Development of a Sensitive and Selective Fluorescent Substrate for the Detection of Chitinase Activity in Entomopathogenic Fungi
Abstract
1. Introduction
2. Materials and Methods
2.1. Tick and Fungal Strains
2.2. Microorganisms and Inoculation
2.3. Chemicals and Reagents
2.4. Synthesis of N-Fluoresceyl Poly-D-Glucosamine
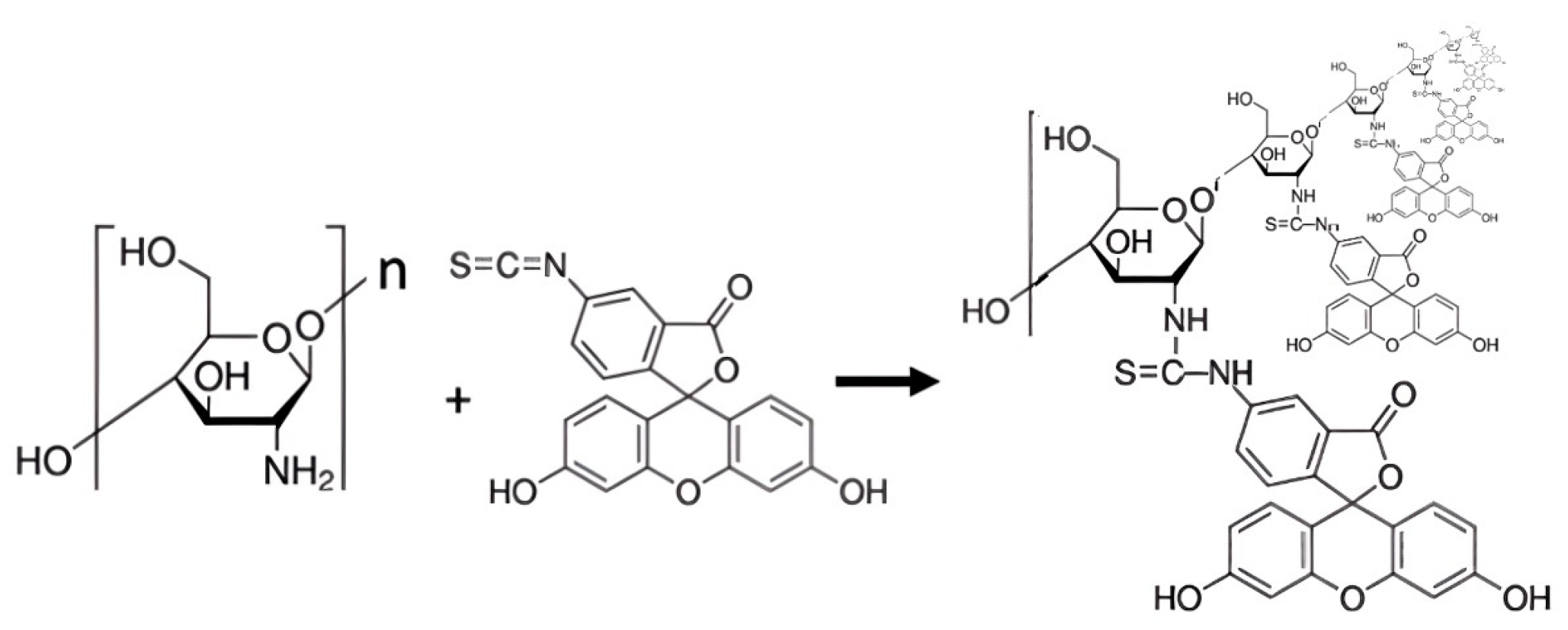
2.5. Preparation of the Fluorogenic Culture Medium Domposition
2.6. Qualitative Detection of Chitinolytic Activity
2.7. Quantitative Analysis of Chitinase Activity
2.8. Statistical Analysis
3. Results
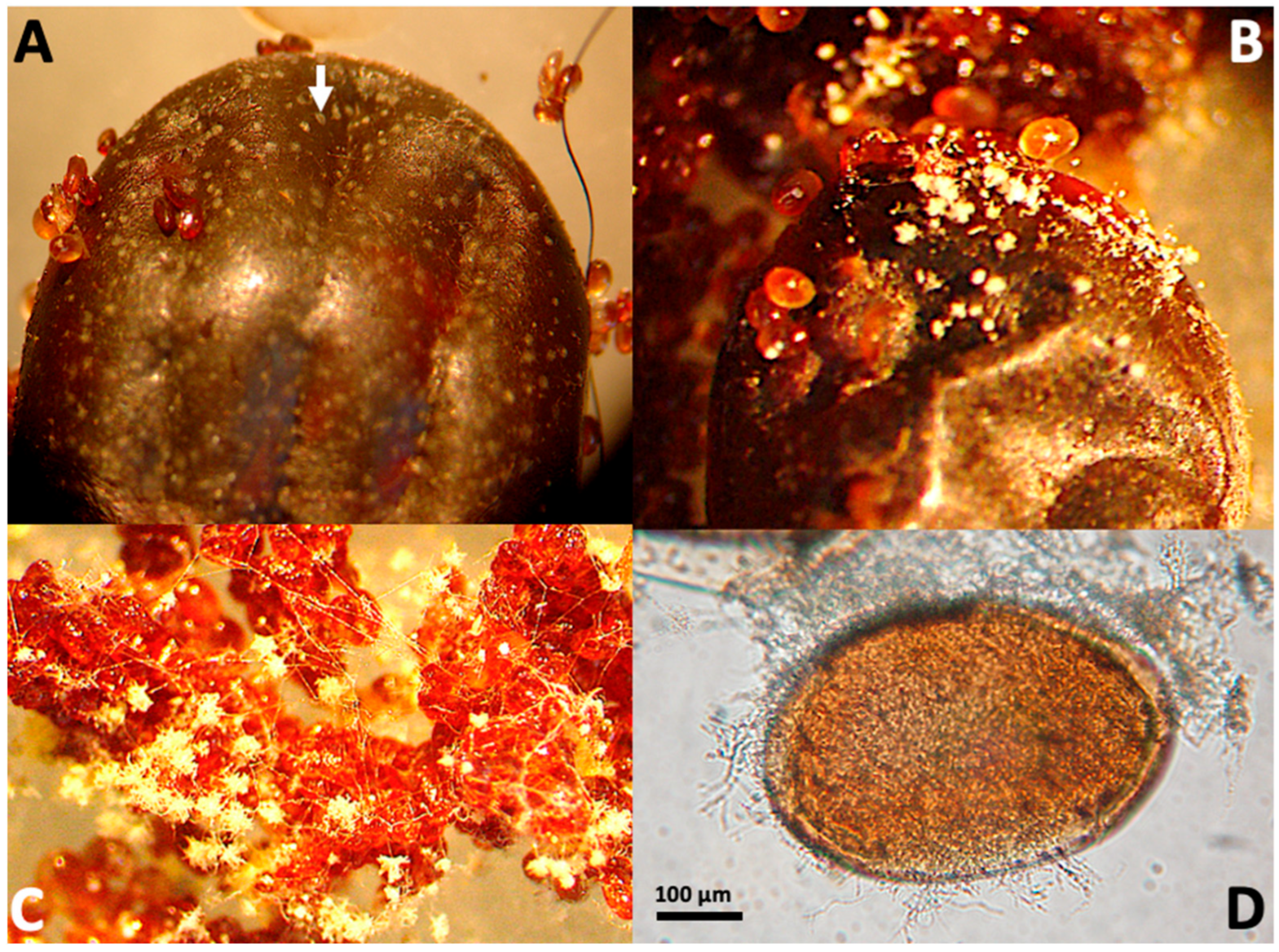
3.1. Experimental Fungal Infection of Engorged Female Ticks
3.2. Visual Detection of Chitinolytic Activity
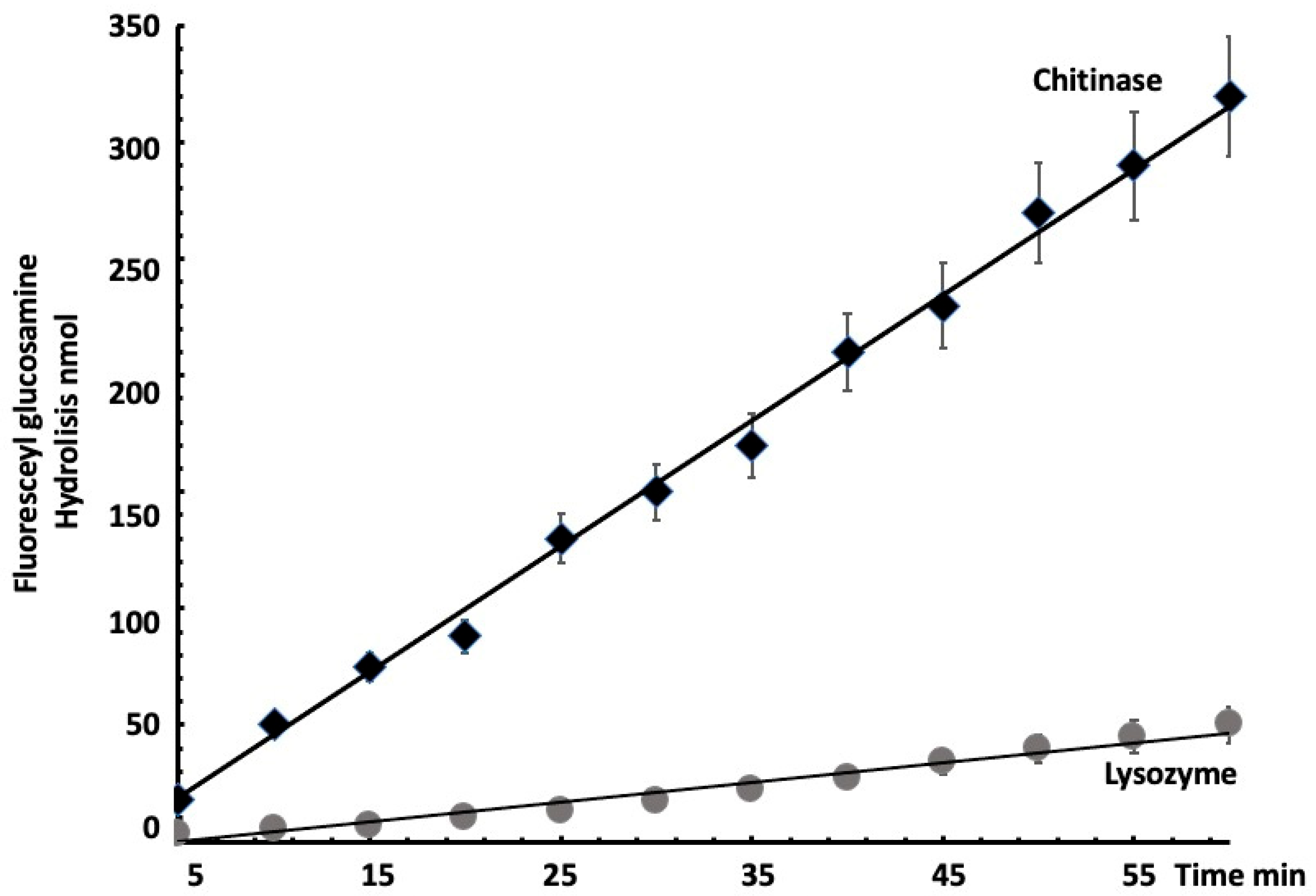
3.3. Quantitative Measurement of Chitinase Activity
3.4. Reproducibility and Sensitivity
4. Discussion
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FITC | Fluorescein isothiocyanate |
| INIFAP | Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias |
| PBS | Phosphate-buffered saline |
| RFU | Relative fluorescence units |
| SD | Standard deviation |
| UV | Ultraviolet |
| CONACYT | Consejo Nacional de Humanidades, Ciencia y Tecnología |
References
