Effect of Tilapia Parvovirus (TiPV) on Fish Health: An In Vitro Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Virus from Infected Samples
2.2. Fish Cell Line Infection
2.3. Confirmation of Virus Presence in Cell Line by PCR
2.4. Cell Viability Assay (MTT Assay) to Determine the Virus Virulence
2.5. Investigation of Cellular Immune Gene Response Against Viral Infection
2.6. Statistical Analysis
3. Results
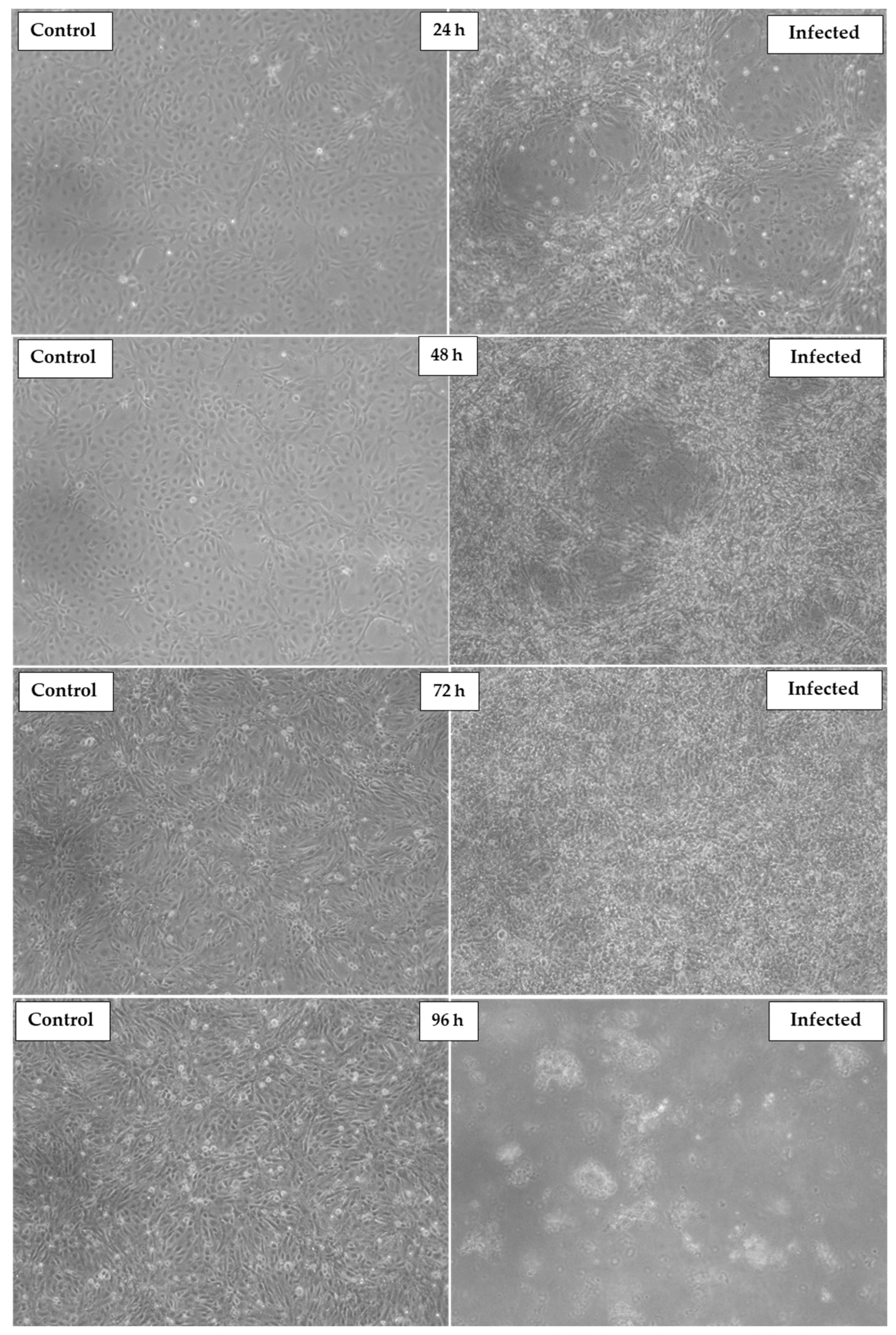
3.1. Virus Isolation in Cell Line
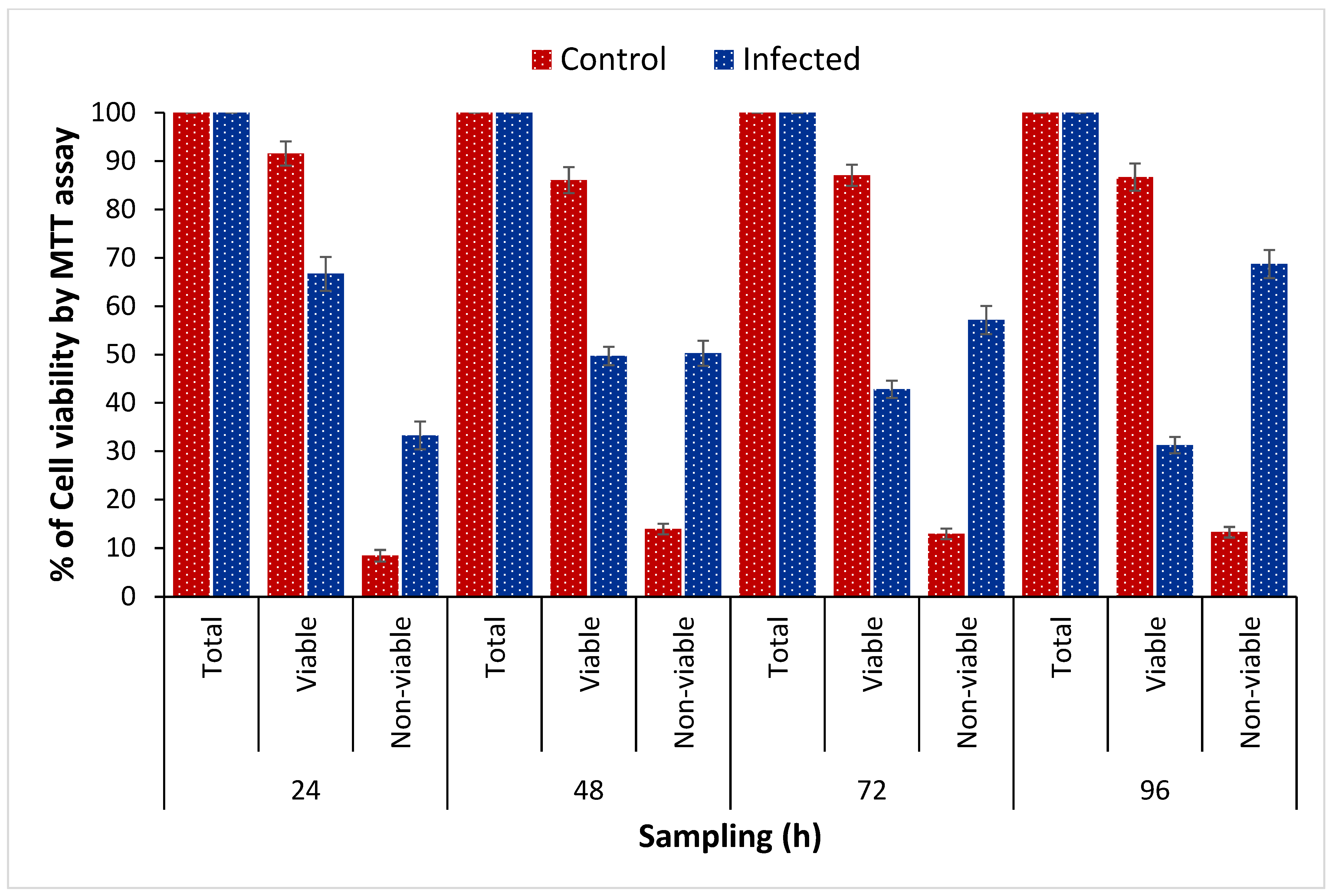
3.2. Assessment of TiPV Virulence by MTT
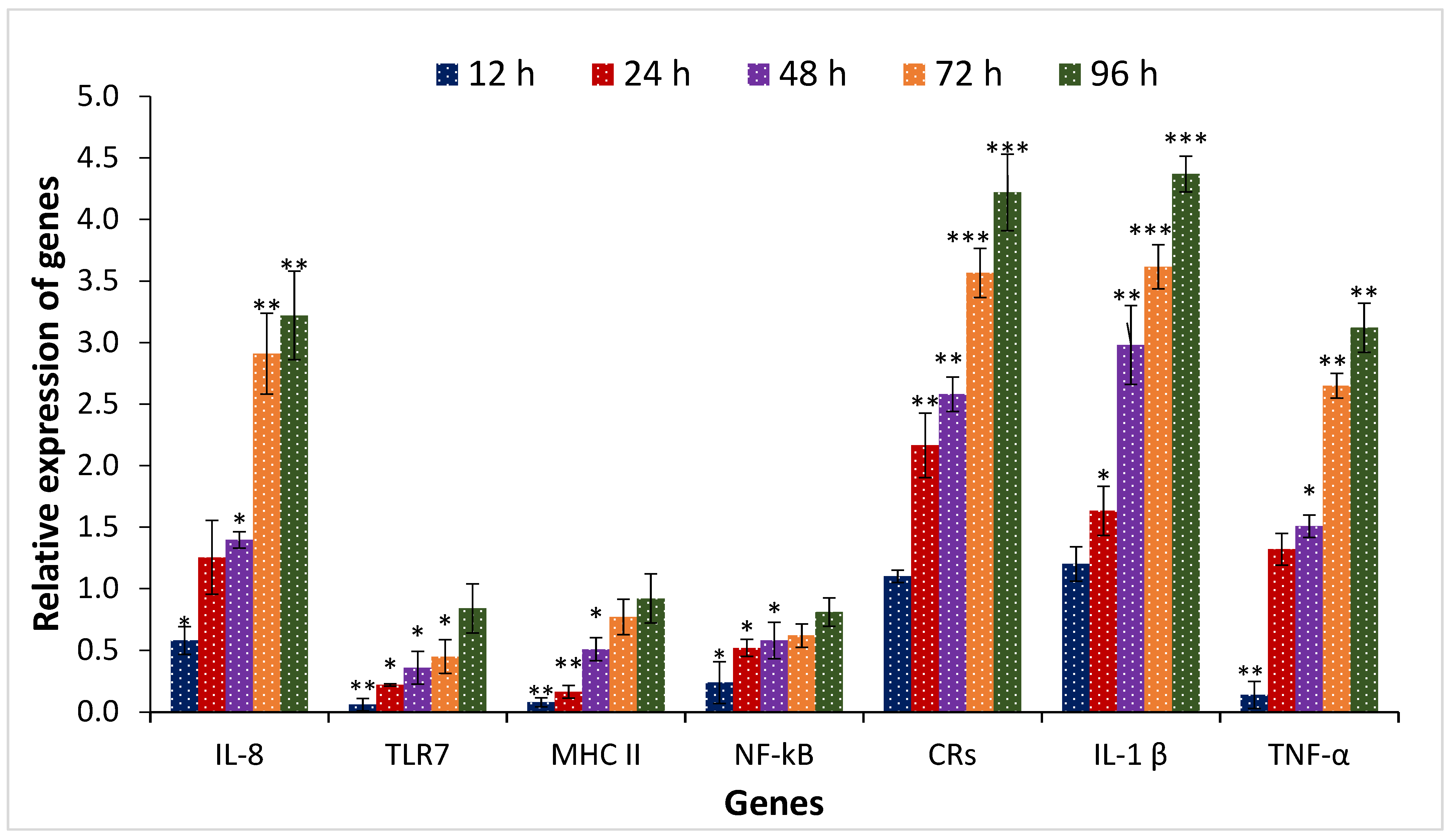
3.3. Effect of TiPV on Gene Expression of Danio rerio Gill (DRG) Cell Samples
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture (SOFIA). In Contributing to Food Security and Nutrition for All; FAO: Rome, Italy, 2016; p. 204. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture. In Sustainability in Action; FAO: Rome, Italy, 2020; p. 244. [Google Scholar]
- Keawcharoen, J.; Techangamsuwan, S.; Ponpornpisit, A.; Lombardini, E.D.; Patchimasiri, T.; Pirarat, N. Genetic characterization of a betanodavirus isolated from a clinical disease outbreak in farm-raised tilapia Oreochromis niloticus (L.) in Thailand. J. Fish Dis. 2015, 38, 49–54. [Google Scholar] [CrossRef]
- Figueiredo, H.C.; Tavares, G.C.; Dorella, F.A.; Rosa, J.C.C.; Marcelino, S.A.C.; Pierezan, F.; Pereira, F.L. First report of infectious spleen and kidney necrosis virus in Nile tilapia in Brazil. Transbound. Emerg. Dis. 2022, 69, 3008–3015. [Google Scholar] [CrossRef]
- Nicholson, P.; Mon-On, N.; Jaemwimol, P.; Tattiyapong, P.; Surachetpong, W. Coinfection of tilapia lake virus and Aeromonas hydrophila synergistically increased mortality and worsened the disease severity in tilapia (Oreochromis sp.). Aquaculture 2020, 520, 734746. [Google Scholar] [CrossRef]
