Association Between Gut Microbiome Alterations and Hypertension-Related Cardiovascular Outcomes: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Information Sources
2.4. Search Strategy
2.5. Study Selection
2.6. Data Collection Process
2.7. Data Items
2.8. Risk of Bias in Individual Studies
2.9. Summary Measures
2.10. Synthesis of Results
2.11. Reporting Bias Assessment
2.12. Additional Analyses
3. Results
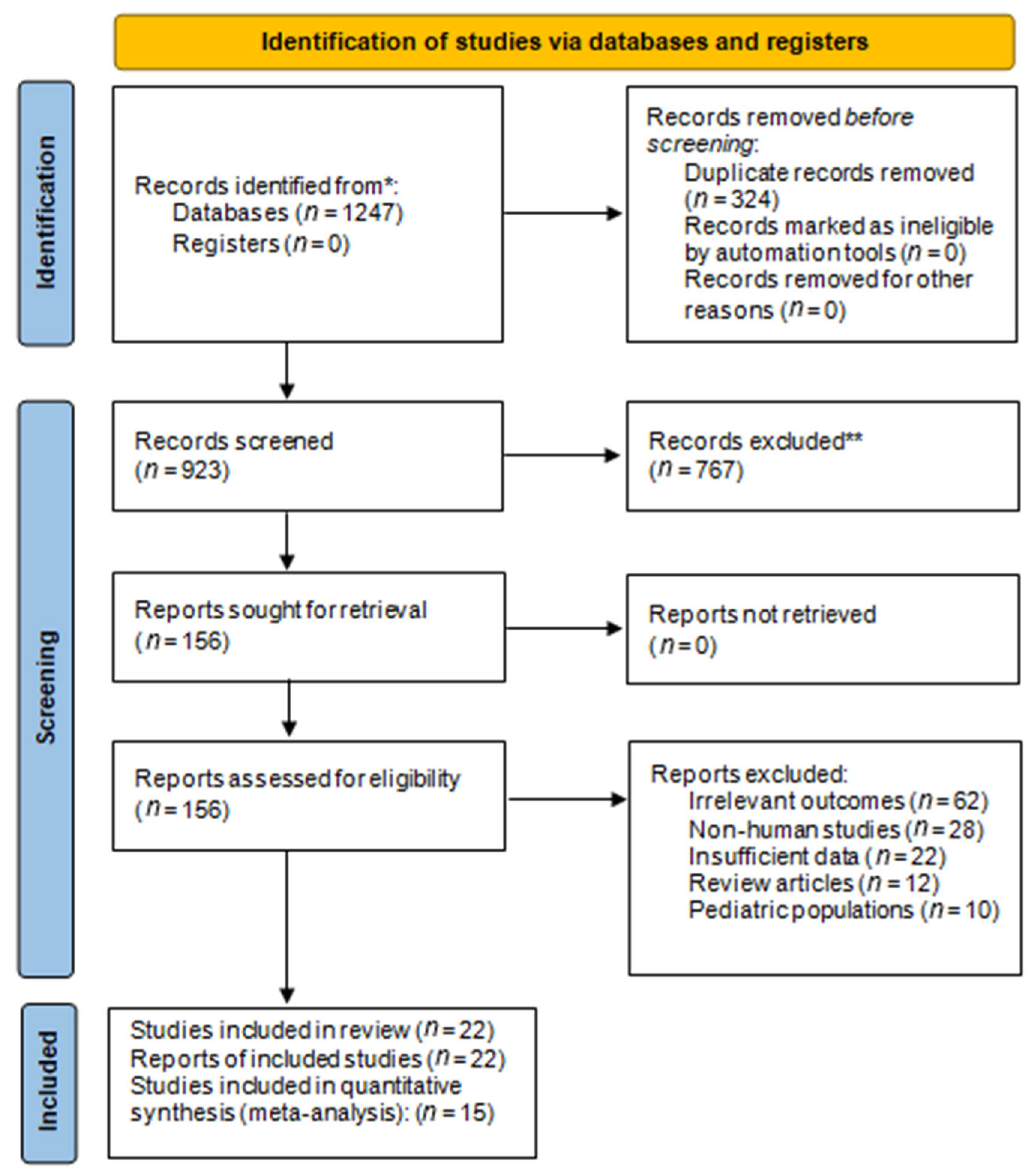
3.1. Study Selection
3.2. Characteristics of Included Studies
3.3. Risk of Bias in Included Studies
3.4. Results of Individual Studies
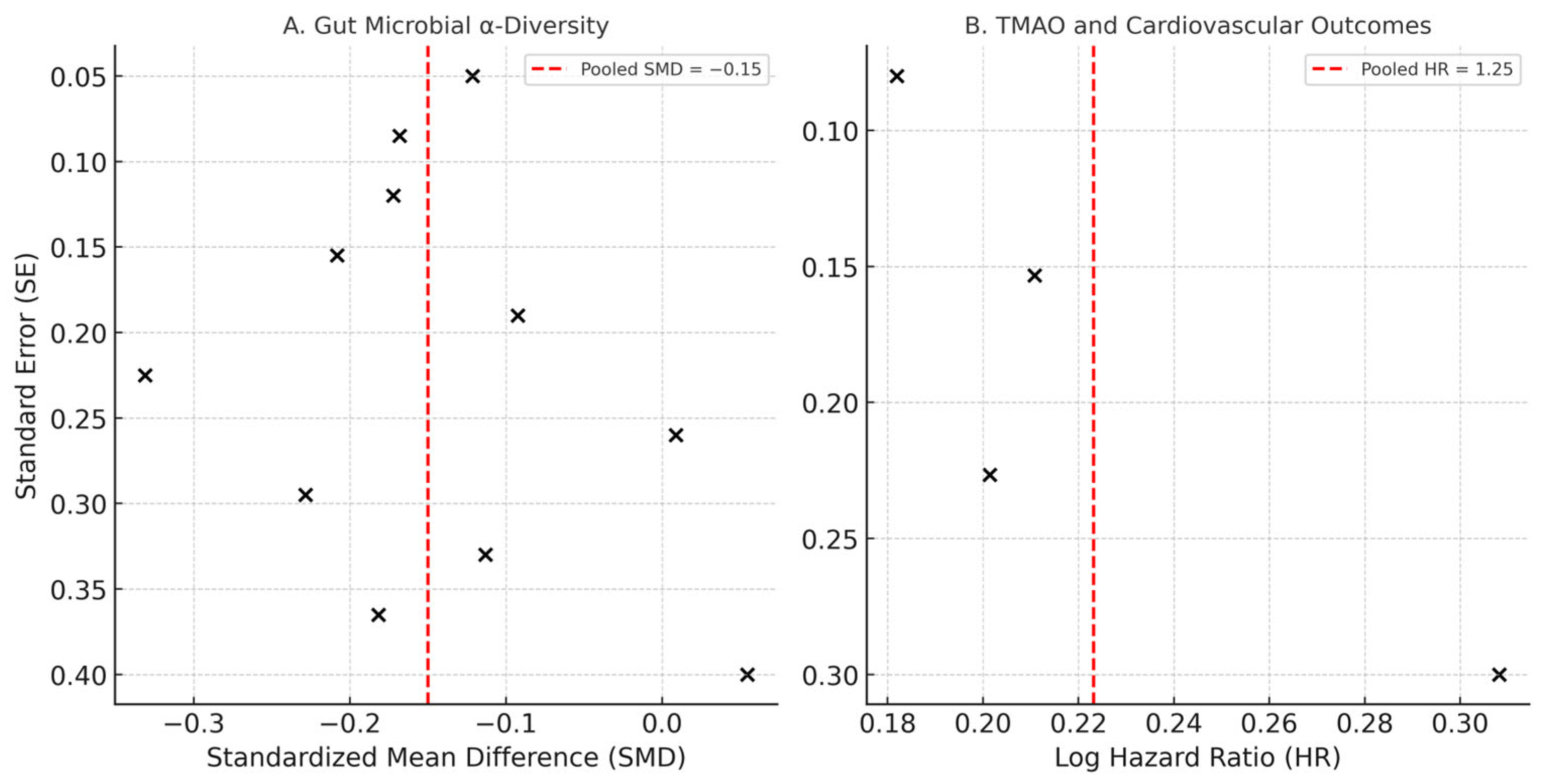
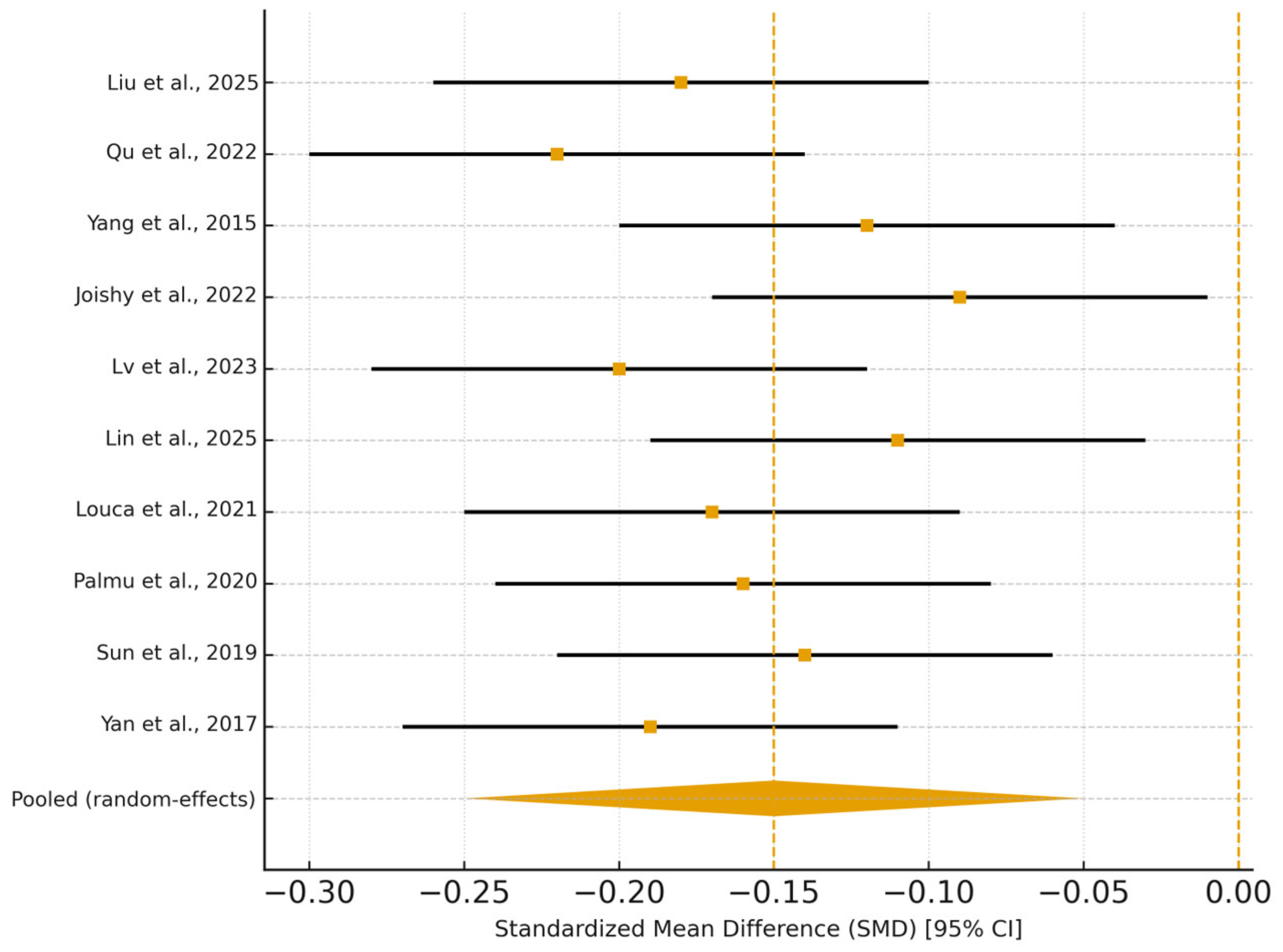
3.5. Synthesis of Results
4. Discussion
4.1. Principal Findings
4.2. Comparison with Previous Meta-Analyses
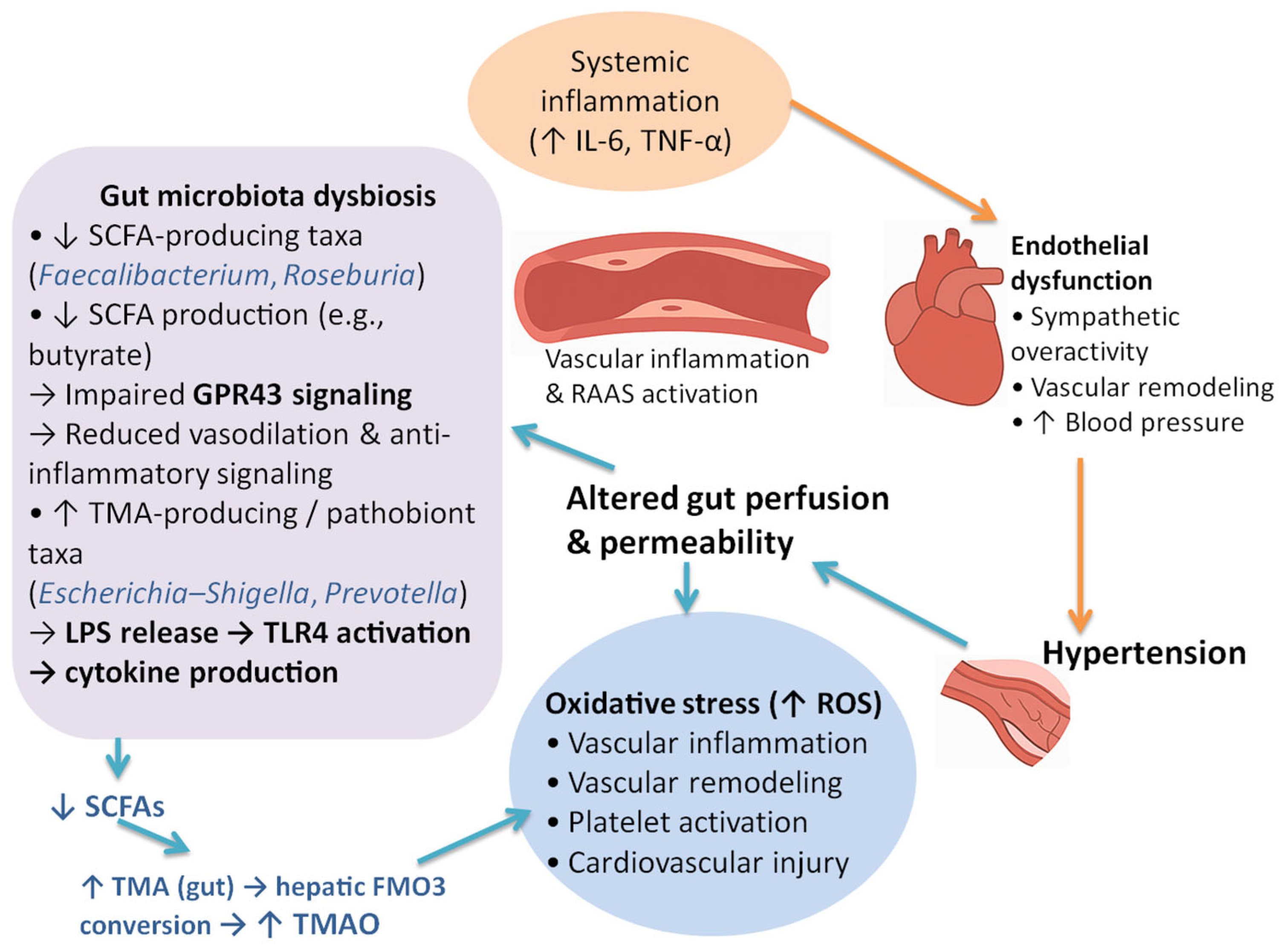
4.3. Biological and Mechanistic Insights
