Microbial Metallophores in the Productivity of Agroecosystems
Abstract
1. Introduction
2. Biosynthesis and Transcription of Metallophores
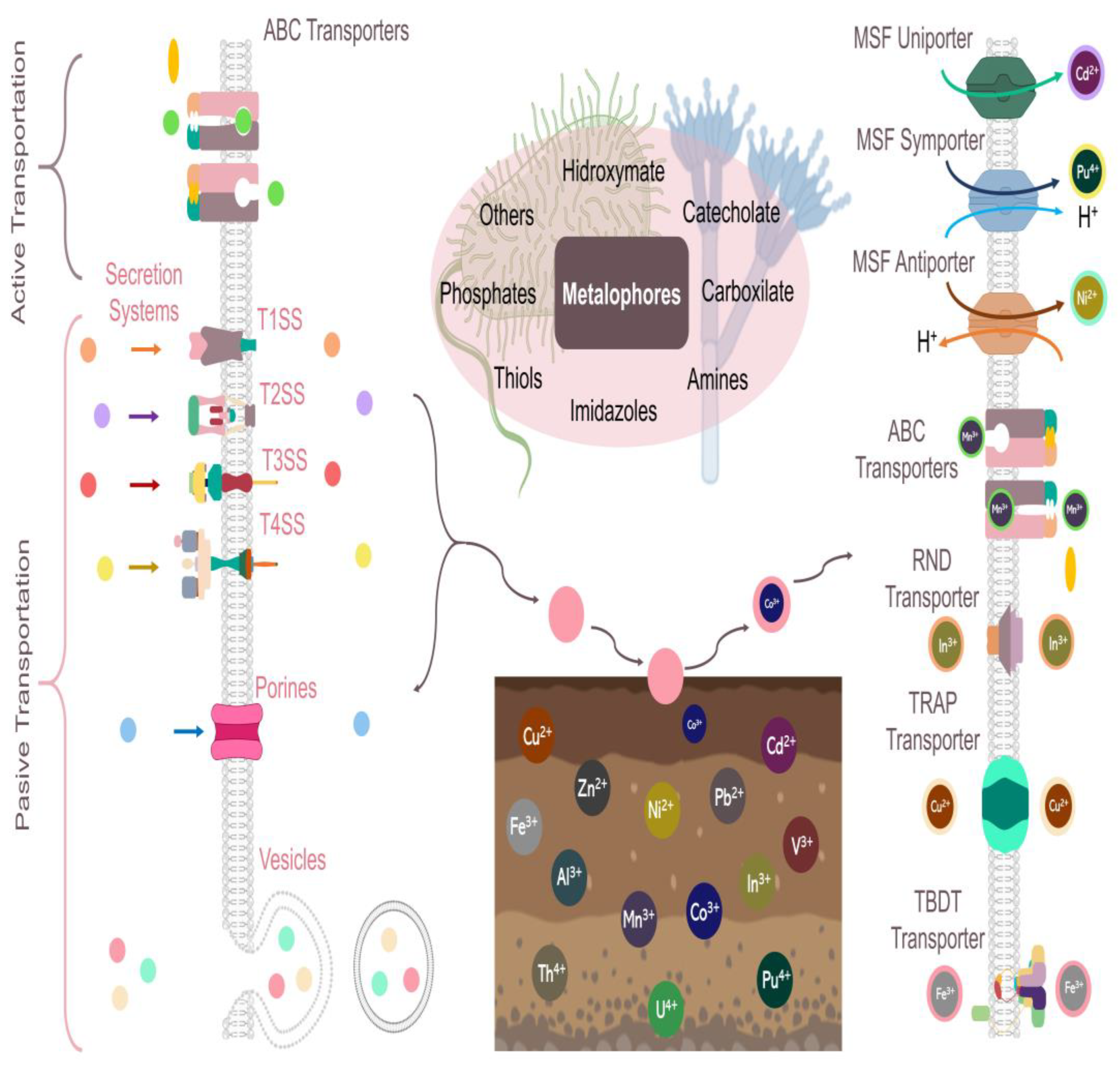
3. Mechanisms of Excretion, Action, and Transport of Microbial Metallophores
4. Metallophore Detection, Extraction, Purification, and Quantification
5. Microbial Metallophores in Microbial Communities
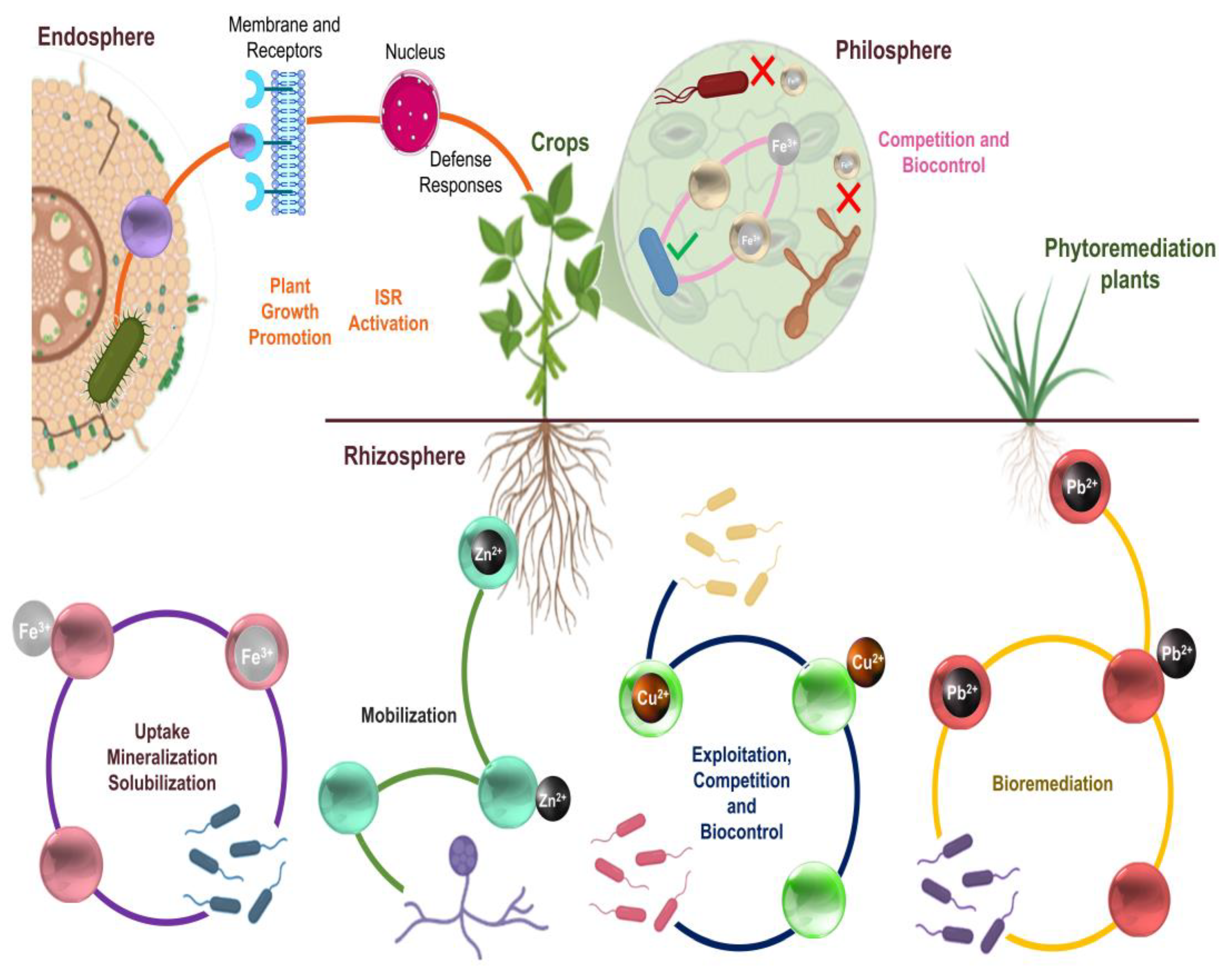
6. The Role of Microbial Metallophores in Promoting Plant Growth
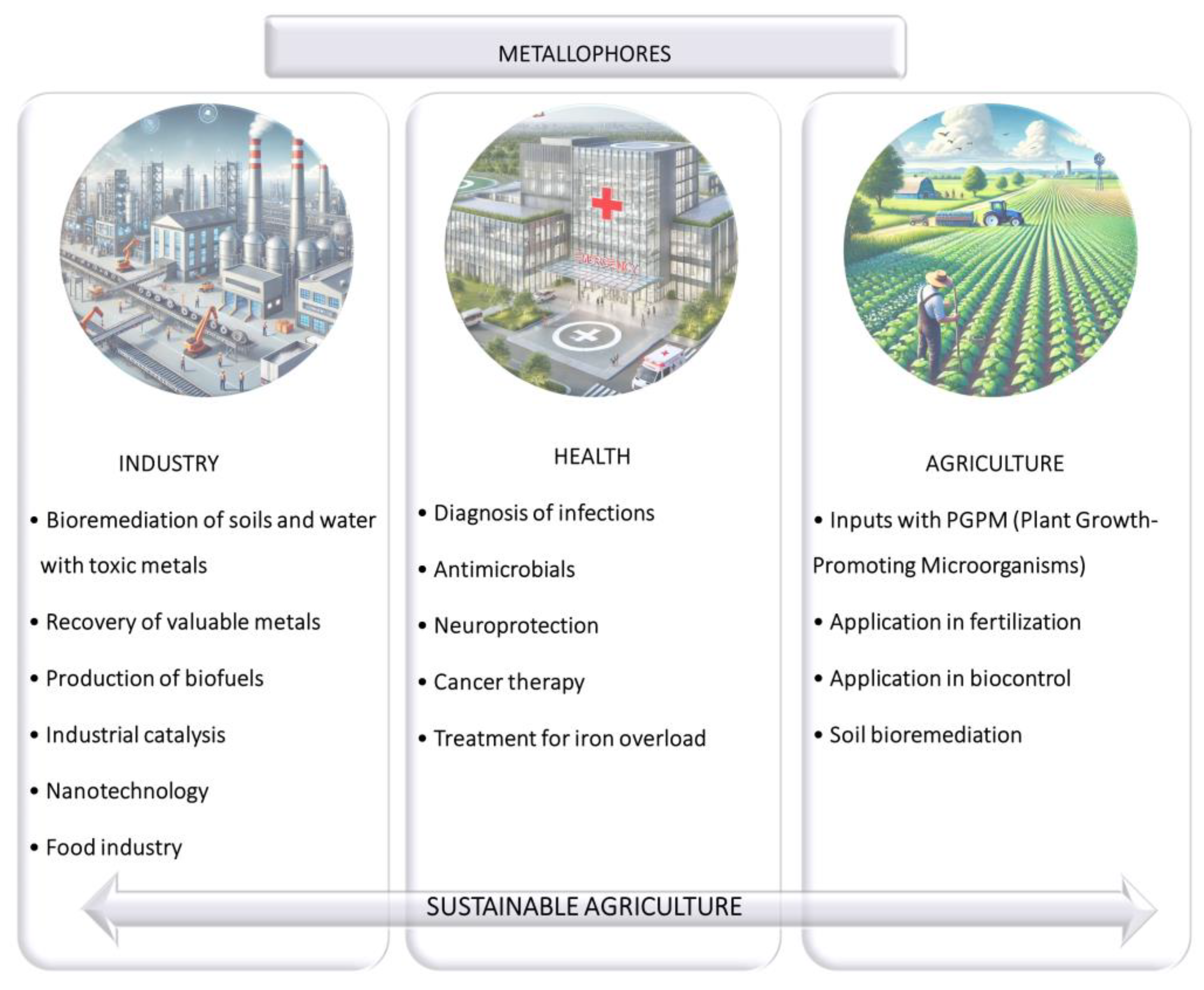
7. Applications of Microbial Metallophores in Agroecosystems
8. The Future of Metallophores
9. Conclusions
Funding
Conflicts of Interest
References
- Anjaria, P.; Vaghela, S. Toxicity of agrochemicals: Impact on environment and human health. J. Toxicol. Stud. 2024, 2, 250. [Google Scholar] [CrossRef]
- Pedraza, R.O.; Teixeira, K.R.S.; Fernández-Scavino, A.; García-de Salamone, I.; Baca, B.E.; Azcón, R.; Baldani, V.L.D.; Bonilla, R. Microorganisms that enhance plant growth and soil quality. Review. Corpoica Cienc. Tecnol. Agropecu. 2010, 11, 155–164. [Google Scholar] [CrossRef]
- Turner, T.R.; James, E.K.; Poole, P.S. The plant microbiome. Genome Biol. 2013, 14, 209. [Google Scholar] [CrossRef]
- Woźniak, M.; Gałązka, A. The rhizosphere microbiome and its beneficial effects on plants–current knowledge and perspectives. Post. Microbiol. 2019, 58, 59–69. [Google Scholar] [CrossRef]
- Kumar, A.; Bahadur, I.; Maurya, B.R.; Raghuwanshi, R.; Meena, V.S.; Singh, D.K.; Dixit, J. Does a plant growth-promoting rhizobacteria enhance agricultural sustainability? J. Pure Appl. Microbiol. 2015, 9, 715–724. [Google Scholar]
- Gouda, S.; Kerry, R.G.; Das, G.; Paramithiotis, S.; Shin, H.S.; Patra, J.K. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol. Res. 2018, 206, 131–140. [Google Scholar] [CrossRef]
- Moreno-Reséndez, A.; Carda-Mendoza, V.; Reyes Carrillo, J.L.; Vásquez Arroyo, J.; Cano Ríos, P. Plant growth promoting rhizobacteria: A biofertilization alternative for sustainable agriculture. Rev. Colomb. Biotecnol. 2018, 20, 68–83. [Google Scholar] [CrossRef]
- Shameer, S.; Prasad, T.N.V.K.V. Plant growth promoting rhizobacteria for sustainable agricultural practices with special reference to biotic and abiotic stresses. Plant Growth Regul. 2018, 84, 603–615. [Google Scholar] [CrossRef]
- Enebe, M.C.; Babalola, O.O. The influence of plant growth-promoting rhizobacteria in plant tolerance to abiotic stress: A survival strategy. Appl. Microbiol. Biotechnol. 2018, 102, 7821–7835. [Google Scholar] [CrossRef]
- Verma, P.P.; Shelake, R.M.; Das, S.; Sharma, P.; Kim, J.Y. Plant growth-promoting rhizobacteria (PGPR) and fungi (PGPF): Potential biological control agents of diseases and pests. In Microbial Interventions in Agriculture and Environment; Singh, D.P., Kumar-Gupta, V., Prabha, R., Eds.; Springer Nature: Singapore, 2019; pp. 281–311. [Google Scholar]
- El-Maraghy, S.S.; Tohamy, A.T.; Hussein, K.A. Plant protection properties of the Plant Growth-Promoting Fungi (PGPF): Mechanisms and potentiality. Curr. Res. Environ. Appl. Mycol. 2021, 11, 391–415. [Google Scholar] [CrossRef]
- Johnston, A.E. Trace elements in soil: Status and management. In Essential Trace Elements for Plants, Animals and Humans; Thorvaldsson, G., Jónsdóttir, R.S., Eds.; Nordic Association of Agricultural Scientists: Reykjavík, Iceland, 2005; pp. 7–14. [Google Scholar]
- Callahan, D.L.; Baker, A.J.; Kolev, S.D.; Wedd, A.G. Metal ion ligands in hyperaccumulating plants. J. Biol. Inorg. Chem. 2006, 11, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Hänsch, R.; Mendel, R.R. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Pesch, M.L.; Hoffmann, M.; Christl, I.; Kraemer, S.M.; Kretzschmar, R. Competitive ligand exchange between Cu-humic acid complexes and methanobactin. Geobiology 2013, 11, 44–54. [Google Scholar] [CrossRef]
- Kraemer, S.M. Iron oxide dissolution and solubility in the presence of siderophores. Aquat. Sci. 2004, 66, 3–18. [Google Scholar] [CrossRef]
- Ghssein, G.; Matar, S.F. Chelating mechanisms of transition metals by bacterial metallophores “pseudopaline and staphylopine”: A quantum chemical assessment. Computation 2018, 6, 56. [Google Scholar] [CrossRef]
- Kramer, J.; Özkaya, Ö.; Kümmerli, R. Bacterial siderophores in community and host interactions. Nat. Rev. Microbiol. 2020, 18, 152–163. [Google Scholar] [CrossRef]
- Kraepiel, A.M.L.; Bellenger, J.P.; Wichard, T.; Morel, F.M.M. Multiples roles of siderophores in free-living nitrogen-fixing bacteria. Biometals 2009, 22, 573–581. [Google Scholar] [CrossRef]
- Schalk, I.J.; Hannauer, M.; Braud, A. New roles for bacterial siderophores in metal transport and tolerance. Environ. Microbiol. 2011, 13, 2844–2854. [Google Scholar] [CrossRef]
- Yu, S.; Teng, C.; Bai, X.; Liang, J.; Song, T.; Dong, L.; Jin, Y.; Qu, J. Optimization of siderophore production by Bacillus sp. PZ-1 and its potential enhancement of phytoextration of Pb from soil. J. Microbiol. Biotechnol. 2017, 27, 1500–1512. [Google Scholar] [CrossRef]
- Ghssein, G.; Ezzeddine, Z. A review of Pseudomonas aeruginosa metallophores: Pyoverdine, pyochelin and pseudopaline. Biology 2022, 11, 1711. [Google Scholar] [CrossRef]
- Gehring, A.M.; Bradley, K.A.; Walsh, C.T. Enterobactin biosynthesis in Escherichia coli: Isochorismate lyase (EntB) is a bifunctional enzyme that is phosphopantetheinylated by EntD and then acylated by EntE using ATP and 2, 3-dihydroxybenzoate. Biochemistry 1997, 36, 8495–8503. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, Y.; Tarchichi, N.; Barakat, R.; Kawtharani, I.; Ghandour, R.; Ezzeddine, Z.; Ghssein, G. The different types of metallophores produced by Salmonella enterica: A review. Microbiol. Res. 2023, 14, 1457–1469. [Google Scholar] [CrossRef]