- Yamkasem, J.; Piewbang, C.; Techangamsuwan, S.; Pierezan, F.; Soto, E.; Surachetpong, W. Susceptibility of ornamental African cichlids Aulonocara spp. to experimental infection with Tilapia lake virus. Aquaculture 2021, 542, 736920. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; Ma, J.; Jiang, N.; Fan, Y.; Zhou, Y.; Cain, K.; Yi, M.; Jia, K.; Wen, H.; et al. Determination of a novel parvovirus pathogen associated with massive mortality in adult tilapia. PLoS Pathog. 2020, 16, e1008765. [Google Scholar] [CrossRef]
- Du, J.; Wang, W.; Chan, J.F.W.; Wang, G.; Huang, Y.; Yi, Y.; Zhu, Z.; Peng, R.; Hu, X.; Wu, Y.; et al. Identification of a Novel Ichthyic Parvovirus in Marine Species in Hainan Island, China. Front. Microbiol. 2019, 10, 2815. [Google Scholar] [CrossRef]
- Das, B.K.; Kumar, V.; Roy, S.; Malick, R.C.; Bisai, K.; Jana, A.K.; Dhar, S. Pathological effects and immune modulation in host during Tilapia Parvovirus (TiPV) outbreak in cage and wetland Tilapia farms. Sci. Rep. 2024, 14, 28689. [Google Scholar] [CrossRef]
- Imajoh, M.; Ikawa, T.; Oshima, S.I. Characterization of a new fibroblast cell line from a tail fin of red sea bream, Pagrus major, and phylogenetic relationships of a recent RSIV isolate in Japan. Virus Res. 2007, 126, 45–52. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, S.J.; Jung, S.J.; Jung, M.H. Effect of rock bream iridovirus (RBIV) contained tissue intake on rock bream (Oplegnathus fasciatus) mortality and blood cell distribution. Fish Shellfish. Immunol. 2024, 153, 109858. [Google Scholar] [CrossRef]
- Dong, H.T.; Senapin, S.; Gangnonngiw, W.; Nguyen, V.V.; Rodkhum, C.; Debnath, P.; Delamare-Deboutteville, J.; Mohan, C.V. Experimental infection reveals transmission of tilapia lake virus (TiLV) from tilapia broodstock to their reproductive organs and fertilized eggs. Aquaculture 2020, 515, 734541. [Google Scholar] [CrossRef]
- Jaemwimol, P.; Rawiwan, P.; Tattiyapong, P.; Saengnual, P.; Kamlangdee, A.; Surachetpong, W. Susceptibility of important warm water fish species to tilapia lake virus (TiLV) infection. Aquaculture 2018, 497, 462–468. [Google Scholar] [CrossRef]
