1. Introduction
Cryptococcosis is an opportunistic mycosis caused by yeasts of the genus
Cryptococcus, which include approximately 70 species widely distributed across various natural environments. However, only two species complexes,
Cryptococcus neoformans and
Cryptococcus gattii, are responsible for the majority of human infections [
1,
2]. Other species, such as
Cryptococcus laurentii and
Cryptococcus albidus, have been reported less frequently, although their incidence appears to be increasing [
2].
Infection by
Cryptococcus primarily occurs through the inhalation of desiccated yeast cells or basidiospores, which establish in the lungs. Without treatment, or following a prolonged period of asymptomatic latency, these organisms can proliferate within pulmonary tissue and disseminate via the bloodstream [
1,
3]. To a lesser extent, cases of infection through direct inoculation have also been documented [
4]. Both
C. neoformans and
C. gattii exhibit a marked tropism for the pulmonary parenchyma and the central nervous system, which are the primary sites of infection. However, to a lesser extent, they may also affect other organs and tissues, including the skin, prostate, eyes, and musculoskeletal system [
1]. In patients with severe immunosuppression, systemic dissemination of
Cryptococcus can involve multiple organs, establishing infectious foci in various anatomical sites and potentially leading to a fatal outcome [
3].
The
C. neoformans complex is the primary causative agent of cryptococcosis worldwide, with most cases attributed to
C. neoformans var.
grubii (serotype A). In contrast,
C. gattii is a relevant pathogen in specific regions such as Australia, Papua New Guinea, and certain areas of the United States and Canada [
5]. This complex is found mainly in vegetation, especially in association with trees and soil rich in organic matter; it was first identified in eucalyptus trees but has since been reported from various other tree species and ecological niches, and has been responsible for outbreaks on Vancouver Island and in the northwestern United States [
6]. Although it was initially considered to be restricted to tropical and subtropical regions, its distribution is now recognized as much broader [
5]. While
C. neoformans infections occur predominantly in individuals with HIV or other immunodeficient conditions,
C. gattii infections have more frequently been reported in apparently immunocompetent individuals [
1,
3,
6].
Mortality associated with
C. neoformans ranges from 41% to 61% in patients with HIV and from 8% to 20% in HIV-negative individuals [
6,
7]. For
C. gattii, reported mortality across different clinical manifestations ranges from 21% to 43% [
6]. Due to their high lethality and global burden, particularly among patients with HIV, both species have been recently included in the World Health Organization (WHO) fungal priority pathogens list [
8].
Pulmonary cryptococcosis is a common manifestation of
Cryptococcus infections, particularly among immunocompromised patients [
6,
9]. In recent years, it has been recognized as an emerging disease in immunocompetent individuals, highlighting the importance of its study and surveillance across different population groups [
6,
10]. Unlike cryptococcal meningitis, pulmonary cryptococcosis remains underdiagnosed due to limitations in available diagnostic tools. Its clinical and radiological presentation can mimic conditions such as lung cancer, pulmonary tuberculosis, bacterial pneumonia, and other pulmonary mycoses, complicating early identification and timely treatment [
9,
11,
12].
In this context, we present the case of a patient with type 2 DM who developed pulmonary cryptococcosis in the absence of severe immunosuppression, highlighting the need to consider this mycosis in the differential diagnosis of pulmonary lesions in diabetic patients. The case also underscores the importance of optimizing diagnostic strategies for early detection and timely management.
Given that culture-based methods using respiratory samples such as sputum and bronchoalveolar lavage have low sensitivity for identifying species within the Cryptococcus complex, this report further advocates for the use of non-culture-based diagnostic tools that allow for faster and more accurate detection—particularly in settings with a high prevalence of invasive mycoses.
As a complement, we include a literature review on the epidemiology and clinical manifestations of, and therapeutic options for, cryptococcosis in patients with diabetes mellitus, based on case reports and case series published over the past 25 years. This case reinforces the need to consider pulmonary cryptococcosis as a possible diagnosis in patients with type 2 DM, even in the absence of apparent immunosuppression, to support early clinical intervention. Additionally, it provides evidence suggesting a potential association between diabetes and susceptibility to invasive fungal infections—an area still not fully understood.
Finally, to the best of our knowledge, this study represents the first molecular characterization of Cryptococcus species associated with infection in Honduras, contributing to epidemiological and microbiological understanding of this mycosis in the region.
2. Case Description
2.1. Prior Medical History
The patient was a 51-year-old female patient with a history of decompensated type 2 diabetes mellitus diagnosed 7 years ago, which was managed with pharmacological monotherapy consisting of metformin once daily, and uncontrolled arterial hypertension treated with amlodipine 10 mg once daily, with poor adherence to both treatments. The patient presented to the emergency department after a four-month history of respiratory symptoms and multiple medical consultations, during which she received various antibiotic regimens for suspected pneumonia and pulmonary tuberculosis. However, diagnostic tests for tuberculosis were negative, and no significant clinical improvement was observed. Poor adherence to the prescribed treatments was also documented.