- Lehner, S.M.; Atanasova, L.; Neumann, N.K.; Krska, R.; Lemmens, M.; Druzhinina, I.S.; Schuhmacher, R. Isotope-assisted screening for iron-containing metabolites reveals a high degree of diversity among known and unknown siderophores produced by Trichoderma spp. Appl. Environ. Microbiol. 2013, 79, 18–31. [Google Scholar] [CrossRef]
- Holinsworth, B.; Martin, J.D. Siderophore production by marine derived fungi. BioMetals 2009, 22, 625–632. [Google Scholar] [CrossRef]
- Saharan, B.S.; Nehra, V. Plant growth promoting rhizobacteria: A critical review. Life Sci. Med. Res. 2011, 21, 1–30. [Google Scholar]
- Ghssein, G.; Brutesco, C.; Ouerdane, L.; Fojcik, C.; Izaute, A.; Wang, S.; Hajjar, C.; Lobinski, R.; Lemaire, D.; Richaud, P.; et al. Biosynthesis of a broad-spectrum nicotianamine-like metallophore in Staphylococcus aureus. Science 2016, 352, 1105–1109. [Google Scholar] [CrossRef]
- Lhospice, S.; Gomez, N.O.; Ouerdane, L.; Brutesco, C.; Ghssein, G.; Hajjar, C.; Liratni, A.; Wang, S.; Richaud, P.; Bleves, S.; et al. Pseudomonas aeruginosa zinc uptake in chelating environment is primarily mediated by the metallophore pseudopaline. Sci. Rep. 2017, 7, 17132. [Google Scholar] [CrossRef]
- Robinson, A.E.; Lowe, J.E.; Koh, E.I.; Henderson, J.P. Uropathogenic enterobacteria use the yersiniabactin metallophore system to acquire nickel. J. Biol. Chem. 2018, 293, 14953–14961. [Google Scholar] [CrossRef]
- Bellenger, J.P.; Wichard, T.; Kraepiel, A.M.L. Vanadium requirements and uptake kinetics in the dinitrogen-fixing bacterium Azotobacter vinelandii. Appl. Environ. Microbiol. 2008, 74, 1478–1484. [Google Scholar] [CrossRef]
- Kenney, G.E.; Rosenzweig, A.C. Chalkophores. Annu. Rev. Biochem. 2018, 87, 645–676. [Google Scholar] [CrossRef]
- Morey, J.R.; Kehl-Fie, T.E. Bioinformatic mapping of opine-like zincophore biosynthesis in bacteria. Msystems 2020, 5, e00554-20. [Google Scholar] [CrossRef]
- Cotruvo, J.A., Jr. The chemistry of lanthanides in biology: Recent discoveries, emerging principles, and technological applications. ACS Cent. Sci. 2019, 5, 1496–1506. [Google Scholar] [CrossRef]
- Johnston, C.W.; Wyatt, M.A.; Li, X.; Ibrahim, A.; Shuster, J.; Southam, G.; Magarvey, N.A. Gold biomineralization by a metallophore from a gold-associated microbe. Nat. Chem. Biol. 2013, 9, 241–243. [Google Scholar] [CrossRef]
- Saito, M.A.; Goepfert, T.J.; Ritt, J.T. Some thoughts on the concept of colimitation: Three definitions and the importance of bioavailability. Limnol. Oceanogr. 2008, 53, 276–290. [Google Scholar] [CrossRef]
- Haas, H.; Eisendle, M.; Turgeon, B.G. Siderophores in fungal physiology and virulence. Annu. Rev. Phytopathol. 2008, 46, 149–187. [Google Scholar] [CrossRef]
- Swinburne, T.R. Iron, Siderophores, and Plant Diseases; Springer: New York, NY, USA, 2012; p. 350. [Google Scholar]
- Butaitė, E.; Baumgartner, M.; Wyder, S.; Kümmerli, R. Siderophore cheating and cheating resistance shape competition for iron in soil and freshwater Pseudomonas communities. Nat. Commun. 2017, 8, 414. [Google Scholar] [CrossRef]
- Xie, P.; Xu, Y.; Tang, J.; Wu, S.; Gao, H. Multifaceted regulation of siderophore synthesis by multiple regulatory systems in Shewanella oneidensis. Commun. Biol. 2024, 7, 498. [Google Scholar] [CrossRef]
- Rodrigues-de Souza Santos, R.E.; Bontempi-Batista, B.; da Silva-Neto, J.F. Ferric uptake regulator Fur coordinates siderophore production and defense against iron toxicity and oxidative stress and contributes to virulence in Chromobacterium violaceum. Appl. Environ. Microbiol. 2020, 86, e01620-20. [Google Scholar]
- Coderre, P.E.; Earhart, C.F. The entD gene of the Escherichia coli K12 enterobactin gene cluster. Microbiology 1989, 135, 3043–3055. [Google Scholar] [CrossRef]
- Dell’Anno, F.; Vitale, G.A.; Buonocore, C.; Vitale, L.; Palma Esposito, F.; Coppola, D.; Della Sala, G.; Tedesco, P.; de Pascale, D. Novel insights on pyoverdine: From biosynthesis to biotechnological application. Int. J. Mol. Sci. 2022, 23, 11507. [Google Scholar] [CrossRef]
- May, J.J.; Wendrich, T.M.; Marahiel, M.A. The dhb operon of Bacillus subtilis encodes the biosynthetic template for the catecholic siderophore 2, 3-dihydroxybenzoate-glycine-threonine trimeric ester bacillibactin. J. Biol. Chem. 2001, 276, 7209–7217. [Google Scholar] [CrossRef]
- El-Maraghy, S.S.; Tohamy, T.A.; Hussein, K.A. Expression of SidD gene and physiological characterization of the rhizosphere plant growth promoting yeasts. Heliyon 2020, 6, e04384. [Google Scholar] [CrossRef]
- McRose, D.L.; Seyedsayamdost, M.R.; Morel, F.M.M. Multiple siderophores: Bug or feature? J. Biol. Inorg. Chem. 2018, 23, 983–993. [Google Scholar] [CrossRef]
- Keating, T.A.; Marshall, C.G.; Walsh, C.T. Vibriobactin biosynthesis in Vibrio cholerae: VibH is an amide synthase homologous to nonribosomal peptide synthetase condensation domains. Biochemistry 2000, 39, 15513–15521. [Google Scholar] [CrossRef]
- Barry, S.M.; Challis, G.L. Recent advances in siderophore biosynthesis. Curr. Opin. Chem. Biol. 2009, 13, 205–215. [Google Scholar] [CrossRef]
- Crosa, J.H.; Walsh, C.T. Genetics and Assembly line enzymology of siderophore biosynthesis in bacteria. Microbiol. Mol. Biol. Rev. 2002, 66, 223–249. [Google Scholar] [CrossRef]
- Khan, A.; Singh, P.; Srivastava, A. Synthesis, nature and utility of universal iron chelator—Siderophore: A review. Microbiol. Res. 2018, 212–213, 103–111. [Google Scholar] [CrossRef]
- Berti, A.D.; Thomas, M.G. Analysis of achromobactin biosynthesis by Pseudomonas syringae pv. syringae B728a. J. Bacteriol. 2009, 191, 4594–4604. [Google Scholar] [CrossRef]
- Schalk, I.J. Siderophore-antibiotic conjugates: Exploiting iron uptake to deliver drugs into bacteria. Clin. Microbiol. Infect. 2019, 24, 801–802. [Google Scholar] [CrossRef]
- Haag, H.; Fiedler, H.P.; Meiwes, J.; Drechsel, H.; Jung, G.; Zähner, H. Isolation and biological characterization of staphyloferrin B, a compound with siderophore activity from staphylococci. FEMS Microbiol. Lett. 1994, 115, 125–130. [Google Scholar] [CrossRef]