- Dai, Y.J.; Jia, Y.F.; Chen, N.; Bian, W.; Li, Q.K.; Ma, Y.B.; Chen, Y.L.; Pei, D.S. Zebrafish as a model system to study toxicology. Environ. Toxicol. Chem. 2014, 33, 11–17. [Google Scholar] [CrossRef]
- MJ, A.W.; S, A.M.; S, M.; V, R.; AS, S.H. A comparative study on targeted gene expression in zebrafish and its gill cell line exposed to chlorpyrifos. Vitr. Cell. Dev. Biol. Anim. 2024, 60, 397–410. [Google Scholar]
- Ajitha, V.; Sarasan, M.; Sreevidya, C.; Aswathy, C.; Kachiprath, B.; Mohandas, A.; Singh, I.S.B.; Hameed, A.S.; Schlenk, D.; Magnuson, J.T.; et al. Cytotoxic impacts of treated electroplating industrial effluent and the comparative effect of their metal components (Zn, Hg, and Zn + Hg) on Danio rerio gill (DrG) cell line. Sci. Total Environ. 2021, 793, 148533. [Google Scholar] [CrossRef]
- Ashfaque, M.; Ahmed, A.N.; Safiullah, S.M.; Taju, G.; Majeed, S.A.; Hameed, A.S.; Basha, K.A. Toxicological assessment of functional polymer with single-walled carbon nanotubes in zebrafish embryos and its gill cell line. Chemosphere 2022, 303, 134891. [Google Scholar] [CrossRef]
- Nga, N.T.H.; Ngoc, T.T.B.; Trinh, N.T.M.; Thuoc, T.L.; Thao, D.T. Optimization and application of MTT assay in determining density of suspension cells. Anal. Biochem. 2020, 610, 113937. [Google Scholar] [CrossRef]
- Rajendran, K.V.; Sood, N.; Rao, B.M.; Valsalam, A.; Bedekar, M.K.; Jeena, K.; Pradhan, K.; Paria, A.; Swaminathan, T.R.; Verma, D.K.; et al. Widespread occurrence of Tilapia parvovirus in farmed Nile tilapia Oreochromis niloticus from India. J. Fish Dis. 2023. [Google Scholar] [CrossRef]
- Badhusha, A.; Mithra, S.; Taju, G.; Rajkumar, V.; Abdul Majeed, S.; Suryakodi, S.; Haridas, L.; Haridas, D.; Sahoo, K.; Mohanty, J.; et al. Detection of Tilapia parvovirus in farm-reared tilapia in India and its isolation using fish cell lines. Vitr. Cell. Dev. Biol. Anim. 2025, 1–13. [Google Scholar] [CrossRef]
- Wang, Z.; Nie, L.; Fei, C.; Chen, J. Establishment of Gill-Derived Primary Cell Cultures from Largemouth Bass (Micropterus salmoides) as an Alternative Platform for Studying Host–Virus Interactions. Fishes 2025, 10, 18. [Google Scholar] [CrossRef]
