Safe and Effective Treatment of Intracranial Infantile Hemangiomas with Beta-Blockers
Abstract
1. Introduction
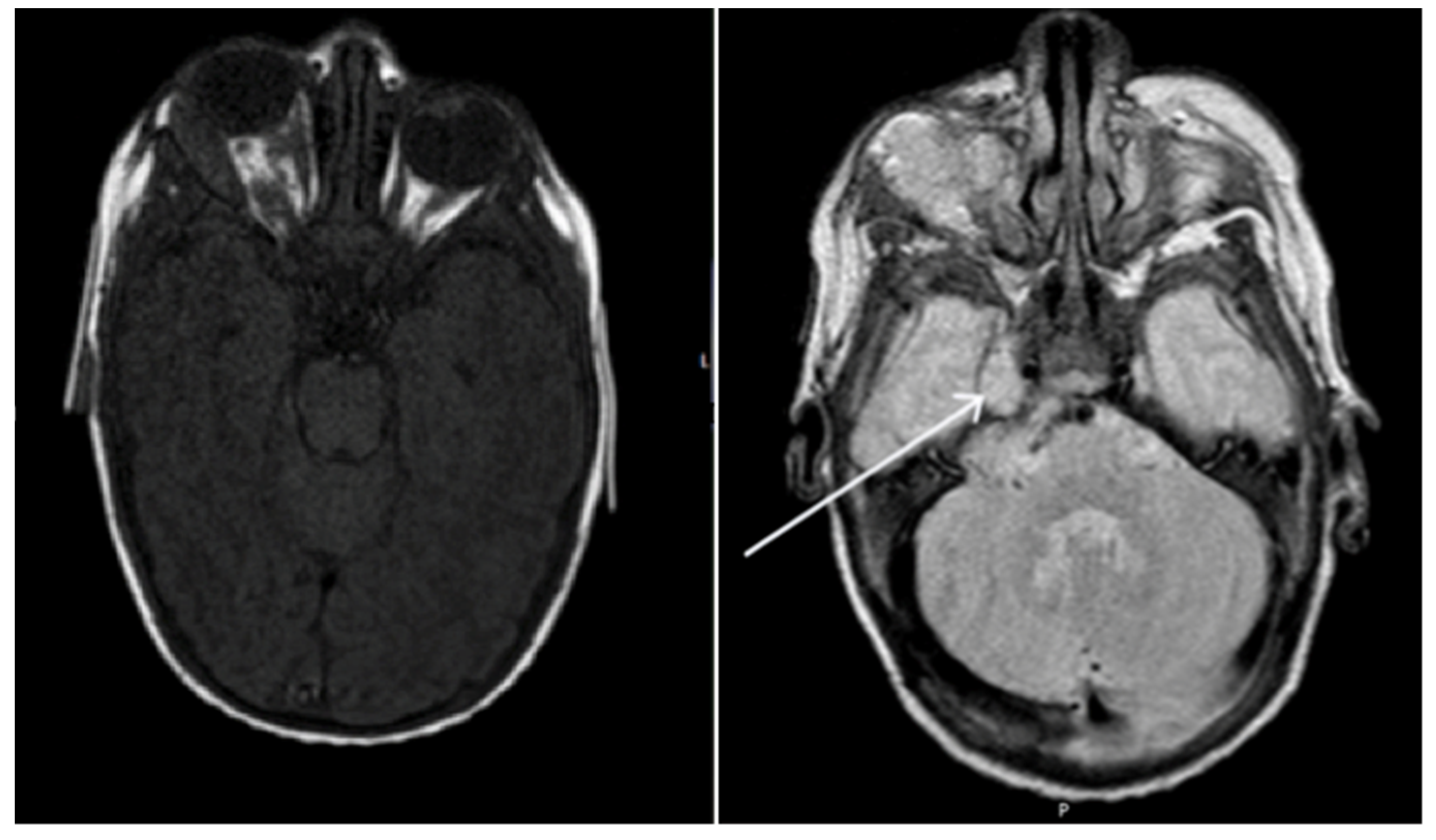
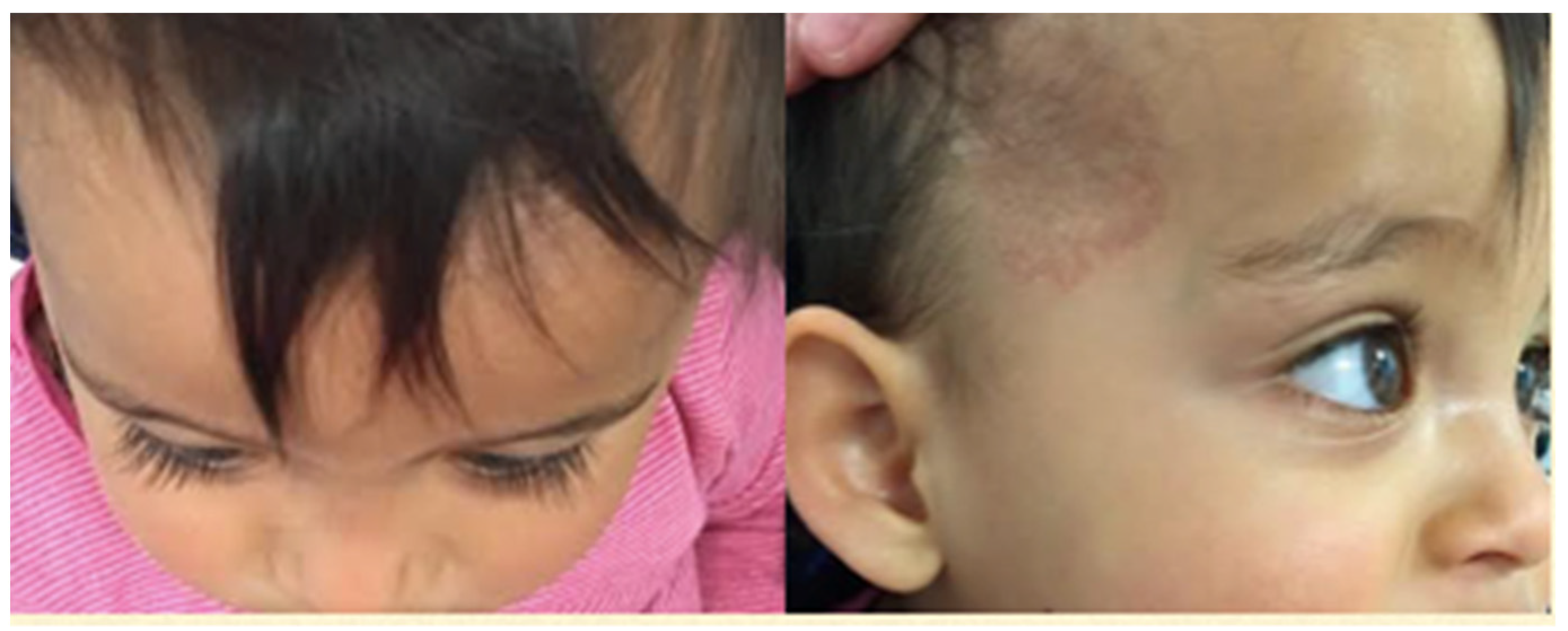
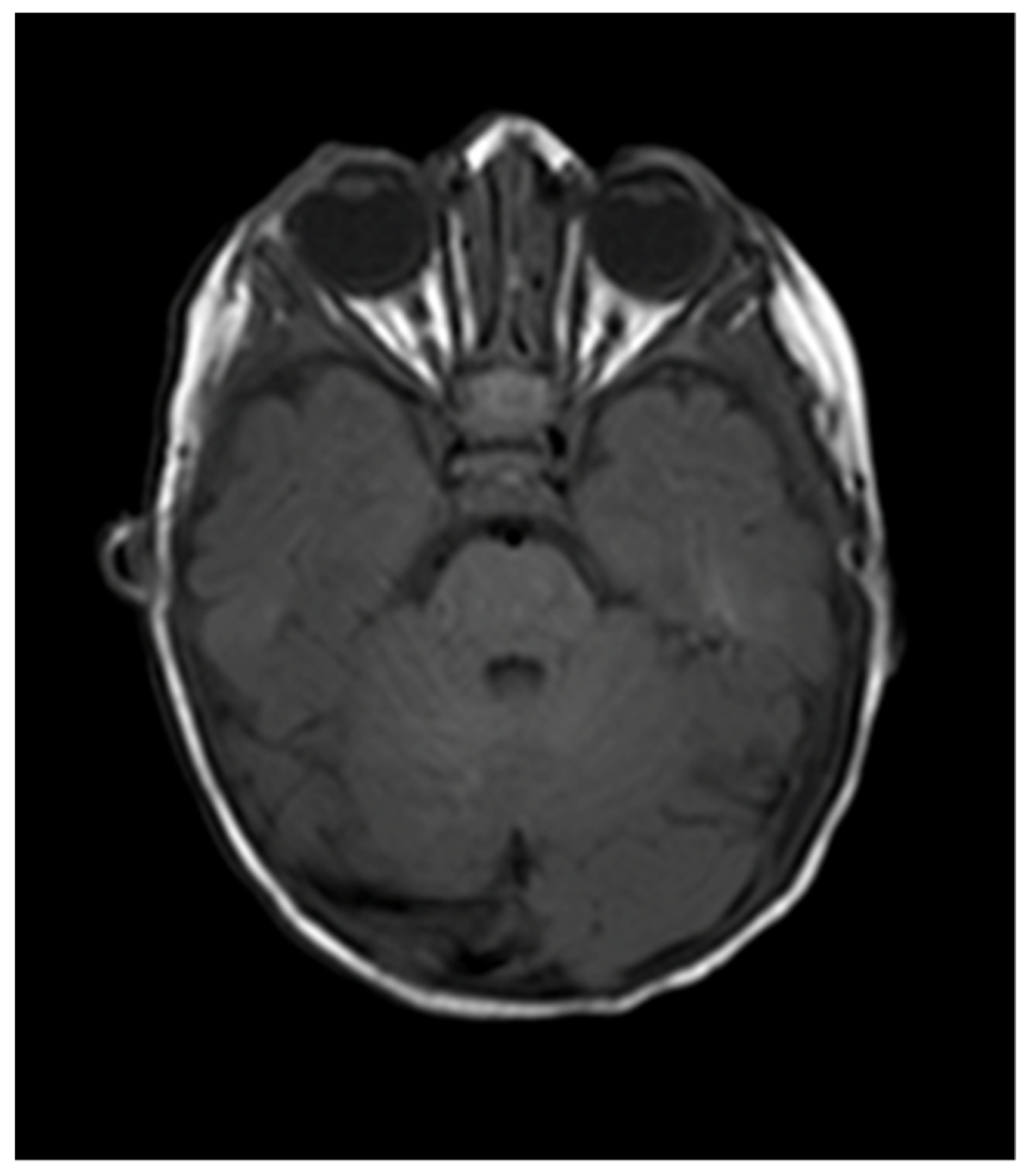
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Further Information
References
- Kilcline, C.; Frieden, I.J. Infantile Hemangiomas: How Common Are They? A Systematic Review of the Medical Literature. Pediatr. Dermatol. 2008, 25, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Haggstrom, A.N.; Drolet, B.A.; Baselga, E.; Chamlin, S.L.; Garzon, M.C.; Horii, K.A.; Lucky, A.W.; Mancini, A.J.; Metry, D.W.; Newell, B.; et al. Prospective Study of Infantile Hemangiomas: Demographic, Prenatal, and Perinatal Characteristics. J. Pediatr. 2007, 150, 291–294. [Google Scholar] [CrossRef]
- Drolet, B.A.; Swanson, E.A.; Frieden, I.J. Infantile Hemangiomas: An Emerging Health Issue Linked to an Increased Rate of Low Birth Weight Infants. J. Pediatr. 2008, 153, 712–715.e1. [Google Scholar] [CrossRef] [PubMed]
- Holland, K.E.; Drolet, B.A. Infantile Hemangioma. Pediatr. Clin. N. Am. 2010, 57, 1069–1083. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.C.; Haggstrom, A.N.; Drolet, B.A.; Baselga, E.; Chamlin, S.L.; Garzon, M.C.; Horii, K.A.; Lucky, A.W.; Mancini, A.J.; Metry, D.W.; et al. Growth Characteristics of Infantile Hemangiomas: Implications for Management. Pediatrics 2008, 122, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Antonov, N.K.; Spence-Shishido, A.; Marathe, K.S.; Tlougan, B.; Kazim, M.; Sultan, S.; Hess, C.P.; Morel, K.D.; Frieden, I.J.; Garzon, M.C. Orbital Hemangioma with Intracranial Vascular Anomalies and Hemangiomas: A New Presentation of PHACE Syndrome? Pediatr. Dermatol. 2015, 32, e267–e272. [Google Scholar] [CrossRef]
- Bar-Sever, Z.; Horev, G.; Lubin, E.; Kornreich, L.; Naor, N.; Ziv, N.; Shimoni, A.; Grunebaum, M. A rare coexistence of a multicentric hepatic hemangioendothelioma with a large brain hemangioma in a preterm infant. Pediatr. Radiol. 1994, 24, 141–142. [Google Scholar] [CrossRef] [PubMed]
