1. Introduction
Psoriasis is a chronic inflammatory dermatosis that affects between 2% and 3% of the world population [
1]. Psoriasis has increasingly been characterized as an autoimmune condition, supported by evidence that microbial dysbiosis and molecular mimicry, particularly between bacterial antigens and host keratinocyte proteins, can precipitate disease exacerbations [
2,
3]. Its etiology is related to genetic, environmental, and immunological factors, and mainly affects young people and middle-aged adults. The disease manifests itself through erythema and scaling and directly impacts patients’ quality of life. The manifestations are varied, with 85% to 90% of cases corresponding to plaque psoriasis, also known as psoriasis vulgaris, characterized by erythematous, scaly, and itchy plaques. Genetic predispositions, immunological and environmental factors can stimulate keratinocytes to secrete pro-inflammatory cytokines [
4].
Although psoriasis is among the most extensively investigated dermatological conditions, its pathogenesis remains complex and multifactorial. The immune system plays a central and critical role in the pathophysiology of psoriasis [
5]. Genetic susceptibility, in conjunction with immunological and environmental triggers, can activate keratinocytes to release pro-inflammatory cytokines that contribute to triggering the disease and progression [
6]. Psoriasis is characterized by chronic inflammation, aberrant differentiation, and the uncontrolled hyperproliferation of keratinocytes. It is predominantly mediated by T helper cell 1 (Th1) lymphocytes, as evidenced by increased levels of interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α) within psoriatic lesions. Furthermore, the identification of Th17 cells and the interleukin (IL)-23/IL-17 axis has underscored their pivotal roles in psoriasis immunopathogenesis [
5]. The disease involves a wide array of immune cell populations, and the induction of keratinocyte hyperproliferation is closely associated with elevated concentrations of key pro-inflammatory mediators, including TNF-α, IL-17, IL-22, IL-23, IL-1β, and IFN-γ. In moderate-to-severe psoriasis, systemic upregulation of these cytokines has been well-documented, and chronic exposure may result in tissue damage and contribute to the onset of comorbid conditions [
7].
Currently, with advancements in studying the skin’s microbiota, its role in the pathology of psoriasis has been widely investigated, as changes in the microbiota have been associated with several dermatological diseases. Skin microbiota plays a fundamental role in maintaining integrity and functions as a protective barrier. It is believed that changes in the local microbiota can contribute significantly to the disease, as psoriatic lesions present substantially different characteristics from healthy skin microbiota [
4]. Several studies show the importance of the composition of the skin and intestinal microbiota in exacerbating psoriasis [
8]. Most studies show high amounts of
Staphylococcus aureus in the skin microbiota of patients with psoriasis, reaching levels approximately 4.5 times higher compared to healthy people [
3].
There is evidence that exacerbation of the disease may be associated with an increase not only in species such as
S. aureus, but also in
Malassezia furfur and
Candida albicans in the skin and intestine of patients affected by the disease [
9].
S. aureus is regarded as one of the most significant human pathogens, associated with a wide range of infections, from superficial skin conditions to severe systemic diseases. Its capacity to invade and persist within host cells, such as keratinocytes, may contribute to the persistence or recurrence of infections and potentially facilitate the progression to deeper tissue involvement or systemic dissemination [
10]. Literature data indicate that
Malassezia species can affect the inflammatory and immune response in psoriasis through mechanisms such as damaging and disrupting the epidermal barrier and inducing greater production of pro-inflammatory cytokines involved in cellular hyperproliferation triggered by the fungus invading keratinocytes and altering cytokine synthesis [
1]. The composition of the microbiota is crucial for triggering adequate inflammatory and immune responses, as changes can exacerbate or increase susceptibility to skin and/or inflammatory diseases [
11].
Treatment is chosen based on the classification of psoriasis (mild, moderate, or severe) and generally begins with topical formulations. It is estimated that around 70 to 80% of patients have mild psoriasis and require only topical treatment [
12]. According to the American Academy of Dermatology, topical medications are the most used agents in the management of mild to moderate psoriasis [
13] and play a crucial role in disease treatment. For mild psoriasis, topical therapy is considered the gold standard. The primary goals of topical treatment are to achieve patient satisfaction and complete or partial disease remission [
14]. Furthermore, patient preferences and experiences should be considered [
15]. The topical agents employed in psoriasis treatment include emollients, keratolytics, topical corticosteroids, vitamin D analogs, topical immunomodulators, coal tar, and anthralin [
16]. In certain cases, such as the involvement of specific lesion areas or failure of topical therapy, combination therapy with multiple agents or phototherapy may be warranted [
17].
Systemic treatment is indicated when topical therapy proves ineffective or in moderate to severe cases of psoriasis [
17]. The main systemic agents include methotrexate, acitretin, cyclosporine A, and biologic immunomodulators. Oral systemic medications may be preferred by patients with limited access to biologics or those who favor non-injectable treatments [
18]; however, reduced efficacy and adverse effects associated with drugs such as methotrexate and cyclosporine often impact patient adherence, increasing the risk of disease relapse [
4]. An effective approach to disease management is the combination of topical treatments, systemic therapies, and phototherapy. Although significant advances have been made in psoriasis management, current treatments still present some limitations. Regarding topical therapy, recent studies have explored novel drugs and strategies to enhance the efficacy of topical agents and, consequently, improve patient adherence [
18].