- Brian, A.; Dawn, A. Bacterial Fish Pathogens; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef]
- Schreck, C.B.; Moyle, B. Methods for Fish Biology; American Fisheries Society: Bethesda, MD, USA, 1990. [Google Scholar]
- Roberts, C. Information structure: Towards an integrated formal theory of pragmatics. Semant. Pragmat. 2012, 5, 1–69. [Google Scholar] [CrossRef]
- Freshney, R.I. Database of misidentified cell lines. Int. J. Cancer 2010, 126, 302. [Google Scholar] [CrossRef] [PubMed]
- George, M.R.; John, K.R.; Mansoor, M.M.; Saravanakumar, R.; Sundar, P.; Pradeep, V. Isolation and characterization of a ranavirus from koi, Cyprinus carpio L., experiencing mass mortalities in India. J. Fish Dis. 2015, 38, 389–403. [Google Scholar] [CrossRef]
- Lorenzen, N.; Lorenzen, E.; Einer-Jensen, K.; Heppell, J.; Wu, T.; Davis, H. Protective immunity to VHS in rainbow trout (Oncorhynchus mykiss, Walbaum) following DNA vaccination. Fish Shellfish. Immunol. 1998, 8, 261–270. [Google Scholar]
- Elbahnaswy, S.; Elshopakey, G.E. Differential gene expression and immune response of Nile tilapia (Oreochromis niloticus) challenged intraperitoneally with Photobacterium damselae and Aeromonas hydrophila demonstrating immunosuppression. Aquaculture 2020, 526, 735364. [Google Scholar]
- Spearman, C. The method of right and wrong cases (constant stimuli) without Gauss’s formulae. Br. J. Psychol. 1908, 2, 227. [Google Scholar] [CrossRef]
- Kärber, G. Contribution to the collective treatment of pharmacological serial experiments. Naunyn Schmiedebergs Arch. Exp. Pathol. Pharmacol. 1931, 162, 480–483. [Google Scholar] [CrossRef]
- Kibenge, F.S.B. Emerging viruses in aquaculture. Curr. Opin. Virol. 2019, 34, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Surachetpong, W.; Roy, S.R.K.; Nicholson, P. Tilapia lake virus: The story so far. J. Fish Dis. 2020, 43, 1115–1132. [Google Scholar] [CrossRef]