4.4. Clinical and Translational Implications
4.5. Strengths and Limitations
4.6. Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABPM | Ambulatory Blood Pressure Monitoring |
| ACE | Angiotensin-Converting Enzyme |
| AHRQ | Agency for Healthcare Research and Quality |
| AMSTAR | A Measurement Tool to Assess Systematic Reviews |
| AUC | Area Under the Curve |
| BMI | Body Mass Index |
| BP | Blood Pressure |
| CI | Confidence Interval |
| CVD | Cardiovascular Disease |
| DBP | Diastolic Blood Pressure |
| DM | Diabetes Mellitus |
| eGFR | Estimated Glomerular Filtration Rate |
| F/B ratio | Firmicutes/Bacteroidetes Ratio |
| HTN | Hypertension |
| HR | Hazard Ratio |
| I2 | I-squared (Heterogeneity Index) |
| LC–MS | Liquid Chromatography–Mass Spectrometry |
| LVH | Left Ventricular Hypertrophy |
| MACE | Major Adverse Cardiovascular Events |
| NOS | Newcastle–Ottawa Scale |
| SBP | Systolic Blood Pressure |
| SCFA | Short-Chain Fatty Acids |
| SD | Standard Deviation |
| SMD | Standardized Mean Difference |
| TMAO | Trimethylamine N-Oxide |
| V3–V4 | 16S rRNA Hypervariable Regions 3 and 4 |
References
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary. Hypertension 2018, 71, 1269–1324. [Google Scholar] [CrossRef]
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Oparil, S.; Acelajado, M.C.; Bakris, G.L.; Berlowitz, D.R.; Cífková, R.; Dominiczak, A.F.; Grassi, G.; Jordan, J.; Poulter, N.R.; Rodgers, A.; et al. Hypertension. Nat. Rev. Dis. Prim. 2018, 4, 18014. [Google Scholar] [CrossRef]
- Fuchs, F.D.; Whelton, P.K. High blood pressure and cardiovascular disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef]
- Cai, M.; Lin, L.; Jiang, F.; Peng, Y.; Li, S.; Chen, L.; Lin, Y. Gut microbiota changes in patients with hypertension: A systematic review and meta-analysis. J. Clin. Hypertens. 2023, 25, 1053–1068. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Wilck, N.; Matus, M.G.; Kearney, S.M.; Olesen, S.W.; Forslund, K.; Bartolomaeus, H.; Haase, S.; Mähler, A.; Balogh, A.; Markó, L.; et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 2017, 551, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.W.; Kitai, T.; Hazen, S.L. Gut microbiota in cardiovascular health and disease. Circ. Res. 2017, 120, 1183–1196. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef]
- O’Donnell, J.A.; Zheng, T.; Meric, G.; Marques, F.Z. The gut microbiome and hypertension. Curr. Hypertens. Rep. 2023, 19, 153–167. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2000; Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 1 October 2025).