- Lv, J.; Lv, X.; Ma, M.; Oh, D.-H.; Jiang, Z.; Fu, X. Chitin and Chitin-Based Biomaterials: A Review of Advances in Processing and Food Applications. Carbohydr. Polym. 2023, 299, 120142. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.S.; Kumar, V. Enzymes in Human and Animal Nutrition; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 978-0-12-805419-2. [Google Scholar]
- Unuofin, J.O.; Odeniyi, O.A.; Majengbasan, O.S.; Igwaran, A.; Moloantoa, K.M.; Khetsha, Z.P.; Iwarere, S.A.; Daramola, M.O. Chitinases: Expanding the Boundaries of Knowledge beyond Routinized Chitin Degradation. Environ. Sci. Pollut. Res. 2024, 31, 38045–38060. [Google Scholar] [CrossRef] [PubMed]
- Dukariya, G.; Kumar, A. Distribution and Biotechnological Applications of Chitinase: A Review. Int. J. Biochem. Biophys. 2020, 8, 17–29. [Google Scholar] [CrossRef]
- Ornela, P.H.; Guimarães, L.H.S. Purification, Characterization and Antifungal Activity of the Aspergillus niveus Chitinase Produced Using Shrimp Shells. Appl. Biosci. 2024, 3, 220–232. [Google Scholar] [CrossRef]
- Dahiya, N.; Tewari, R.; Hoondal, G.S. Biotechnological Aspects of Chitinolytic Enzymes: A Review. Appl. Microbiol. Biotechnol. 