- Behera, B.K.; Pradhan, K.; Swaminathan, T.R.; Sood, N.; Paria, P.; Das, A.; Verma, D.K.; Kumar, R.; Yadav, M.K.; Dev, A.K.; et al. Emergence of Tilapia Lake Virus associated with mortalities of farmed Nile Tilapia Oreochromis niloticus (Linnaeus 1758) in India. Aquaculture 2018, 484, 168–174. [Google Scholar] [CrossRef]
- Acharya, V.; Chakraborty, H.J.; Rout, A.K.; Balabantaray, S.; Behera, B.K.; Das, B.K. Structural Characterization of Open Reading Frame-Encoded Functional Genes from Tilapia Lake Virus (TiLV). Mol. Biotechnol. 2019, 61, 945–957. [Google Scholar] [CrossRef]
- Ramírez-Paredes, J.G.; Paley, R.K.; Hunt, W.; Feist, S.W.; Stone, D.M.; Field, T.R.; Haydon, D.J.; Ziddah, A.; Nkansa, M.; Guilder, J.; et al. First detection of infectious spleen and kidney necrosis virus (ISKNV) associated with massive mortalities in farmed tilapia in Africa. Transbound. Emerg. Dis. 2021, 68, 1550–1563. [Google Scholar] [CrossRef]
- Piewbang, C.; Tattiyapong, P.; Khemthong, M.; Lachroje, S.; Boonrungsiman, S.; Kasantikul, T.; Surachetpong, W.; Techangamsuwan, S. Dual infections of tilapia parvovirus (TiPV) and tilapia lake virus (TiLV) in multiple tilapia farms: Their impacts, genetic diversity, viral tropism, and pathological effects. Aquaculture 2022, 550, 737887. [Google Scholar] [CrossRef]
- Kembou-Ringert, J.E.; Steinhagen, D.; Thompson, K.D.; Daly, J.M.; Adamek, M. Immune responses to Tilapia lake virus infection: What we know and what we don’t know. Front. Immunol. 2023, 14, 1240094. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.T.; Chaijarasphong, T.; Barnes, A.C.; Delamare-Deboutteville, J.; Lee, A.; Senapin, S.; Mohan, C.V.; Tang, K.F.; McGladdery, S.E.; Bondad-Reantaso, M.G. From the basics to emerging diagnostic technologies: What is on the horizon for tilapia disease diagnostics? Rev. Aquac. 2023, 15, 186–212. [Google Scholar] [CrossRef]