- Cavalheiro, S.; Campos, H.G.D.A.; da Costa, M.D.S. A case of giant fetal intracranial capillary hemangioma cured with propranolol. J. Neurosurg. Pediatr. 2016, 17, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Daenekindt, T.; Weyns, F.; Kho, K.H.; Peuskens, D.; Engelborghs, K.; Wuyts, J. Giant intracranial capillary hemangioma associated with enlarged head circumference in a newborn. J. Neurosurg. Pediatr. 2008, 1, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Ersoy, S.; Mancini, A.J. Hemifacial infantile hemangioma with intracranial extension: A rare entity. Pediatr Dermatol. 2005, 22, 309–313. [Google Scholar] [CrossRef]
- Frei-Jones, M.; McKinstry, R.C.; Perry, A.; Leonard, J.R.; Park, T.S.; Rubin, J.B. Use of thalidomide to diminish growth velocity in a life-threatening congenital intracranial hemangioma. J. Neurosurg. Pediatr. 2008, 2, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Haine, E.; Sevely, A.; Boetto, S.; Delisle, M.-B.; Cances, C. Infantile Hemangioma of the Posterior Fossa in a Newborn: Early Management and Long-Term Follow-up. Neuropediatrics 2017, 48, 378–381. [Google Scholar] [CrossRef]
- Heyer, G.L.; Garzon, M.C. An Infant with a Facial Hemangioma and More. Semin. Pediatr. Neurol. 2008, 15, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Jalloh, I.; Dean, A.F.; O’Donovan, D.G.; Cross, J.; Garnett, M.R.; Santarius, T. Giant intracranial hemangioma in a neonate. Acta Neurochir. 2014, 156, 1151–1154. [Google Scholar] [CrossRef] [PubMed]
- Judd, C.; Chapman, P.; Koch, B.; Shea, C. Intracranial Infantile Hemangiomas Associated With PHACE Syndrome. Am. J. Neuroradiol. 2007, 28, 25–29. [Google Scholar] [PubMed]
- Kang, E.; Friedman, N.; Mamoun, I.; Tamburro, J.; Golden, A. Beta Blockade as Treatment for Intracranial Infantile Hemangioma: Case Report and Literature Review. Pediatr. Neurol. 2016, 59, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Karikari, I.O.; Selznick, L.A.; Cummings, T.J.; George, T.M. Capillary hemangioma of the fourth ventricle in an infant. J. Neurosurgery: Pediatr. 2006, 104, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Le Bihannic, A.; Michot, C.; Heckly, A.; Loget, P.; Beucher, A.; Brassier, G.; Hamlat, A. Capillary haemangioma arising from the anterior choroidal artery. Childs Nerv Syst. 2005, 21, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Philpott, C.; Wray, A.; MacGregor, D.; Coleman, L. Dural Infantile Hemangioma Masquerading as a Skull Vault Lesion. Am. J. Neuroradiol. 2011, 33, E85–E87. [Google Scholar] [CrossRef] [PubMed]