2.2. At Admission
The patient had a history of decompensated type 2 DM treated with Metformin 850 mg once daily with inconsistent adherence, and uncontrolled arterial hypertension managed with Amlodipine 10 mg once daily, also with irregular adherence. She presented to the emergency department (Day 1) with a four-month history of respiratory symptoms. The illness had an insidious onset with a non-productive cough lacking a specific pattern, which worsened with physical exertion, and was accompanied by progressive dyspnea with use of lower intercostal accessory muscles, noticeable tachypnea, and intermittent subjectively high fevers partially relieved by antipyretics.
On physical examination, the patient was in poor general condition but fully alert (Glasgow Coma Scale score of 15/15). Her vital signs were a blood pressure of 140/80 mmHg, heart rate of 112 bpm, respiratory rate of 30 breaths/minute, temperature of 37 °C, and oxygen saturation of 95% on 36% FiO2. Pulmonary auscultation revealed decreased breath sounds in both lung bases without crackles or wheezing. The remainder of the examination was unremarkable.
Laboratory findings revealed leukocytosis (15.0 × 10
3/µL), hemoglobin at 11 g/dL, blood glucose at 191 mg/dL, HbA1c at 9.5%, and creatinine at 0.8 mg/dL. Liver function tests and electrolytes were within normal limits. Additional biochemical and hematological parameters recorded throughout the patient’s clinical course are detailed in
Table 1. HIV serology was negative, and the GeneXpert
® molecular test for
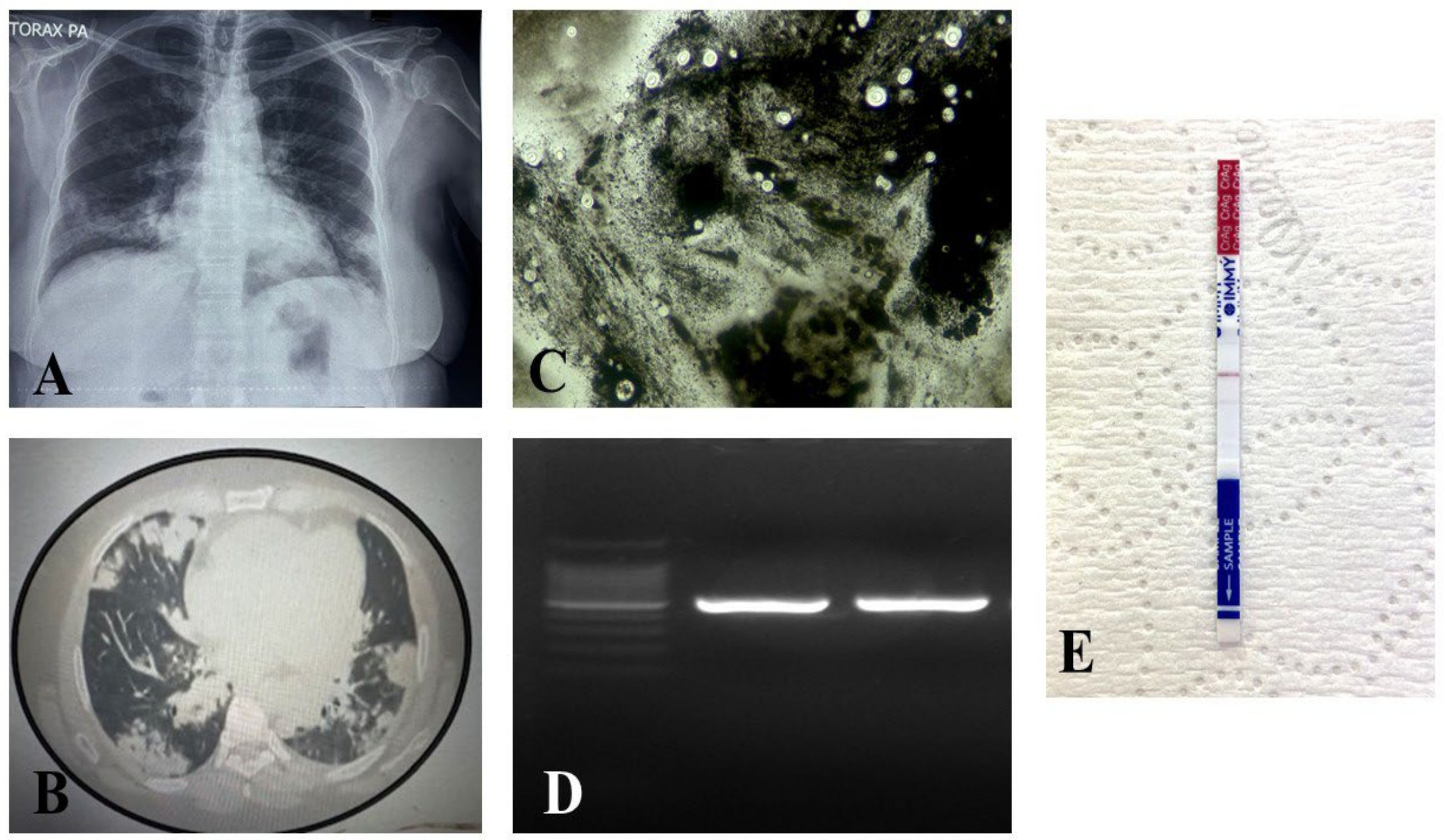
Mycobacterium tuberculosis was negative. Chest imaging revealed multiple cavitated consolidations with bilateral distribution (
Figure 1).