- Xue, S.; Liu, X.; Liu, Y.; Lu, C.; Jia, L.; Yu, Y.; Liu, H.; Yang, S.; Zeng, Z.; Li, H.; et al. Determination and Characterization of Novel Papillomavirus and Parvovirus Associated with Mass Mortality of Chinese Tongue Sole (Cynoglossus semilaevis) in China. Viruses 2024, 16, 705. [Google Scholar] [CrossRef]
- Zhao, H.; Zhou, Y.; Fan, Y.; Jiang, N.; Meng, Y.; Li, Y.; Xue, M.; Xu, C.; Guo, W.; Liu, W. Development and application of a sensitive droplet digital PCR-based method to detect tilapia parvovirus. J. Fish Dis. 2023, 46, 239–245. [Google Scholar] [CrossRef]
- Suresh, T.; Nithin, M.S.; Kushala, K.B.; Girisha, S.K.; Shivakumar, V.B.; Dheeraj, S.B.; Puneeth, T.G.; Kishan, K.; Vinay, T.N. Largescale mortality of Oreochromis mossambicus in lakes and reservoirs of Karnataka due to coinfection of Tilapia Lake virus (TiLV) and multidrug-resistant Aeromonas veronii: An emerging fish disease in India. Aquaculture 2023, 565, 739077. [Google Scholar] [CrossRef]
- Phusantisampan, T.; Yamkasem, J.; Tattiyapong, P.; Sriariyanun, M.; Surachetpong, W. Specific and rapid detection of tilapia parvovirus using loop-mediated isothermal amplification (LAMP) method. J. Fish Dis. 2022, 45, 1893–1898. [Google Scholar] [CrossRef]
- Raksaseri, P.; Lertwanakarn, T.; Tattiyapong, P.; Kijtawornrat, A.; Klomkleaw, W.; Surachetpong, W. Tilapia lake virus causes mitochondrial damage: A proposed mechanism that leads to extensive death in fish cells. PeerJ 2023, 11, e16190. [Google Scholar] [CrossRef]
- Johny, T.K.; Swaminathan, T.R.; Sood, N.; Pradhan, K.; Lal, K.K. A panoptic review of techniques for finfish disease diagnosis: The status quo and future perspectives. J. Microbiol. Methods 2022, 196, 106477. [Google Scholar] [CrossRef]
- Ranjitha, H.B.; Ramesh, M.; Behera, S.; ValiyaValappil, D.; Basagoudanavar, S.H.; Sherasiya, A. Genetic engineering tools and techniques in livestock production. In Sustainable Agriculture Reviews 57: Animal Biotechnology for Livestock Production 2; Springer International Publishing: Cham, Switzerland, 2022; pp. 175–207. [Google Scholar]
| Gene | Primer Sequence 5′ to 3′ | References |
|---|---|---|