- Ghssein, G.; Ezzeddine, Z. The key element role of metallophores in the pathogenicity and virulence of Staphylococcus aureus: A Review. Biology 2022, 11, 1525. [Google Scholar] [CrossRef]
- Buettner, H.; Hoerl, J.; Krabbe, J.; Hertweck, C. Discovery and biosynthesis of anthrochelin, a growth-promoting metallophore of the human pathogen Luteibacter anthropi. ChemBioChem 2023, 24, e202300322. [Google Scholar]
- Buglino, J.A.; Ozakman, Y.; Xu, Y.; Chowdhury, F.; Tan, D.S.; Glickman, M.S. Diisonitrile lipopeptides mediate resistance to copper starvation in pathogenic mycobacteria. mBio 2022, 13, e0251322. [Google Scholar] [CrossRef]
- Semrau, J.D.; DiSpirito, A.A.; Obulisamy, P.K.; Kang-Yun, C.S. Methanobactin from methanotrophs: Genetics, structure, function and potential applications. FEMS Microbiol. Lett. 2020, 367, fnaa045. [Google Scholar] [CrossRef]
- Reitz, Z.L.; Medema, M.H. Genome mining strategies for metallophore discovery. Curr. Opin. Biotechnol. 2022, 77, 102757. [Google Scholar] [CrossRef]
- Leprevost, L.; Jünger, S.; Lippens, G.; Guillaume, C.; Sicoli, G.; Oliveira, L.; Falcone, E.; de-Santis, E.; Rivera-Millot, A.; Billon, G.; et al. A widespread family of ribosomal peptide metallophores involved in bacterial adaptation to metal stress. Proc. Natl. Acad. Sci. USA 2024, 121, e2408304121. [Google Scholar] [CrossRef]
- Antelo, G.T.; Vila, A.J.; Giedroc, D.P.; Capdevila, D.A. Molecular evolution of transition metal bioavailability at the host–pathogen interface. Trends Microbiol. 2021, 29, 441–457. [Google Scholar] [CrossRef]
- Hider, R.C.; Kong, X. Chemistry and biology of siderophores. Nat. Prod. Rep. 2010, 27, 637–657. [Google Scholar] [CrossRef]
- Johnstone, T.C.; Nolan, E.M. Beyond iron: Non-classical biological functions of bacterial siderophores. Dalton Trans. 2015, 44, 6320–6339. [Google Scholar] [CrossRef]
- Bellotti, D.; Rowińska-Żyrek, M.; Remelli, M. How zinc-binding systems, expressed by human pathogens, acquire zinc from the colonized host environment: A critical review on zincophores. Curr. Med. Chem. 2021, 28, 7312. [Google Scholar]
- Kusakizako, T.; Miyauchi, H.; Ishitani, R.; Nureki, O. Structural biology of the multidrug and toxic compound extrusion superfamily transporters. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183154. [Google Scholar] [CrossRef]
- Kraemer, S.M.; Duckworth, O.W.; Harrington, J.M.; Schenkeveld, W.D. Metallophores and trace metal biogeochemistry. Aquat. Geochem. 2015, 21, 159–195. [Google Scholar] [CrossRef]
- Laschat, S.; Bilitewski, U.; Blodgett, J.; Duhme-Klair, A.K.; Dallavalle, S.; Routledge, A.; Schobert, R. Chemical and biological aspects of nutritional immunity-perspectives for new antiinfectives targeting iron uptake systems. Angew. Chem. Int. Ed. 2017, 56, 14360. [Google Scholar]
- Hofmann, M.; Retamal-Morales, G.; Tischler, D. Metal binding ability of microbial natural metal chelators and potential applications. Nat. Prod. Rep. 2020, 37, 1262. [Google Scholar] [CrossRef]
- Mohr, J.F.; Baldeweg, F.; Deicke, M.; Morales-Reyes, C.F.; Hoffmeister, D.; Wichard, T. Frankobactin metallophores produced by nitrogen-fixing Frankia actinobacteria function in toxic metal sequestration. J. Nat. Prod. 2021, 84, 1216–1225. [Google Scholar] [CrossRef]
- Maknun, L.; Kińska, K.; González-Álvarez, I.; Ouerdane, L.; Lauga, B.; Siripinyanond, A.; Szpunar, J.; Lobinski, R. Quantitative determination of iron–siderophore complexes in peat by isotope-exchange size-exclusion UPLC–electrospray ionization high-resolution accurate mass (HRAM) mass spectrometry. Anal. Chem. 2023, 95, 9182–9190. [Google Scholar] [CrossRef]
- Ramesh, B.; Kameswaran, S.; Venkatrayulu, C.; Silpa, S.; Chandra, M.S.; Reddy, G.V.S.; Kumar, K.N. Microbial interaction with metals and metalloids. In Innovations in Biotechnology for a Sustainable Future; Maddela, N.R., García, L.C., Eds.; Elsevier: Cham, Switzerland, 2021; pp. 243–272. [Google Scholar]
- Ramke, R.; Jeyaraman, A. Microbial interaction with metals and metalloids. In Microbes and Microbial Biotechnology for Green Remediation; Malik, J.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 29–46. [Google Scholar]
- Wilson, B.R.; Bogdan, A.R.; Miyazawa, M.; Hashimoto, K.; Tsuji, Y. Siderophores in Iron metabolism: From mechanism to therapy potential. Trends Mol. Med. 2016, 22, 1077–1090. [Google Scholar] [CrossRef]
- Timofeeva, A.M.; Galyamova, M.R.; Sedykh, S.E. Bacterial siderophores: Classification, biosynthesis, perspectives of use in agriculture. Plants 2022, 11, 3065. [Google Scholar] [CrossRef]
- Puja, H.; Mislin, G.L.A.; Rigouin, C. Engineering siderophore biosynthesis and regulation pathways to increase diversity and availability. Biomolecules 2023, 13, 959. [Google Scholar] [CrossRef]
- Furrer, J.L.; Sanders, D.N.; Hook-Barnard, I.G.; McIntosh, M.A. Export of the siderophore enterobactin in Escherichia coli: Involvement of a 43 kDa membrane exporter. Mol. Microbiol. 2002, 44, 1225–1234. [Google Scholar] [CrossRef]
- Salvail, H.; Lanthier-Bourbonnais, P.; Sobota, J.M.; Caza, M.; Benjamin, J.A.M.; Mendieta, M.E.S.; Lépine, F.; Dozois, C.M.; Imlay, J.; Massé, E. A small RNA promotes siderophore production through transcriptional and metabolic remodeling. Proc. Natl. Acad. Sci. USA 2010, 107, 15223–15228. [Google Scholar] [CrossRef]
- Schubert, S.; Fischer, D.; Heesemann, J. Ferric enterochelin transport in Yersinia enterocolitica: Molecular and evolutionary aspects. J. Bacteriol. 1999, 181, 6387–6395. [Google Scholar] [CrossRef]
- Endicott, N.P.; Rivera, G.S.M.; Yang, J.; Wencewicz, T.A. Emergence of ferrichelatase activity in a siderophore-binding protein supports an iron shuttle in bacteria. ACS Cent. Sci. 2020, 6, 493–506. [Google Scholar] [CrossRef]
- Trapet, P.; Avoscan, L.; Klinguer, A.; Pateyron, S.; Citerne, S.; Chervin, C.; Mazurier, S.; Lemanceau, P.; Wendehenne, D.; Besson-Bard, A. The Pseudomonas fluorescens siderophore pyoverdine weakens Arabidopsis thaliana defense in favor of growth in iron-deficient conditions. Plant Physiol. 2016, 171, 675–693. [Google Scholar] [CrossRef]