- Poetke, M.; Frommeld, T.; Berlien, H.P. PHACE Syndrome: New Views on Diagnostic Criteria. Eur. J. Pediatr. Surg. 2002, 12, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Poindexter, G.; Metry, D.W.; Barkovich, A.J.; Frieden, I.J. PHACE Syndrome with Intracerebral Hemangiomas, Heterotopia, and Endocrine Dysfunction. Pediatr. Neurol. 2007, 36, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, R.M.; Shakir, H.J.; McBride, P. Dural-based infantile hemangioma of the posterior fossa: Case report. Surg. Neurol. Int. 2016, 7, 52. [Google Scholar] [CrossRef][Green Version]
- Tortori-Donati, P.; Fondelli, M.P.; Rossi, A.; Bava, G.L. Intracranial contrast-enhancing masses in infants with capillary haemangioma of the head and neck: Intracranial capillary haemangioma? Neuroradiology 1999, 41, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Uyama, A.; Kawamura, A.; Akiyama, H.; Nakamizo, S.; Yamamoto, K.; Nagashima, T.; Uetani, T.; Takeda, H.; Yoshida, M. A Case of Cerebellar Capillary Hemangioma with Multiple Cysts. Pediatr. Neurosurg. 2008, 44, 344–349. [Google Scholar] [CrossRef]
- Viswanathan, V.; Smith, E.; Mulliken, J.; Fishman, S.; Kozakewich, H.; Burrows, P.; Orbach, D. Infantile Hemangiomas Involving the Neuraxis: Clinical and Imaging Findings. Am. J. Neuroradiol. 2009, 30, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Willing, S.J.; Faye-Petersen, O.; Aronin, P.; Faith, S. Radiologic-pathologic correlation. Capillary hemangioma of the meninges. Am. J. Neuroradiol. 1993, 14, 529–536. [Google Scholar] [PubMed]
- Zheng, S.-P.; Ju, Y.; You, C. Giant intracranial capillary hemangioma in a 3-year-old child: Case report and literature review. Clin. Neurol. Neurosurg. 2012, 114, 1270–1273. [Google Scholar] [CrossRef] [PubMed]
- Benvenisti, H.; Ben-Sira, L.; Constantini, S.; Roth, J. Giant cranial and cerebellar hemangioma treated with propranolol. Child’s Nerv. Syst. 2014, 31, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Dalsin, M.; Silva, R.S.; Chaves, J.P.G.; Oliveira, F.H.; Antunes, Á.C.M.; Vedolin, L.M. Intracranial extra-axial hemangioma in a newborn: A case report and literature review. Surg. Neurol. Int. 2016, 7, 314–316. [Google Scholar] [CrossRef][Green Version]