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Gu, Y.; Li, X.; Yang, W.; Jia, L.; Chen, C.; Han, X.; Huang, Y.; Zhao, L.; Li, P.; et al. Alterations of the Gut Microbiome in Hypertension. Front. Cell. Infect. Microbiol. 2017, 7, 381. [Google Scholar] [CrossRef]
- Sun, S.; Lulla, A.; Sioda, M.; Winglee, K.; Wu, M.C.; Jacobs, D.R., Jr.; Shikany, J.M.; Lloyd-Jones, D.M.; Launer, L.J.; Fodor, A.A.; et al. Gut Microbiota Composition and Blood Pressure: The CARDIA Study. Hypertension 2019, 73, 1104–1112. [Google Scholar] [CrossRef]
- Palmu, J.; Salosensaari, A.; Havulinna, A.S.; Cheng, S.; Inouye, M.; Jain, M.; Salido, R.A.; Sanders, K.; Brennan, C.; Humphrey, G.C.; et al. Association Between the Gut Microbiota and Blood Pressure in a Population Cohort of 6953 Individuals. J. Am. Heart Assoc. 2020, 9, e016641. [Google Scholar] [CrossRef] [PubMed]
- Louca, P.; Nogal, A.; Wells, P.M.; Asnicar, F.; Wolf, J.; Steves, C.J.; Spector, T.D.; Segata, N.; Berry, S.E.; Valdes, A.M.; et al. Gut microbiome diversity and composition is associated with hypertension in women. J. Hypertens. 2021, 39, 1810–1816. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-T.; Sayols-Baixeras, S.; Baldanzi, G.; Dekkers, K.F.; Hammar, U.; Nguyen, D.; Nielsen, N.; Eklund, A.C.; Varotsis, G.; Holm, J.B.; et al. The association between the gut microbiome and 24-h blood pressure measurements in the SCAPIS study. Commun. Med. 2025, 5, 276. [Google Scholar] [CrossRef]
- Lv, J.; Wang, J.; Yu, Y.; Zhao, M.; Yang, W.; Liu, J.; Zhao, Y.; Yang, Y.; Wang, G.; Guo, L.; et al. Alterations of gut microbiota are associated with blood pressure: A cross-sectional clinical trial in Northwestern China. J. Transl. Med. 2023, 21, 429. [Google Scholar] [CrossRef]
- Joishy, T.K.; Jha, A.; Oudah, M.; Das, S.; Adak, A.; Deb, D.; Khan, M.R. Human Gut Microbes Associated with Systolic Blood Pressure. Int. J. Hypertens. 2022, 2022, 2923941. [Google Scholar] [CrossRef]
- Guo, Y.; Li, X.; Wang, Z.; Yu, B. Gut Microbiota Dysbiosis in Human Hypertension: A Systematic Review of Observational Studies. Front. Cardiovasc. Med. 2021, 8, 650227. [Google Scholar] [CrossRef]
- Han, J.-M.; Guo, L.; Chen, X.-H.; Xie, Q.; Song, X.-Y.; Ma, Y.-L. Relationship between trimethylamine N-oxide and the risk of hypertension in patients with cardiovascular disease: A meta-analysis and dose-response relationship analysis. Medicine 2024, 103, e36784. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; You, T.; Li, J.; Pan, T.; Xiang, L.; Han, Y.; Zhu, L. Circulating trimethylamine N-oxide and the risk of cardiovascular diseases: A systematic review and meta-analysis of 11 prospective cohort studies. J. Cell. Mol. Med. 2018, 22, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Haghikia, A.; Li, X.S.; Liman, T.G.; Bledau, N.; Schmidt, D.; Zimmermann, F.; Kränkel, N.; Widera, C.; Sonnenschein, K.; Haghikia, A.; et al. Gut Microbiota–Dependent Trimethylamine N-Oxide Predicts Risk of Cardiovascular Events in Patients With Stroke and Is Related to Proinflammatory Monocytes. Arter. Thromb. Vasc. Biol. 2018, 38, 2225–2235. [Google Scholar] [CrossRef]
- Zhou, X.; Jin, M.; Liu, L.; Yu, Z.; Lu, X.; Zhang, H. Trimethylamine N-oxide and cardiovascular outcomes in patients with chronic heart failure after myocardial infarction. ESC Heart Fail. 2020, 7, 188–193. [Google Scholar] [CrossRef]
