Abstract
The cacao trade and export industry has been impacted by cadmium (Cd2+) accumulation in soils, as the metal is absorbed by plants and transferred to the tissues. Consequently, cacao beans and their derivatives can become contaminated, sometimes exceeding permissible limits. In this study, the capacity of native Trichoderma strains to reduce Cd accumulation in cacao was evaluated. Twelve Trichoderma strains were analyzed to assess their cadmium removal capacity through in vitro assays and their ability to reduce Cd concentration in cacao plants under controlled in vivo conditions. The in vitro results showed that several Trichoderma strains could remove cadmium and accumulate it in their biomass. However, this process is complex as it depends on metal concentration and environmental conditions. Notably, T. afroharzianum UCF18-M1 and CP24-6 exhibited high removal efficiencies at 100 ppm (61.79 ± 2.98% and 57.93 ± 4.14%, respectively). In contrast, the in vivo assays revealed that, contrary to expectations, some strains—including those with the highest removal efficiency—stimulated Cd uptake in plants, even at toxic levels, such as T. orientale BLPF1-C1. However, T. longibrachiatum UCF17-M4 and Trichoderma sp. UCPF2-C1 significantly reduced Cd accumulation in the stem. These findings highlight the potential of these strains to mitigate Cd contamination in cacao.
1. Introduction
Cacao (Theobroma cacao L.), native to the South American Amazon region [1], is an economically important crop [2]. In Peru, cacao is mainly produced on small farms (1–5 ha) in regions such as San Martín, Pasco, Ucayali, Junín, Cusco, Ayacucho, Huánuco, and Amazonas, reaching an export volume of 142,390 tons in 2024, valued at USD 1.17 billion [3]. However, high cadmium (Cd2+) levels in cacao-growing soils and international regulatory limits threaten Peruvian cacao production [4,5].
Cadmium (Cd2+) is a non-essential heavy metal that can be naturally present in the soil or introduced through external sources. Although typically found in small concentrations, its bio-availability can increase depending on soil physicochemical conditions such as pH and organic matter content [6,7,8]. This increased availability, combined with its well-documented toxicity [9], represents an environmental and food safety concern [10]. The latter is particularly relevant for certain crops, such as cacao, which can absorb Cd from the soil, translocate it to its vegetative and reproductive organs, and introduce it into the food chain [11,12].
Cadmium contamination in cacao-growing soils has increased over time. Several studies have reported Cd presence in soils [13,14,15], cacao beans, and final products [16,17]. Furthermore, recent findings indicate a direct relationship between soil–Cd levels and cacao products [18,19,20,21]. In Peru, high Cd levels have been detected in samples from the main cocoa-producing regions. A characterization analysis of leaves and beans collected from eight cacao-growing regions revealed Cd concentrations exceeding 0.96 μg/g in samples from Piura, Tumbes, and Huánuco [15]. Similarly, Cd was detected in samples of roots, leaves, testa, and cotyledons collected from various districts in Bagua Province (Amazonas region), with concentrations ranging from 0.49 μg/g to 2.53 μg/g [22], exceeding, in some cases, the maximum permissible limit (0.80 μg/g) established by Commission Regulation (EU) No. 488/2014 [4].
Some mitigation strategies have been developed to address this issue. A georeferenced analysis of Cd content in soils led to the creation of a risk map for the Amazonas region. This study identified that 39% of the territory exceeds the tolerable limit for agricultural soil (1.4 μg/g), while 19% presents levels close to but still within the permitted range (1.0–1.4 μg/g) [23]. Although this initiative aims to guide producers in selecting more suitable cultivation areas, the reduction in arable land could negatively impact cacao production. In this context, the development of complementary sustainable strategies to reduce Cd accumulation in cacao beans without compromising soil use is essential.
It is well known that several Trichoderma species establish beneficial interactions with various plants, including cacao [24,25]. This saprophytic fungus is commonly found colonizing soil and rhizospheric ecosystems. As described by [26], members of this genus are generally not considered pathogenic or harmful to plant development. Furthermore, some are regarded as strategic partners in agriculture and industry, mainly due to their roles as biocontrol agents, their plant growth-promoting effects, and their physiological capacity to produce industrially important metabolites [27,28,29,30]. In addition, certain Trichoderma strains have also been reported to exhibit heavy metal tolerance and uptake capabilities [31,32,33,34,35]. These properties are attributed to several physiological and biochemical mechanisms, such as adsorption, heavy metal flux across the cellular membrane, and intracellular chelation by metallothionein (MT) and specific peptides [36]. Although several studies have demonstrated the cadmium-removal capacity of Trichoderma strains [37,38], little is known about their effectiveness in association with cacao plants. Therefore, this study aims to determine the Cd-uptake capacity of 12 native Trichoderma strains isolated from cacao-growing soils in Amazonas under in vitro conditions and to evaluate their effectiveness in reducing Cd levels in cacao plants.
2. Methods
2.1. Biological Material and Strain Reactivation
Twelve native Trichoderma strains (Table 1) from cacao agroecosystems in five provinces of the Amazonas region were used in this study. These strains were obtained from rhizospheric soil samples, using the serial dilution method on Potato Dextrose Agar (PDA) medium. The selection was based on previously reported positive effects on cacao plants [39,40]. All strains are stored in the fungal collection of the Laboratorio de Investigación en Sanidad Vegetal (LABISANV) at the Instituto de Investigación para el Desarrollo Sustentable de Ceja de Selva of the Universidad Nacional Toribio Rodríguez de Mendoza (UNTRM). For each biological assay, the strains were reactivated using PDA medium by puncture inoculation, and incubated at 28 °C for seven days until visible mycelial growth was observed.

Table 1.
Native Trichoderma strains.
2.2. In Vitro Cadmium Removal and Biomass Accumulation Assay
The capacity of each Trichoderma strain to remove cadmium was evaluated under in vitro conditions. For this, Potato Dextrose Broth (PDB) was supplemented with 100, 200, and 300 ppm of cadmium chloride (CdCl2), prepared from a previously sterilized 1000 ppm stock solution. A control treatment consisting of cadmium-free culture medium was included. Each treatment was inoculated with ten 7 mm PDA disks, containing uniform mycelial growth, and incubated at 28 °C and 210 rpm for 15 days. After the incubation period, the cultures were transferred to sterile 50 mL Falcon tubes and centrifuged at 4000 rpm for 15 min. The supernatants were filtered using 2.5 µm Whatman filter paper. The filtered cultures were diluted in sterile ultrapure water at a 1:10 ratio. Cadmium quantification was performed using atomic absorption spectrometry (Agilent Technologies, USA), following the validated protocol of the Laboratorio de Investigación en Suelos y Aguas at UNTRM. Cadmium removal efficiency was calculated using the formula R = ((Ci − Cf)/Ci) × 100 [41], where
R = Cadmium removal percentage (%);
Ci = Initial cadmium concentration (ppm);
Cf = Final cadmium concentration (ppm).
