Therapeutic Potential of Clerodendrum glabrum and Gardenia volkensii Acetone Extracts: Antioxidant, Antibacterial, and Anti-Virulence Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Collection
2.3. Extraction
2.4. Quantitative Phytochemical Screening
2.4.1. Total Phenolic Content
2.4.2. Total Flavonoid Content
2.4.3. Total Flavonol Content
2.4.4. Total Tannin Content
2.5. Antioxidant Activity
2.5.1. DPPH Free Radical Scavenging Assay
2.5.2. Hydrogen Peroxide Scavenging Assay
2.6. Antibacterial Activity
2.6.1. Preparation of Microorganisms
2.6.2. Broth Microdilution Assay
2.6.3. Determination of Minimum Bactericidal Concentration (MBC)
2.6.4. Total Activity (TA)
2.6.5. Evaluation of Growth Kinetics During Treatment
2.6.6. Combinational Effects
2.7. Anti-Virulence Activity
2.7.1. Antibiofilm Activity (Biomass)
2.7.2. Inhibition of Initial Cell Attachment
2.7.3. Eradication of Preformed Biofilms
2.7.4. Inhibition of Biofilm Formation
2.7.5. Crystal Violet Staining Assay
2.7.6. Evaluation of Metabolic Activity
2.7.7. Antimotility Activity
2.8. Statistical Analysis
3. Results
3.1. Extraction of Plant Material
3.2. Quantitative Phytochemical Analysis
3.3. Evaluation of the Antioxidant Activity of the Extracts
3.3.1. Free Radical Scavenging Activity Assay
3.3.2. Hydrogen Peroxide Assay
3.4. Antibacterial Activity Analysis
3.5. Combinational Studies
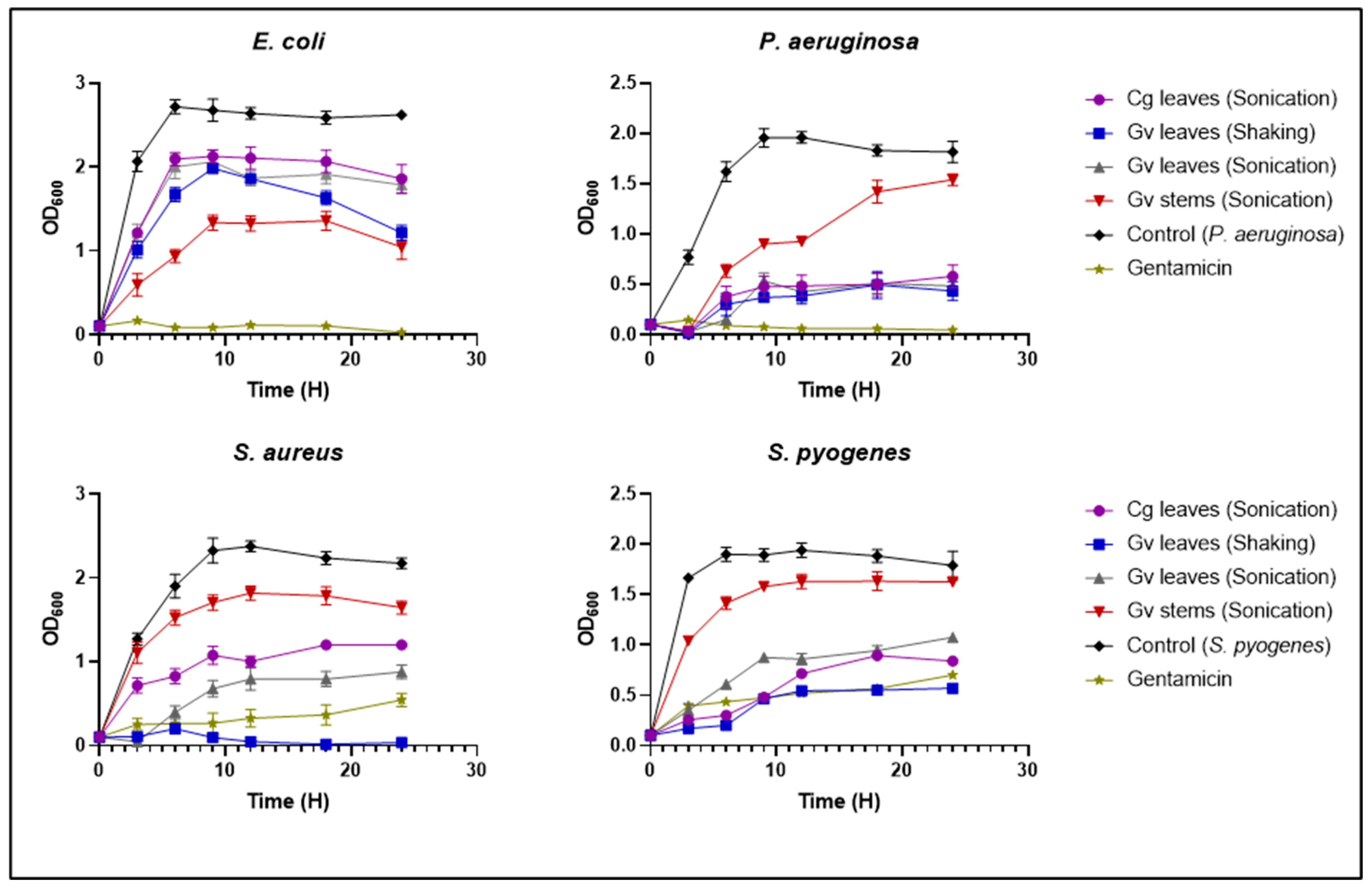
3.6. Growth Kinetics
3.7. Anti-Virulence Activity Analysis
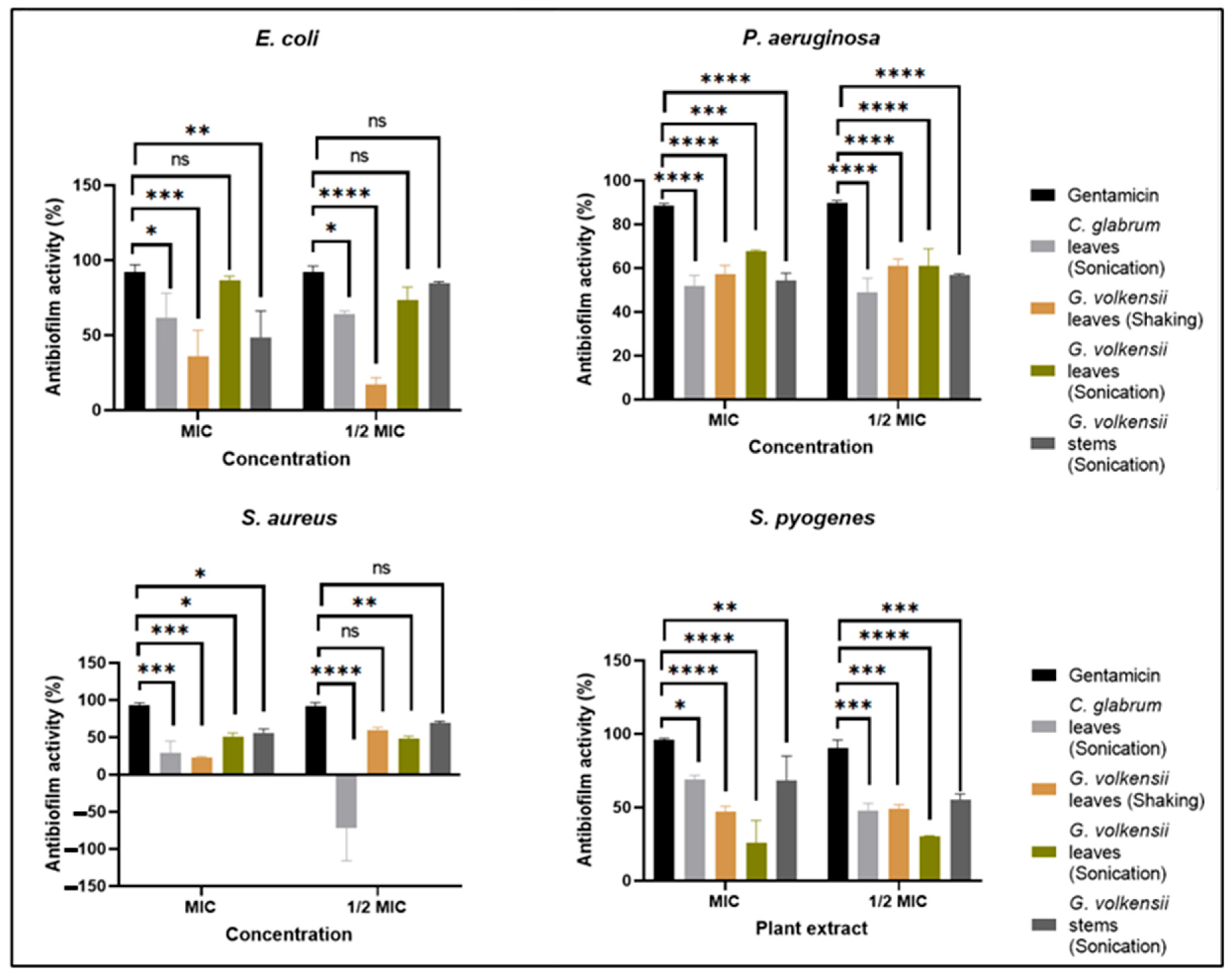
3.7.1. Antibiofilm Biomass Activity
Inhibition of Initial Cell Attachment of the Test Microorganisms
Inhibition of Biofilm Formation of the Test Microorganisms
Eradication of Preformed Biofilms of the Test Microorganisms
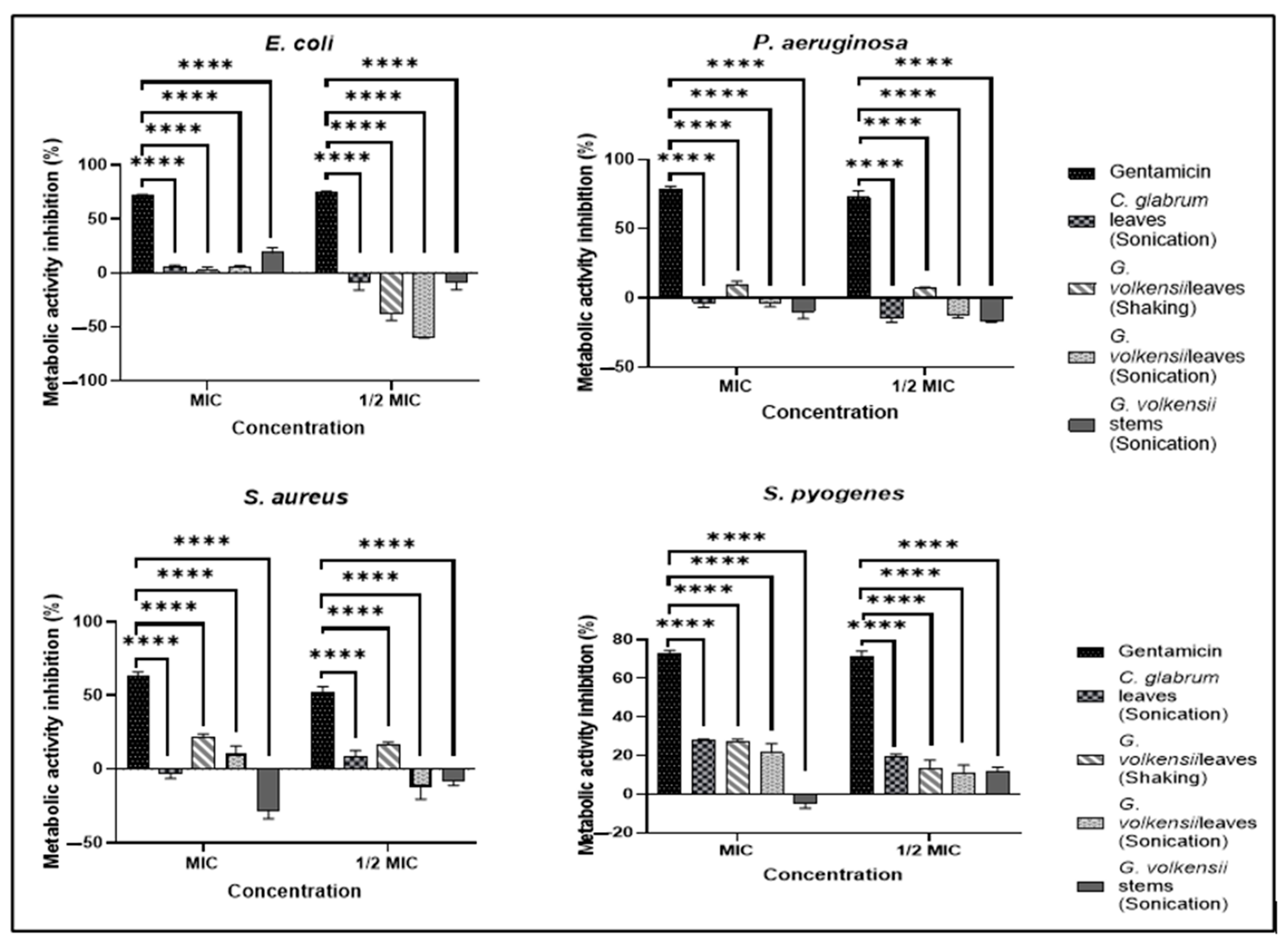
3.7.2. Metabolic Activity
3.7.3. Antimotility Activity
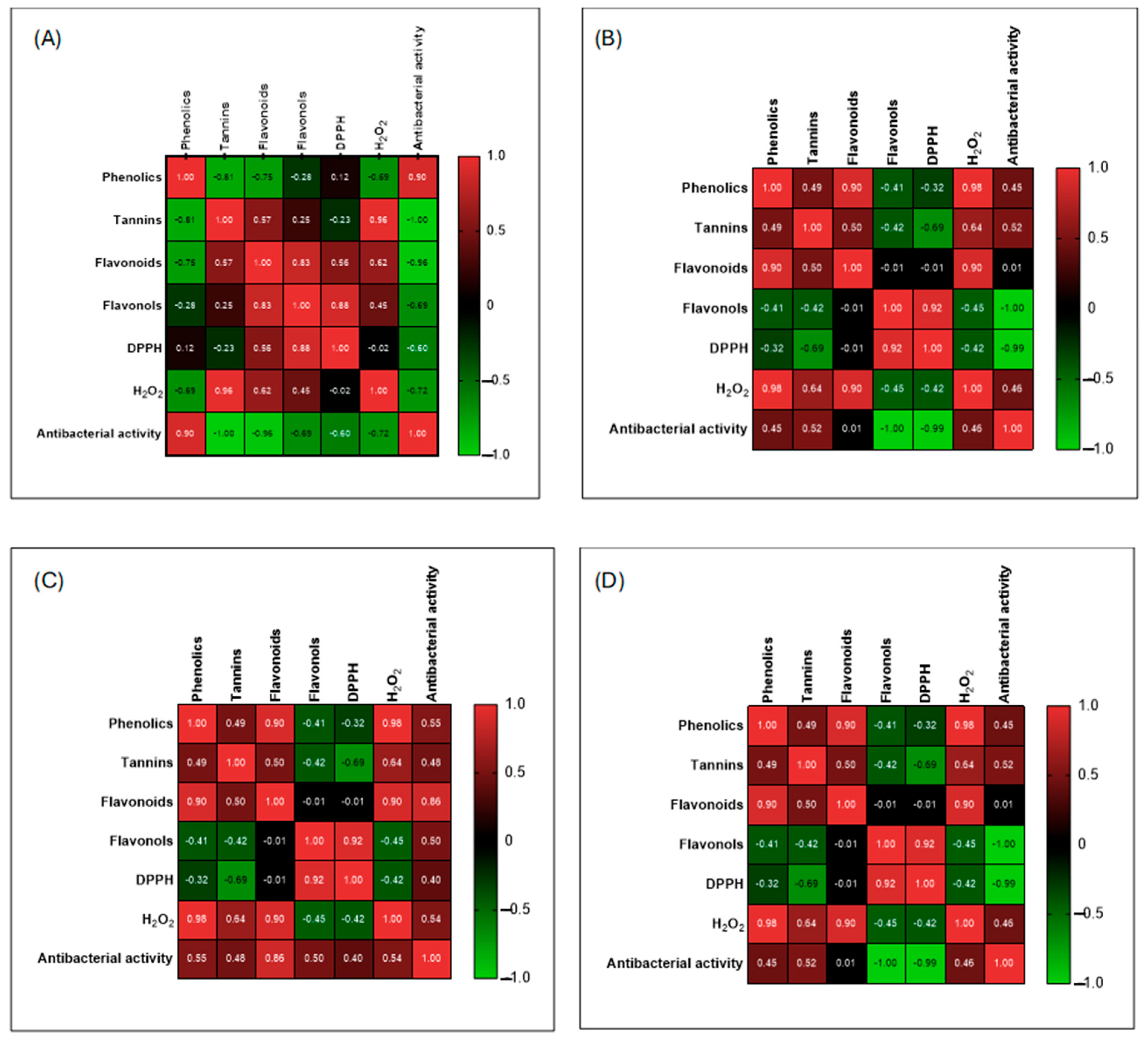