- Yang, T.; Santisteban, M.M.; Rodriguez, V.; Li, E.; Ahmari, N.; Carvajal, J.M.; Zadeh, M.; Gong, M.; Qi, Y.; Zubcevic, J.; et al. Gut dysbiosis is linked to hypertension. Hypertension 2015, 65, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Dong, Z.; Ma, S.; Liu, Y.; Zhou, W.; Wang, Z.; Wu, C.; Ma, R.; Jiang, X.; Zu, T.; et al. Gut Microbiome Signatures Are Predictive of Cognitive Impairment in Hypertension Patients—A Cohort Study. Front. Microbiol. 2022, 13, 841614. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, Q.; Xu, T.; Deng, Q.; Sun, Y.; Fu, J.; Chen, M.; Chen, X.; Ma, Z.; Dong, Q.; et al. Non-differential gut microbes contribute to hypertension and its severity through co-abundances: A multi-regional prospective cohort study. iMeta 2025, 4, e268. [Google Scholar] [CrossRef]
- Wang, Z.; Bergeron, N.; Levison, B.S.; Li, X.S.; Chiu, S.; Jia, X.; Koeth, R.A.; Li, L.; Wu, Y.; Tang, W.H.W.; et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur. Heart J. 2019, 40, 583–594. [Google Scholar] [CrossRef]
- Janeiro, M.H.; Ramírez, M.J.; Milagro, F.I.; Martínez, J.A.; Solas, M. Implication of Trimethylamine N-Oxide (TMAO) in Disease: Potential Biomarker or New Therapeutic Target. Nutrients 2018, 10, 1398. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Warrier, M. Trimethylamine N-Oxide, the Microbiome, and Heart and Kidney Disease. Annu. Rev. Nutr. 2017, 37, 157–181. [Google Scholar] [CrossRef]
- Li, Z.; Wu, Z.; Yan, J.; Liu, H.; Liu, Q.; Deng, Y.; Ou, C.; Chen, M. Gut microbe-derived metabolite trimethylamine N-oxide induces cardiac hypertrophy and fibrosis. Lab. Investig. 2019, 99, 346–357. [Google Scholar] [CrossRef]
- Chen, M.-L.; Zhu, X.-H.; Ran, L.; Lang, H.-D.; Yi, L.; Mi, M.-T. Trimethylamine-N-Oxide Induces Vascular Inflammation by Activating the NLRP3 Inflammasome Through the SIRT3-SOD2-mtROS Signaling Pathway. J. Am. Heart Assoc. 2017, 6, e006347. [Google Scholar] [CrossRef]
- Organ, C.L.; Otsuka, H.; Bhushan, S.; Wang, Z.; Bradley, J.; Trivedi, R.; Polhemus, D.J.; Tang, W.W.; Wu, Y.; Hazen, S.L.; et al. Choline Diet and Its Gut Microbe–Derived Metabolite, Trimethylamine N-Oxide, Exacerbate Pressure Overload–Induced Heart Failure. Circ. Heart Fail. 2016, 9, e002314. [Google Scholar] [CrossRef]
- Chen, K.; Zheng, X.; Feng, M.; Li, D.; Zhang, H. Gut Microbiota-Dependent Metabolite Trimethylamine N-Oxide Contributes to Cardiac Dysfunction in Western Diet-Induced Obese Mice. Front. Physiol. 2017, 8, 139. [Google Scholar] [CrossRef]
- Sun, X.; Jiao, X.; Ma, Y.; Liu, Y.; Zhang, L.; He, Y.; Chen, Y. Trimethylamine N-oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS-TXNIP-NLRP3 inflammasome. Biochem. Biophys. Res. Commun. 2016, 481, 63–70. [Google Scholar] [CrossRef]
- Seldin, M.M.; Meng, Y.; Qi, H.; Zhu, W.; Wang, Z.; Hazen, S.L.; Lusis, A.J.; Shih, D.M. Trimethylamine N-Oxide Promotes Vascular Inflammation Through Signaling of Mitogen-Activated Protein Kinase and Nuclear Factor-κB. J. Am. Heart Assoc. 2016, 5, e002767. [Google Scholar] [CrossRef]
- Yang, S.; Li, X.; Yang, F.; Zhao, R.; Pan, X.; Liang, J.; Tian, L.; Li, X.; Liu, L.; Xing, Y.; et al. Gut Microbiota-Dependent Marker TMAO in Promoting Cardiovascular Disease: Inflammation Mechanism, Clinical Prognostic, and Potential as a Therapeutic Target. Front. Pharmacol. 2019, 10, 1360. [Google Scholar] [CrossRef]
| ID | Author (Year) [Ref] | Design | n (HTN Cases) | Population/ Setting | Exposure Measure | Outcome(s) | Effect Size (95% CI) | Key Findings/ Adjustments |
|---|---|---|---|---|---|---|---|---|
| 1 | Yan et al. (2017) [16] | Cross-sectional | 120 (60) | Chinese adults, mean age 57 yrs | Whole-metagenome shotgun; Shannon index | HTN prevalence, BP | AUC 0.78 (0.73–0.82) | ↓ alpha diversity; ↑ pathogenic taxa; ↓ SCFA producers; TMAO–CV link. Adj: age, gender, BW. |
| 2 | Sun et al. (2019) [17] | Cross-sectional | 529 (183) | US (CARDIA); 46% male, age 55.3 ± 3.4 yrs | 16S rRNA V3–V4; Shannon, richness | HTN, SBP | OR 0.75 (0.60–0.94); β −1.52 (−2.92–−0.12) | Inverse diversity–BP; BMI attenuates; adj: age, sex, race, education, activity, smoking, diet, meds, BMI. |
