Gallbladder Interleukins in Children with Calculous Cholecystitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Characteristics of Subjects
2.2. Immunohistochemical Analysis
2.3. Statistical Analysis
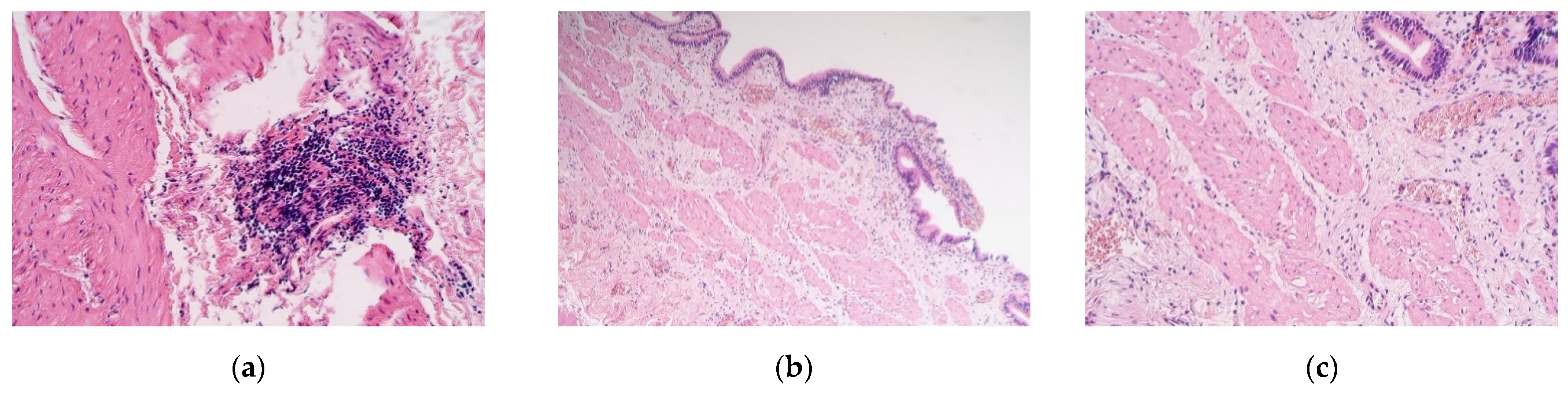
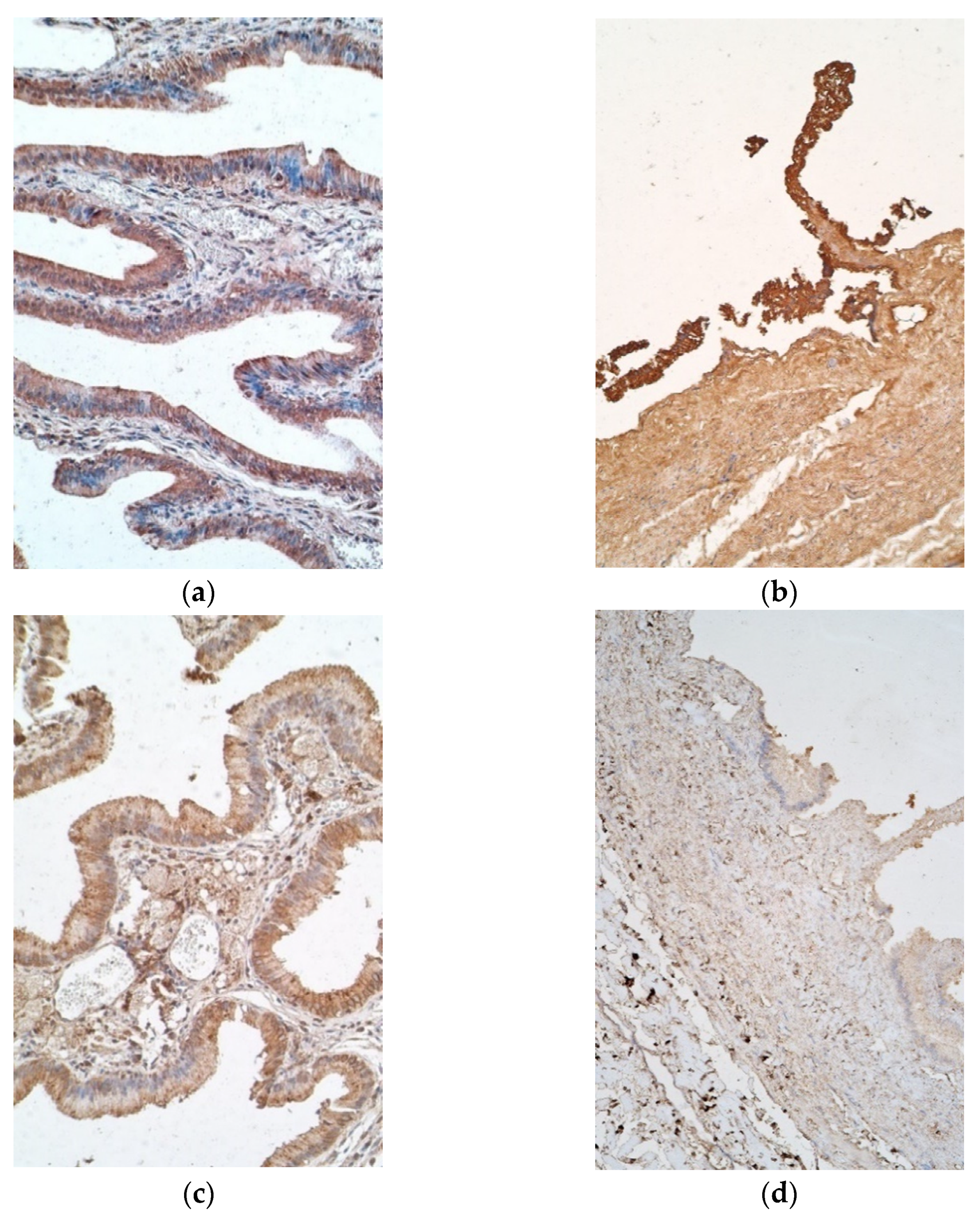
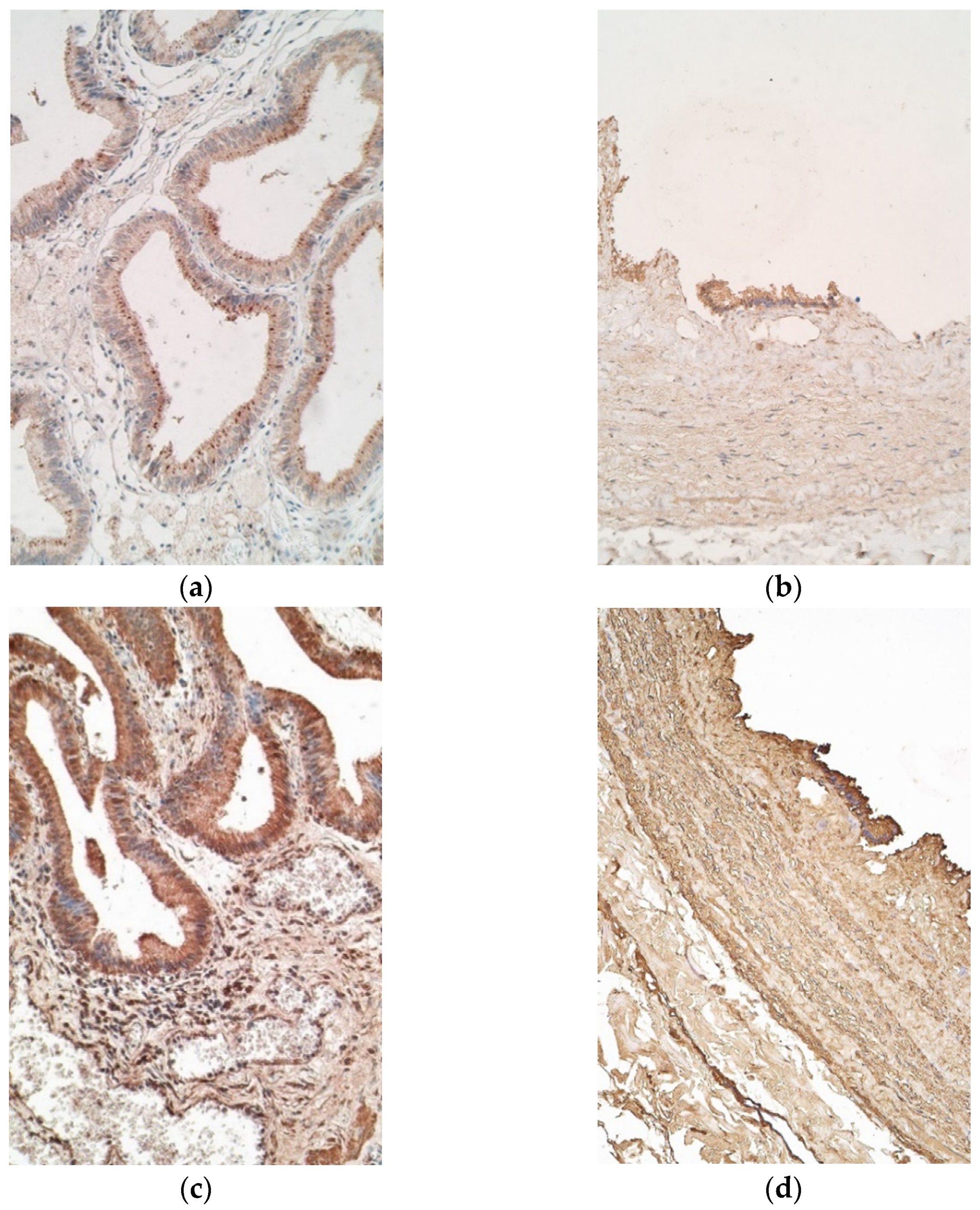
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Strasberg, S.M. Acute calculous cholecystitis. N. Engl. J. Med. 2008, 358, 2804–2811. [Google Scholar] [CrossRef]
- Akhtar-Danesh, G.G.; Doumouras, A.G.; Bos, C.; Flageole, H.; Hong, D. Factors associated with outcomes and costs after pediatric laparoscopic cholecystectomy. JAMA Surg. 2018, 153, 551–557. [Google Scholar] [CrossRef]
- Khoo, A.K.; Cartwright, R.; Berry, S.; Davenport, M. Cholecystectomy in English children: Evidence of an epidemic (1997–2012). J. Pediatr. Surg. 2014, 49, 284–288. [Google Scholar] [CrossRef]
- Mehta, S.; Lopez, M.E.; Chumpitazi, B.P.; Mazziotti, M.V.; Brandt, M.L.; Fishman, D.S. Clinical characteristics and risk factors for symptomatic pediatric gallbladder disease. Pediatrics 2012, 129, 82–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenberger, N.J.; Paumgartner, G. Diseases of the gallbladder and bile ducts. In Harrison’s Principles of Internal Medicine, 20th ed.; Jameson, J., Fauci, A.S., Kasper, D.L., Hauser, S.L., Longo, D.L., Loscalzo, J., Eds.; McGraw-Hill: New York, NY, USA, 2018. [Google Scholar]
- Shengelia, M.; Intskirveli, N.; Gogebashvili, N. Inflammatory markers of gallstones disease in menopausal women. Georgian Med. News 2012, 52, 208–209. [Google Scholar]
- Issa, A.H.; Muthena, M. Serum levels of several types of cytokines associated with Chronic Cholecystitis in Basrah/south of Iraq. Basrah J. Sci. 2014, 32, 248–280. [Google Scholar]
- Liu, Z.; Kemp, T.J.; Gao, Y.-T.; Corbel, A.; McGee, E.E.; Wang, B.; Shen, M.-C.; Rashid, A.; Hsing, A.W.; Hildesheim, A.; et al. Association of circulating inflammation proteins and gallstone disease. J. Gastroenterol. Hepatol. 2018, 33, 1920–1924. [Google Scholar] [CrossRef] [PubMed]