3.8. Pearson Correlation Between Phytochemicals and Biological Activities of the Plant Extracts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| ATCC | American Type Culture Collection |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| EC50 | Half maximal effective concentration |
| ESBL | Extended-spectrum beta-lactamase |
| FIC | Fractional inhibitory concentration |
| FICI | Fractional inhibitory concentration index |
| INT | p-Iodonitrotetrazolium chloride |
| mg GAE/g | Milligrams of gallic acid equivalents per gram of extract |
| mg QE/g | Milligrams of quercetin equivalents per gram of extract |
| MIC | Minimum inhibitory concentration |
| MBC | Minimum bactericidal concentration |
| NHLS | National Health Laboratory Service |
| OD600 | Optical density at 600 nanometres |
| TA | Total activity |
References
- Sionov, R.V.; Steinberg, D. Targeting the Holy Triangle of Quorum Sensing, Biofilm Formation, and Antibiotic Resistance in Pathogenic Bacteria. Microorganisms 2022, 10, 1239. [Google Scholar] [CrossRef] [PubMed]
- Famuyide, I.M.; Aro, A.O.; Fasina, F.O.; Eloff, J.N.; McGaw, L.J. Antibacterial and antibiofilm activity of acetone leaf extracts of nine under-investigated South African Eugenia and Syzygium (Myrtaceae) species and their selectivity indices. BMC Complement. Altern. Med. 2019, 19, 141. [Google Scholar] [CrossRef] [PubMed]
- Vivas, R.; Barbosa, A.A.T.; Dolabela, S.S.; Jain, S. Multidrug-Resistant Bacteria and Alternative Methods to Control Them: An Overview. Microb. Drug Resist. 2019, 25, 890–908. [Google Scholar] [CrossRef] [PubMed]
- Uruén, C.; Chopo-Escuin, G.; Tommassen, J.; Mainar-Jaime, R.C.; Arenas, J. Biofilms as Promoters of Bacterial Antibiotic Resistance and Tolerance. Antibiotics 2020, 10, 3. [Google Scholar] [CrossRef]
- National Department of Health (South Africa). Surveillance for Antimicrobial Resistance and Consumption of Antibiotics in South Africa 2018–2022; South African AMR Surveillance Report; National Department of Health: Pretoria, South Africa, 2024. [Google Scholar]