The transthoracic echocardiogram revealed no structural abnormalities. Empirical antibiotic therapy was initiated with vancomycin 500 mg every 6 h and ceftriaxone 2 g daily.
In addition, subcutaneous NPH insulin was initiated at a dose of 30 IU in the morning and 15 IU in the evening. On the third day of hospitalization, due to the lack of clinical response and the suspicion of a fungal etiology underlying the slowly resolving pneumonia, a bronchoscopy with bronchoalveolar lavage was performed. The samples were sent to the Pathology and Microbiology Departments for further evaluation. On the same day, an infectious diseases consultation was requested, and empirical antifungal therapy was initiated with amphotericin B deoxycholate at a dose of 60 mg daily (1 mg/kg/day) for a planned 14-day course, together with intravenous fluconazole 200 mg daily as part of the induction phase.
Laboratory studies performed on the bronchoalveolar lavage sample (Day 4) included a wet mount and India ink staining, both of which revealed abundant yeast cells. The India ink stain demonstrated structures consistent with encapsulated yeasts. Additionally, a lateral flow assay (LFA) for cryptococcal antigen detection yielded a positive result with a titer of 1:32. The samples were also cultured at 37 °C for 48 h.
Simultaneously, in the Pathology Department, histological analysis of the bronchoalveolar lavage was conducted. Cytopathological examination revealed a severe, organized chronic inflammatory response associated with recent hemorrhage. Papanicolaou (PAP) staining revealed numerous large yeasts with a clear halo and single budding, which were morphologically consistent with Cryptococcus spp.
On Day 6, culture plates were examined, and no growth was observed. Therefore, incubation was extended for an additional three days (until Day 9), but cultures remained negative. Since both microscopic and serological findings suggested the presence of an encapsulated yeast, DNA was extracted directly from the sample following a previously published protocol [
13], and the internal transcribed spacer (ITS) region of ribosomal DNA was subsequently amplified using the ITS1 and ITS4 primers.
Briefly, PCR reactions were carried out in a final volume of 50 μL, consisting of 25 μL of PCR Master Mix (Promega Corp., Madison, WI, USA), 1 μL of each primer ITS1 and ITS4 (10 μM; 5′-TCCGTAGGTGAACCTGCGG-3′/5′-TCCTCCGCTTATTGATATGC-3′), and 1 μL of DNA (20–40 ng/μL). The thermal cycling program included an initial denaturation at 95 °C for 5 min, followed by 37 cycles of 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s, with a final extension at 72 °C for 10 min. Amplicons were visualized on a 1.5% agarose gel stained with ethidium bromide. The ITS (Internal Transcribed Spacer) region was amplified and sequenced bidirectionally following the protocols of Psomagen (
https://www.psomagen.com, accessed on 2 January 2025).