| Tilapia Parvovirus-F | GAGATGGTGTGAAAATGAACGGG | [7] |
| Tilapia Parvovirus-R | CTATCTCCTCGTTGCTCGGTGTATC | |
| Tilapia Parvovirus-Fq | GCACCACAGCTGAGTACAAC | |
| Tilapia Parvovirus-Rq | AACTGCTCGGCTATCTCCTC | |
| Beta-Actin-F | CAGCAAGCAGGAGTACGATGAG | [28] |
| Beta-Actin-R | TGTGTGGTGTGTGGTTGTTTTG | |
| Interleukin8-F | GCACTGCCGCTGCATTAAG | |
| Interleukin88-R | GCAGTGGGAGTTGGGAAGAA | |
| Toll-like receptor7-F | TCAGCAGGGTGAGAGCATAC | |
| Toll-like receptor7-R | ACATATCCCAGCCGTAGAGG | |
| Major Histocompatibility Complex-II-F | TGGCCCTGACTGAACCACTG | |
| Major Histocompatibility Complex-II-R | TCAGACCCACGCCACAGAAC | |
| Nuclear factorκB-F | AACGACGGTGATGACAACGAC | |
| Nuclear factorκB-R | AAATTCAGGCTCCACACTGACC | |
| CRs-chemokine receptors -F | ACAGAGCCGATCTTGGGTTACTTG | |
| CRs-chemokine receptors-R | TGAAGGAGAGGCGGTGGATGTTAT | |
| Interleukin1β -F | TGCACTGTCACTGACAGCCAA | |
| Interleukin1β -R | ATGTTCAGGTGCACTATGCGG | |
| Tumor necrosis factor-α -F | CCAGAAGCACTAAAGGCGAAGA | |
| Tumor necrosis factor-α -R | CCTTGGCTTTGCTGCTGATC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, V.; Das, B.K.; Adhikari, A.; Bisai, K.; Mandal, B. Effect of Tilapia Parvovirus (TiPV) on Fish Health: An In Vitro Approach. Microbiol. Res. 2025, 16, 68. https://doi.org/10.3390/microbiolres16030068
Kumar V, Das BK, Adhikari A, Bisai K, Mandal B. Effect of Tilapia Parvovirus (TiPV) on Fish Health: An In Vitro Approach. Microbiology Research. 2025; 16(3):68. https://doi.org/10.3390/microbiolres16030068
Chicago/Turabian StyleKumar, Vikash, Basanta Kumar Das, Anupam Adhikari, Kampan Bisai, and Biswajit Mandal. 2025. "Effect of Tilapia Parvovirus (TiPV) on Fish Health: An In Vitro Approach" Microbiology Research 16, no. 3: 68. https://doi.org/10.3390/microbiolres16030068
APA StyleKumar, V., Das, B. K., Adhikari, A., Bisai, K., & Mandal, B. (2025). Effect of Tilapia Parvovirus (TiPV) on Fish Health: An In Vitro Approach. Microbiology Research, 16(3), 68. https://doi.org/10.3390/microbiolres16030068