- Corbel, A.F.; Kemp, T.J.; Pinto, L.A.; Koshiol, J. High sensitivity multiplex cytokine panel used to identify unique signatures in serum associated with gallbladder cancer. J. Immunol. 2016, 196, 20. [Google Scholar]
- Zweers, S.; Shiryaev, A.; Komuta, M.; Vesterhus, M.; Hov, J.R.; Perugorria, M.J.; Schaap, F.G. Elevated interleukin-8 in Bile of Patients With Primary Sclerosing Cholangitis. Liver Int. 2016, 36, 1370–1377. [Google Scholar] [CrossRef]
- Su, P.-Y.; Liu, S.-J.; Chen, Y.-H.; Wu, S.-S.; Chen, Y.-L.; Ke, J.-R.; Sher, Y.-P. Increased IL-8 and IL-1β in the bile of acute cholecystitis patients. BioMedicine 2013, 131, 181–185. [Google Scholar] [CrossRef]
- Harada, K.; Shimoda, S.; Sato, Y.; Isse, K.; Ikeda, H.; Nakanuma, Y. Periductal interleukin-17 production in association with biliary innate immunity contributes to the pathogenesis of cholangiopathy in primary biliary cirrhosis. Clin. Exp. Immunol. 2009, 157, 261–270. [Google Scholar] [CrossRef]
- Contassot, E.; Beer, H.-D.; French, L.E. Interleukin-1, inflammasomes, autoinflammation and the skin. Swiss Med. Wkly. 2012, 142, w13590. [Google Scholar] [CrossRef] [Green Version]
- Paolo, N.C.; Shayakhmetov, D.M. Interleukin 1α and the inflammatory process. Nat. Immunol. 2016, 17, 906–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedersen, B.K.; Fabbraio, M.A. Muscle as an endocrine organ. In Muscle and Exercise Physiology; Zoladz, J.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 285–307. [Google Scholar]
- Chandramohan, V.; Sampson, J.H.; Pastan, I.; Bigner, D.D. Toxin-Based Targeted Therapy for Malignant Brain Tumors. Clin. Dev. Immunol. 2012, 2012, 480429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, V.L.; Szilvassy, S.J.; Daugherty, A. Interleukin-4 deficiency promotes gallstone formation. J. Lipid Res. 2002, 43, 768–771. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Keystone, E.; Omair, M.A. Interleukin-6 inhibition. In Rheumatology, 6th ed.; Hochberg, M.C., Silman, A.J., Smolen, J.S., Weinblatt, M.E., Weisman, M.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 1, pp. 485–491. [Google Scholar]
- Khan, U.; Ghazanfar, H. T Lymphocytes and Autoimmunity. Int. Rev. Cell. Mol. Biol. 2018, 341, 125–168. [Google Scholar] [CrossRef] [PubMed]
- Jamwal, S.; Kumar, P. Animal Models of Inflammatory Bowel Disease. In Animal Models for the Study of Human Disease, 2nd ed.; Conn, M.P., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 467–477. [Google Scholar]
- Wong, H.R.; Nowak, J.E.; Standage, S.W.; Oliveira, C.F. Sepsis and septic shock. In Pediatric Critical Care, 4th ed.; Fuhrman, B., Zimmerman, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 1413–1429. [Google Scholar]
- Rascher-Eggstein, G.; Liebner, S.; Wolburg, H. The Blood–Brain Barrier in the Human Glioma. In Blood-Spinal Cord and Brain Barriers in Health and Disease; Sharma, H.S., Westman, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 561–576. [Google Scholar]
- Deng, Y.; Tsao, B.P. Genetics of Human SLE. In Dubois’ Lupus Erythematosus and Related Syndromes, 8th ed.; Wallace, D.J., Hahn, B.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 35–40. [Google Scholar]
- Parker, K.H.; Beury, D.W. Ostrand-Rosenberg, SMyeloid-Derived Suppressor Cells: Critical Cells Driving Immune Suppression in the Tumor Microenvironment. In Immunotherapy of Cancer; Wang, X.-Y., Fisher, P.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 128, pp. 95–139. [Google Scholar]
- Zenobia, C.; Hajishengallis, G. Basic biology and role of interleukin-17 in immunity and inflammation. Periodontology 2000 2016, 69, 142–159. [Google Scholar] [CrossRef]
- Hsu, S.M.; Raine, L.; Fanger, H. The use of antiavidin antibody and avidin-biotin-peroxidase complex in immunoperoxidase technics. Am. J. Clin. Pathol. 1981, 75, 816–821. [Google Scholar] [CrossRef]