2006, 71, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Sunny, N.E.; Kumar, S.R.; Kumar, S.V. A Review on Chitinase Synthesis from Varied Sources and Its Applications towards Environment. Res. J. Pharm. Technol. 2018, 11, 4200. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, Z.; Song, L.; Farag, M.A. Maximizing Crustaceans (Shrimp, Crab, and Lobster) by-Products Value for Optimum Valorization Practices: A Comparative Review of Their Active Ingredients, Extraction, Bioprocesses and Applications. J. Adv. Res. 2024, 57, 59–76. [Google Scholar] [CrossRef] [PubMed]
- Deka, B.; Baruah, C.; Babu, A. Entomopathogenic Microorganisms: Their Role in Insect Pest Management. Egypt. J. Biol. Pest Control. 2021, 31, 121. [Google Scholar] [CrossRef]
- Arreguin-Perez, C.A.; Miranda-Miranda, E.; Folch-Mallol, J.; Ferrara-Tijera, E.; Cossio-Bayugar, R. Complete Genome Sequence Dataset of Enthomopathogenic Aspergillus Flavus Isolated from a Natural Infection of the Cattle-Tick Rhipicephalus microplus. Data Brief 2023, 48, 109053. [Google Scholar] [CrossRef] [PubMed]
- Homthong, M.; Kubera, A.; Srihuttagum, M.; Hongtrakul, V. Isolation and Characterization of Chitinase from Soil Fungi, Paecilomyces sp. Agric. Nat. Resour. 2016, 50, 232–242. [Google Scholar] [CrossRef][Green Version]