- Rajkumar, M.; Ae, N.; Prasad, M.N.V.; Freitas, H. Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol. 2010, 28, 142–149. [Google Scholar] [CrossRef]
- Albelda-Berenguer, M.; Monachon, M.; Joseph, E. Siderophores: From natural roles to potential applications. Adv. Appl. Microbiol. 2019, 106, 193–225. [Google Scholar]
- Gomes, A.F.; Almeida, M.C.; Sousa, E.; Resende, D.I. Siderophores and metallophores: Metal complexation weapons to fight environmental pollution. Sci. Total Environ. 2024, 932, 173044. [Google Scholar] [CrossRef]
- Francis, M.S.; Thomas, C.J. Mutants in the CtpA copper transporting P-type ATPase reduce virulence of Listeria monocytogenes. Microb. Pathog. 1997, 22, 67–78. [Google Scholar] [CrossRef]
- Renshaw, J.C.; Robson, G.D.; Trinci, A.P.J.; Wiebe, M.G.; Livens, F.R.; Collison, D.; Taylor, R.J. Fungal siderophores: Structures, functions and applications. Mycol. Res. 2002, 106, 1123–1142. [Google Scholar] [CrossRef]
- Müller, G.; Isowa, Y.; Raymond, K.N. Stereospecificity of siderophore-mediated iron uptake in Rhodotorula pilimanae as probed by enantiorhodotorulic acid and isomers of chromic rhodotorulate. J. Biol. Chem. 1985, 260, 13921–13926. [Google Scholar] [CrossRef]
- Fekete, F.A.; Chandhoke, V.; Jellison, J. Iron-binding compounds produced by wood-decaying basidiomycetes. Appl. Environ. Microbiol. 1989, 55, 2720–2722. [Google Scholar] [CrossRef] [PubMed]
- Sushma, J.; Neeti, K.; Sarrah, R.; Pratibha, S.; Hajra, G.A.S. Optimization of universal chrome Azurol S (CAS) siderophore detecting media with an economical dye substitute using Pseudomonas fluorescens as a test organism. Res. J. Chem. Environ. 2021, 25, 27–34. [Google Scholar]
- Dimkpa, C. Microbial siderophores: Production, detection and application in agriculture and environment. Endocytobiosis Cell Res. 2016, 27, 7–16. [Google Scholar]
- Andrews, M.Y.; Santelli, C.M.; Duckworth, O.W. Layer plate CAS assay for the quantitation of siderophore production and determination of exudation patterns for fungi. J. Microbiol. Methods 2016, 121, 41–43. [Google Scholar] [CrossRef]
- Murakami, C.; Tanaka, A.R.; Sato, Y.; Kimura, Y.; Morimoto, K. Easy detection of siderophore production in diluted growth media using an improved CAS reagent. J. Microbiol. Methods 2021, 189, 106310. [Google Scholar] [CrossRef]
- Bhattacharya, S.; John, P.J.; Ledwani, L. Microbial siderophores an envisaged tool for asbestos bioremediation—A microcosm approach. Mater. Today Proc. 2021, 43, 3110–3116. [Google Scholar] [CrossRef]
- Arora, N.K.; Verma, M. Modified microplate method for rapid and efficient estimation of siderophore produced by bacteria. 3 Biotech 2017, 7, 381. [Google Scholar] [CrossRef]
- Gu, S.; Wan, W.; Shao, Z.; Zhong, W. High-throughput method for detecting siderophore production by rhizosphere bacteria. Bio Protoc. 2021, 11, e4001. [Google Scholar] [CrossRef]
- Yoder, M.F.; Kisaalita, W.S. Fluorescence of pyoverdin in response to iron and other common well water metals. J. Environ. Sci. Health A 2006, 41, 369–380. [Google Scholar] [CrossRef]
- Chen, J.; Sun, Z.; Jin, J.; Wang, F.; Yang, Q.; Yu, H.; Yu, J.; Wang, Y. Role of siderophore in Pseudomonas fluorescens biofilm formation and spoilage potential function. Food Microbiol. 2023, 109, 104151. [Google Scholar] [CrossRef]
- Sayyed, R.Z.; Chincholkar, S.B. Purification of siderophores of Alcaligenes feacalis on Amberlite XAD. Bioresour. Technol. 2006, 97, 1026–1029. [Google Scholar] [CrossRef] [PubMed]
- Sayyed, R.Z.; Chincholkar, S.B.; Reddy, M.S.; Gangurde, N.S.; Patel, P.R. Siderophore producing PGPR for crop nutrition and phytopathogen suppression. In Bacteria in Agrobiology: Disease Management; Sayyed, R.Z., Chincholkar, S.B., Reddy, M.S., Gangurde, N.S., Patel, P.R., Eds.; Springer: Berlin, Germany, 2012; pp. 449–471. [Google Scholar]
- Schwabe, R.; Senges, C.H.R.; Bandow, J.E.; Heine, T.; Lehmann, H.; Wiche, O.; Schlömanna, M.; Levicáne, G.; Tischler, D. Cultivation dependent formation of siderophores by Gordonia rubripertincta CWB2. Microbiol. Res. 2020, 238, 126481. [Google Scholar] [CrossRef] [PubMed]
- Meesungnoen, O.; Chantiratikul, P.; Thumanu, K.; Nuengchamnong, N.; Hokura, A.; Nakbanpote, W. Elucidation of crude siderophore extracts from supernatants of Pseudomonas sp. ZnCd2003 cultivated in nutrient broth supplemented with Zn, Cd, and Zn plus Cd. Arch. Microbiol. 2021, 203, 2863–2874. [Google Scholar] [CrossRef]
- Mazari, H.E.; Meliani, A.; Berkat, S.; Aliane, S.; Djibaoui, R.; Bouderoua, K. Washing of heavy metal-contaminated soils using pyoverdine extracted from plant growth-promoting bacteria Pseudomonas lactis and P. atacamensis. Carpathian J. Earth Environ. Sci. 2024, 19, 169–178. [Google Scholar] [CrossRef]
- Schwabe, R.; Anke, M.K.; Szymańska, K.; Wiche, O.; Tischler, D. Analysis of desferrioxamine-like siderophores and their capability to selectively bind metals and metalloids: Development of a robust analytical RP-HPLC method. Res. Microbiol. 2018, 169, 598–607. [Google Scholar] [CrossRef]
- Doyama, K.; Haruma, T.; Hishiyama, S.; Kato, A.; Masuya, H.; Yamaji, K. Isoavenaciol and 7-hydroxy-isoavenaciol: Zn-chelating metallophores produced by root-endophytic Pezicula ericae in a Zn-accumulating plant, Aucuba japonica. Phytochemistry 2023, 206, 113547. [Google Scholar] [CrossRef]
- Deicke, M.; Bellenger, J.P.; Wichard, T. Direct quantification of bacterial molybdenum and iron metallophores with ultra-high-performance liquid chromatography coupled to time-of-flight mass spectrometry. J. Chromatogr. A 2013, 1298, 50–60. [Google Scholar] [CrossRef]
- Franco-Sierra, N.D.; Posada, L.F.; Santa-María, G.; Romero-Tabarez, M.; Villegas-Escobar, V.; Álvarez, J.C. Bacillus subtilis EA-CB0575 genome reveals clues for plant growth promotion and potential for sustainable agriculture. Funct. Integr. Genomics 2020, 20, 575–589. [Google Scholar] [CrossRef]