- Guesdon, J.L.; Ternynck, T.; Avrameas, S. The use of avidin-biotin interaction in immunoenzymatic techniques. J. Histochem. Cytochem. 1979, 27, 1131–1139. [Google Scholar] [CrossRef]
- Pilmane, M.; Rumba, I.; Sundler, F.; Luts, A. Patterns of distribution and occurrence of neuroendocrine elements in lungs of humans with chronic lung disease. Proc. Latv. Acad. Sci. 1998, 52, 144–152. [Google Scholar]
- Housset, C.; Chrétien, Y.; Debray, D.; Chignard, N. Functions of the Gallbladder. Compr. Physiol. 2016, 6, 1549–1577. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; He, X.; Li, X.; Qian, Y. The roles and functional mechanisms of interleukin-17 family cytokines in mucosal immunity. Cell Mol. Immunol. 2016, 13, 418–431. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, K.A.; Manieri, N.A.; Liu, T.; Stappenbeck, T.S. IL-6 stimulates intestinal epithelial proliferation and repair after injury. PLoS ONE 2014, 9, e114195. [Google Scholar] [CrossRef] [Green Version]
- Gagnon, J.; Ramanathan, S.; Leblanc, C.; Cloutier, A.; McDonald, P.P.; Ilangumaran, S. IL-6, in synergy with IL-7 or IL-15, stimulates tcr-independent proliferation and functional differentiation of CD8+ T Lymphocytes. J. Immunol. 2008, 180, 7958–7968. [Google Scholar] [CrossRef] [Green Version]
- Webster, K.E.; Kim, H.-O.; Kyparissoudis, K.; Corpuz, M.T.; Pinget, G.V.; Uldrich, A.P.; Brink, R.; Belz, G.T.; Cho, J.-H.; Goddfrey, D.I.; et al. IL-17-producing NKT cells depend exclusively on IL-7 for homeostasis and survival. Mucosal Immunol. 2014, 7, 1058–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, Y.; Yang, J.; Ouyang, X.; Liu, W.; Li, H.; Yang, J.; Bromberg, J.; Chen, S.-H.; Mayer, L.; Unkeless, J.C.; et al. Interleukin 10 suppresses Th17 cytokines secreted by macrophages and T cells. Eur. J. Immunol. 2008, 38, 1807–1813. [Google Scholar] [CrossRef] [Green Version]