- Gonfa, T.G.; Negessa, A.K.; Bulto, A.O. Isolation, Screening, and Identification of Chitinase-Producing Bacterial Strains from Riverbank Soils at Ambo, Western Ethiopia. Heliyon 2023, 9, e21643. [Google Scholar] [CrossRef] [PubMed]
- Boller, T.; Mauch, F. Colorimetric Assay for Chitinase. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1988; Volume 161, pp. 430–435. ISBN 978-0-12-182062-6. [Google Scholar]
- Ferrari, A.R.; Gaber, Y.; Fraaije, M.W. A Fast, Sensitive and Easy Colorimetric Assay for Chitinase and Cellulase Activity Detection. Biotechnol. Biofuels 2014, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Arreguin-Perez, C.A.; Miranda-Miranda, E.; Folch-Mallol, J.L.; Cossío-Bayúgar, R. Identification of Virulence Factors in Entomopathogenic Aspergillus flavus Isolated from Naturally Infected Rhipicephalus microplus. Microorganisms 2023, 11, 2107. [Google Scholar] [CrossRef] [PubMed]
- Tikhonov, V.E.; Lopez-Llorca, L.V.; Salinas, J.; Monfort, E. Endochitinase Activity Determination Using N-Fluorescein-Labeled Chitin. J. Biochem. Biophys. Methods 2004, 60, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Ibañez, F.; Miranda-Miranda, E.; Jasso-Villazul, C.E.; Cossio-Bayugar, R. Chapter 9. Reference Tick Strains as an Important Biological Material for