Despite available therapeutic options, patients report dissatisfaction with topical formulations due to skin irritation, stains on clothes, excessive stickiness, unpleasant odor, and problems related to formulation tolerance, which directly affect adherence to treatment and remission of the disease [
12,
17]. In this scenario of new strategies for treating the skin’s microbiota, there is growing interest in prebiotics and postbiotics. Prebiotics are used as food by microorganisms and can have beneficial effects on human health. Studies have demonstrated the beneficial effects of prebiotics on the gut microbiota, such as the reduction in toxic metabolites in the colon [
19]. Dietary prebiotics and probiotics have the potential to promote healthy microbiota and are of interest in the management of autoimmune inflammatory diseases, as they can modulate the immune system to regulate mechanisms related to the production of pro-inflammatory cells [
20].
β-glucans are natural polysaccharides that serve as structural components of the cell walls of plants, fungi, and bacteria, and are considered potential prebiotics. β-glucans extracted from yeast, filamentous fungi, seaweed, or derived from oats exhibit immunomodulatory activities [
21]. Beyond their immunomodulatory properties, β-glucans may confer additional health benefits, including effects on diabetes, infections, and cholesterol reduction [
22]. Regarding skin health, potential benefits have been described due to their antioxidant, antimicrobial, anti-aging, and wound-healing activities, as well as their role in immune system modulation [
23].
Oat-derived β-(1,3;1,4)-glucan functions as a dietary fiber and may play a significant role in conferring resistance to infectious diseases, while yeast-derived β-glucans exhibit immunomodulatory activity. Studies suggest that dietary components such as β-glucans may participate in defense against pathogenic microorganisms and modulate the functions of neutrophils and macrophages [
24]. Fahlquist-Hagert et al. (2022) evaluated the activity of three β-glucan variants (1,6-β-glucan, 1,3-β-glucan, and 1,3;1,6-β-glucan) in the development of psoriasis and psoriatic arthritis using an in vivo model. The study showed that β-glucans negatively regulate disease progression through anti-inflammatory actions and may improve symptoms of psoriasis and psoriatic arthritis, representing a novel therapeutic avenue to be explored for clinical management of these conditions [
25].
Like prebiotics, postbiotics do not contain live microorganisms and exert beneficial effects akin to those of probiotics, while minimizing the risks associated with ingestion [
26]. Postbiotics are substances released or produced through the metabolic activity of bacteria and fungi, offering benefits to human health. Examples of postbiotics include exopolysaccharides, short-chain fatty acids, and antioxidant enzymes. Studies have shown the effectiveness of postbiotics produced by probiotic species like
Lactobacillus and
Saccharomyces cerevisiae [
27,
28], with promising results for topical formulations containing postbiotics aiding in the healing of skin wounds [
29,
30].
These compounds are primarily obtained through fermentations conducted by lactic acid bacteria and yeasts [
31]. Postbiotics are predominantly produced by bacteria of the genus
Lactobacillus and yeast
S. cerevisiae [
32]. Postbiotics exhibit antimicrobial, immunomodulatory, antioxidant, and anti-inflammatory activities. Their mechanisms of action may involve direct effects on host cells or modulation of the microbiota by promoting the growth of beneficial microorganisms and inhibiting pathogenic species [
31]. Current literature indicates that the use of prebiotics and postbiotics represents a promising strategy for restoring skin microbiota balance, thereby benefiting patients with dermatological conditions through such therapeutic approaches [
29,
30]. One study showed that the incorporation of postbiotics into cosmetic formulations can modulate skin microbiota by altering the diversity and composition of facial microbial communities compared to baseline, contributing to overall skin health [
33]. Additional research supports the efficacy of postbiotics derived from probiotic species such as
Lactobacillus and
S. cerevisiae [
27].
The application of probiotics and postbiotics in dermatological conditions is still in its early stages, and more extensive studies are required to evaluate their clinical applications, potential risks, and mechanisms of action on the skin. Nevertheless, research indicates that certain probiotic strains and their metabolites can enhance skin barrier function and improve skin appearance. Additionally, improvements in skin hydration, prevention of wrinkle formation, and alleviation of symptoms associated with dermatological diseases have been reported [
34]. Therefore, further investigations are needed to identify and characterize the metabolites produced by probiotics [
31], as well as to elucidate their activities within the skin microbiota and potential incorporation into topical formulations. Such research is essential for the development of products aimed at benefiting skin microbiota and managing associated dermatological disorders.
Given the limitations of current topical treatments for psoriasis [
17], there is a growing need to develop new formulations for topical application that meet patient needs and provide safety and efficacy in treatment [
23]. One study reports the development of a silver-containing wound gel composed of polyvinyl alcohol and a functional aryloxycyclotriphosphazene derivative. Antimicrobial testing showed that the gel inhibits several key skin pathogens, including
S. aureus. The gel presented significant wound healing capabilities in vivo. Additionally, the level of C-reactive protein in the blood serum of animals treated with the gel was 1.3 times lower (
p < 0.05), indicating reduced inflammation and enhanced tissue regeneration and epithelialization in the treatment group. The gel’s ability to absorb wound exudate and maintain a moist environment further supports its effectiveness as a wound dressing, highlighting its rapid epidermal regeneration and absence of secondary infections during the healing process [
35]. The development of a hydrogel incorporating a methanol extract from mango (
Mangifera indica) leaves was achieved by utilizing Carbopol as a gelling agent. This formulation showed substantial antibacterial activity against
S. aureus strains, including clinical isolates, both in vitro and ex vivo on porcine skin models. The gel’s composition included phytochemicals (tannins, saponins, flavonoids, phenols, and coumarins), which contributed to its antimicrobial properties [
36].