Following the previous step, the cadmium uptake capacity of microbial biomass was determined. The previously obtained fungal pellet (biomass) was dried in an oven at 70 °C for 24 h. The dry mycelial weight was measured using an analytical precision balance.
Cadmium uptake capacity was calculated using the formula q = ((Ci − Cf)/m) × V [41], where
q = Cadmium absorption (mg g−1);
Ci = Initial cadmium concentration (ppm);
Cf = Final cadmium concentration (ppm);
m = Dry fungal biomass weight (g);
V = Volumen (L).
2.3. Inoculum Preparation
To evaluate the capacity of Trichoderma strains to reduce cadmium concentration in cacao plants, inoculations were performed in Cd-contaminated soil where CCN51 cacao seedlings were cultivated. The Trichoderma strains were first formulated using rice as a substrate. A mixture of boiled rice and water (5:1 ratio) was prepared and left to rest for 30 min. The excess water was then drained, and the rice was distributed into 400 g polypropylene bags. These bags were sterilized at 121 °C and 1 atm for 15 min, then inoculated with 10 mL of a Trichoderma spore suspension after seven days of growth. The inoculated bags were incubated at 27 °C for ten days. After incubation, spores were harvested by washing the 400 g of colonized rice with 2 L of sterile distilled water. The resulting solution was supplemented with 2 mL of 5% chloramphenicol and sedimented for 24 h. The sediment was then dried at 30 °C for 48 h. The dried Trichoderma spores were used as active compounds for plant inoculation. For this, the spores were resuspended in 250 mL of sterile distilled water and the concentration was determined using a Neubauer chamber. Finally all the inocula concentrations were adjusted to a 1 × 106 spores mL−1.
2.4. In Vivo Cd Reduction Assay in CCN51 Cacao Plants
The in vivo experiment was conducted in the experimental greenhouse of INDESCES (UNTRM), located in Bagua Province (5°44′02.65″ S, 78°25′00.91″ W). Polyethylene nursery bags (1 Kg capacity) were filled with a prepared substrate composed of agricultural soil (Cd < 0.0001 ppm) from cacao-growing fields (pH = 7.82 ± 0.34; CE = 1.20 ± 0.20), sand, and rice-husk (as an organic matter source) at a 2:1:1 rate. The substrate was previously sterilized through solarization by covering it with plastic and exposing it to direct sunlight for seven consecutive days. During this period, daily temperatures ranged between 15.50 and 30.03 °C, according to data from the Bagua Chica Meteorological Station [42]. The prepared bags were irrigated with 250 mL of a 5 ppm Cd solution and left to rest for 15 days to ensure cadmium adsorption to the soil. Three days before planting, CCN51 cacao seeds were germinated. This clone was selected for its widespread cultivation, economic significance, and genetic uniformity, which make it a suitable model for evaluating plant responses under controlled experimental conditions. To this, the cacao pods were washed with clean water and disinfected using a sodium hypochlorite (NaClO) solution (5%) for 5 min. The seeds, previously separated from the mucilage, were planted in each bag and inoculated with 250 mL of Trichoderma spore suspension at 1 × 106 spores mL−1. This was applied directly to the soil around each seed to ensure close contact between the inoculum and the emerging radicle.
2.5. Growth Variables
Twelve weeks after Trichoderma inoculation, the cacao plants were harvested. The plants were carefully removed from the substrate, and the plant height and number of leaves were measured. The stem was separated and weighed on an analytical balance to determine the fresh weight. The samples were dried in an oven at 37 °C for 48 h. After drying, the final dry weight was recorded.
2.6. Cadmium Determination in Cacao Plants
Cadmium quantification was performed using stem tissue samples. To obtain ash from plant material, 1 g of a previously pulverized sample was incinerated in a muffle furnace at 450 °C for 8 h. The resulting ash was mixed with 1 mL of hydrochloric acid (HCl) and left to rest for 1 h. After this period, 1 mL of water and 1 mL of HCl were added, and the mixture was left to rest for another hour. The final solution was filtered and brought to a total volume of 25 mL. Cadmium concentrations were determined using atomic absorption spectrometry, following the procedure described by [43].
2.7. Data Analysis
Data processing and analysis were performed using R-Studio (v. 2024.12.1). When normality and homoscedasticity assumptions were met, the variables were analyzed using parametric ANOVA followed by Dunnett’s post hoc test, using the packages car, rstatix, and multcomp. Non-parametric factorial ANOVA analyses were performed using the ARTool package (v 0.11.2), followed by the “art.con” function with Holm corrections for multiple comparisons. Graphs were generated using the ggplot2 package (v. 3.5.2), with color, typography, and label alignment adjustments made using Illustrator (v.23.0.05), while preserving the original graphical data.
3. Results
3.1. Cadmium Removal Efficiency and Biomass Accumulation Under In Vitro Conditions
In this study, we evaluated Cd removal efficiency and its accumulation in biomass for 12 native Trichoderma strains isolated from the Amazonian cacao agroecosystem. The analysis was conducted using three biological replicates, cultivated under different metal concentrations under in vitro conditions. The results are presented in Table 2, where the data represent the percentage of Cd removal relative to the initial concentration and the Cd content (mg g−1) accumulated in the biomass.

Table 2.
Cadmium removal efficiency and biomass accumulation of Trichoderma strains under in vitro conditions.
Regarding removal efficiency, the data suggest that strains of the same species tend to exhibit similar behavior, while variations exist among species. For instance, the T. longibrachiatum group, including strains UCF10A-C1, UCF5A-C1, and UCF17-M4, achieved the lowest efficiencies (8.26 ± 5.06%, 7.27 ± 0.87%, and 6.75 ± 4.53%, respectively). Conversely, the T. afroharzianum group, including strains CP24-6 and UCF18-M1 (except UCF12-M3), exhibited the highest efficiencies, exceeding 33.35 ± 3.22% and 33.93 ± 1.28%, respectively. The T. orientale group, represented by strains CRSF1-C1, BM18-C1, and BLPF1-C1, showed moderate efficiencies ranging from 11.41 ± 1.14% to 20.97 ± 6.96%. On the other hand, in some cases, such as strains CNF20-C1 and UCPF2-C1, efficiency values could not be determined due to unreliable readings, and these cases were indicated as “not determined” (nd) in Table 2.