- Bhandari, S.; Khadayat, K.; Poudel, S.; Shrestha, S.; Shrestha, R.; Devkota, P.; Khanal, S.; Marasini, B.P. Phytochemical analysis of medicinal plants of Nepal and their antibacterial and antibiofilm activities against uropathogenic Escherichia coli. BMC Complement. Med. Ther. 2021, 21, 116. [Google Scholar] [CrossRef]
- Uc-Cachón, A.H.; Dzul-Beh, A.; Palma-Pech, G.; Jiménez-Delgadillo, B.; Flores-Guido, J.S.; Gracida-Osorno, C.; Molina-Salinas, G.M. Antibacterial and antibiofilm activities of Mayan medicinal plants against Methicillin-susceptible and -resistant strains of Staphylococcus aureus. J. Ethnopharmacol. 2021, 279, 114369. [Google Scholar] [CrossRef]
- Adeyemo, R.O.; Famuyide, I.M.; Dzoyem, J.P.; McGaw, L.J. Anti-Biofilm, Antibacterial, and Anti-Quorum Sensing Activities of Selected South African Plants Traditionally Used to Treat Diarrhoea. Evid. Based Complement. Altern. Med. 2022, 2022, 1307801. [Google Scholar] [CrossRef]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef]
- Salmerón-Manzano, E.; Garrido-Cardenas, J.A.; Manzano-Agugliaro, F. Worldwide Research Trends on Medicinal Plants. Int. J. Environ. Res. Public Health 2020, 17, 3376. [Google Scholar] [CrossRef]
- Kebede, T.; Gadisa, E.; Tufa, A. Antimicrobial activities evaluation and phytochemical screening of some selected medicinal plants: A possible alternative in the treatment of multidrug-resistant microbes. PLoS ONE 2021, 16, e0249253. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards Advances in Medicinal Plant Antimicrobial Activity: A Review Study on Challenges and Future Perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef] [PubMed]
- Manandhar, S.; Luitel, S.; Dahal, R.K. In Vitro Antimicrobial Activity of Some Medicinal Plants against Human Pathogenic Bacteria. J. Trop. Med. 2019, 2019, 1895340. [Google Scholar] [CrossRef] [PubMed]
- Calixto, J.B. The role of natural products in modern drug discovery. An. Acad. Bras. Cienc. 2019, 91, e20190105. [Google Scholar] [CrossRef] [PubMed]