- El Rassi, E.; MacArthur, C.J. Propranolol-responsive cranial nerve palsies in a patient with PHACES syndrome. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 1778–1781. [Google Scholar] [CrossRef] [PubMed]
- Friedland, R.; Ben Amitai, D.; Zvulunov, A. Screening for Brain Involvement in Infants with Multifocal Cutaneous Infantile Hemangiomas. Dermatology 2017, 233, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Holland, K.E.; Frieden, I.J.; Frommelt, P.C.; Mancini, A.J.; Wyatt, D.; Drolet, B.A. Hypoglycemia in Children Taking Propranolol for the Treatment of Infantile Hemangioma. Arch. Dermatol. 2010, 146, 775–778. [Google Scholar] [CrossRef]
- Enjolras, O.; Riche, M.C.; Merland, J.J.; Escande, J.P. Management of alarming hemangiomas in infancy: A review of 25 cases. Pediatrics 1990, 85, 491–498. [Google Scholar]
- Barrio, V.R.; Drolet, B.A. Treatment of hemangiomas of infancy. Dermatol. Ther. 2005, 18, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Léauté-Labrèze, C.; De La Roque, E.D.; Hubiche, T.; Boralevi, F.; Thambo, J.B.; Taïeb, A. Propranolol for severe hemangiomas of infancy. N. Engl. J. Med. 2008, 358, 2649–2651. [Google Scholar] [CrossRef] [PubMed]
- Painter, S.L.; Hildebrand, G.D. Review of topical beta blockers as treatment for infantile hemangiomas. Surv. Ophthalmol. 2016, 61, 51–58. [Google Scholar] [CrossRef]
- Sipkova, Z.; Xue, K.; Mudhar, H.S.; Wagner, B.; Hildebrand, G.D. Early and Late Histological and Ultrastructural Findings in Resected Infantile Capillary Hemangiomas Following Treatment with Topical Beta-Blocker Timolol Maleate 0.5%. Ocul. Oncol. Pathol. 2017, 4, 100–106. [Google Scholar] [CrossRef]
- Marqueling, A.L.; Oza, V.; Frieden, I.J.; Puttgen, K.B. Propranolol and Infantile Hemangiomas Four Years Later: A Systematic Review. Pediatr. Dermatol. 2013, 30, 182–191. [Google Scholar] [CrossRef] [PubMed]

| Author, Year | Age at Presentation, Gender | Neurological or Ophthalmic Complaint | Intracranial Location of Hemangioma | Treatment | Outcome |
|---|---|---|---|---|---|
| Willing et al., 1993 | 17 months, male | Focal seizures, mild developmental delay | Right temporal dura | Surgical excision | Resolution |