Gels based on hydroxyethyl cellulose (HEC) are cost-effective and can tolerate a wide range of pH values. They are easy to use, capable of absorbing active ingredients, and have a lower potential for staining clothes [
17,
23], making them an attractive alternative for incorporating active ingredients in the treatment of psoriasis. The objective of this study is to evaluate the activity of prebiotics based on β-glucans and postbiotics from
L. paracasei and
S. cerevisiae, as well as topical formulations containing these active ingredients, against
S. aureus and
M. furfur in vitro. This is aimed at providing new, effective strategies to help rebalance the microbiota of skin affected by psoriasis.
3. Results
3.1. Growth Curve of Microorganisms
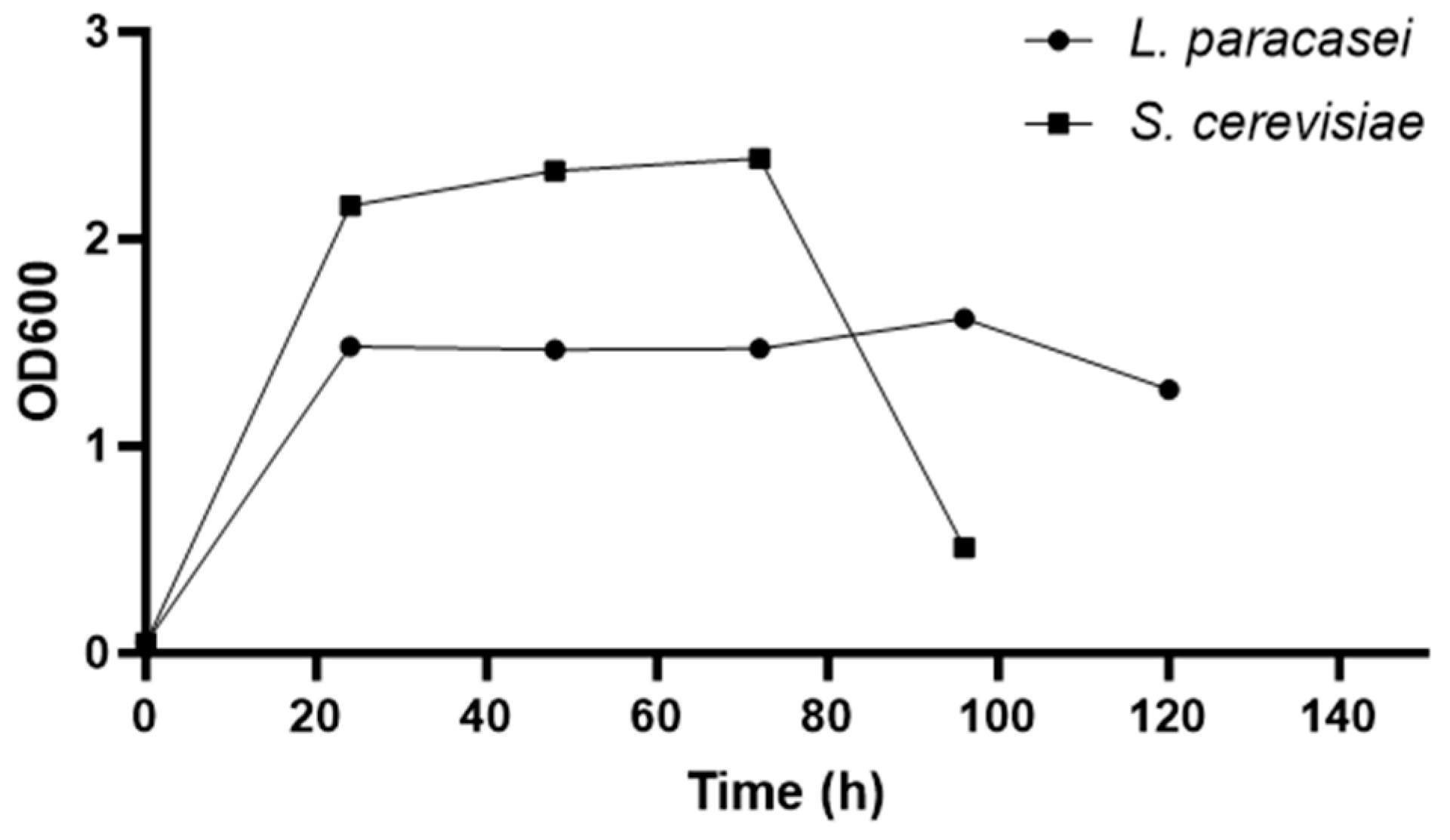
The growth curve of the microorganisms was determined based on measurements of their absorbance over time. As shown in
Figure 1, the log phase (exponential phase) of
L. paracasei occurs within the first 24 h. After this period, the stationary phase begins and continues until approximately 96 h. For
S. cerevisiae, the exponential phase also occurs within the first 24 h, and the stationary phase extends from 24 to 72 h after incubation of the microorganism. To obtain extracts at the onset of the stationary phase, the probiotics were incubated for 48 h prior to conducting the experiments.