Additionally, we observed that, for almost all evaluated strains, the highest removal efficiency was achieved at 100 ppm compared to 200 and 300 ppm CdCl2, except for T. reesei BIF3-C1, which reached maximum efficiency at 300 ppm (10.24 ± 5.70%). Notably, the strains T. afroharzianum CP24-6 and UCF18-M1 exhibited specific variations in removal efficiency at different Cd concentrations, suggesting the influence of multiple factors on this process. These effects were confirmed through a non-parametric factorial analysis of variance (Aligned Rank Transformed Data), which demonstrated a significant interaction effect between strain and concentration (p < 2.22 × 10−16). This pattern is illustrated in Figure S1, where the convergence of continuous lines indicates the interaction between both variables. Furthermore, post hoc analysis revealed significant differences in 636 out of 1128 interactions (Figure S2). Moreover, as shown in Figure 1, the multiple comparisons showed significant differences in 254 of 630 comparisons, with purple highlights indicating the most significant differences (p < 0.05, indicated by asterisk). These findings suggest that removal efficiency variability depends not only on the strain but also on the metal concentration in the culture medium.
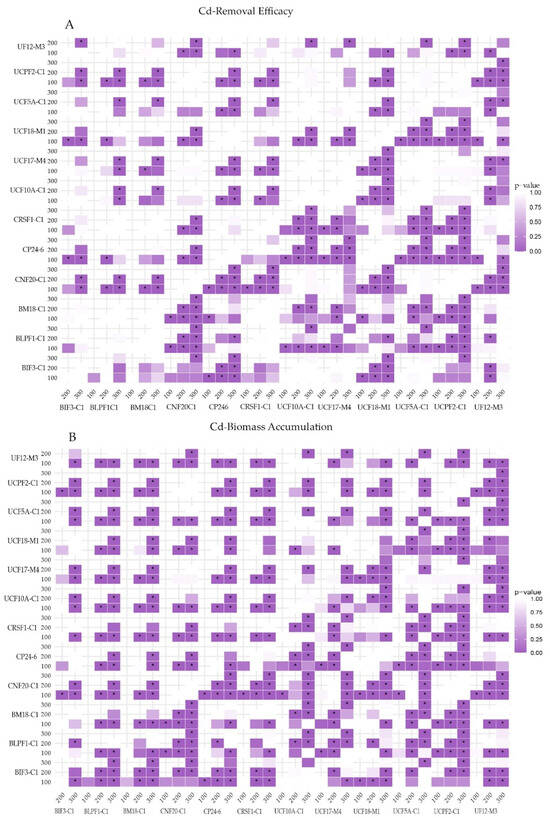
Figure 1.
Significance of multiple comparisons of cadmium removal (A) and biomass accumulations (B). For both datasets, the data were analyzed using a non-parametric factorial ANOVA with the ARTool package, followed by the “art.con” function with Holm corrections. The results indicate significant differences among multiple comparisons of different combinations of strain and Cd concentrations, with purple highlights denoting the most significant differences (p < 0.05, indicated by asterisks). Graphs were generated using the ggplot2 package, and adjustments to color, typography, and label alignment were made in Illustrator software while preserving the integrity of the original graphical data.
On the other hand, cadmium accumulation in cellular biomass increased progressively with higher metal concentrations in the culture medium. These effects were particularly evident in the T. afroharzianum and T. orientale groups, which exhibited high and moderate removal efficiencies, respectively (Table 2). Notably, T. orientale BLPF1-C1 displayed the highest Cd accumulation in biomass, with values of 10.78 ± 2.14, 82.17 ± 8.23, and 111.65 ± 37.58 mg g−1 for 100, 200, and 300 ppm conditions, respectively.
Similarly to removal efficiency, the non-parametric factorial variance test revealed significant strain–concentration interaction effects (p = 2.22 × 10−16) on Cd accumulation in biomass. Additionally, post hoc analysis identified significant differences in 828 out of 1128 interactions (Figure S2) and 372 of 630 multiples comparisons (Figure 1), further suggesting that this response depends on both the Trichoderma strain and Cd concentration in the culture medium. A Pearson correlation analysis was also conducted to determine the relationship between removal efficiency and biomass accumulation. The results indicated a moderate, significant positive correlation for strains BIF3-C1, BLPF1-C1, BM18-C1, CRSF1-C1, UCF10A-C1, UCF18-M1, and UF12-M3, while strain CP24-6 showed no significant correlation. This suggests that, for these strains, higher Cd concentrations in the culture medium lead to greater accumulation in biomass.
3.2. In Vivo Determination of Cadmium-Reducing Capacity in CCN51 Cacao Plants
Prior to the plant assay, Trichoderma strains were formulated on a rice substrate and inoculated at a concentration of 106 spores mL−1. Spore germination was performed and the results indicated germination percentages above 69.99 ± 5.26% (Table S1). After normalizing the spore concentrations, the strains were inoculated into the seedlings immediately after sowing. Twelve weeks post-inoculation, the cacao plants were harvested and transported to the laboratory. Cadmium quantification in the stem revealed a significant reduction effect (p < 2 × 10−16 ***) in cadmium content for treatments inoculated with T. longibrachiatum UCF17-M4 and Trichoderma sp. UCPF2-C1 compared to the positive control inoculated with Cd (Figure 2). These results suggest that these strains could contribute to reducing cadmium accumulation in plant tissues.
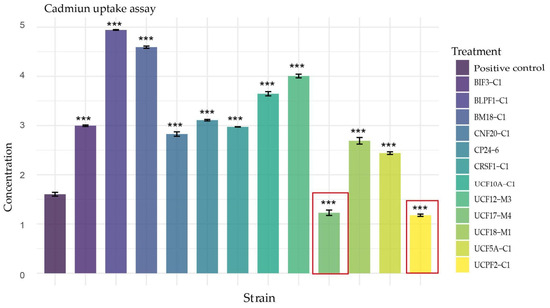
Figure 2.
Cadmium-reducing capacity in CCN51 cacao plants by Trichoderma strains. The bars represent the means of four to five replicates ± SD. Cadmium accumulation was analyzed using a one-way ANOVA, followed by Dunnett’s test for post hoc comparisons. Statistically significant differences are indicated as follows: p-value < 0 (***). Most Trichoderma strains induced cadmium accumulation, except for Trichoderma sp. UCPF2-C1 and T. longibrachiatum UCF17-M4, which significantly reduced cadmium concentration in leaves. These strains are highlighted with a red rectangle.
Similarly, significant effects were observed for strains BIF3-C1, BLPF1-C1, BM18-C1, CNF20-C1, CP24-6, CRSF1-C1, UCF10A-C1, UCF12-M3, UCF18-M1, and UCF5A-C1. However, contrary to expectations, these strains stimulated Cd uptake in the plant, reaching concentrations exceeding 2.83 ppm (Figure 2). Finally, no cadmium was detected in the uninoculated, Cd-free treatment.
3.3. Promotive Effect on Cacao Plants Under Heavy Metal Stress
Additionally, the growth-promoting effect of Trichoderma strains was evaluated in cacao plants grown under heavy metal stress. For this purpose, plant height, number of leaves, fresh weight, and dry weight were measured (Table 3). Statistical analyses revealed no significant differences between Trichoderma-inoculated treatments and the Cd control for plant height and number of leaves, suggesting that these strains do not affect these variables under metal stress conditions. Conversely, in some cases, inoculation proved detrimental; notably, the T. orientale strain BLPF1-C1 significantly reduced both the dry and fresh weight of the plants (Figure 2).