- Raja, R.R. Medicinally Potential Plants of Labiatae (Lamiaceae) Family: An Overview. Res. J. Med. Plant 2012, 6, 203–213. [Google Scholar] [CrossRef]
- Teclegeorgish, Z.W.; Mokgalaka, N.S.; Vukea, N.; de la Mare, J.; Tembu, V.J. Cytotoxicity of triterpenoids from Clerodendrum glabrum against triple-negative breast cancer cells in vitro. S. Afr. J. Bot. 2020, 133, 144–150. [Google Scholar] [CrossRef]
- Ogundajo, A.L.; Ashafa, A.O.T. Medicinal properties of Clerodendrum glabrum leaf extracts: Phytochemical constituents, antioxidant, cytotoxicity, and carbohydrate-metabolizing enzyme inhibitory potentials. Comp. Clin. Pathol. 2019, 28, 927–936. [Google Scholar] [CrossRef]
- Maroyi, A. Gardenia volkensii K. Schum. (Rubiaceae): Review of Medicinal Uses, Phytochemistry, and Biological Activities. J. Pharm. Nutr. Sci. 2020, 10, 175–181. [Google Scholar] [CrossRef]
- Kinuthia, E.W.; Langat, M.K.; Mwangi, E.M.; Cheplogoi, P.K. Constituents of Kenyan Gardenia volkensii. Nat. Prod. Commun. 2012, 7, 13–14. [Google Scholar] [CrossRef]
- Matotoka, M.M.; Mashabela, G.T.; Masoko, P. Phytochemical content, antibacterial activity, and antioxidant, anti-inflammatory, and cytotoxic effects of traditional medicinal plants against respiratory tract bacterial pathogens. Evid. Based Complement. Altern. Med. 2023, 2023, 1243438. [Google Scholar] [CrossRef]
- Mnisi, T.J.; Matotoka, M.M.; Masoko, P. Antioxidant, antibacterial, and anti-biofilm activities of selected indigenous plant species against nosocomial bacterial pathogens. Lett. Appl. Microbiol. 2024, 77, ovae080. [Google Scholar] [CrossRef]
- Wanjiru, K.E. Screening for Antimicrobial Compounds in Gardenia volkensii and Meyna tetraphylla (Rubiaceae). Afribary. Published 17 May 2021. Available online: https://afribary.com/works/screening-for-antimicrobial-compounds-in-gardenia-volkensii-and-meyna-tetraphylla-rubiaceae (accessed on 1 June 2025).