| Bar-Sever et al., 1994 | 2 weeks, female | Nil | Right middle fossa, extending into the right orbit and suprasellar cistern | Oral prednisolone (for 2 months—no response) Subsequent interferon | Marked reduction with interferon treatment |
| Tortori et al., 1999 | 1 month, female | Nil | Right uncohippocampal | Observation | Resolution |
| 1.5 months, female | Nil | Left CPA, leptomeningeal enhancement at cerebellar surface | Observation | Resolution | |
| 18 months, female | Nil at presentation, developed ataxia | Right CPA, hypothalamus | Systemic steroids, endovascular treatment with contour particles | Unchanged following steroids. Partial resolution following endovascular intervention | |
| 1 month, female | Nil | Left CPA, persistent trigeminal artery | Systemic steroids | Lost to follow-up | |
| Poetke et al., 2002 | 10 weeks, male | Nil | CPA, leptomeningeal enhancement on cerebellar surface | Nil | Unknown |
| Le Bihannic et al., 2005 | 6 weeks, male | Vomiting, disturbance in consciousness, seizures | Anterior choroidal artery, right temporal lobe | Nil | Intracranial haemorrhage, death |
| Ersoy et al., 2005 | 8 months, female | Nil | Lateral medullary cistern, IAC, fourth ventricle | Oral prednisolone | Marked reduction in lesion size |
| Karikari et al., 2006 | 3 months, male | Central hypotonia | Fourth ventricle, left CPA | Surgical resection | Resolution |
| Judd et al., 2007 | 3 weeks, female | Nil | IAC/CPA, | Oral prednisolone | Resolution |
| 3 weeks, female | Right facial paresis | IAC/CPA, fourth ventricle | Oral prednisolone | Resolution | |
| 6 weeks, female | Nil | IAC/CPA | Intralesional triamcinolone | Resolution | |
| 8 weeks, female | Nil | IAC, Meckel’s cave, cavernous sinus | Intralesional triamcinolone | Resolution | |
| Poindexter et al., 2007 | 2.5 months, female | Reduced truncal tone | Left IAC | Observation | Partial involution, developmental delay, diffuse hypotonia |
| Daenekindt et al., 2008 | 7 weeks, male | Enlarged head circumference | Right temporal fossa | Biopsy, Endovascular embolization and surgical resection | Resolution |
| Frei-Jones et al., 2008 | Newborn, female | Left CNVII palsy, left sensorineural hearing loss | Middle cranial fossa, temporal bone, posterior fossa | Biopsy, Thalidomide | Partial Resolution |