Table 3.
Growth variables of Theobroma cacao CCN51.
4. Discussion
The extensive metabolic variability of microorganisms allows them to interact with diverse substrates through multiple mechanisms, which can be leveraged for the development of increasingly efficient bioremediation strategies. Fungi, in particular, can tolerate and accumulate high concentrations of heavy metals and have been widely used for metal ion adsorption [44]. In this study, the results suggest that strains of the same species tend to exhibit similar removal efficiency, while variations exist among species. Moreover, removal efficiency depends not only on the strain but also on the metal concentration in the culture medium. This phenotypic similarity may be explained by the conservation of certain stress signaling pathways within the genomes of microorganisms from the same species [45]. However, due to their high genetic variability, these microorganisms do not always exhibit identical responses [46]. Fungal genomes, in particular, display remarkable architectural diversity, and their genetic plasticity enables adaptation to environmental changes [47]. The complexity of these interactions has been reported in a comparative study involving three Trichoderma species (T. asperellum, T. harzianum, and T. tomentosum), grown under different Cd concentrations and pH levels, which demonstrated a species-specific relationship with cadmium removal [33]. Other factors strongly influencing removal efficiency include environmental conditions such as medium pH, which affects metal solubility in aqueous solutions [36].
Various Trichoderma species isolated from different environments have been reported for their ability to reduce Cd and other heavy metals [31,32,33,34,35,36]. Consistent with our findings, evidence suggests that some Trichoderma strains exhibit high tolerance to different metal concentrations, often accompanied by high removal capacity. This behavior may be influenced by both environmental and biological factors [34,35,36]
In association with cacao, multiple Trichoderma strains have been primarily studied for their well-known antagonistic activity against phytopathogenic fungi [48,49,50]. However, little is known about the bioremediation potential of native Trichoderma strains from the cacao-agroecosystem. Although this activity has been mainly reported for bacteria [43,51,52,53,54], a recent study has demonstrated the Cd removal capacity of certain native Trichoderma strains [55]. That study reported in vitro removal efficiencies of 83.1%, 67.0%, and 65.8% for T. brevicompactum M43D, T. harzianum M1P, and T. spirale M55SM, respectively. Beyond Trichoderma, other Cd-tolerant fungi associated with the cacao agroecosystem include Talaromyces santanderensis, Periconia igniaria, Metarhizium sp., and Annulohypoxylon sp. [56,57].
Regarding the cadmium-reducing uptake in plants by Trichoderma strains, T. longibrachiatum UCF17-M4 and Trichoderma sp. UCPF2-C1 reduced the Cd concentration in cacao plants. Previous studies have shown that T. longibrachiatum has bioremediation potential for heavy metals. Furthermore, in addition to metal removal [58], strains of this species have been associated with enhanced plant stress tolerance and improved soil quality [59,60,61].
In response to cadmium toxicity, Trichoderma has been shown to induce the expression of genes related to reactive oxygen species (ROS) synthesis and detoxification. A recent study reported that T. reesei overexpressed genes associated with the MAPK signaling pathway, thereby enhancing the detoxification of ROS. Additionally, genes related to ABC transporters, viral myocarditis, and the ErbB signaling pathway were also upregulated [62]. Beyond the fungal mechanisms involved in the response to cadmium stress, it is also important to consider that environmental conditions play a critical role in the detoxification process, as they influence the bioavailability of the metal. For instance, cadmium concentrations tend to increase with higher total Cd levels and lower pH values. Moreover, in some cases, Cd concentrations also increase with decreasing organic matter content (%OM) [6]. Although soil physicochemical properties were not measured in this study, their potential influence should be considered in future experiments.
Though not extensively studied in cacao, Trichoderma inoculation has demonstrated cadmium-reducing effects in other plant models. For example, in Vigna radiata, inoculation with the hyper-tolerant strain Trichoderma sp. TF-13 reduced lead (Pb) and Cd uptake in root and aerial tissues by 34/39% and 47/38%, respectively, while also improving plant growth and physiological parameters [37]. Similar effects were observed in Cicer arietinum plants inoculated with a consortium of Pseudomonas fluorescens PGPR-7 and Trichoderma sp. T-4, where joint inoculation reduced Cd uptake in roots by 38% in plants exposed to 25 μg kg−1 Cd in soil [38]. In addition to a reduction in Cd accumulation, the improvement of plant growth and physiological parameters was also reported [37,63]. Furthermore, a recent metabolomic analysis identified the production of 43 key metabolites, including nicotinic acid, succinic acid, and fumaric acid, involved in Cd detoxification in Nicotiana plants inoculated with T. nigricans T32781 [63]. These findings suggest that the use of Trichoderma, either alone or in consortium, is a promising strategy for reducing cadmium uptake and enhancing plant growth in Cd-contaminated environments. In this context, further experimentation with Trichoderma strains UCF17-M4 and UCPF2-C1 must be performed. Particularly as part of a fungal consortium considering the natural complexity of soil environments and the potential for synergistic interactions among microorganisms.
While Cd reduction in plants has been documented in some studies, the opposite effect—the stimulation of metal accumulation—has also been widely studied due to its potential for phytoremediation and the effective removal of specific contaminants. Among the most recent evidence, inoculation of T. harzianum in combination with biochar significantly increased Cd accumulation by 187.49–308.92% and arsenic (As) by 125.74–221.43% in Brassica juncea, reducing the bioavailability of both metals in the soil [64]. Similarly, T. harzianum enhanced Cd bioavailability for Arachis hypogaea, significantly increasing its bioaccumulation in both roots and leaves, consequently improving the plant’s phytoextraction capacity [65].
The heavy metal phytoremediation effect mediated by Trichoderma can be explained by different mechanisms. Trichoderma inoculation has been shown to stimulate soil enzymatic activity, primarily related to soil fertility, allowing for the establishment of metal-accumulating plants [66]. It can also enhance the activity of antioxidant enzymes such as superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), and ascorbate peroxidase [67]. These findings align with the results of this study, where Trichoderma BIF3-C1, BLPF1-C1, BM18-C1, CNF20-C1, CP24-6, CRSF1-C1, UCF10A-C1, UCF12-M3, UCF18-M1, and UCF5A-C1 strains stimulated Cd accumulation in the cacao stem, suggesting a potential role in promoting Cd uptake. Among these, BLPF1-C1 exhibited the highest Cd uptake, which corresponded with a significant reduction in plant biomass. While numerous studies have reported beneficial effects of Trichoderma on plant growth, its capacity to enhance heavy metal uptake, which may lead to toxicity, has also been documented. Although this capacity is desirable in phytoremediation strategies, excessive accumulation of metals in plants can result in adverse effects. The toxicity of cadmium (Cd) in plants is well documented. According to [68], Cd toxicity can cause growth retardation, alterations in photosynthetic activity, changes in stomatal movement, modifications in enzymatic activity and protein metabolism, and disruptions in cell membrane function. In cacao, morphological, molecular, and physiological changes have been reported in response to Cd toxicity, in addition to reduced absorption of essential micronutrients such as Zn and Fe [69].