- Bitwell, C.; Indra, S.S.; Luke, C.; Kakoma, M.K. A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants. Sci. Afr. 2023, 19, e01585. [Google Scholar] [CrossRef]
- Tambe, V.D.; Bhambar, R.S. Estimation of total phenol, tannin, alkaloid and flavonoid in Hibiscus tiliaceus Linn. wood extracts. Res. Rev. J. Pharmacogn. Phytochem. 2014, 2, 41–47. [Google Scholar]
- Madjid, A.; Amoussa, O.; Sanni, A.; Lagnika, L. Antioxidant activity and total phenolic, flavonoid and flavonol contents of the bark extracts of Acacia ataxacantha. J. Pharmacogn. 2015, 4, 172–178. [Google Scholar]
- Chigayo, K.; Mojapelo, P.E.L.; Mnyakeni-Moleele, S.; Misihairabgwi, J.M. Phytochemical and antioxidant properties of different solvent extracts of Kirkia wilmsii tubers. Asian Pac. J. Trop. Biomed. 2016, 6, 1037–1043. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Jaganmohan Rao, L.; Sakariah, K.K. Antioxidant activities of flavidin in different in vitro model systems. Bioorg Med. Chem. 2004, 12, 5141–5146. [Google Scholar] [CrossRef]
- Eloff, J.N. A Sensitive and Quick Microplate Method to Determine the Minimal Inhibitory Concentration of Plant Extracts for Bacteria. Planta Med. 1998, 64, 711–713. [Google Scholar] [CrossRef]
- Senhaji, S.; Lamchouri, F.; Toufik, H. Phytochemical content, antibacterial and antioxidant potential of Anabasis aretioïdes (Chenopodiaceae). Biomed. Res. Int. 2020, 2020, 6152932. [Google Scholar] [CrossRef]
- Jiang, T.; He, L.; Zhan, Y.; Zang, S.; Ma, Y.; Zhao, X.; Zhang, C.; Xin, Y. The effect of MSMEG_6402 gene disruption on the cell wall structure of Mycobacterium smegmatis. Microb. Pathog. 2011, 51, 156–160. [Google Scholar] [CrossRef]
- van Vuuren, S.; Viljoen, A. Plant-based antimicrobial studies—Methods and approaches to study the interaction between natural products. Planta Med. 2011, 77, 1168–1182. [Google Scholar] [CrossRef]
- Sandasi, M.; Leonard, C.M.; Viljoen, A.M. The effect of five common essential oil components on Listeria monocytogenes biofilms. Food Control. 2008, 19, 1070–1075. [Google Scholar] [CrossRef]
- Caigoy, J.C.; Xedzro, C.; Kusalaruk, W.; Nakano, H. Antibacterial, antibiofilm, and antimotility signatures of some natural antimicrobials against Vibrio cholerae. FEMS Microbiol. Lett. 2022, 369, fnac076. [Google Scholar] [CrossRef] [PubMed]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Soxhlet extraction of phenolic compounds from Vernonia cinerea leaves and its antioxidant activity. J. Appl. Res. Med. Aromat. Plants 2018, 11, 12–17. [Google Scholar] [CrossRef]
- Rutkowska, M.; Namieśnik, J.; Konieczka, P. Ultrasound-Assisted Extraction. In The Application of Green Solvents in Separation Processes; Pena-Pereira, F., Tobiszewski, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 301–324. [Google Scholar] [CrossRef]
- Osorio-Tobón, J.F. Recent advances and comparisons of conventional and alternative extraction techniques of phenolic compounds. J. Food Sci. Technol. 2020, 57, 4299–4315. [Google Scholar] [CrossRef] [PubMed]
- Shekwa, W.; Maliehe, T.S.; Masoko, P. Antimicrobial, antioxidant, and cytotoxic activities of the leaf and stem extracts of Carissa bispinosa used for dental healthcare. BMC Complement. Med. Ther. 2023, 23, 462. [Google Scholar] [CrossRef] [PubMed]
- Ameer, K.; Shahbaz, H.M.; Kwon, J. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef]
- Nawaz, H.; Shad, M.A.; Rehman, N.; Andaleeb, H.; Ullah, N. Effect of solvent polarity on extraction yield and antioxidant properties of phytochemicals from bean (Phaseolus vulgaris) seeds. Braz. J. Pharm. Sci. 2020, 56, e17129. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Hsieh, Y.H.; Hsu, P.H.; Hu, A.; Cheng, Y.J.; Shih, T.L.; Chen, J.J. Synthesis of Flavonols and Assessment of Their Biological Activity as Anticancer Agents. Molecules 2024, 29, 2041. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, P.; Cheng, G.; Zhang, Y. A Brief Review of Phenolic Compounds Identified from Plants: Their Extraction, Analysis, and Biological Activity. Nat. Prod. Commun. 2022, 17, 1934578X211069721. [Google Scholar] [CrossRef]