- Williams, L.M.; Ricchetti, G.; Sarma, U.; Smallie, T.; Foxwell, B.M.J. Interleukin-10 suppression of myeloid cell activation—A continuing puzzle. Immunology 2004, 113, 281–292. [Google Scholar] [CrossRef]
- Roh, J.S.; Sohn, D.H. Damage-associated molecular patterns in inflammatory diseases. Immune Netw. 2018, 18, e27. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhao, L.; Wan, Y.Y.; Zhu, B. Mechanism of Action of IL-7 and Its Potential Applications and Limitations in Cancer Immunotherapy. Int. J. Mol. Sci. 2015, 16, 267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, N.; Qin, X. New insights into IL-7 signaling pathways during early and late T cell development. Cell Mol. Immunol. 2013, 10, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Rincón, M.; Anguita, J.; Nakamura, T.; Fikrig, E.; Flavell, R.A. Interleukin (IL)-6 Directs Differentiation of IL-4 producing CD4+ T cells. J. Exp. Med. 1997, 185, 461–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| IL’s/ Patients | IL-1α | IL-4 | IL-6 | IL-7 | IL-8 | IL-10 | IL-17A | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E | CT | E | CT | E | CT | E | CT | E | CT | E | CT | E | CT | |
| 1. | ++ | + | ++ | ++ | ++ | ++ | ++/+++ | ++ | 0 | 0/+ | +/++ | 0/+ | +++ | ++/+++ |
| 2. | +/++ | + | +++ | ++ | ++ | ++ | ++/+++ | ++ | 0/+ | + | +/++ | 0 | +/++ | +/++ |
| 3. | ++ | + | No | No | +/++ | ++ | +/++ | ++/+++ | +/++ | + | ++/+++ | 0/+ | ++ | ++ |
| 4. | +++ | ++ | +++ | ++/+++ | ++/+++ | ++/+++ | ++ | ++/+++ | 0 | +/++ | ++ | + | ++ | +/++ |
| 5. | N | ++ | N | ++/+++ | N | ++ | N | ++/+++ | N | 0/+ | N | 0/+ | N | ++/+++ |
| 6. | N | +/++ | N | ++ | N | ++ | N | ++/+++ | N | + | N | 0/+ | N | ++ |
| 7. | ++/+++ | +/++ | ++/+++ | ++/+++ | +++ | ++/+++ | +++ | ++/+++ | 0 | ++ | ++ | 0/+ | +++ | +/++ |
| 8. | ++/+++ | +/++ | ++ | +/++ | ++/+++ | +/++ | +++ | ++ | +++ | +/++ | ++/+++ | 0 | +++ | + |