3.2. Broth Microdilution Tests
The crude
L. paracasei extract showed antibacterial action against 10
6 CFU/mL of
S. aureus. The concentration of 2.1 mg/mL led to an average inhibition of bacterial growth by 16% compared to the control (
p = 0.0075). No antibacterial activity was observed against 10
6 CFU/mL of
S. aureus within the concentration range of 2.1 mg/mL to 0.03281 mg/mL of the lyophilized
L. paracasei extract.
Figure 2A illustrates the performance of the higher concentrations of the lyophilized extract of
L. paracasei against
S. aureus, specifically in the range from 500 mg/mL to 3.9 mg/mL.
Figure 2B shows that the concentration range between 500 mg/mL and 125 mg/mL of the lyophilized extract of
L. paracasei reduced the growth of the fungus.
The results indicated that the concentration range between 500 mg/mL and 7.8 mg/mL of the lyophilized extract was able to significantly interfere with bacterial growth due to the decrease in absorbance compared to the positive control. Concentrations of 500 mg/mL, 250 mg/mL, and 125 mg/mL exhibited absorbances close to the negative control and significantly inhibited the growth of S. aureus by 97.6% (0.577 versus 0.014, p < 0.0001), 98.3% (0.577 versus 0.01, p < 0.0001), and 96.4% (0.577 versus 0.021, p < 0.0001), respectively. This suggests that unknown molecules with antibacterial action, produced by the secondary metabolism of L. paracasei, are present in greater quantities in the highest concentrations of the extract and, consequently, demonstrate greater activity compared to other concentrations.
The crude L. paracasei extract showed antimycotic action against 105 CFU/mL of M. furfur. Among the concentrations assessed, 2.1 mg/mL of the extract inhibited the growth of M. furfur by 33.5% (p = 0.0230), and 1.05 mg/mL inhibited it by 35.81% (p = 0.0422). Concentrations of 2.1 mg/mL and 1.05 mg/mL of the lyophilized extract of L. paracasei interfered with the growth of M. furfur by approximately 26.57% (p = 0.0418) and 28.7% (p = 0.0373), respectively. Concentrations of 500 mg/mL, 250 mg/mL, and 125 mg/mL of the extract significantly inhibited the growth of 105 CFU/mL of M. furfur by 89.63%, 51.68%, and 34.4%, respectively. Concentrations starting from 62.5 mg/mL showed no significant difference compared to the positive control in this test. The lower concentrations of the lyophilized extract showed greater variability in response against M. furfur.
Concentrations ranging from 2.1 mg/mL to 0.03281 mg/mL of the lyophilized extract of
S. cerevisiae also did not show antibacterial action against 10
6 CFU/mL of
S. aureus.
Figure 3A indicates that the concentration range between 500 mg/mL and 7.8 mg/mL of the lyophilized extract of
S. cerevisiae was capable of interfering with the growth of the bacteria.
Figure 3B illustrates that the concentration range between 500 mg/mL and 125 mg/mL of the lyophilized extract of
S. cerevisiae was capable of interfering with the growth of the fungus.
The concentration of 500 mg/mL exhibited an absorbance close to that of the negative control and inhibited the growth of
S. aureus by approximately 97% (
p < 0.0001). Concentrations of 250 mg/mL, 125 mg/mL, 62.5 mg/mL, 31.25 mg/mL, 15.625 mg/mL, and 7.813 mg/mL also significantly interfered with and inhibited the growth of the bacteria by approximately 77.78%, 42.02%, 19.52%, 23.31%, 23.19%, and 17.05% (
p < 0.05), respectively. However, 3.9 mg/mL was not able to interfere with the growth of the microorganism. As observed in the results for
L. paracasei, it is proposed that unknown molecules produced by the secondary metabolism of
S. cerevisiae are present in greater quantities in the highest concentrations of the extract and, consequently, may exhibit greater antibacterial activity against
S. aureus at other concentrations. These findings suggest that high concentrations of
S. cerevisiae extract manifest antimycotic and antibacterial properties. While the molecules produced by the studied strain have yet to be identified, they demonstrate the capacity to prevent the growth of pathogenic microorganisms, such as
S. aureus and
M. furfur, as evidenced in previous studies [
28].
Concentrations ranging from 2.1 mg/mL to 0.03281 mg/mL of the lyophilized extract of S. cerevisiae did not demonstrate antimycotic action against 105 CFU/mL of M. furfur. The extract concentration of 500 mg/mL significantly inhibited the growth of 105 CFU/mL of M. furfur by 94.9% (p < 0.0001). Concentrations of 250 mg/mL and 125 mg/mL inhibited fungal growth by 65.11% (p = 0.0001) and 26.56% (p = 0.0257), respectively. Concentrations starting from 62.5 mg/mL were not able to interfere with the growth of the fungus. Both lyophilized extracts showed greater antimycotic activity at concentrations between 500 mg/mL and 125 mg/mL, indicating the presence of molecules with potential antimycotic activity in the mixture of metabolites obtained from the extracts of L. paracasei and S. cerevisiae. This activity appears to be concentration-dependent, with greater antimycotic potential observed at higher concentrations.