- Fraga-Corral, M.; Otero, P.; Echave, J.; Garcia-Oliveira, P.; Carpena, M.; Jarboui, A.; Nuñez-Estevez, B.; Simal-Gandara, J.; Prieto, M.A. By-Products of Agri-Food Industry as Tannin-Rich Sources: A Review of Tannins’ Biological Activities and Their Potential for Valorization. Foods 2021, 10, 137. [Google Scholar] [CrossRef]
- Saifullah, M.; McCullum, R.; McCluskey, A.; Vuong, Q. Comparison of conventional extraction technique with ultrasound assisted extraction on recovery of phenolic compounds from lemon scented tea tree (Leptospermum petersonii) leaves. Heliyon 2020, 6, e03666. [Google Scholar] [CrossRef] [PubMed]
- Enke, C.G.; Nagels, L.J. Undetected Components in Natural Mixtures: How Many? What Concentrations? Do They Account for Chemical Noise? What Is Needed to Detect Them? Anal. Chem. 2011, 83, 2539–2546. [Google Scholar] [CrossRef] [PubMed]
- Tohma, H.; Gülçin, İ.; Bursal, E.; Gören, A.C.; Alwasel, S.H.; Köksal, E. Antioxidant activity and phenolic compounds of ginger (Zingiber officinale Rosc.) determined by HPLC-MS/MS. J. Food Meas. Charact. 2017, 11, 556–566. [Google Scholar] [CrossRef]
- Rahman, M.J.; Ambigaipalan, P.; Shahidi, F. Biological activities of Camelina and Sophia seeds phenolics: Inhibition of LDL oxidation, DNA damage, and pancreatic lipase and α-glucosidase activities. J. Food Sci. 2018, 83, 237–245. [Google Scholar] [CrossRef]
- Pabón Baquero, L.C.; Rendón, F.M.R.; Chaparro, G.M.P.; Otálvaro Álvarez, Á.M. Plant Extracts as Antioxidant Additives for Food Industry. In Antioxidants in Foods and Its Applications; Shalaby, E., Azzam, G.M., Eds.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- de Almada-Vilhena, A.O.; dos Santos, O.V.M.; Machado, M.d.A.; Nagamachi, C.Y.; Pieczarka, J.C. Prospecting Pharmacologically Active Biocompounds from the Amazon Rainforest: In Vitro Approaches, Mechanisms of Action Based on Chemical Structure, and Perspectives on Human Therapeutic Use. Pharmaceuticals 2024, 17, 1449. [Google Scholar] [CrossRef]
- Kuete, V. Potential of Cameroonian plants and derived products against microbial infections: A review. Planta Med. 2010, 76, 1479–1491. [Google Scholar] [CrossRef]
- Erhabor, R.C.; Erhabor, J.O.; Nkadimeng, S.M.; McGaw, L.J. In vitro antimicrobial, antibiofilm, and antioxidant activities of six South African plants with efficacy against selected foodborne pathogens. S. Afr. J. Bot. 2022, 146, 643. [Google Scholar] [CrossRef]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial activity of polyphenols and alkaloids in Middle Eastern plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef]
- Abdoulahi, M.I.I.; Yanick, K.M.; Lauve, R.T.; Habibou, H.H.; Sahabi, B.; Abdelkader, A.S.; Boyom, F.F.; Tidjani, I.A.; Borquaye, L.S. Antibacterial activity of eight medicinal plants from the traditional pharmacopoeia of Niger. J. Trop. Med. 2023, 2023, 6120255. [Google Scholar] [CrossRef]
- Twaij, B.M.; Hasan, M.N. Bioactive secondary metabolites from plant sources: Types, synthesis, and their therapeutic uses. Int. J. Plant Biol. 2022, 13, 3. [Google Scholar] [CrossRef]
- Naqvi, S.A.R.; Nadeem, S.; Komal, S.; Naqvi, S.A.A.; Mubarik, M.S.; Qureshi, S.Y.; Ahmad, S.; Abbas, A.; Zahid, M.; Khan, N.-U.-H.; et al. Antioxidants: Natural Antibiotics; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Singh, S.K.; Mohammed, A.; Alghamdi, O.A.; Husain, S.M. New approaches for targeting drug resistance through drug combination. In Combination Therapy Against Multidrug Resistance; Wani, M.Y., Ahmad, A., Eds.; Academic Press: Waltham, MA, USA, 2020; pp. 221–246. [Google Scholar]