- Kenney, G.E.; Rosenzweig, A.C. Methanobactins: Maintaining copper homeostasis in methanotrophs and beyond. J. Biol. Chem. 2018, 293, 4606–4615. [Google Scholar] [CrossRef]
- Dassama, L.M.; Kenney, G.E.; Rosenzweig, A.C. Methanobactins: From genome to function. Metallomics 2017, 9, 7–20. [Google Scholar] [CrossRef]
- Calderón-Celis, F.; González-Álvarez, I.; Fabjanowicz, M.; Godin, S.; Ouerdane, L.; Lauga, B.; Łobiński, R. Unveiling the pool of metallophores in native environments and correlation with their potential producers. Environ. Sci. Technol. 2023, 57, 17302–17311. [Google Scholar] [CrossRef] [PubMed]
- Garber, A.I.; Nealson, K.H.; Okamoto, A.; McAllister, S.M.; Chan, C.S.; Barco, R.A.; Merino, N. FeGenie: A comprehensive tool for the identification of iron genes and iron gene neighborhoods in genome and metagenome assemblies. Front. Microbiol. 2020, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Khan, A.; Kumar, R.; Ojha, K.K.; Singh, V.K.; Srivastava, A. In silico analysis of comparative affinity of phytosiderophore and bacillibactin for iron uptake by YSL15 and YSL18 receptors of Oryza sativa. J. Biomol. Struct. Dyn. 2023, 41, 2733–2746. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Verges, M.C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar]
- Hassani, M.A.; Durán, P.; Hacquard, S. Microbial interactions within the plant holobiont. Microbiome 2018, 6, 58. [Google Scholar] [CrossRef]
- Cordero, O.X.; Ventouras, L.A.; DeLong, E.F.; Polz, M.F. Public good dynamics drive evolution of iron acquisition strategies in natural bacterioplankton populations. Proc. Natl Acad. Sci. USA 2012, 109, 20059–20064. [Google Scholar] [CrossRef]
- Leinweber, A.; Inglis, R.F.; Kümmerli, R. Cheating fosters species co-existence in well-mixed bacterial communities. ISME J. 2017, 11, 1179–1188. [Google Scholar] [CrossRef]
- MacLean, R.C. The tragedy of the commons in microbial populations: Insights from theoretical, comparative and experimental studies. Heredity 2008, 100, 471–477. [Google Scholar] [CrossRef]
- Özkaya, Ö.; Xavier, K.B.; Dionisio, F.; Balbontin, R. Maintenance of microbial cooperation mediated by public goods in single and multiple traits scenarios. J. Bacteriol. 2017, 199, e00297-17. [Google Scholar] [CrossRef]
- Lin, H.; Liu, C.; Li, B.; Dong, Y. Trifolium repens L. regulated phytoremediation of heavy metal contaminated soil by promoting soil enzyme activities and beneficial rhizosphere associated microorganisms. J. Hazard. Mater. 2021, 402, 123829. [Google Scholar] [CrossRef]
- Oberegger, H.; Schoeser, M.; Zadra, I.; Abt, B.; Haas, H. SREA is involved in regulation of siderophore biosynthesis, utilisation and uptake in Aspergillus nidulans. Mol. Microbiol. 2001, 41, 1077–1089. [Google Scholar] [CrossRef]
- Fiedler, H.P.; Krastel, P.; Muller, J.; Gebhardt, K.; Zeeck, A. Enterobactin: The characteristic catecholate siderophore of Enterobacteriaceae is produced by Streptomyces species. FEMS Microbiol. Lett. 2001, 115, 125–130. [Google Scholar] [CrossRef]
- Haas, D.; Keel, C. Regulation of antibiotic production in root-colonizing Pseudomonas spp and relevance for biological control of plant disease. Annu. Rev. Phytopathol. 2003, 41, 117–153. [Google Scholar] [CrossRef]
- O’Brien, S.; Hodgson, D.J.; Buckling, A. Social evolution of toxic metal bioremediation in Pseudomonas aeruginosa. Proc. Biol. Sci. 2014, 281, 20140858. [Google Scholar] [CrossRef]
- Gonzales, D.T.; Suraritdechachai, S.; Zechner, C.; Tang, T.Y.D. Bidirectional communication between droplet interface bilayers driven by cell-free quorum sensing gene circuits. ChemSystemsChem 2023, 5, e202300029. [Google Scholar] [CrossRef]
- Lynch, J.M. Beneficial interactions between micro-organisms and roots. Biotechnol. Adv. 1990, 8, 335–346. [Google Scholar] [CrossRef]
- Sørensen, J. The rhizosphere as a habitat for soil microorganisms. In Modern Soil Microbiology; van Elsas, J.D., Trevors, J.T., Wellington, E.M.H., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 1997; pp. 21–45. [Google Scholar]
- Kennedy, A.C.; De Luna, L.Z. Rhizosphere. In Encyclopedia of Soil as in the Environment; Hillel, D., Ed.; Elsevier: Oxford, UK, 2004; pp. 399–406. [Google Scholar]
- Ngullie, E.; Singh, A.K.; Sema, A.; Srivastava, A.K. Citrus growth and rhizosphere properties. Commun. Soil Sci. Plant Anal. 2015, 46, 1540–1550. [Google Scholar] [CrossRef]
- Curl, E.A.; Truelove, B. The Rhizosphere; Springer: New York, NY, USA, 1986; p. 289. [Google Scholar]
- DeAngelis, K.M.; Silver, W.L.; Thompson, A.W.; Firestone, M.K. Microbial communities acclimate to recurring changes in soil redox potential status. Environ. Microbiol. 2010, 12, 3137–3149. [Google Scholar] [CrossRef]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef]
- Haichar, Z.F.; Marol, C.; Berge, O.; Rangel-Castro, J.I.; Prosser, J.I.; Balesdent, J.; Heulin, T.; Achouak, W. Plant host habitat and root exudates shape soil bacterial community structure. ISME J. 2008, 2, 1221. [Google Scholar] [CrossRef]
- Dennis, P.G.; Miller, A.J.; Hirsch, P.R. Are root exudates more important than other sources of rhizodeposits in structuring rhizosphere bacterial communities? FEMS Microbiol. Ecol. 2010, 72, 313–327. [Google Scholar] [CrossRef]
- Yadav, A. Exploring the potential of endophytes in agriculture: A minireview. Adv. Plants Agric. Res. 2017, 6, 102–106. [Google Scholar] [CrossRef]
- Bhardwaj, D.; Ansari, M.W.; Sahoo, R.K.; Tuteja, N. Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb. Cell Fact. 2014, 13, 66. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, G.; Kulasooriya, S.A. Reinstating soil microbial diversity in agroecosystems: The need of the hour for sustainability and health. Agric. Ecosyst. Environ. 2013, 164, 181–182. [Google Scholar] [CrossRef]
- Rout, G.R.; Sahoo, S. Role of iron in plant growth and metabolism. Rev. Agric. Sci. 2015, 3, 1–24. [Google Scholar] [CrossRef]