In the case of SymGlucan
®, the presence of sodium benzoate in the formulation did not affect the growth of the microorganism.
Figure 4A illustrates the effect of different concentrations of SymGlucan
® against
S. aureus.
Figure 4B illustrates the growth of
M. furfur in response to different concentrations of SymGlucan
®.
Concentrations of 0.5% and 0.25% of oat-derived β-glucan demonstrated significant antibacterial action within this concentration range. β-glucan at a concentration of 0.5% was able to inhibit the growth of the bacteria by 97.2% compared to the positive control (p = 0.0012). The lowest concentration capable of interfering with the growth of 106 CFU/mL of S. aureus was 0.25% β-glucan, which achieved an average inhibition of 57.8% (p = 0.0094). Concentrations below 0.25% β-glucan were not effective in interfering with bacterial growth and, therefore, did not exhibit antibacterial action. Concentrations ranging from 0.5% to 0.0625% of β-glucan were effective in interfering with the growth of the fungus, indicating potential antimycotic action within this concentration range. β-glucan at concentrations of 0.5%, 0.25%, 0.125%, and 0.0625% significantly inhibited the growth of 105 CFU/mL of M. furfur by 97.86% (0.327 versus 0.007, p = 0.0002), 90.33% (0.327 versus 0.031, p = 0.0003), 70.57% (0.327 versus 0.096, p = 0.0010), and 48.17% (0.327 versus 0.169, p = 0.0281) compared to the positive control, respectively. In comparison to the results obtained against S. aureus, lower concentrations of SymGlucan® were effective in interfering with the growth of the fungus. Moreover, 0.5% β-glucan demonstrated more than 97% inhibition of the growth of both microorganisms, indicating the antimicrobial potential of β-glucan in the concentration range studied.
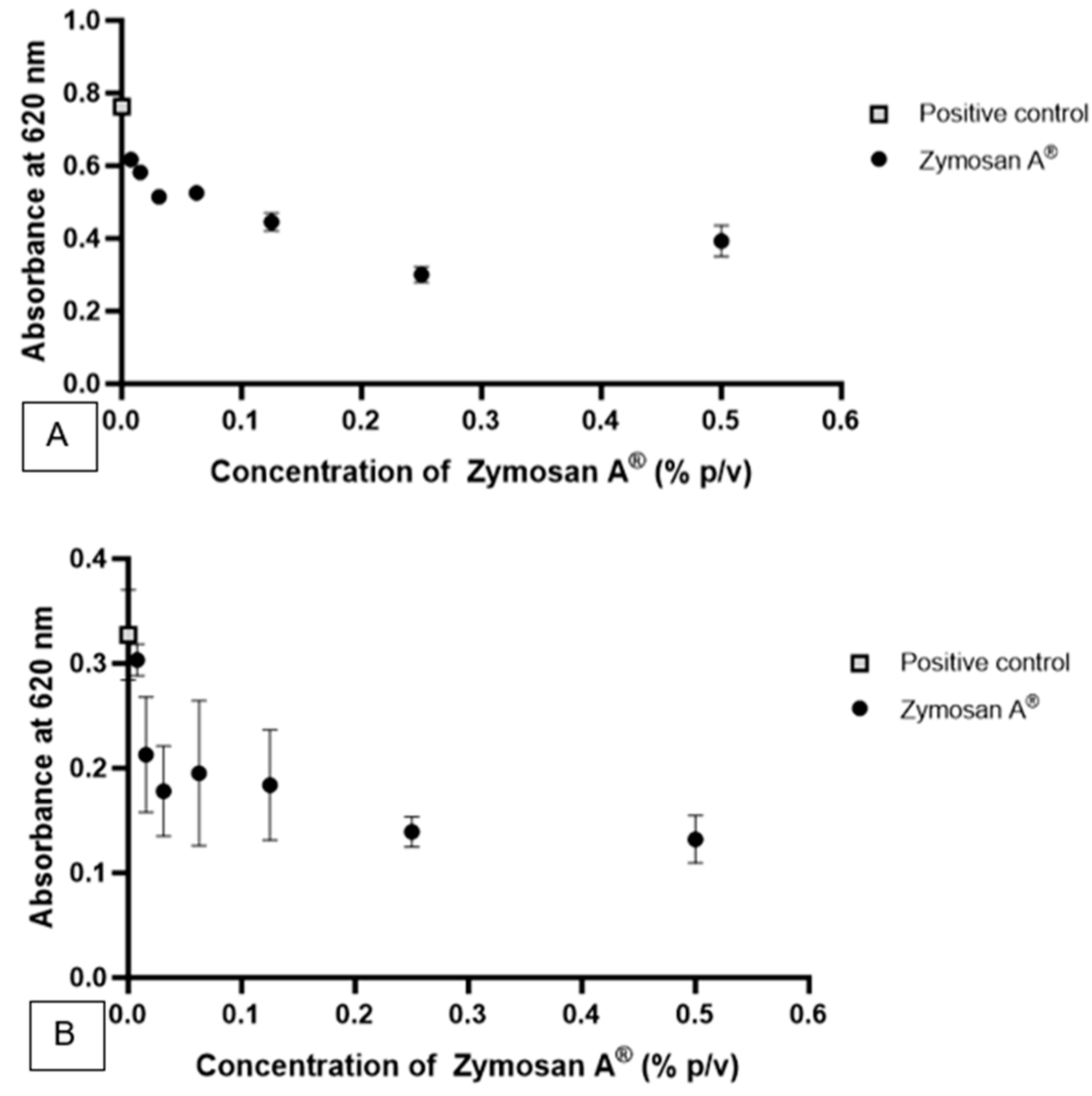
Zymosan A
® was utilized in the form of a suspension following dispersion in an aqueous system.