| Heyer et al., 2008 | 6 months, female | Nil | Left IAC | Observation | Unchanged |
| Uyama et al., 2008 | 4 months, female | Hydrocephalus | Left cerebellar hemisphere | Neuroendoscopic fenestration of cysts, Surgical resection of lesion | Resolution |
| Viswanathan et al., 2009 | 3 weeks, female | Hydrocephalus | Quad plate cistern, pineal region, left CPA | Corticosteroids | Reduction in lesion size |
| 9 weeks, female | Nil | Left cavernous sinus, Meckel’s cave, IAC | Corticosteroids | Lost to follow-up | |
| 4 months, female | Hydrocephalus | Fourth ventricle, left IAC, CPA | Corticosteroids | Reduction in lesion size | |
| 3 months, female | Left ptosis | Fourth ventricle, left foramen of Luschka, quad plate cistern | Interferon, OK432, subsequent corticosteroids | Reduction in lesion size | |
| 7 weeks, female | Right proptosis | Right temporal fossa, cavernous sinus, Meckel’s cave, sella, quad plate cisterns | Corticosteroids | Reduction in lesion size | |
| 7 weeks, male | Nil | Fourth ventricle, right foramen of Luschka, IAC | Reduction in lesion size | ||
| 3 weeks, male | Nil | Right CPA, foramen of Luschka, fourth ventricle | Corticosteroids, Interferon | Minimal response to corticosteroids, reduction in lesion size with Interferon | |
| Infancy, female | Nil | Peri-mesencephalic cistern, sella, cavernous sinus, left CPA | Interferon | Reduction in lesion size | |
| 3 months, female | Nil at presentation, subsequent stroke and hydrocephalus | Right cavernous sinus, CPA | Corticosteroids | Reduction in lesion size | |
| Philpott et al., 2012 | 12 months, female | Enlarged head circumference | Dura of right parietal lobe | Surgical resection | Resolution |
| Zheng et al., 2012 | 3 years, male | Somnolence, right CNIII palsy | Middle cranial fossa | Surgical resection | Resolution |
| Jalloh et al., 2014 | 2 weeks, Male | Tense anterior fontanelle, enlarging head circumference, seizures | Left middle cranial fossa | Biopsy, Surgical resection | Residual cyst, no recurrence |
| Benvenisti et al., 2014 | 4 weeks, female | Nil | Left posterior fossa | Oral propranolol | Reduction in lesion size, maintained at 12 months |
| Antonov et al., 2015 | 3 months, female | Nil | Middle cranial fossa, right cavernous sinus, prepontine cistern, infratemporal fossa | Oral propranolol | Resolution |