Finally, whether used independently or in association with other organisms (e.g., legumes), the study and application of fungi with bioremediation potential present several advantages, including their great diversity, adaptability, rapid growth, and high biomass production [70]. Moreover, as discussed throughout this work, some of these organisms have demonstrated excellent capacities for capturing and bio-removing Cd [71,72]; however, the bioremediation effect depends on a variety of factors and therefore requires detailed study and characterization.
5. Conclusions
The results indicate that Cd removal efficiency tends to be similar within the same species but varies between species. Additionally, higher cadmium concentrations in the medium lead to increased accumulation in fungal biomass. Furthermore, Cd removal efficiency and biomass accumulation are directly correlated.
The in vitro results do not always correspond to in vivo conditions. While some strains promoted Cd accumulation in the stem, Trichoderma sp. UCPF2-C1 and T. longibrachiatum UCF17-M4 significantly reduced cadmium levels compared to other Trichoderma strains, suggesting their potential for mitigating heavy metal uptake.
Finally, these Trichoderma strains did not enhance plant growth under cadmium-stress soil conditions. Moreover, in some cases, such as with strain BLPF1-C1, inoculation had adverse effects, reducing both the dry and fresh weight of the plants.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/microbiolres16060130/s1: Figure S1: The interaction results of cadmium removal and biomass accumulations. The results represent the means of four to five replicates ± SD. The variables were analyzed using a non-parametric factorial analysis of variance (Aligned Rank Transform). The lines indicate the cadmium removal efficiency (A) and biomass accumulation (B), where the convergence of continuous lines denotes the interaction for both the factors Strains and Concentrations; Figure S2: The significance of the interaction results of cadmium removal (A) and biomass accumulations (B). For both datasets, the data were analyzed using a non-parametric factorial ANOVA with the ARTool package in the R environment, followed by the “art.con” function with Holm corrections. The results indicate significant interactions among treatments and strains, with purple denoting the most significant differences (p-value < 0.05, indicated by asterisks) and white representing the least significant ones. Graphs were generated using the ggplot2 package, and adjustments to color, typography, and label alignment were made in Illustrator software while preserving the integrity of the original graphical data; Table S1: Inoculum quality evaluation.
Author Contributions
R.Y.M.-C.: Methodology, Data Curation, Methodology, Formal analysis, Writing—original draft. C.-L.A.A.: Writing—review and editing. C.-C.J.I.: Writing—review and editing. A.-I.M.: Conceptualization and Methodology. C.-V.L.M.: Conceptualization, Formal Analysis, Supervision, Writing—original draft, Writing—review and editing, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Program for Scientific Research and Advanced Studies (PROCIENCIA; Contract PE501082633-2023-PROCIENCIA), the Ministry of Economy and Finance (MEF) (CEINCACAO, CUI No. 2315081), the International Centre of Genetic Engineering and Biotechnology (Project CRP-24-010), and the Universidad Nacional Toribio Rodriguez de Mendoza de Amazonas (UNTRM-A).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors thank field technician Marco Pasapera, as well as all the technical staff at the Plant Health Research Laboratory of INDESCES—UNTRM, for their technical assistance and for providing the strains used in this study.
Conflicts of Interest
The authors have no relevant financial or non-financial interests to disclose.
References
- Zarrillo, S.; Gaikwad, N.; Lanaud, C.; Powis, T.; Viot, C.; Lesur, I.; Fouet, O.; Argout, X.; Guichoux, E.; Salin, F.; et al. The Use and Domestication of Theobroma cacao during the Mid-Holocene in the Upper Amazon. Nat. Ecol. Evol. 2018, 2, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Desarrollo Agrario y Riego. Análisis de La Cadena Productiva Del Cacao Con Enfoque En Los Pequeños Productores de Limitado Acceso Al Mercado; Ministerio de Agricultura y Riego: Lima, Peru, 2018.
- Cámara Peruana de Café y Cacao Cacao Peruano, Datos Generales de Comercio. Available online: https://camcafeperu.com.pe/ES/cacao-peruano.php (accessed on 20 February 2025).
- MERCOSUR. Reglamento Técnico Mercosur Sobre Límites Máximos de Contaminantes Inorgánicos En Alimentos (Derogación De Las Res. GMC N° 102/94 y N° 36/96). 2011. Available online: https://normas.mercosur.int/public/normativas/2474 (accessed on 20 February 2025).
- European Food Safety Authority Commission Regulation (EU) N° 488/2014. 2014. Available online: https://eur-lex.europa.eu/eli/reg/2014/488/oj/eng (accessed on 20 February 2025).
- Smolders, E.; Mertens, J. Chapter 3: Cadmium. In Heavy Metals in Soils; Springer: Dordrecht, The Netherlands, 2013; pp. 283–311. [Google Scholar] [CrossRef]
- Khan, M.A.; Khan, S.; Khan, A.; Alam, M. Soil Contamination with Cadmium, Consequences and Remediation Using Organic Amendments. Sci. Total Environ. 2017, 601–602, 1591–1605. [Google Scholar] [CrossRef] [PubMed]
- Maddela, N.R.; Kakarla, D.; García, L.C.; Chakraborty, S.; Venkateswarlu, K.; Megharaj, M. Cocoa-Laden Cadmium Threatens Human Health and Cacao Economy: A Critical View. Sci. Total Environ. 2020, 720, 137645. [Google Scholar] [CrossRef] [PubMed]
- USDA. Foreign Agricultural Service China Releases the Standard for Maximum Levels of Contaminants in Foods; USDA: Beijing, China, 2018. Available online: https://www.fsis.usda.gov/sites/default/files/media_file/2021-02/GB-2762-2017.pdf (accessed on 20 February 2025).