| 3 | Palmu et al. (2020) [18] | Cross-sectional | 6953 (3291) | Finnish cohort, age 49.2 ± 12.9 yrs, 45% male, BMI 27 ± 4.7 | Shotgun metagenomics; Shannon, Bray–Curtis | HTN, SBP/DBP | OR 0.91 (0.86–0.96); β −0.54 (−0.96–−0.12) | 45 genera ↔ BP; Lactobacillus ↓ BP; adj: age, sex, BMI, smoking, exercise, DM, meds. |
| 4 | Louca et al. (2021) [19] | Cross-sectional | 1319 (454) | UK females, mean age 56 ± 11.3 yrs | 16S rRNA; ASVs/UniFrac | HTN, BP levels | β −0.05 (−0.095–−0.004) | ↓ Ruminiclostridium 6; ↑ Erysipelotrichaceae; linked to thiamine/tryptophan pathways. Adj: age, BMI, NSP intake. |
| 5 | Lin et al. (2025) [20] | Cross-sectional | 3695 (NS) | Scandinavian adults, age 57.3 ± 4.4 yrs | Shotgun metagenomics; Shannon | 24 h ABPM (SBP/DBP var) | β −0.32 (−0.59–−0.06) | Lower diversity ↔ DBP variability; ↑ Streptococcus, ↓ Intestinimonas. Adj: age, sex, birth country, smoking, fiber/Na/energy, meds, BMI. |
| 6 | Lv et al. (2023) [21] | Cross-sectional | 132 (87) | China (NW), untreated HTN, mean age ~54 yrs | 16S rRNA + metagenomic; Shannon | BP, HTN status | AUC 0.80 (0.62–0.92) | ↑ Diversity in females; sex-dimorphic pathways; ↑ signal transduction. Adj: age, BMI, waist, glucose, lipids. |
| 7 | Joishy et al. (2022) [22] | Cross-sectional | 71 (34) | Rural India, Assamese, age 35.9 ± 11.4 yrs | 16S rRNA; Shannon/richness | SBP, high BP ≥ 120 mmHg | p = 0.02 (richness) | ↑ Prevotella/Megasphaera in high BP; classifier AUC 0.93. Adj: age, sex, location, milk, gastric, meds, DBP, BMI, labs. |
| 8 | Guo et al. (2021) [23] | Systematic review | 9085 (~4279) | Multi-country | 16S rRNA (various) | HTN, inflammation | — | ↓ Diversity/inflammation ↑ in HTN; inconsistent alpha results. Adj: varies (age, BMI, etc.). |
| 9 | Han et al. (2024) [24] | Meta-analysis (cohort) | 15,498 (NS) | CVD patients, age 59–80 yrs | Circulating TMAO (μmol/L) | HTN risk in CVD | RR 1.14 (1.08–1.20) | ↑ TMAO → HTN risk (+1%/μmol L); endothelial injury. Adj: country, disease, n, age, sex, BMI, smoking, DM, lipids, meds. |
| 10 | Qi et al. (2018) [25] | Meta-analysis (11 cohorts) | 10,245 (NS) | CAD patients, mean age 63 ± 11 yrs | Plasma TMAO (LC–MS) | CV events, mortality | HR 1.23 (1.07–1.42); 1.55 (1.19–2.02) | ↑ TMAO ↔↑ CV events/mortality; platelet activation pathway. Adj: age, gender, eGFR, NT-proBNP, CVD risks. |
| 11 | Haghikia et al. (2018) [26] | Prospective cohort | 671 (NS) | Post-stroke patients | Plasma TMAO | CV events | HR 3.3 (1.2–10.9) | ↑ TMAO predicts CV events; corr. proinflammatory monocytes (r = 0.70). Adj: HTN, DM, LDL, eGFR, stroke severity/etiology. |
| 12 | Zhou et al. (2020) [27] | Prospective cohort | 1208 (NS) | CHF post-MI, median age 73 yrs | Plasma TMAO (HPLC–MS) | MACE, mortality | HR 2.31 (1.42–3.59); 2.15 (1.37–3.24) | Independent predictor MACE/mortality; improves risk prediction. Adj: age, gender, BMI, HTN, DM, lipids, NT-proBNP, eGFR, hsCRP. |
| 13 | Yang et al. (2015) [28] | Cross-sectional | 17 (7) | Adults, SBP 144 ± 9 vs. 119 ± 2 mmHg | 16S rRNA; Chao/Shannon | BP, HTN | p < 0.05 | ↓ Richness & evenness; ↑ F/B ratio; ↓ butyrate producers. Adj: NS. |
| 14 | Qu et al. (2022) [29] | Cohort | 97 (63) | Chinese HTN patients, mean age 59.9 yrs | 16S rRNA; Shannon/Simpson | Cognitive impairment | AUC 0.94 (0.89–1.00) | ↑ Escherichia–Shigella; ↓ Prevotella; LPS–neuroinflammation link. Adj: age, gender, education, BMI. |
| 15 | Liu et al. (2025) [30] | Prospective cohort | 6999 (2355) | Guangdong Gut Project | Co-abundances (188 genera) | HTN prevalence, severity | 61% ↑ co-abundances (FDR < 0.05) | Microbial networks ↔ HTN severity; tryptophan/androgen pathways. Adj: covariates (Kruskal–Wallis, linear reg.). |