| 9. | +/++ | + | +/++ | ++ | ++ | ++ | +/++ | ++ | 0 | +/++ | + | 0 | +/++ | +/++ |
| 10. | ++ | + | ++/+++ | ++/+++ | +++ | ++ | ++/+++ | ++ | ++/+++ | +/++ | +/++ | 0 | ++/+++ | ++ |
| 11. | +/++ | + | +++ | ++ | +/++ | ++ | +/++ | +/++ | +/++ | +/++ | 0/+ | 0 | +/++ | +/++ |
| 12. | +/++ | + | +/++ | ++ | ++ | +/++ | +/++ | +/++ | +/++ | +/++ | 0/+ | 0 | +/++ | +/++ |
| 13. | +/++ | + | ++/+++ | ++ | ++/+++ | ++ | ++ | ++/+++ | ++/+++ | +/++ | ++ | 0/+ | ++/+++ | ++ |
| 14. | +/++ | + | ++ | ++/+++ | ++/+++ | +++ | +/++ | +++ | ++/+++ | ++ | 0/+ | 0/+ | ++/+++ | ++/+++ |
| 15. | + | +/++ | ++ | ++ | ++ | +++ | ++ | ++/+++ | 0 | 0/+ | + | 0 | ++ | ++/+++ |
| 16. | ++ | +/++ | +++ | ++ | +++ | ++/+++ | ++/+++ | ++/+++ | 0/+ | +/++ | ++ | + | ++/+++ | ++/+++ |
| 17. | ++/+++ | +/++ | +++ | +/++ | +/++ | ++ | +/++ | ++ | + | +/++ | 0/+ | 0/+ | +/++ | ++/+++ |
| 18. | ++/+++ | +/++ | +++ | ++ | +++ | +/++ | +++ | +/++ | +++ | +/++ | ++ | 0 | +++ | ++ |
| 19. | + | + | ++/+++ | ++/+++ | ++/+++ | ++/+++ | ++ | ++ | 0/+ | 0/+ | + | 0 | ++/+++ | ++ |
| 20. | N | + | N | ++ | N | ++/+++ | N | ++/+++ | N | + | N | 0 | N | ++ |
| Avg | ++ | +/++ | ++/+++ | ++ | ++/+++ | ++ | ++/+++ | ++ | 0/+ | +/++ | +/++ | 0/+ | +++ | ++ |
| IL’s/ Controls | IL-1α | IL-4 | IL-6 | IL-7 | IL-8 | IL-10 | IL-17A | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E | CT | E | CT | E | CT | E | CT | E | CT | E | CT | E | CT | |
| 1. | N | 0/+ | N | + | + | + | +++ | 0 | ++ | + | 00/+ | 0 | +++ | + |
| 2. | 0 | 0 | +++ | + | +++ | + | +++ | 0 | +++ | 0 | +++ | 0 | +++ | + |
| 3. | 0 | 0 | +++ | 0 | +++ | 0/+ | +++ | 0 | +++ | 0 | ++ | 0 | +++ | + |
| 4. | N | 00/+ | N | + | N | + | +++ | + | N | + | 0/+ | 0/+ | +++ | +/++ |
| 5. | 0 | 0 | +++ | + | +++ | + | ++ | 00/+ | 0 | 0 | +++ | 0 | +++ | + |
| 6. | N | 0 | N | + | No | No | ++ | 0 | N | + | +++ | + | +++ | + |
| 7. | N | 0 | N | + | N | + | N | 0 | N | + | N | 0/+ | +++ | + |