- Hebbern, C.A.; Laursen, K.H.; Ladegaard, A.H.; Schmidt, S.B.; Pedas, P.; Bruhn, D.; Schjoerring, J.K.; Wulfsohn, D.; Husted, S. Latent manganese deficiency increases transpiration in barley (Hordeum vulgare). Physiol. Plant. 2009, 135, 307–316. [Google Scholar] [CrossRef]
- Schwarz, G.; Mendel, R.R. Molybdenum cofactor biosynthesis and molybdenum enzymes. Annu. Rev. Plant Biol. 2006, 57, 623–647. [Google Scholar] [CrossRef]
- Follmer, C. Insights into the role and structure of plant ureases. Phytochemistry 2008, 69, 18–28. [Google Scholar] [CrossRef]
- Kawagashira, N.; Ohtomo, Y.; Murakami, K.; Matsubara, K.; Kawai, J.; Carninci, R.; Hayashizaki, P.; Kikuchi, S.; Higo, K. Multiple zinc finger motifs with comparison of plant and insects. Genom. Inform. 2001, 12, 368–369. [Google Scholar]
- Kramer, U.; Clemens, S. Function and homeostasis of zinc, copper, and nickel in plants. In Molecular Biology of Metal Homeostasis and Detoxification: From Microbes to Man; Tamas, M.J., Martinoia, E., Eds.; Springer-Verlag: Berlin, Germany, 2006; pp. 215–271. [Google Scholar]
- Constantin, M.; Chioncel, M.F.; Petrescu, L.; Vrancianu, C.O.; Paun, M.; Cristian, R.E.; Sidoroff, M.; Dionisie, M.V.; Chifiriuc, M.C. From rock to living systems: Lanthanides toxicity and biological interactions. Ecotoxicol. Environ. Saf. 2025, 289, 117494. [Google Scholar] [CrossRef]
- Sharma, A.; Johri, B. Growth promoting influence of siderophore-producing Pseudomonas strains GRP3A and PRS9 in maize (Zea mays L.) under iron limiting conditions. Microbiol. Res. 2003, 158, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Sah, S.; Singh, N.; Singh, R. Iron acquisition in maize (Zea mays L.) using Pseudomonas siderophore. 3 Biotech 2017, 7, 121. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, Y.; Kong, S. Effects of Trichoderma asperellum and its siderophores on endogenous auxin in Arabidopsis thaliana under iron-deficiency stress. Int. Microbiol. 2020, 23, 501–509. [Google Scholar] [CrossRef]
- Cui, K.; Xu, T.; Chen, J.; Yang, H.; Liu, X.; Zhuo, R.; Peng, Y.; Tang, W.; Wang, R.; Chen, L.; et al. Siderophores, a potential phosphate solubilizer from the endophyte Streptomyces sp. CoT10, improved phosphorus mobilization for host plant growth and rhizosphere modulation. J. Clean. Prod. 2022, 367, 133110. [Google Scholar] [CrossRef]
- Singh, P.; Kumar, R.; Khan, A.; Singh, A.; Srivastava, A. Bacillibactin siderophore induces iron mobilisation responses inside aerobic rice variety through YSL15 transporter. Rhizosphere 2023, 27, 100724. [Google Scholar] [CrossRef]
- Wensing, A.; Braun, S.D.; Büttner, P.; Expert, D.; Völksch, B.; Ullrich, M.S.; Weingart, H. Impact of siderophore production by Pseudomonas syringae pv. syringae 22d/93 on epiphytic fitness and biocontrol activity against Pseudomonas syringae pv. glycinea 1a/96. Appl. Environ. Microbiol. 2010, 76, 2704–2711. [Google Scholar] [CrossRef]
- Sha, Z.; Watanabe, T.; Chu, Q.; Oka, N.; Osaki, M.; Shinano, T. A reduced phosphorus application rate using a mycorrhizal plant as the preceding crop maintains soybean seeds’ nutritional quality. J. Agric. Food Chem. 2019, 67, 32–42. [Google Scholar] [CrossRef]
- Bellenger, J.P.; Wichard, T.; Kustka, A.B.; Kraepiel, A.M.L. Nitrogen fixing soil bacterium uses catechol siderophores for molybdenum and vanadium acquisition. Nat. Geosci. 2008, 1, 243–246. [Google Scholar] [CrossRef]
- Bellenger, J.P.; Wichard, T.; Xu, Y.; Kraepiel, A.M. Essential metals for nitrogen fixation in a free-living N2-fixing bacterium: Chelation, homeostasis and high use efficiency. Environ. Microbiol. 2011, 13, 1395–1411. [Google Scholar] [CrossRef]
- Holler, T.; Wegener, G.; Knittel, K.; Boetius, A.; Brunner, B.; Kuypersm, M.M.M.; Widdel, F. Substantial 13C/12C and D/H fractionation during anaerobic oxidation of methane by marine consortia enriched in vitro. Environ. Microbiol. Rep. 2009, 1, 370–376. [Google Scholar] [CrossRef]
- Wichard, T.; Mishra, B.; Myneni, S.C.B.; Bellenger, J.P.; Kraepiel, A.M.L. Storage and bioavailability of molybdenum in soils increased by organic matter complexation. Nat. Geosci. 2009, 2, 625. [Google Scholar] [CrossRef]
- Srivastava, S.; Dong, H.; Baars, O.; Sheng, Y. Bioavailability of mineral-associated trace metals as cofactors for nitrogen fixation by Azotobacter vinelandii. Geobiology 2023, 21, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Kaushik, R.; Saxena, A.K.; Arora, D.K. Diversity and phylogeny of plant growth-promoting bacilli from moderately acidic soil. J. Basic Microbiol. 2011, 51, 98–106. [Google Scholar] [CrossRef]
- Van Scholl, L.; Kuyper, T.W.; Smits, M.M.; Landeweert, R.; Hoffland, E.; van Breemen, N. Rock-eating mycorrhizas: Their role in plant nutrition and biogeochemical cycles. Plant Soil 2008, 303, 35–47. [Google Scholar] [CrossRef]
- Loper, J.E.; Buyer, J.S. Siderophores in microbial interactions on plant surfaces. Mol. Plant Microbe Interact. 1991, 4, 5–13. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Sluis, L.V.D.; Bakker, P.A.; Schippers, B.; Koster, M.; Weisbeek, P.J. Utilisation of heterologous siderophores and rhizosphere competence of fluorescent Pseudomonas spp. Can. J. Microbiol. 1995, 41, 126–135. [Google Scholar] [CrossRef]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Ma, Y.; Oliveira, R.S.; Freitas, H.; Zhang, C. Biochemical and molecular mechanisms of plant-microbe-metal interactions: Relevance for phytoremediation. Front. Plant Sci. 2016, 7, 918. [Google Scholar] [CrossRef]
- Gu, S.; Yang, T.; Shao, Z.; Wang, T.; Cao, K.; Jousset, A.; Friman, V.P.; Mallon, C.; Mei, X.; Wei, Z.; et al. Siderophore-mediated interactions determine the disease suppressiveness of microbial consortia. mSystems 2020, 5, e00811-19. [Google Scholar] [CrossRef]