| ID | Author (Year) [Ref] | Study Design | Tool Applied | Selection | Comparability | Outcome/ Exposure | Total Score/Quality | Risk of Bias | Main Limitations |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Yan et al. (2017) [16] | Cross-sectional | AHRQ | ✔✔ | ✔ | ✔✔ | 9/11 (Good) | Low | Limited temporality, moderate confounder adjustment. |
| 2 | Sun et al. (2019) [17] | Cross-sectional | AHRQ | ✔✔✔ | ✔✔ | ✔✔ | 10/11 (Good) | Low | Dietary recall bias. |
| 3 | Palmu et al. (2020) [18] | Cross-sectional | AHRQ | ✔✔✔ | ✔✔✔ | ✔✔ | 10/11 (Good) | Low | Self-reported BP medication use. |
| 4 | Louca et al. (2021) [19] | Cross-sectional | AHRQ | ✔✔✔ | ✔✔ | ✔✔ | 9/11 (Good) | Low–Moderate | Female-only cohort limits generalizability. |
| 5 | Lin et al. (2025) [20] | Cross-sectional | AHRQ | ✔✔✔ | ✔✔ | ✔✔ | 9/11 (Good) | Low | Potential unmeasured confounding (diet/smoking). |
| 6 | Lv et al. (2023) [21] | Cross-sectional | AHRQ | ✔✔ | ✔ | ✔✔ | 8/11 (Moderate) | Moderate | Small sample, regional selection bias. |
| 7 | Joishy et al. (2022) [22] | Cross-sectional | AHRQ | ✔✔ | ✔ | ✔✔ | 8/11 (Moderate) | Moderate | Limited adjustments, rural sample. |
| 8 | Guo et al. (2021) [23] | Systematic review | AMSTAR-2 | ✔✔ | ✔✔ | ✔✔ | 10/11 (High) | Low | Heterogeneous data, secondary extraction. |
| 9 | Han et al. (2024) [24] | Meta-analysis (cohort) | NOS/AMSTAR | ✔✔✔ | ✔✔✔ | ✔✔✔ | 8/9 (High) | Low | Publication bias possible; dose–response variation. |
| 10 | Qi et al. (2018) [25] | Meta-analysis (cohort) | NOS/AMSTAR | ✔✔✔ | ✔✔✔ | ✔✔✔ | 8/9 (High) | Low | Residual confounding (renal function). |
| 11 | Haghikia et al. (2018) [26] | Prospective cohort | NOS | ✔✔✔ | ✔✔ | ✔✔✔ | 8/9 (Good) | Low | Small sample size; no dietary data. |
| 12 | Zhou et al. (2020) [27] | Prospective cohort | NOS | ✔✔✔ | ✔✔✔ | ✔✔✔ | 9/9 (High) | Low | Well-adjusted, minimal bias. |
| 13 | Yang et al. (2015) [28] | Cross-sectional | AHRQ | ✔✔ | ✔ | ✔ | 7/11 (Moderate) | Moderate | Small n; limited sequencing depth. |
| 14 | Qu et al. (2022) [29] | Cohort | NOS | ✔✔✔ | ✔✔ | ✔✔✔ | 8/9 (Good) | Low | Modest sample size, cognitive bias possible. |
| 15 | Liu et al. (2025) [30] | Prospective cohort | NOS | ✔✔✔ | ✔✔✔ | ✔✔✔ | 8/9 (High) | Low | Variable adjustment across regions. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avram, A.-C.; Craciun, M.-L.; Pah, A.-M.; Buleu, F.; Cotet, I.-G.; Mateescu, D.-M.; Iurciuc, S.; Crisan, S.; Belei, O.; Militaru, A.G.; et al. Association Between Gut Microbiome Alterations and Hypertension-Related Cardiovascular Outcomes: A Systematic Review and Meta-Analysis. Microbiol. Res. 2025, 16, 244. https://doi.org/10.3390/microbiolres16110244
Avram A-C, Craciun M-L, Pah A-M, Buleu F, Cotet I-G, Mateescu D-M, Iurciuc S, Crisan S, Belei O, Militaru AG, et al. Association Between Gut Microbiome Alterations and Hypertension-Related Cardiovascular Outcomes: A Systematic Review and Meta-Analysis. Microbiology Research. 2025; 16(11):244. https://doi.org/10.3390/microbiolres16110244
Chicago/Turabian StyleAvram, Adina-Cristiana, Maria-Laura Craciun, Ana-Maria Pah, Florina Buleu, Ioana-Georgiana Cotet, Diana-Maria Mateescu, Stela Iurciuc, Simina Crisan, Oana Belei, Anda Gabriela Militaru, and et al. 2025. "Association Between Gut Microbiome Alterations and Hypertension-Related Cardiovascular Outcomes: A Systematic Review and Meta-Analysis" Microbiology Research 16, no. 11: 244. https://doi.org/10.3390/microbiolres16110244
APA StyleAvram, A.-C., Craciun, M.-L., Pah, A.-M., Buleu, F., Cotet, I.-G., Mateescu, D.-M., Iurciuc, S., Crisan, S., Belei, O., Militaru, A. G., & Avram, C. (2025). Association Between Gut Microbiome Alterations and Hypertension-Related Cardiovascular Outcomes: A Systematic Review and Meta-Analysis. Microbiology Research, 16(11), 244. https://doi.org/10.3390/microbiolres16110244






