Benjamin, J.; Myers, E.H.; Qiu, M.; Schneider, K.C.; Rothman, U.L.; Constable, R.; Ment, L.R. GABA, resting-state connectivity and the developing brain. Neonatology 2014, 106, 149–155. [Google Scholar] [CrossRef] [Green Version]
- Demarque, M.; Represa, A.; Becq, H.; Khalilov, I.; Ben-Ari, Y.; Aniksztejn, L. Paracrine Intercellular Communication by a Ca2+- and SNARE-Independent Release of GABA and Glutamate Prior to Synapse Formation. Neuron 2002, 36, 1051–1061. [Google Scholar] [CrossRef] [Green Version]
- McDonald, J.W.; Johnston, M.V. Pharmacology of N-methyl-D-aspartate-induced brain injury in an in vivo perinatal rat model. Synapse 1990, 6, 179–188. [Google Scholar] [CrossRef]
- Luján, R.; Shigemoto, R.; López-Bendito, G. Glutamate and GABA receptor signalling in the developing brain. Neuroscience 2005, 130, 567–580. [Google Scholar] [CrossRef]
- Kaindl, A.M.; Ikonomidou, C. Glutamate antagonists are neurotoxins for the developing brain. Neurotox. Res. 2007, 11, 203–218. [Google Scholar] [CrossRef]
- Desfeux, A.; El Ghazi, F.; Jégou, S.; Legros, H.; Marret, S.; Laudenbach, V.; Gonzalez, B.J. Dual Effect of Glutamate on GABAergic Interneuron Survival during Cerebral Cortex Development in Mice Neonates. Cereb. Cortex 2010, 20, 1092–1108. [Google Scholar] [CrossRef] [Green Version]
- Liguz-Lecznar, M.; Lehner, M.; Kaliszewska, A.; Zakrzewska, R.; Sobolewska, A.; Kossut, M. Altered glutamate/GABA equilibrium in aged mice cortex influences cortical plasticity. Brain Struct. Funct. 2015, 220, 1681–1693. [Google Scholar] [CrossRef]
- Basu, S.K.; Pradhan, S.; du Plessis, A.J.; Ben-Ari, Y.; Limperopoulos, C. GABA and glutamate in the preterm neonatal brain: In-vivo measurement by magnetic resonance spectroscopy. NeuroImage 2021, 238, 118215. [Google Scholar] [CrossRef]
- Kilb, W. Development of the GABAergic System from Birth to Adolescence. Neuroscientist 2012, 18, 613–630. [Google Scholar] [CrossRef]
- Cancedda, L.; Fiumelli, H.; Chen, K.; Poo, M.-M. Excitatory GABA Action Is Essential for Morphological Maturation of Cortical Neurons In Vivo. J. Neurosci. 2007, 27, 5224–5235. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.D.; Kriegstein, A.R. GABA Regulates Excitatory Synapse Formation in the Neocortex via NMDA Receptor Activation. J. Neurosci. 2008, 28, 5547–5558. [Google Scholar] [CrossRef]
- Sipilä, S.T.; Huttu, K.; Soltesz, I.; Voipio, J.; Kaila, K. Depolarizing GABA Acts on Intrinsically Bursting Pyramidal Neurons to Drive Giant Depolarizing Potentials in the Immature Hippocampus. J. Neurosci. 2005, 25, 5280–5289. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavaliere, S.; Lori, S.; Bastianelli, M.; Cossu, C.; Gabbanini, S.; Dani, C.; Bertini, G. Unilateral Transient Enhanced SEP during Integrated Multiparameter Neurophysiological Monitoring in a Newborn with Symptomatic Seizure. Pediatr. Rep. 2022, 14, 254-261. https://doi.org/10.3390/pediatric14020033
Cavaliere S, Lori S, Bastianelli M, Cossu C, Gabbanini S, Dani C, Bertini G. Unilateral Transient Enhanced SEP during Integrated Multiparameter Neurophysiological Monitoring in a Newborn with Symptomatic Seizure. Pediatric Reports. 2022; 14(2):254-261. https://doi.org/10.3390/pediatric14020033
Chicago/Turabian StyleCavaliere, Sara, Silvia Lori, Maria Bastianelli, Cesarina Cossu, Simonetta Gabbanini, Carlo Dani, and Giovanna Bertini. 2022. "Unilateral Transient Enhanced SEP during Integrated Multiparameter Neurophysiological Monitoring in a Newborn with Symptomatic Seizure" Pediatric Reports 14, no. 2: 254-261. https://doi.org/10.3390/pediatric14020033
APA StyleCavaliere, S., Lori, S., Bastianelli, M., Cossu, C., Gabbanini, S., Dani, C., & Bertini, G. (2022). Unilateral Transient Enhanced SEP during Integrated Multiparameter Neurophysiological Monitoring in a Newborn with Symptomatic Seizure. Pediatric Reports, 14(2), 254-261. https://doi.org/10.3390/pediatric14020033