- Huaraca-Fernandez, J.N.; Pérez-Sosa, L.; Bustinza-Cabala, L.S.; Pampa-Quispe, N.B. Enmiendas Orgánicas En La Inmovilización de Cadmio En Suelos Agrícolas Contaminados: Una Revisión. Inf. Tecnol. 2020, 31, 139–152. [Google Scholar] [CrossRef]
- Blommaert, H.; Aucour, A.M.; Wiggenhauser, M.; Moens, C.; Telouk, P.; Campillo, S.; Beauchêne, J.; Landrot, G.; Testemale, D.; Pin, S.; et al. From Soil to Cacao Bean: Unravelling the Pathways of Cadmium Translocation in a High Cd Accumulating Cultivar of Theobroma cacao L. Front. Plant Sci. 2022, 13, 1055912. [Google Scholar] [CrossRef]
- Vanderschueren, R.; De Mesmaeker, V.; Mounicou, S.; Isaure, M.P.; Doelsch, E.; Montalvo, D.; Delcour, J.A.; Chavez, E.; Smolders, E. The Impact of Fermentation on the Distribution of Cadmium in Cacao Beans. Food Res. Int. 2020, 127, 108743. [Google Scholar] [CrossRef]
- Scaccabarozzi, D.; Castillo, L.; Aromatisi, A.; Milne, L.; Castillo, A.B.; Muñoz-Rojas, M. Soil, Site, and Management Factors Affecting Cadmium Concentrations in Cacao-Growing Soils. Agronomy 2020, 10, 806. [Google Scholar] [CrossRef]
- Lewis, C.; Lennon, A.M.; Eudoxie, G.; Umaharan, P. Genetic Variation in Bioaccumulation and Partitioning of Cadmium in Theobroma cacao L. Sci. Total Environ. 2018, 640–641, 696–703. [Google Scholar] [CrossRef]
- Arévalo-Gardini, E.; Arévalo-Hernández, C.O.; Baligar, V.C.; He, Z.L. Heavy Metal Accumulation in Leaves and Beans of Cacao (Theobroma cacao L.) in Major Cacao Growing Regions in Peru. Sci. Total Environ. 2017, 605–606, 792–800. [Google Scholar] [CrossRef]
- Gramlich, A.; Tandy, S.; Andres, C.; Chincheros Paniagua, J.; Armengot, L.; Schneider, M.; Schulin, R. Cadmium Uptake by Cocoa Trees in Agroforestry and Monoculture Systems under Conventional and Organic Management. Sci. Total Environ. 2017, 580, 677–686. [Google Scholar] [CrossRef]
- Manton, W.I. Chapter 6: Nonnutritive Constituents in Chocolate and Cocoa. In Chocolate in Health and Nutrition; Humana: Totowa, NJ, USA, 2013; pp. 1–553. [Google Scholar] [CrossRef]
- Vanderschueren, R.; Argüello, D.; Blommaert, H.; Montalvo, D.; Barraza, F.; Maurice, L.; Schreck, E.; Schulin, R.; Lewis, C.; Vazquez, J.L.; et al. Mitigating the Level of Cadmium in Cacao Products: Reviewing the Transfer of Cadmium from Soil to Chocolate Bar. Sci. Total Environ. 2021, 781, 146779. [Google Scholar] [CrossRef]
- Bertoldi, D.; Barbero, A.; Camin, F.; Caligiani, A.; Larcher, R. Multielemental Fingerprinting and Geographic Traceability of Theobroma cacao Beans and Cocoa Products. Food Control 2016, 65, 46–53. [Google Scholar] [CrossRef]
- Abt, E.; Fong Sam, J.; Gray, P.; Robin, L.P. Cadmium and Lead in Cocoa Powder and Chocolate Products in the US Market. Food Addit. Contam. Part B Surveill. 2018, 11, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Fechner, C.; Greiner, M.; Heseker, H.; Lindtner, O. Dietary Exposure Assessment of Aluminium and Cadmium from Cocoa in Relation to Cocoa Origin. PLoS ONE 2019, 14, e0217990. [Google Scholar] [CrossRef]
- Oliva, M.; Rubio, K.; Epquin, M.; Marlo, G.; Leiva, S. Cadmium Uptake in Native Cacao Trees in Agricultural Lands of Bagua, Peru. Agronomy 2020, 10, 1551. [Google Scholar] [CrossRef]
- Rojas-Briceño, N.B.; Oliva-Cruz, M.; Rascón, J. Idoneidad Del Territorio Para El Cultivo Sostenible de Cacao (Theobroma cacao L.) Según Presencia de Cadmio En Suelos de Amazonas. Rev. Investig. Agroproducción Sustentable 2021, 5, 77. [Google Scholar] [CrossRef]
- Bailey, B.A.; Strem, M.D.; Wood, D. Trichoderma Species Form Endophytic Associations within Theobroma cacao Trichomes. Mycol. Res. 2009, 113, 1365–1376. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma Species—Opportunistic, Avirulent Plant Symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Abdul-Halim, A.M.A.A.; Shivanand, P.; Krishnamoorthy, S.; Taha, H. A Review on the Biological Properties of Trichoderma spp. as a Prospective Biocontrol Agent and Biofertilizer. J. Appl. Biol. Biotechnol. 2023, 11, 34–46. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Horwitz, B.A.; Herrera-Estrella, A.; Schmoll, M.; Kenerley, C.M. Trichoderma Research in the Genome Era. Annu. Rev. Phytopathol. 2013, 51, 105–129. [Google Scholar] [CrossRef]
- Chakroun, H.; Mechichi, T.; Martinez, M.J.; Dhouib, A.; Sayadi, S. Purification and Characterization of a Novel Laccase from the Ascomycete Trichoderma atroviride: Application on Bioremediation of Phenolic Compounds. Process Biochem. 2010, 45, 507–513. [Google Scholar] [CrossRef]
- Mejía, L.C.; Rojas, E.I.; Maynard, Z.; Van Bael, S.; Arnold, A.E.; Hebbar, P.; Samuels, G.J.; Robbins, N.; Herre, E.A. Endophytic Fungi as Biocontrol Agents of Theobroma cacao Pathogens. Biol. Control 2008, 46, 4–14. [Google Scholar] [CrossRef]
- Rubini, M.R.; Silva-Ribeiro, R.T.; Pomella, A.W.V.; Maki, C.S.; Araújo, W.L.; Dos Santos, D.R.; Azevedo, J.L. Diversity of Endophytic Fungal Community of Cacao (Theobroma cacao L.) and Biological Control of Crinipellis perniciosa, Causal Agent of Witches’ Broom Disease. Int. J. Biol. Sci. 2005, 1, 24–33. [Google Scholar] [CrossRef]
- López-Errasquín, E.; Vázquez, C. Tolerance and Uptake of Heavy Metals by Trichoderma atroviride Isolated from Sludge. Chemosphere 2003, 50, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.; Mandal, A.; Thakur, J.; Manna, M.C.; Rao, A.S. Exploring Bioaccumulation Efficacy of Trichoderma viride: An Alternative Bioremediation of Cadmium and Lead. Natl. Acad. Sci. Lett. 2012, 35, 299–302. [Google Scholar] [CrossRef]