Acaricide-Resistance Characterization. In The Entomological Guide to Rhipicephalus; Insects and Other Terrestrial Arthopods: Biology, Chemistry and Behavior; Nova Science Publishers, Inc.: New York, NY, USA, 2021; pp. 177–200. ISBN 978-1-5361-9635-1. [Google Scholar]
- Miranda-Miranda, E.; Cossio-Bayugar, R.; Martínez-Ibañez, F.; Casasanero-Orduña, R.; Folch-Mallol, J. Natural Occurrence of Lethal Aspergillosis in the Cattle Tick Rhipicephalus (Boophilus) microplus (Acari:Ixodidae). Parasitology 2012, 139, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Veliz, E.A.; Martínez-Hidalgo, P.; Hirsch, A.M. Chitinase-Producing Bacteria and Their Role in Biocontrol. AIMS Microbiol. 2017, 3, 689–705. [Google Scholar] [CrossRef] [PubMed]
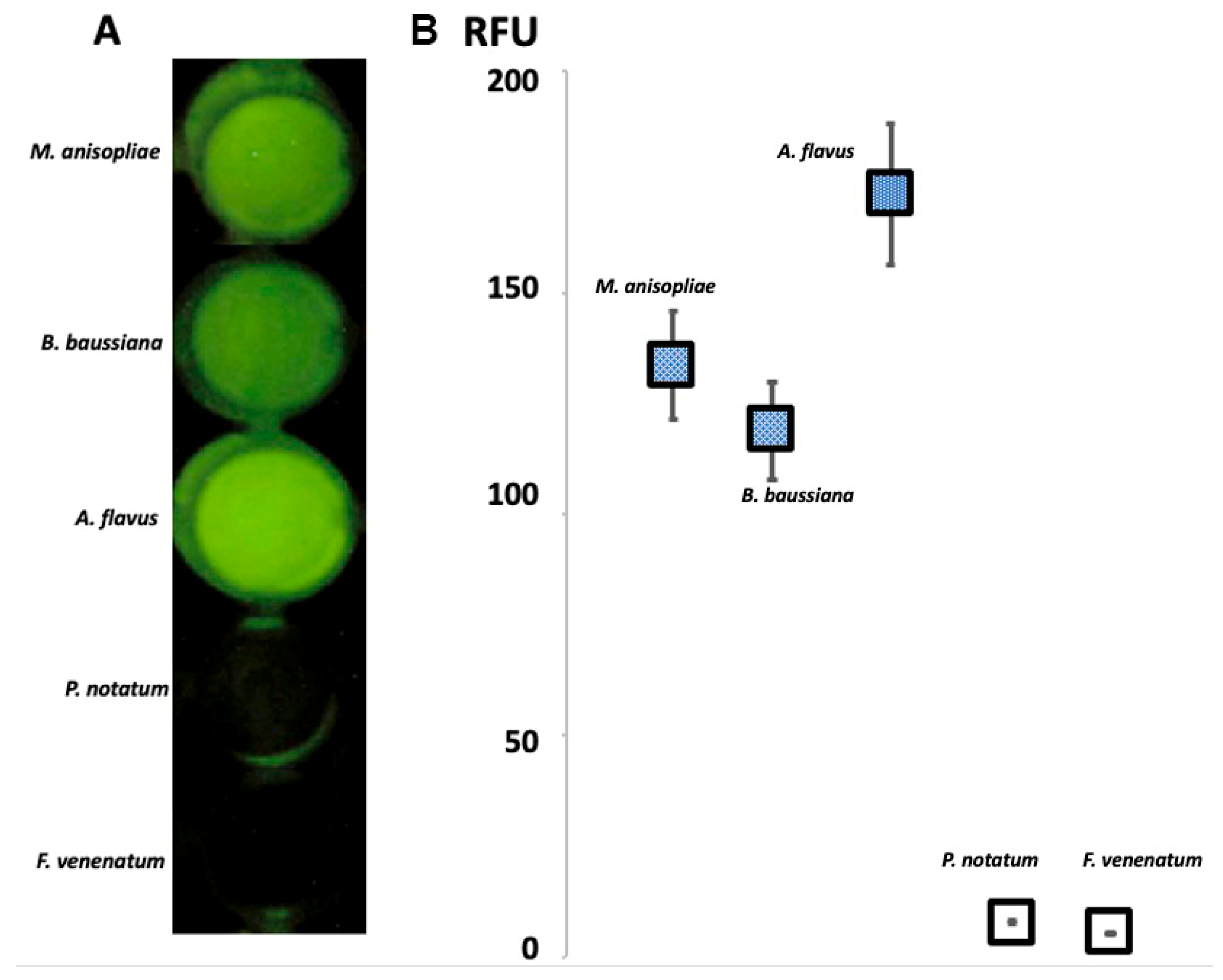
| Inoculum Type | Fluorescence Relative Units | Optical Density at 493 nm (±SD) | Chitinase Activity (U/mL) |
|---|---|---|---|
| Aspergillus flavus | 176 | 0.36 ± 0.012 | 5.14 |
| Bauveria bassiana | 135 | 0.32 ± 0.011 | 4.02 |
| Metarhizium anisopliae | 122 | 0.29 ± 0.010 | 3.77 |
| Penicillum notatum | 3 | 0.005 ± 0.004 | 0.02 |
| Fusarium venenatum | 1 | 0.002 ± 0.001 | 0.01 |