- Gu, S.; Wei, Z.; Shao, Z.; Friman, V.P.; Cao, K.; Yang, T.; Kramer, J.; Wang, X.; Li, M.; Mei, X.; et al. Competition for iron drives phytopathogen control by natural rhizosphere microbiomes. Nat. Microbiol. 2020, 5, 1002–1010. [Google Scholar] [CrossRef]
- Maurhofer, M.; Hase, C.; Meuwly, P.; Metraux, J.P.; Defago, G. Induction of systemic resistance of tobacco to tobacco necrosis virus by the root-colonizing Pseudomonas fluorescens strain CHA0: Influence of the gacA gene and of pyoverdine production. Phytopathology 1994, 84, 139–146. [Google Scholar] [CrossRef]
- Leeman, M.; Den Ouden, F.M.; Van Pelt, J.A.; Dirkx, F.P.M.; Steijl, H.; Bakker, P.A.H.M.; Schippers, B. Iron availability affects induction of systemic resistance to Fusarium wilt of radish by Pseudomonas fluorescens. Phytopathology 1996, 86, 149–155. [Google Scholar] [CrossRef]
- Michavila, G.; Adler, C.; De Gregorio, P.R.; Lami, M.J.; Caram-Di Santo, M.C.; Zenoff, A.M.; de Cristobal, R.E.; Vincent, P.A. Pseudomonas protegens CS 1 from the lemon phyllosphere as a candidate for citrus canker biocontrol agent. Plant Biol. 2017, 19, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Dhanya, S.; Sherin, V.; Divya, K.; Sreekumar, J.; Jisha, M.S. Pseudomonas taiwanensis (MTCC11631) mediated induction of systemic resistance in Anthurium andreanum L. against blight disease and visualization of defense related secondary metabolites using confocal laser scanning microscopy. Biocatal. Agric. Biotechnol. 2020, 24, 101561. [Google Scholar] [CrossRef]
- Ghazy, N.; El-Nahrawy, S. Siderophore production by Bacillus subtilis MF497446 and Pseudomonas koreensis MG209738 and their efficacy in controlling Cephalosporium maydis in maize plant. Arch. Microbiol. 2020, 203, 1195–1209. [Google Scholar] [CrossRef]
- Gupta, V.; Kumar, G.N.; Buch, A. Colonization by multi-potential Pseudomonas aeruginosa P4 stimulates peanut (Arachis hypogea L.) growth, defense physiology and root system functioning to benefit the root-rhizobacterial interface. J. Plant Physiol. 2020, 248, 153144. [Google Scholar] [CrossRef]
- Arora, N.; Kang, S.; Maheshwari, D. Isolation of siderophore-producing strains of Rhizobium meliloti and their biocontrol potential against Macrophomina phaseolina that causes charcoal rot of groundnut. Curr. Sci. 2001, 81, 673–677. [Google Scholar]
- Verma, V.C.; Singh, S.K.; Prakash, S. Bio-control and plant growth promotion potential of siderophore producing endophytic Streptomyces from Azadirachta indica A. Juss. J. Basic Microbiol. 2011, 51, 550–556. [Google Scholar] [CrossRef]
- Myo, E.M.; Liu, B.; Ma, J.; Shi, L.; Jiang, M.; Zhang, K.; Ge, B. Evaluation of Bacillus velezensis NKG-2 for bio-control activities against fungal diseases and potential plant growth promotion. Biol. Control 2019, 134, 23–31. [Google Scholar] [CrossRef]
- Di Francesco, A.; Sciubba, L.; Bencivenni, M.; Marzadori, C.; Baraldi, E. Application of Aureobasidium pullulans in iron-poor soil. Can the production of siderophores improve iron bioavailability and yeast antagonistic activity? Ann. Appl. Biol. 2022, 180, 398–406. [Google Scholar] [CrossRef]
- Seregin, I.V.; Kozhevnikova, A.D. Low-molecular-weight ligands in plants: Role in metal homeostasis and hyperaccumulation. Photosynth. Res. 2021, 150, 51–96. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Murata, J.; Namba, K. Unraveling the new biological roles and possible applications of phytosiderophores in plants and mammals. Met. Res. 2022, 2, MR202202. [Google Scholar]
- Carrillo, J.T.; Borthakur, D. Methods for metal chelation in plant homeostasis: Review. Plant Physiol. Biochem. 2021, 163, 95–107. [Google Scholar] [CrossRef]
- Delaporte-Quintana, P.; Lovalsa, N.C.; Raspisarda, V.A.; Pedraza, R.O. The plant growth promoting bacteria Glauconobacter diazotrophicus and Azospirillum brasiliense contribute to the iron nutrition of strawberry plants through siderophores production. Plant Growth Regul. 2020, 91, 185–199. [Google Scholar] [CrossRef]
- Engelbrecht, A.; Saad, H.; Gross, H.; Kaysser, L. Natural products from Nocardia and their role in pathogenicity. Microb. Physiol. 2021, 31, 217–232. [Google Scholar] [CrossRef]
- Barra-Caracciolo, A.; Terenzi, V. Rhizosphere microbial communities and heavy metals. Microorganisms 2021, 9, 1462. [Google Scholar] [CrossRef]
- Ahmed, E.; Holmström, S.J. Siderophores in environmental research: Roles and applications. Microb. Biotechnol. 2014, 7, 196–208. [Google Scholar] [CrossRef]
- Mohanty, S.K.; Gonneau, C.; Salamatipour, A.; Pietrofesa, R.A.; Casper, B.; Christofidou-Solomidou, M.; Willenbring, J.K. Siderophore-mediated iron removal from chrysotile: Implications for asbestos toxicity reduction and bioremediation. J. Hazard. Mater. 2018, 341, 290–296. [Google Scholar] [CrossRef]
- Roskova, Z.; Skarohlid, R.; McGachy, L. Siderophores: An alternative bioremediation strategy? Sci. Total Environ. 2022, 819, 153144. [Google Scholar] [CrossRef]
- Tripathi, M.; Munor, H.P.; Shouch, Y.; Meyer, J.M. Isolation and functional characterization of siderophore producing lead and cadmium resistant Pseudomonas putida KNP9. Curr. Microbiol. 2005, 50, 233–237. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Merten, D.; Svatoš, A.; Büchel, G.; Kothe, E. Metal-induced oxidative stress impacting plant growth in contaminated soil is alleviated by microbial siderophores. Soil Biol. Biochem. 2009, 41, 154–162. [Google Scholar] [CrossRef]
- Singh, A.; Kaushik, M.S.; Srivastava, M.; Tiwari, D.N.; Mishra, A.K. Siderophore mediated attenuation of cadmium toxicity by paddy field cyanobacterium Anabaena oryzae. Algal Res. 2016, 16, 63–68. [Google Scholar] [CrossRef]
- Whiting, S.N.; de Souza, M.P.; Terry, N. Rhizosphere bacteria mobilize Zn for hyperaccumulation by Thlaspi caerulescens. Environ. Sci. Technol. 2001, 35, 3144–3150. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Kim, D.D.; Semrau, J.D.; Lee, J.Y.; Heo, H.; Gu, W.; Yoon, S. Enhancement of nitrous oxide emissions in soil microbial consortia via copper competition between proteobacterial methanotrophs and denitrifiers. Appl. Environ. Microbiol. 2021, 87, e02301. [Google Scholar] [CrossRef]