- Mohsenzadeh, F.; Shahrokhi, F. Biological Removing of Cadmium from Contaminated Media by Fungal Biomass of Trichoderma Species. J. Environ. Heal. Sci. Eng. 2014, 12, 102. [Google Scholar] [CrossRef]
- Bazrafshan, E.; Zarei, A.A.; Mostafapour, F.K. Biosorption of Cadmium from Aqueous Solutions by Trichoderma Fungus: Kinetic, Thermodynamic, and Equilibrium Study. Desalin. Water Treat. 2016, 57, 14598–14608. [Google Scholar] [CrossRef]
- Rahman, N.N.N.A.; Shahadat, M.; Omar, F.M.; Chew, A.W.; Kadir, M.O.A. Dry Trichoderma Biomass: Biosorption Behavior for the Treatment of Toxic Heavy Metal Ions. Desalin. Water Treat. 2016, 57, 13106–13112. [Google Scholar] [CrossRef]
- Hoseinzadeh, S.; Shahabivand, S.; Aliloo, A.A. Toxic Metals Accumulation in Trichoderma asperellum and T. harzianum. Microbiology 2017, 86, 728–736. [Google Scholar] [CrossRef]
- Altaf, M.; Ilyas, T.; Shahid, M.; Shafi, Z.; Tyagi, A.; Ali, S. Trichoderma Inoculation Alleviates Cd and Pb-Induced Toxicity and Improves Growth and Physiology of Vigna radiata (L.). ACS Omega 2023, 9, 8557–8573. [Google Scholar] [CrossRef]
- Syed, A.; Elgorban, A.M.; Bahkali, A.H.; Eswaramoorthy, R.; Iqbal, R.K.; Danish, S. Metal-Tolerant and Siderophore Producing Pseudomonas Fluorescence and Trichoderma spp. Improved the Growth, Biochemical Features and Yield Attributes of Chickpea by Lowering Cd Uptake. Sci. Rep. 2023, 13, 4471. [Google Scholar] [CrossRef] [PubMed]
- Leiva, S.; Oliva, M.; Hernández, E.; Chuquibala, B.; Rubio, K.; García, F.; de la Cruz, M.T.M.T.; Torres de la Cruz, M.; de la Cruz, M.T.M.T. Assessment of the Potential of Trichoderma spp. Strains Native to Bagua (Amazonas, Peru) in the Biocontrol of Frosty Pod Rot (Moniliophthora roreri). Agronomy 2020, 10, 1376. [Google Scholar] [CrossRef]
- Leiva, S.; Rubio, K.; Díaz-Valderrama, J.R.; Granda-Santos, M.; Mattos, L. Phylogenetic Affinity in the Potential Antagonism of Trichoderma spp. against Moniliophthora Roreri. Agronomy 2022, 12, 2052. [Google Scholar] [CrossRef]
- Yaghoubian, Y.; Siadat, S.A.; Moradi Telavat, M.R.; Pirdashti, H.; Yaghoubian, I. Bio-Removal of Cadmium from Aqueous Solutions by Filamentous Fungi: Trichoderma spp. and Piriformospora indica. Environ. Sci. Pollut. Res. 2019, 26, 7863–7872. [Google Scholar] [CrossRef]
- Servicio Nacional de Meteorología e Hidrología del Perú Datos Hidrometeorológicos a Nivel Nacional. Available online: https://www.senamhi.gob.pe/?p=estaciones (accessed on 4 June 2025).
- Arce-Inga, M.; González-Pérez, A.R.; Hernandez-Diaz, E.; Chuquibala-Checan, B.; Chavez-Jalk, A.; Llanos-Gomez, K.J.; Leiva-Espinoza, S.T.; Oliva-Cruz, S.M.; Cumpa-Velasquez, L.M. Bioremediation Potential of Native Bacillus sp. Strains as a Sustainable Strategy for Cadmium Accumulation of Theobroma Cacao in Amazonas Region. Microorganisms 2022, 10, 2108. [Google Scholar] [CrossRef]
- Yin, K.; Wang, Q.; Lv, M.; Chen, L. Microorganism Remediation Strategies towards Heavy Metals. Chem. Eng. J. 2019, 360, 1553–1563. [Google Scholar] [CrossRef]
- Gasch, A.P. Comparative Genomics of the Environmental Stress Response in Ascomycete Fungi. Yeast 2007, 24, 961–976. [Google Scholar] [CrossRef]
- Nikolaou, E.; Agrafioti, I.; Stumpf, M.; Quinn, J.; Stansfield, I.; Brown, A.J. Phylogenetic Diversity of Stress Signalling Pathways in Fungi. BMC Evol. Biol. 2009, 9, 44. [Google Scholar] [CrossRef]
- Naranjo-Ortiz, M.A.; Gabaldón, T. Fungal Evolution: Cellular, Genomic and Metabolic Complexity. Biol. Rev. 2020, 95, 1198–1232. [Google Scholar] [CrossRef]
- Bailey, B.A.; Bae, H.; Strem, M.D.; Crozier, J.; Thomas, S.E.; Samuels, G.J.; Vinyard, B.T.; Holmes, K.A. Antibiosis, Mycoparasitism, and Colonization Success for Endophytic Trichoderma Isolates with Biological Control Potential in Theobroma cacao. Biol. Control 2008, 46, 24–35. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Yu, C.; Dou, K.; Wang, M.; Li, Y.; Chen, J. Synergistic Effect of Trichoderma-Derived Antifungal Metabolites and Cell Wall Degrading Enzymes on Enhanced Biocontrol of Fusarium oxysporum f. sp. Cucumerinum. Biol. Control 2016, 94, 37–46. [Google Scholar] [CrossRef]
- Walid, N.; Al-Jaramany, L.; Elbenay, A.; Al-Mhethawi, R. Biological Control of Tomato Damping-off and Potato Black Scurf by Seed Treatment with Trichoderma harzianum. Jordan J. Biol. Sci. 2022, 15, 373–380. [Google Scholar] [CrossRef]
- Crisostomo-Panuera, J.S.; del Valle Nieva, A.S.; Ix-Balam, M.A.; Díaz-Valderrama, J.R.; Alviarez-Gutierrez, E.; Oliva-Cruz, S.M.; Cumpa-Velásquez, L.M. Diversity and Functional Assessment of Indigenous Culturable Bacteria Inhabiting Fine-Flavor Cacao Rhizosphere: Uncovering Antagonistic Potential against Moniliophthora roreri. Heliyon 2024, 10, e28453. [Google Scholar] [CrossRef] [PubMed]
- González-Reguero, D.; Robas-Mora, M.; Fernández-Pastrana, V.M.; Agustin Probanza-Lobo, P.J.-G. Reduced Antibiotic Resistance in the Rhizosphere of Lupinus albus in Mercury-Contaminated Soil Mediated by the Addition of PGPB. Biology 2023, 12, 801. [Google Scholar] [CrossRef]