Figure 5A illustrates the effect of different concentrations of Zymosan A
® against
S. aureus.
Figure 5B illustrates the effect of different concentrations of Zymosan A
® against
M. furfur.
The concentrations studied, ranging from 0.5% to 0.0078% of β-glucan derived from the cell wall of S. cerevisiae, were able to significantly interfere with the growth of the bacteria, indicating the potential antibacterial action of β-glucan within this concentration range. Concentrations of 0.5%, 0.25%, 0.125%, 0.0625%, 0.03125%, 0.0156%, and 0.0078% inhibited the growth of 106 CFU/mL of S. aureus by approximately 48.47%, 60.66%, 41.70%, 31.18%, 32.58%, 23.8%, and 19.18% (p < 0.05), respectively. Concentrations ranging from 0.5% to 0.0156% of β-glucan significantly interfered with the growth of the fungus, indicating potential antimycotic action within this concentration range. β-glucan at concentrations of 0.5%, 0.25%, 0.125%, 0.0625%, and 0.03125% inhibited the growth of 105 CFU/mL of M. furfur by 59.67%, 57.43%, 43.79%, 40.33%, and 45.62% compared to the positive control (p < 0.05), respectively. Compared to the results against S. aureus, the highest concentrations of Zymosan A® showed greater inhibition of fungal growth. Additionally, relative to SymGlucan®, the oat-derived β-glucan exhibited greater antimycotic activity, achieving over 90% inhibition of fungal growth at concentrations of 0.5% and 0.25%.
3.3. HPLC Analysis
The main results were obtained at a wavelength of 360 nm.
Figures S1 and S2 indicate that the
L. paracasei extract has some substance or group of substances that generated the peaks in the chromatogram at 360 nm.
Figure S1 shows that, as observed in previous studies [
51],
L. paracasei produces molecules that are different from those observed in the culture medium and that have a greater affinity for ethyl acetate, which makes it of great interest to study these substances to determine the mechanisms involved in their therapeutic properties.
Figures S3 and S4 also indicate that the
S. cerevisiae extract has some substance or group of substances that generated the peaks in the chromatogram at 360 nm.
Figure S4 shows that there are 4 different peaks produced by
S. cerevisiae at the retention times of 2.1, 4.0 min, 34.2 min, and 43.2 min. Only the peak of 4.0 min was not observed in the chromatogram of the negative control. The peaks at 2.1 min, 34.2 min, and 43.2 min were observed in samples of both extracts and the negative control, which indicates the presence of some substance in the culture media that can be used as a basic component of the microorganisms’ metabolism.
3.4. Agar Diffusion Test—Postbiotics and Prebiotics
For prebiotics, concentrations of 0.25% to 1% were initially tested. A 1% concentration of SymGlucan® resulted in the formation of 9 mm halos around the wells for both microorganisms. In tests with Zymosan A®, no inhibition halo was formed in the concentration range between 2.5 mg/mL and 100 mg/mL, corresponding to concentrations between 0.25% and 10% of β-glucan. Zymosan A® is a product with a solid formulation that can be used in suspension after dispersion in an aqueous system. The sample dispersion may have impaired diffusion in the agar and, consequently, underestimated the antimicrobial activity of the product. As higher concentrations contained more suspended particles, Zymosan A® was tested up to a concentration of 10% and reserved for later incorporation into the topical formulation.
Regarding postbiotics, concentrations between 2.1 mg/mL and 32.03 mg/mL of crude and lyophilized extracts of
L. paracasei, and between 2.1 mg/mL and 11.04 mg/mL of crude and lyophilized extracts of
S. cerevisiae, did not show activity against the microorganisms. The concentrations of the crude extracts were calculated from the freeze-drying process, and since no activity was observed against microorganisms up to the maximum obtained concentrations, higher concentrations of the freeze-dried extracts, between 50 mg/mL and 1 g/mL, were tested. The results show that the concentration range between 50 mg/mL and 250 mg/mL of the lyophilized extract of
L. paracasei did not inhibit bacterial growth, and no inhibition halo was formed. Following the incorporation of TTC,
Figure S5 displays inhibition halos against
S. aureus corresponding to extract concentrations of 375 mg/mL and 500 mg/mL. These concentrations inhibited bacterial proliferation, forming inhibition zones with total diameters of 12 mm and 15 mm, respectively. These findings indicate that lyophilized
L. paracasei extract, at concentrations between 375 mg/mL and 500 mg/mL, can inhibit the growth of
S. aureus at 10
6 CFU/mL in the agar diffusion assay, as per the described methodology. At the highest concentration tested (1 g/mL), the lyophilized extract also exhibited activity against
M. furfur, producing a 13 mm inhibition halo observed both before and after TTC staining (
Figure S6). These results suggest that a concentration of 1 g/mL of
L. paracasei extract is effective in inhibiting
M. furfur growth at 10
5 CFU/mL under the same test conditions. The inhibition zone produced by the
S. cerevisiae extract measured only 9 mm against both microorganisms. Compared to the results with the lyophilized extract of
L. paracasei, the extract of
S. cerevisiae exhibited less activity against
S. aureus and
M. furfur.
3.5. Preparation of Gels and Agar Diffusion Test
To prepare the formulations, the samples were incorporated at a 1:1 ratio with the gel. The HEC-based gel was mixed with an equal volume of sterile distilled water (1:1). The formulations used in the test are described in
Table 1.