| 3 weeks, female | Nil | Right lateral ventricular trigone | Oral propranolol | Resolution | |
| El Rassi et al., 2015 | 5 weeks, female | Left CN V and VII palsy (PHACE syndrome) | Left CPA, IAC | Oral propranolol | Improvement in facial lesion, status of intracranial hemangioma not described |
| Cavalheiro et al., 2016 | 33 weeks gestation, male | Nil | Posterior fossa | Oral propranolol | Resolution |
| Kang et al., 2016 | 1 month, male | Nil | CPA | Oral propranolol | Resolution |
| Shakir et al., 2016 | 2 weeks, female | Hydrocephalus | Posterior fossa | Surgical resection | Resolution Post operative enlarging head circumference requiring VP shunt |
| Dalsin et al., 2016 | 37 weeks gestation, female | Diagnosed on antenatal ultrasound | Left middle cranial fossa | Surgical resection | Resolution, no neurological deficits |
| Haine et al., 2017 | 3 weeks, male | Symptoms of raised ICP | Posterior fossa | Surgical decompression Oral prednisolone | Resolution on imaging |
| Friedland et al., 2017 | 1 week, male | Nil | Not specified | Observation | Spontaneous resolution |
| Naughton et al., 2020 (this paper) | 6 weeks, female | Right CNVII palsy | Right orbit, right CPA and Meckel’s cave | Oral propranolol and topical timolol maleate 0.5% | Resolution |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naughton, A.; Ong, A.Y.; Hildebrand, G.D. Safe and Effective Treatment of Intracranial Infantile Hemangiomas with Beta-Blockers. Pediatr. Rep. 2021, 13, 347-356. https://doi.org/10.3390/pediatric13030043
Naughton A, Ong AY, Hildebrand GD. Safe and Effective Treatment of Intracranial Infantile Hemangiomas with Beta-Blockers. Pediatric Reports. 2021; 13(3):347-356. https://doi.org/10.3390/pediatric13030043
Chicago/Turabian StyleNaughton, Aoife, Ariel Yuhan Ong, and Goran Darius Hildebrand. 2021. "Safe and Effective Treatment of Intracranial Infantile Hemangiomas with Beta-Blockers" Pediatric Reports 13, no. 3: 347-356. https://doi.org/10.3390/pediatric13030043
APA StyleNaughton, A., Ong, A. Y., & Hildebrand, G. D. (2021). Safe and Effective Treatment of Intracranial Infantile Hemangiomas with Beta-Blockers. Pediatric Reports, 13(3), 347-356. https://doi.org/10.3390/pediatric13030043