- Phitaktim, S.; Chomnawang, M.; Sirichaiwetchakoon, K.; Dunkhunthod, B.; Hobbs, G.; Eumkeb, G. Synergism and the mechanism of action of the combination of α-mangostin isolated from Garcinia mangostana L. and oxacillin against an oxacillin-resistant Staphylococcus saprophyticus. BMC Microbiol. 2016, 16, 195. [Google Scholar] [CrossRef] [PubMed]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Biharee, A.; Sharma, A.; Kumar, A.; Jaitak, V. Antimicrobial flavonoids as a potential substitute for overcoming antimicrobial resistance. Fitoterapia 2020, 146, 104720. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Zang, X.; He, M.; Pan, S.; Xu, X. Structure–Activity Relationship of Flavonoids on Their Anti-Escherichia coli Activity and Inhibition of DNA Gyrase. J. Agric. Food Chem. 2013, 61, 8185–8190. [Google Scholar] [CrossRef]
- Saleem, M.; Nazir, M.; Ali, M.S.; Hussain, H.; Lee, Y.S.; Riaz, N.; Jabbar, A. Antimicrobial natural products: An update on future antibiotic drug candidates. Nat. Prod. Rep. 2010, 27, 238–254. [Google Scholar] [CrossRef]
- Savoia, D. Plant-derived antimicrobial compounds: Alternatives to antibiotics. Future Microbiol. 2012, 7, 979–990. [Google Scholar] [CrossRef]
- Tsolis, R.M.; Bäumler, A.J. Gastrointestinal host-pathogen interaction in the age of microbiome research. Curr. Opin. Microbiol. 2020, 53, 78–89. [Google Scholar] [CrossRef]
- Rosenberg, C.R.; Fang, X.; Allison, K.R. Potentiating aminoglycoside antibiotics to reduce their toxic side effects. PLoS ONE 2020, 15, e0237948. [Google Scholar] [CrossRef]
- Dubey, A.; Ghosh, N.; Saxena, G.K.; Purohit, D.; Patel, S.; Singh, S. Management implications for neurotoxic effects associated with antibiotic use. NeuroQuantology 2022, 20, 1303–5150. [Google Scholar] [CrossRef]
- Bhagwat, A.; Haldar, T.; Kanojiya, P.; Saroj, S.D. Bacterial metabolism in the host and its association with virulence. Virulence 2025, 16, 2459336. [Google Scholar] [CrossRef]
- Kearns, D.B. A field guide to bacterial swarming motility. Nat. Rev. Microbiol. 2010, 8, 634–644. [Google Scholar] [CrossRef]
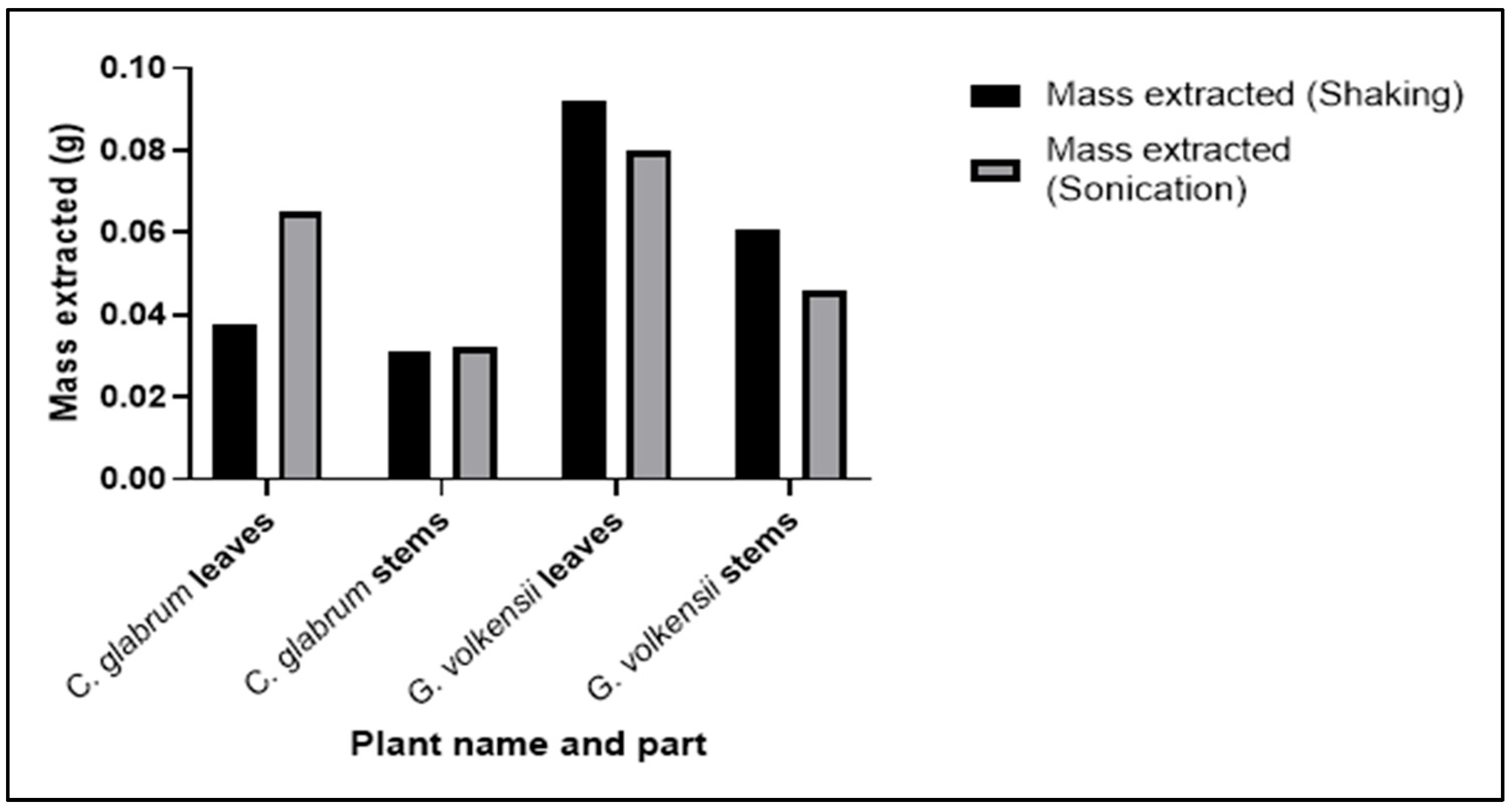




| Total Phenolics (mg GAE/g) | Total Tannins (mg GAE/g) | Total Flavonoids (mg QE/g) | Total Flavonols (mg QE/g) | |||||
|---|---|---|---|---|---|---|---|---|
| Extraction Method | ||||||||
| Plants | Shaking | Sonication | Shaking | Sonication | Shaking | Sonication | Shaking | Sonication |
| C. glabrum leaves | 29.89 ± 1.72 a | 125.34 ± 2.82 e | 7.83 ± 0.12 a | 34.71 ± 2.72 c | 6.17 ± 0.03 a,b | 24.52 ± 0.06 c | 43.31 ± 6.10 a | 51.40 ± 3.68 b |
| C. glabrum stems | 27.14 ± 0.42 a | 61.45 ± 0.80 d | 18.73 ± 0.32 b | 55.84 ± 0.39 d | 6.86 ± 0.65 a,b | 5.87 ± 0.12 a | 49.08 ± 0.51 a,b | 39.12 ± 1.13 a |
| G. volkensii leaves | 35.81 ± 0.36 b | 38.85 ± 0.88 b,c | 7.56 ± 0.35 a | 11.35 ± 0.23 a,b | 7.41 ± 1.07 b | 6.26 ± 0.13 a,b | 61.33 ± 3.99 b,c | 40.58 ± 4.06 a |
| G. volkensii stems | 122.50 ± 2.21 e | 59.81 ± 0.33 d | 18.13 ± 0.13 b | 14.53 ± 0.34 a,b | 8.69 ± 0.03 b | 7.15 ± 0.22 a,b | 43.27 ± 4.02 a | 37.62 ± 1.47 a |
| Free Radical Scavenging Activity | Hydrogen Peroxide Assay | |||
|---|---|---|---|---|
| EC50 (µg/mL) | ||||
| Plants | Shaking | Sonication | Shaking | Sonication |
| C. glabrum Leaves | 800.43 ± 4.32 e | 61.59 ± 5.41 d | 131.39 ± 1.99 c | 72.40 ± 10.48 b |
| C. glabrum Stems | 22.84 ± 1.52 b | 55.97 ± 1.60 d | 1140.16 ± 8.52 e | 2480.61 ± 8.59 g |
| G. volkensii Leaves | 6199.59 ± 1.63 g | 4750.34 ± 8.02 f | 454.10 ± 5.53 d | 3712.43 ± 1.23 h |
| G. volkensii Stems | 32.35 ± 1.83 b | 36.57 ± 6.11 b,c | 6920.30 ± 5.06 i | 1849.97 ± 5.86 f |
| Positive control | ||||
| L-Ascorbic acid | 0.002 ± 1.10 a | 45.61 ± 4.47 a | ||
| Plant Extract | Microorganism | E. coli | P. aeruginosa | S. aureus | S. pyogenes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Extraction Method | MIC | MBC | TA | MIC | MBC | TA | MIC | MBC | TA | MIC | MBC | TA | |
| CgL | Shaking | 0.16 | 0.63 | 237.50 | 1.25 | - | 30.40 | 0.31 | 1.25 | 122.58 | 0.63 | 2.5 | 60.32 |
| Sonication | 0.16 | 0.63 | 406.25 | 0.63 | 2.5 | 103.17 | 0.31 | 1.25 | 209.68 | 0.63 | 2.5 | 103.17 | |