The gels were tested in the agar diffusion test against 106 CFU/mL of S. aureus and 105 CFU/mL of M. furfur. The growth of pathogenic microorganisms occurred on all plates, without the presence of inhibition halos in the areas where the gels were applied, except for the sample containing 1% SymGlucan®, which led to the formation of halos for both microorganisms, as observed in previous tests. The results indicated that HEC-based gels containing 0.5% to 5% of postbiotics and 500 mg/mL of lyophilized extracts did not exhibit antibacterial activity against 106 CFU/mL of S. aureus and 105 CFU/mL of M. furfur in the agar diffusion test.
4. Discussion
According to the database research, this is the first study to evaluate the antimicrobial activity of crude and lyophilized extracts of
L. paracasei,
S. cerevisiae, and β-glucans derived from oats and the cell wall of
S. cerevisiae against
S. aureus and
M. furfur in vitro, with the aim of using them in the development of topical formulations that show activity against microorganisms considered pathogenic in the skin microbiota. Pathogenic microorganisms such as
S. aureus and
M. furfur are present in greater quantity in the skin microbiota of patients with psoriasis, as well as in other dermatological diseases [
9,
51], making it necessary to conduct studies with molecules capable of interfering with the growth of these pathogens and, consequently, contributing to microbiota rebalancing and improvement of patient symptoms.
The growth kinetics of the probiotics were studied, and the results showed that the stationary phases of the
L. paracasei and
S. cerevisiae strains occur from the first 24 h of incubation. Extracts were obtained during the stationary phases of the probiotics, after 48 h of incubation, as compounds of interest are produced during this stage and have shown activity against pathogens [
27]. Currently, there is great interest in studying molecules produced by probiotics, as postbiotics offer health benefits and reduce the adverse effects that may arise from ingesting live microorganisms [
26]. In this study, it was not possible to identify the molecules produced by the strains, but they were characterized by HPLC chromatogram analysis, and the activity of the substances produced—with different polarities—was evaluated through extractions using ethyl acetate and butanol.
Despite the compounds observed in the HPLC results being unknown and thus making comparisons with the literature unfeasible, it is evident that the crude extract of
L. paracasei can interfere with the growth of pathogenic microorganisms at the studied concentrations, particularly against the fungus. In addition to studying the crude extract of the probiotic, the same tests were carried out with lyophilized extracts. Higher concentrations of the lyophilized extract resulted in greater activity against the pathogenic microorganisms. The relationship between concentration and activity was also observed in studies with Aloe vera extract against
M. furfur strains resistant to clotrimazole and isolated from patients with seborrheic dermatitis, where higher concentrations resulted in greater antimycotic activity [
52]. Concentrations of 500 mg/mL, 250 mg/mL, and 125 mg/mL of the extract inhibited the growth of 10
6 CFU/mL
S. aureus by more than 90%, while the highest concentration inhibited the growth of 10
5 CFU/mL
M. furfur by 89.63%. These results indicate that antibacterial and antimycotic molecules are produced by this
L. paracasei strain during the stationary growth phase and are present in the mixture of secondary metabolites.
Broth microdilution assays using the crude S. cerevisiae extract against M. furfur show that, although the compounds identified by HPLC analysis remain unknown, they may exert a synergistic effect and exhibit potential antimycotic activity at the tested concentrations. Crude extracts from both probiotics, as well as prebiotics, showed greater variability in results against M. furfur when compared to those observed for S. aureus. These findings reflect the inherent complexity of studying microbiota interactions and emphasize the need for standardized testing protocols involving Malassezia species.
Regarding
S. aureus, no inhibitory activity was observed with the crude
S. cerevisiae extract, possibly due to the absence of known antibacterial substances such as β-glucan and the concentration range studied. The
S. cerevisiae extract obtained in this study did not contain β-glucan because the polysaccharide is present in the yeast cell wall, and no experiments were conducted to break down the cell wall and obtain the active compound. Based on this study’s results and previous publications, β-glucan shows potential in inhibiting
S. aureus growth [
53], and its absence may explain the lack of antibacterial activity in the
S. cerevisiae extract. Additionally, studies with higher concentrations of yeast extract have shown promising antibacterial activity [
28,
41]. A study by Saidi et al. (2019) showed promising results when comparing the effects of
S. cerevisiae S3 extract against 10
5 CFU/mL of methicillin-resistant (MRSA) and methicillin-sensitive (MSSA)
S. aureus strains. The extract had a minimum inhibitory concentration of 4.096 mg/mL. Compounds produced by the yeast reduced bacterial biofilm formation, showing potential for controlling
S. aureus-caused infections [
41]. Another study showed that the
S. cerevisiae extract exhibited significant antibacterial activity against
S. aureus and
S. epidermidis at concentrations of 100 and 200 mg/mL, respectively [
28]. Therefore, higher concentrations of
S. cerevisiae extracts may have greater potential to inhibit bacterial growth. Therefore, it is suggested that higher concentrations of