| Avg | 0 | 0 | +++ | + | +++ | + | ++/+++ | 0 | ++/+++ | + | ++ | 0/+ | +++ | + |
| Interleukins | IL-1α E | IL-1α CT | IL-4 CT | IL-6 CT | IL-7 CT | IL-8 CT | IL-17A E | IL-17A CT |
|---|---|---|---|---|---|---|---|---|
| p-Value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.008 | 0.002 | <0.001 |
| Mean | 1.8824 | 1.2750 | 2.105 | 2.150 | 2.2000 | 1.250 | 2.235 | 1.950 |
| St. Deviation | 0.57362 | 0.34317 | 0.3153 | 0.4323 | 0.41039 | 0.4730 | 0.5894 | 0.4560 |
| Factor 1 | Factor 2 | R | p-Value |
|---|---|---|---|
| Strong Positive Correlation (0.60–0.79) | |||
| IL-1α E | IL-1α CT | 0.643 | 0.005 |
| IL-6 E | IL-7 E | 0.699 | 0.002 |
| IL-6CT | IL-7 CT | 0.692 | 0.001 |
| IL-7E | IL-10 E | 0.625 | 0.007 |
| Il-7CT | IL-10 CT | 0.63 | 0.003 |
| IL-6E | IL-17A E | 0.709 | 0.001 |
| IL-7E | IL-17A E | 0.749 | 0.001 |
| Moderate Positive Correlation (0.40–0.59) | |||
| IL-1α E | IL-10 E | 0.543 | 0.024 |
| IL-4 CT | IL-6 E | 0.501 | 0.048 |
| IL-4CT | IL-6 CT | 0.492 | 0.032 |
| IL-10 E | IL-17A E | 0.561 | 0.019 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deņisova, A.; Pilmane, M.; Eņģelis, A.; Pētersons, A. Gallbladder Interleukins in Children with Calculous Cholecystitis. Pediatr. Rep. 2021, 13, 470-482. https://doi.org/10.3390/pediatric13030054
Deņisova A, Pilmane M, Eņģelis A, Pētersons A. Gallbladder Interleukins in Children with Calculous Cholecystitis. Pediatric Reports. 2021; 13(3):470-482. https://doi.org/10.3390/pediatric13030054
Chicago/Turabian StyleDeņisova, Arina, Māra Pilmane, Arnis Eņģelis, and Aigars Pētersons. 2021. "Gallbladder Interleukins in Children with Calculous Cholecystitis" Pediatric Reports 13, no. 3: 470-482. https://doi.org/10.3390/pediatric13030054
APA StyleDeņisova, A., Pilmane, M., Eņģelis, A., & Pētersons, A. (2021). Gallbladder Interleukins in Children with Calculous Cholecystitis. Pediatric Reports, 13(3), 470-482. https://doi.org/10.3390/pediatric13030054