- Kumar, S.; Choudhary, A.K.; Suyal, D.C.; Makarana, G.; Goel, R. Leveraging Arsenic Resistant Plant Growth-Promoting Rhizobacteria for Arsenic Abatement in Crops. J. Hazard. Mater. 2022, 425, 127965. [Google Scholar] [CrossRef]
- Liu, Y.R.; Delgado-Baquerizo, M.; Bi, L.; Zhu, J.; He, J.Z. Consistent Responses of Soil Microbial Taxonomic and Functional Attributes to Mercury Pollution across China. Microbiome 2018, 6, 183. [Google Scholar] [CrossRef]
- Cayotopa-Torres, J.; Arévalo-López, L.; Pichis-García, R.; Olivera-Cayotopa, D.; Rimachi-Valle, M.; Márquez-Dávila, K. New Cadmium Bioremediation Agents: Trichoderma species Native to the Rhizosphere of Cacao Trees. Sci. Agropecu. 2021, 24, 155–160. [Google Scholar] [CrossRef]
- Cordoba-Novoa, H.A.; Cáceres-Zambrano, J.; Torres-Rojas, E. Isolation of Native Cadmium-Tolerant Bacteria and Fungi from Cacao (Theobroma cacao L.)—Cultivated Soils in Central Colombia. Heliyon 2023, 9, e22489. [Google Scholar] [CrossRef]
- Guerra Sierra, B.E.; Arteaga-Figueroa, L.A.; Sierra-Pelaéz, S.; Alvarez, J.C. Talaromyces Santanderensis: A New Cadmium-Tolerant Fungus from Cacao Soils in Colombia. J. Fungi 2022, 8, 1042. [Google Scholar] [CrossRef]
- Firincă, C.; Zamfir, L.G.; Constantin, M.; Răut, I.; Capră, L.; Popa, D.; Jinga, M.L.; Baroi, A.M.; Fierăscu, R.C.; Corneli, N.O.; et al. Microbial Removal of Heavy Metals from Contaminated Environments Using Metal-Resistant Indigenous Strains. J. Xenobiotics 2024, 14, 51–78. [Google Scholar] [CrossRef]
- Cheng, H.; Gao, M.; Yang, W.; Sun, H.; Kong, T.; Xu, H. Combined Application of Organic Wastes and Trichoderma longibraciatum to Promote Vegetation Restoration and Soil Quality on Mining Waste Dump Sites. Plant Soil 2024, 508, 567–588. [Google Scholar] [CrossRef]
- Qian, X.; Dong, Y.; Yu, D.; Cao, Y.; Sarsaiya, S.; Chen, J. Cobalt Stress Enhanced Dendrobine-Type Total Alkaloids Biosynthesis of Trichoderma longibrachiatum UN32 through Reactive Oxygen Species Formation. World J. Microbiol. Biotechnol. 2024, 40, 328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gan, Y.; Xu, B. Application of Plant-Growth-Promoting Fungi Trichoderma longibrachiatum T6 Enhances Tolerance of Wheat to Salt Stress through Improvement of Antioxidative Defense System and Gene Expression. Front. Plant Sci. 2016, 7, 1405. [Google Scholar] [CrossRef] [PubMed]
- Azam, T.; Dai, X.; Chen, X.; Ali, I.; Chen, S.; Noor, F.; Haider, S.Z. Comparative Transcriptomic and Physiological Analysis of Extremophilic and Non-Extremophilic Fungi in Bioremediation of Cadmium (Cd) and Strontium (Sr). Environ. Pollut. 2025, 367, 125678. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, C.; Gao, Z.F.; Qiu, C.W.; Shi, S.H.; Chen, Z.H.; Ali, M.A.; Wang, F.; Wu, F. Integrated Physiological and Omics Analyses Reveal the Mechanism of Beneficial Fungal Trichoderma sp. Alleviating Cadmium Toxicity in Tobacco (Nicotiana tabacum L.). Ecotoxicol. Environ. Saf. 2023, 267. [Google Scholar] [CrossRef]
- Yao, S.; Zhou, B.; Duan, M.; Cao, T.; Wen, Z.; Chen, X.; Wang, H.; Wang, M.; Cheng, W.; Zhu, H.; et al. Combination of Biochar and Trichoderma harzianum Can Improve the Phytoremediation Efficiency of Brassica Juncea and the Rhizosphere Micro-Ecology in Cadmium and Arsenic Contaminated Soil. Plants 2023, 12, 2939. [Google Scholar] [CrossRef]
- Xiong, J.; Zou, D.; Kang, J.; Mo, Y.; Li, L.; Zhan, L.; Wu, Q.; Xiao, Z. Improving Peanut Growth and Cadmium Phytoextraction Capacity by Inoculating Bacillus megaterium and Trichoderma harzianum. J. Environ. Manag. 2024, 370, 122758. [Google Scholar] [CrossRef]
- Govarthanan, M.; Mythili, R.; Selvankumar, T.; Kamala-Kannan, S.; Kim, H. Myco-Phytoremediation of Arsenic- and Lead-Contaminated Soils by Helianthus annuus and Wood Rot Fungi, Trichoderma sp. Isolated from Decayed Wood. Ecotoxicol. Environ. Saf. 2018, 151, 279–284. [Google Scholar] [CrossRef]
- Chen, D.W.; Wang, Y.H.; Li, N.; Huang, Y.L.; Mao, Y.F.; Liu, X.J.; Du, Y.R.; Sun, K. Transcriptomic and Physiological Analyses of Trichoderma citrinoviride HT-1 Assisted Phytoremediation of Cd Contaminated Water by Phragmites australis. BMC Microbiol 2024, 24, 93. [Google Scholar] [CrossRef]
- Shanmugaraj, B.M.; Malla, A.; Ramalingam, S. Cadmium Stress and Toxicity in Plants: An Overview; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128148655. [Google Scholar]
- Castro, A.V.; de Almeida, A.A.F.; Pirovani, C.P.; Reis, G.S.M.; Almeida, N.M.; Mangabeira, P.A.O. Morphological, Biochemical, Molecular and Ultrastructural Changes Induced by Cd Toxicity in Seedlings of Theobroma cacao L. Ecotoxicol. Environ. Saf. 2015, 115, 174–186. [Google Scholar] [CrossRef]
- Fazli, M.M.; Soleimani, N.; Mehrasbi, M.; Darabian, S.; Mohammadi, J.; Ramazani, A. Highly Cadmium Tolerant Fungi: Their Tolerance and Removal Potential. J. Environ. Heal. Sci. Eng. 2015, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Singh, A.; Kumar, V. Recent Advancements in Cadmium-Microbe Interactive Relations and Their Application for Environmental Remediation: A Mechanistic Overview. Environ. Sci. Pollut. Res. 2023, 30, 17009–17038. [Google Scholar] [CrossRef] [PubMed]
- Ng, I.S.; Wu, X.; Yang, X.; Xie, Y.; Lu, Y.; Chen, C. Synergistic Effect of Trichoderma reesei Cellulases on Agricultural Tea Waste for Adsorption of Heavy Metal Cr(VI). Bioresour. Technol. 2013, 145, 297–301. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).