- Chang, J.; Peng, P.; Ul-Haque, M.F.; Hira, A.; Dispirito, A.A.; Semrau, J.D. Inhibition of nitrous oxide reduction in forest soil microcosms by different forms of methanobactin. Environ. Microbiol. 2023, 25, 2338–2350. [Google Scholar] [CrossRef]
- Ferousi, C.; Majer, S.H.; DiMucci, I.M.; Lancaster, K.M. Biological and bioinspired inorganic N–N bond-forming reactions. Chem. Rev. 2020, 120, 5252–5307. [Google Scholar] [CrossRef]
- Chang, J.; Gu, W.; Park, D.; Semrau, J.D.; Dispirito, A.A.; Yoon, S. Methanobactin from Methylosinus trichosporium OB3b inhibits N2O reduction in denitrifiers. ISME J. 2018, 12, 2086–2089. [Google Scholar] [CrossRef]
- Chang, J.; Peng, P.; DiSpirito, A.A.; Semrau, J.D. Variable inhibition of nitrous oxide reduction in denitrifying bacteria by different forms of methanobactin. Appl. Environ. Microbiol. 2022, 88, e02346. [Google Scholar] [CrossRef]
- Lee, A.; Winther, M.; Priemé, A.; Blunier, T.; Christensen, S. Hot spots of N2O emission move with the seasonally mobile oxic-anoxic interface in drained organic soils. Soil Biol. Biochem. 2017, 115, 178–186. [Google Scholar] [CrossRef]
- Sultana, S.; Alamb, S.; Karimc, M.M. Screening of siderophore-producing salt-tolerant rhizobacteria suitable for supporting plant growth in saline soils with iron limitation. J. Agri. Food Res. 2021, 4, 100150. [Google Scholar] [CrossRef]
- Sadeghi, A.; Karimi, E.; Dahaji, P.A.; Javid, M.G.; Dalvand, Y.; Askar, H. Plant growth promoting activity of an auxin and siderophore producing isolate of Streptomyces under saline soil conditions. World J. Microbiol. Biotechnol. 2012, 28, 1503–1509. [Google Scholar] [CrossRef] [PubMed]
- Córdova-Albores, L.C.; Zelaya-Molina, L.X.; Ávila-Alistac, N.; Valenzuela-Ruíz, V.; Cortés-Martínez, N.E.; Parra-Cota, F.I.; Burgos-Canul, Y.Y.; Chávez-Díaz, I.F.; Fajardo-Franco, M.L.; de los Santos-Villalobos, S. Omics sciences potential on bioprospecting of biological control microbial agents: The case of Mexican agro-biotechnology. Mex. J. Phytopathol. 2021, 39, 147–184. [Google Scholar] [CrossRef]
- Kong, H.; Cheng, W.; Wei, H.; Yuan, Y.; Yang, Z.; Zhang, X. An overview of recent progress in siderophore-antibiotic conjugates. Eur. J. Med. Chem. 2019, 182, 111615. [Google Scholar] [CrossRef] [PubMed]
- Negash, K.H.; Norris, J.K.S.; Hodgkinson, J.T. Siderophore-antibiotic conjugate design: New drugs for bad bugs? Molecules 2019, 24, 3314. [Google Scholar] [CrossRef]
- Ezzeddine, Z.; Ghssein, G. Towards new antibiotics classes targeting bacterial metallophores. Microb. Pathog. 2023, 182, 106221. [Google Scholar] [CrossRef]
- Ezzeddine, Z.; Ghssein, G. Metallophores as promising chelates for heavy metals removal from polluted water. Bioremediat. J. 2024, 1–3. [Google Scholar] [CrossRef]
- Wang, L.; Yan, W.; Chen, J.; Huang, F.; Gao, P. Function of the ironbinding chelator produced by Coriolus versicolor in lignin biodegradation. Sci. China Ser. C Life Sci. 2008, 51, 214–221. [Google Scholar] [CrossRef]
- Winkelmann, G. The history of siderophores. Curr. Trends Microbiol. 2022, 16, 87–92. [Google Scholar]
- Atugoda, D.R.A.M.T.R.; Mandakini, L.L.U.; Bandara, N.J.G.J.; Gunaguardana, D. How to taxonomically-ambiguous cyanobiont and vanadete assist in the phytoremediation of cadmium by Azolla pinnata: Implications for CKDu. Environ. Pollut. 2018, 7, 53–65. [Google Scholar] [CrossRef]
- Moscatello, N.; Swayambhu, G.; Jones, C.H.; Xu, J.; Dai, N.; Pfeifer, B.A. Continuous removal of copper, magnesium and nickel from industrial wastewater utilizing the natural product yersiniabactin immobilized withing a packed-bed column. Chem. Eng. 2018, 343, 173–179. [Google Scholar] [CrossRef]
- Wong, M.Y.; Neill, C.; Marino, R.; Silvério, D.; Howarth, R.W. Molybdenum phosphorus, and pH constratin nitrogen fixation in a tropical forest in the southeastern Amazon. Ecology. 2021, 102, e03211. [Google Scholar] [CrossRef] [PubMed]
- Sidiqi, M.H.; Erber, W.N. Red blood cell disorders. In Clinical Pharmacology, 12th ed.; Brown, M.J., Sharma, P., Mir, F.A., Bennett, P.N., Eds.; Elsevier: Edinburgh, UK, 2018; pp. 528–542. [Google Scholar]
- El-Lababidi, R.M.; Rizk, J.G. Cefiderocol: A siderophore cephalosporin. Ann. Pharmacother. 2020, 54, 1215–1231. [Google Scholar] [CrossRef] [PubMed]
- Soares, E.V. Perspective on the biotechnological production of bacterial siderophores and their use. Appl. Microbiol. Biotechnol. 2022, 106, 3985–4004. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zelaya-Molina, L.X.; Chávez-Díaz, I.F.; Urrieta-Velázquez, J.A.; Aragón-Magadan, M.A.; Puente-Valenzuela, C.O.; Blanco-Camarillo, M.; de los Santos-Villalobos, S.; Ramos-Garza, J. Microbial Metallophores in the Productivity of Agroecosystems. Microbiol. Res. 2025, 16, 67. https://doi.org/10.3390/microbiolres16030067
Zelaya-Molina LX, Chávez-Díaz IF, Urrieta-Velázquez JA, Aragón-Magadan MA, Puente-Valenzuela CO, Blanco-Camarillo M, de los Santos-Villalobos S, Ramos-Garza J. Microbial Metallophores in the Productivity of Agroecosystems. Microbiology Research. 2025; 16(3):67. https://doi.org/10.3390/microbiolres16030067
Chicago/Turabian StyleZelaya-Molina, Lily X., Ismael F. Chávez-Díaz, José A. Urrieta-Velázquez, Marco A. Aragón-Magadan, Cristo O. Puente-Valenzuela, Mario Blanco-Camarillo, Sergio de los Santos-Villalobos, and Juan Ramos-Garza. 2025. "Microbial Metallophores in the Productivity of Agroecosystems" Microbiology Research 16, no. 3: 67. https://doi.org/10.3390/microbiolres16030067
APA StyleZelaya-Molina, L. X., Chávez-Díaz, I. F., Urrieta-Velázquez, J. A., Aragón-Magadan, M. A., Puente-Valenzuela, C. O., Blanco-Camarillo, M., de los Santos-Villalobos, S., & Ramos-Garza, J. (2025). Microbial Metallophores in the Productivity of Agroecosystems. Microbiology Research, 16(3), 67. https://doi.org/10.3390/microbiolres16030067