| Negative Control (no inoculum) | – | 0.001 ± 0.0001 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda-Miranda, E.; Arreguín-Pérez, C.A.; Aguilar-Díaz, H.; Cossío-Bayúgar, R. Development of a Sensitive and Selective Fluorescent Substrate for the Detection of Chitinase Activity in Entomopathogenic Fungi. Microbiol. Res. 2025, 16, 243. https://doi.org/10.3390/microbiolres16110243
Miranda-Miranda E, Arreguín-Pérez CA, Aguilar-Díaz H, Cossío-Bayúgar R. Development of a Sensitive and Selective Fluorescent Substrate for the Detection of Chitinase Activity in Entomopathogenic Fungi. Microbiology Research. 2025; 16(11):243. https://doi.org/10.3390/microbiolres16110243
Chicago/Turabian StyleMiranda-Miranda, Estefan, César A. Arreguín-Pérez, Hugo Aguilar-Díaz, and Raquel Cossío-Bayúgar. 2025. "Development of a Sensitive and Selective Fluorescent Substrate for the Detection of Chitinase Activity in Entomopathogenic Fungi" Microbiology Research 16, no. 11: 243. https://doi.org/10.3390/microbiolres16110243
APA StyleMiranda-Miranda, E., Arreguín-Pérez, C. A., Aguilar-Díaz, H., & Cossío-Bayúgar, R. (2025). Development of a Sensitive and Selective Fluorescent Substrate for the Detection of Chitinase Activity in Entomopathogenic Fungi. Microbiology Research, 16(11), 243. https://doi.org/10.3390/microbiolres16110243