S. cerevisiae extracts may have greater potential to inhibit bacterial growth.
Higher concentrations of the lyophilized extract—500 mg/mL, 250 mg/mL, and 125 mg/mL—were able to inhibit the growth of 10
5 CFU/mL
M. furfur by 94.9%, 65.11%, and 26.56%, respectively. For
S. aureus, the 500 mg/mL concentration inhibited bacterial growth by approximately 97%. The results of this study indicate that higher concentrations of
S. cerevisiae extract exhibit antimycotic and antibacterial activities and, despite the molecules produced by the studied strain being unknown, they have the potential to aid in the inhibition or interference of the growth of pathogenic microorganisms such as
S. aureus and
M. furfur, as observed in previous studies [
28].
The product SymGlucan
®, containing 1% β-glucan derived from oats [
54], showed promising in vitro activity against
S. aureus and
M. furfur. Oat-derived β-glucan at a 0.5% concentration inhibited over 90% of microbial growth. The product’s antimicrobial activity was concentration-dependent, with higher concentrations resulting in greater efficacy. Previous research has shown that β-glucans extracted from yeast, fungi, or oats exhibit immunomodulatory activities capable of stimulating immune functions. The β-(1,3;1,4)-glucan from oats may also play an important role in providing resistance to infectious diseases [
55], stimulating collagen, balancing extracellular matrix proteins, and reducing skin roughness [
54]. Zymosan A
®, a natural β-1,3-glucan polysaccharide present in the yeast cell wall [
56], also showed antibacterial and antimycotic activity. Unlike oat-derived β-glucan, the product did not inhibit more than 90% of the growth of 10
6 CFU/mL
S. aureus. Bacterial growth inhibition was also concentration-dependent. Unlike SymGlucan
®, the lowest studied concentration (0.0078%) of β-glucan was still able to interfere with bacterial growth, inhibiting it by 19.18% compared to the positive control. Although the product did not inhibit microbial growth above 90%, as observed with lyophilized extracts at concentrations above 125 mg/mL and SymGlucan
® at β-glucan concentrations above 0.25%, the results are still considered promising. According to the literature, fungal β-glucans have antitumor, immunomodulatory, and anti-inflammatory properties [
22]. Based on these findings, there is an interest in incorporating
S. cerevisiae β-glucans into topical formulations to evaluate their activity against pathogenic microorganisms.
The agar diffusion test results indicate that, as observed in the broth microdilution test, the
L. paracasei extract showed greater activity against both microorganisms compared to the
S. cerevisiae extract. SymGlucan
®, containing 1% β-glucan, formed inhibition zones against both microorganisms, demonstrating the compound’s potential to interfere with the growth of pathogenic microorganisms. A limitation of the agar diffusion test was observed with Zymosan
®, which, due to its viscous dispersion, was unable to diffuse in the agar; even after 48 h of incubation, the product remained in the wells. Fozouni et al. (2018) evaluated the activity of aloe vera extract against
M. furfur strains resistant to clotrimazole and isolated from seborrheic dermatitis patients. In the well diffusion method, the inhibition zone diameter increased with extract concentration, with the largest diameter observed at 250 mg/mL, similar to the relationship between high concentrations and inhibition zone diameters observed in this study [
52].
Despite the difficulties encountered with Zymosan
® in the test, all actives were incorporated into gels to assess whether this would aid diffusion in agar and to evaluate the release capacity of the actives from the gel and their ability to interfere with the growth of 10
6 CFU/mL
S. aureus and 10
5 CFU/mL
M. furfur. Although the broth microdilution and agar diffusion tests showed promising results with the isolated actives, hydroxyethylcellulose-based gels containing 0.5% oat β-glucan, 0.5–5% yeast β-glucan, and 500 mg/mL of
L. paracasei and
S. cerevisiae extracts did not produce inhibition zones and, consequently, showed no antibacterial or antimycotic activity. Mehdi-Alamdarloo et al. (2016) conducted a study to evaluate the activity of a hydroxypropyl methylcellulose (HPMC)-based gel containing
Lactobacillus casei extract against four species of dermatophytes. The formulation containing 5% probiotic extract exhibited significantly greater activity than gels containing 3% or 4% extract, indicating that HPMC-based gel formulations with these concentrations of extracts and β-glucans are suitable for incorporating the extracts [
29].
The actives tested in this work formed inhibition zones when tested alone, and none were observed when incorporated into gels. It is suggested that the active compounds were not available, possibly due to interaction with the cellulose polymer, as the compounds in the extracts are unknown, making it difficult to select an appropriate gel base. Additionally, for the prebiotics, only the highest concentration of SymGlucan® showed activity on its own, and the formulation of Zymosan® as a dispersion may have affected gel homogeneity and active incorporation. The results of this study are promising and show that the compounds tested can interfere with the growth of S. aureus and M. furfur in vitro with increasing concentrations. Based on the findings, further studies are warranted to identify the molecules produced by the probiotics with antimicrobial activity, as well as to evaluate their incorporation into various topical formulations and their release potential.