| CgS | Shaking | - | - | - | - | - | - | - | - | - | - | - | - |
| Sonication | 1.25 | - | 25.60 | 2.5 | - | 12.80 | 1.25 | - | 25.6 | 1.25 | - | 25.60 | |
| GvL | Shaking | 0.16 | 0.63 | 575.00 | 0.63 | 2.5 | 146.03 | 0.63 | 1.25 | 146.03 | 0.63 | 2.5 | 146.03 |
| Sonication | 0.16 | 0.63 | 500.00 | 0.31 | 1.25 | 258.06 | 0.31 | 0.63 | 258.06 | 0.31 | 1.25 | 258.06 | |
| GvS | Shaking | 0.63 | - | 96.83 | 1.25 | - | 48.80 | 0.63 | 2.5 | 96.83 | 0.63 | - | 96.83 |
| Sonication | 0.31 | 2.5 | 148.39 | 0.31 | 2.5 | 148.39 | 0.16 | 0.31 | 287.50 | 0.31 | - | 148.39 | |
| Antibiotic | |||||||||||||
| Amp | 0.125 | 0.16 | 0.008 | 0.008 | |||||||||
| Gent | 0.16 | 0.004 | 0.008 | 0.008 | |||||||||
| MIC (mg/)mL | FIC(A) | FIC(B) | FIC Index | Interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gram-negative | ||||||||||
| E. coli | P. aeruginosa | E. coli | P. aeruginosa | E. coli | P. aeruginosa | E. coli | P. aeruginosa | E. coli | P. aeruginosa | |
| CgGv | - | - | - | - | - | - | - | - | Antagonism | Antagonism |
| CgAmp | 0.29 | - | 1.78 | - | 2.28 | - | 4.06 | - | Antagonism | Antagonism |
| CgGent | - | 0.16 | - | 0.26 | - | 51.87 | - | 52.13 | Antagonism | Antagonism |
| GvAmp | 0.44 | - | 1.40 | - | 3.48 | - | 4.88 | - | Antagonism | Antagonism |
| GvGent | - | - | - | - | - | - | - | - | Antagonism | Antagonism |
| Gram-positive | ||||||||||
| S. aureus | S. pyogenes | S. aureus | S. pyogenes | S. aureus | S. pyogenes | S. aureus | S. pyogenes | S. aureus | S. pyogenes | |
| CgGv | - | - | - | - | - | - | - | - | Antagonism | Antagonism |
| CgAmp | - | - | - | - | - | - | - | - | Antagonism | Antagonism |
| CgGent | 0.021 | 0.020 | 0.066 | 0.032 | 2.563 | 2.531 | 2.629 | 2.563 | Indifference | Indifference |
| GvAmp | - | - | - | - | - | - | - | - | Antagonism | Antagonism |
| GvGent | 0.005 | 0.010 | 0.035 | 0.033 | 0.656 | 1.281 | 0.691 | 1.314 | Additivity | Indifference |
| Anti-Swarming Activity | ||||||
|---|---|---|---|---|---|---|
| Plant Extract | E. coli | P. aeruginosa | S. aureus | |||
| MIC | ½ MIC | MIC | ½ MIC | MIC | ½ MIC | |
| C. glabrum leaves (sonication) | 20.83 ± 2.95 | 13.54 ± 1.47 | 50.09 ± 0.00 | 20.68 ± 1.26 | −23.64 ± 10.29 | 5.45 ± 0.00 |
| G. volkensii leaves (shaking) | 20.83 ± 0.00 | 20.83 ± 11.79 | 47.42 ± 1.26 | 44.74 ± 2.52 | −12.73 ± 5.14 | −9.09 ± 10.29 |
| G. volkensii leaves (sonication) | 16.67 ± 5.89 | 14.58 ± 2.95 | 37.61 ± 2.52 | 26.92 ± 2.52 | −12.73 ± 5.14 | 0.00 ± 12.86 |
| G. volkensii stem (sonication) | 20.83 ± 11.79 | 20.83 ± 5.89 | 45.63 ± 3.78 | 21.57 ± 5.04 | 12.73 ± 0.00 | 16.36 ± 5.14 |
| Gentamicin | 100.00 ± 0.00 | 100.00 ± 0.00 | 84.85 ± 1.26 | 12.66 ± 1.26 | 100.00 ± 0.00 | 100.00 ± 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sithole, N.; Matotoka, M.M.; Masoko, P. Therapeutic Potential of Clerodendrum glabrum and Gardenia volkensii Acetone Extracts: Antioxidant, Antibacterial, and Anti-Virulence Activities. Microbiol. Res. 2025, 16, 129. https://doi.org/10.3390/microbiolres16060129
Sithole N, Matotoka MM, Masoko P. Therapeutic Potential of Clerodendrum glabrum and Gardenia volkensii Acetone Extracts: Antioxidant, Antibacterial, and Anti-Virulence Activities. Microbiology Research. 2025; 16(6):129. https://doi.org/10.3390/microbiolres16060129
Chicago/Turabian StyleSithole, Ndzalama, Mashilo Mash Matotoka, and Peter Masoko. 2025. "Therapeutic Potential of Clerodendrum glabrum and Gardenia volkensii Acetone Extracts: Antioxidant, Antibacterial, and Anti-Virulence Activities" Microbiology Research 16, no. 6: 129. https://doi.org/10.3390/microbiolres16060129
APA StyleSithole, N., Matotoka, M. M., & Masoko, P. (2025). Therapeutic Potential of Clerodendrum glabrum and Gardenia volkensii Acetone Extracts: Antioxidant, Antibacterial, and Anti-Virulence Activities. Microbiology Research, 16(6), 129. https://doi.org/10.3390/microbiolres16060129