Amplification of the ITS region produced a 535 bp product, which was subsequently sequenced. The resulting sequences were trimmed and edited using Geneious
® software version 2024.0.5 and subsequently deposited in GenBank (
https://www.ncbi.nlm.nih.gov/genbank/) under accession number PV710882. Sequence analysis performed with the NCBI BLAST tool identified the isolate as
Cryptococcus neoformans (
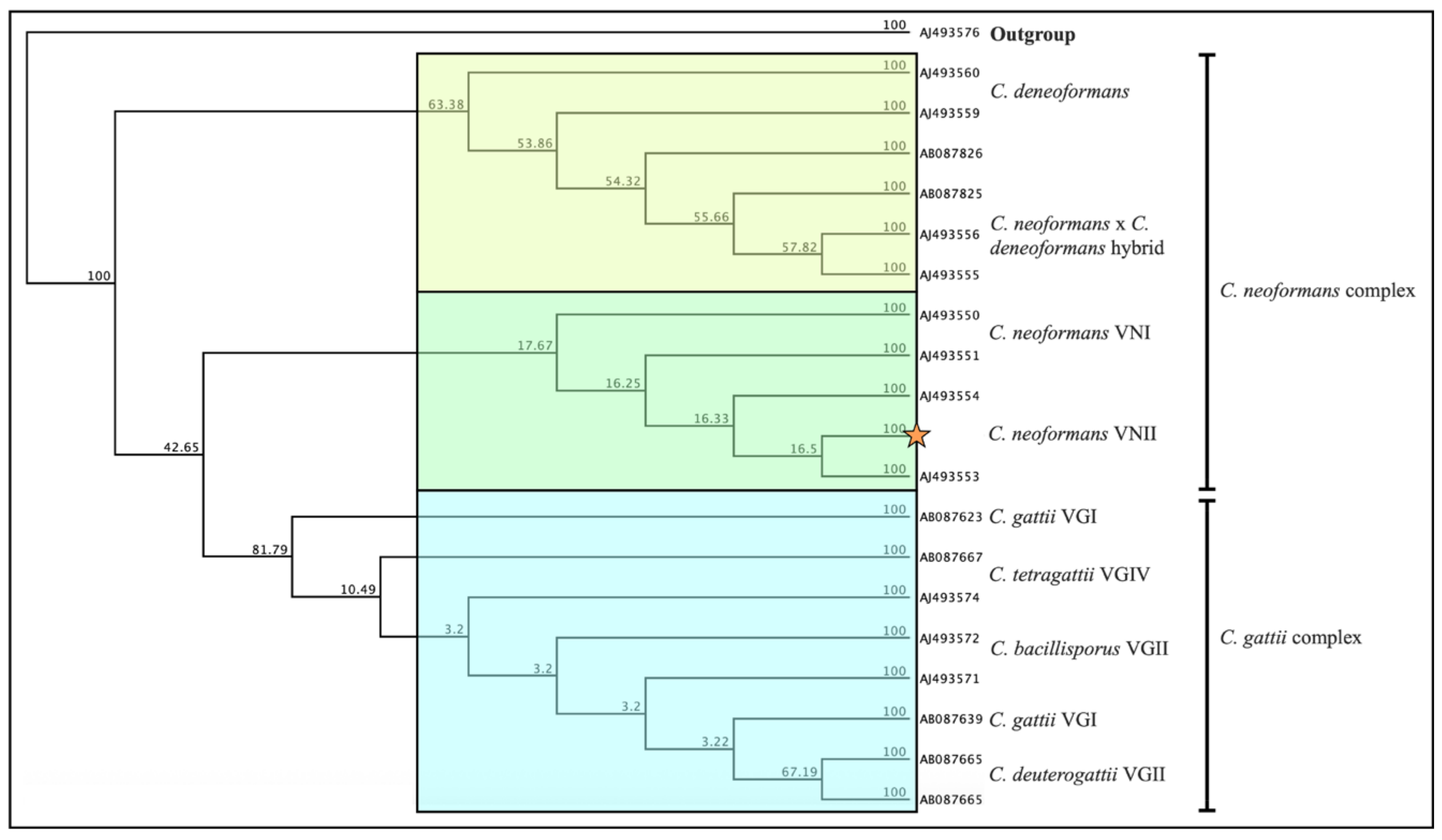
Figure 2). A phylogenetic tree was constructed from our sequence following the methodology described by Weiss et al. 2022 and compared with previously deposited sequences in GenBank, as well as with representative strains from the
Cryptococcus neoformans and
Cryptococcus gattii species complexes [
14]. This analysis positioned our isolate within the
C. neoformans VNII molecular type.
Despite supportive measures, including adequate hydration and administration of amphotericin B deoxycholate, the patient developed renal impairment, as evidenced by a progressive rise in nitrogenous waste markers: serum creatinine increased to 2.5 mg/dL, and the estimated glomerular filtration rate (eGFR) declined to 23 mL/min. After consultation with the infectious diseases team, antifungal therapy was discontinued due to suspected amphotericin B-induced nephrotoxicity, and fluconazole was initiated at a dose of 400 mg IV every 12 h. This change stabilized renal function, although hepatic transaminases (AST, ALT) progressively increased. On the eighth day of fluconazole therapy (Day 17), gradual improvement in renal function was noted, with eGFR rising to 43 mL/min and bilirubin levels decreasing. Given this favorable clinical evolution, amphotericin B deoxycholate was reintroduced at a daily dose of 1 mg/kg/day. However, metabolic and inflammatory abnormalities persisted, including leukocytosis (WBC: 11.07 ×103/µL) and elevated blood glucose (289 mg/dL). The induction phase with amphotericin B deoxycholate was completed by Day 30, and maintenance therapy with fluconazole at 400 mg IV every 24 h was initiated. Despite comprehensive management, the clinical course remained unfavorable. The patient developed persistent hyperglycemia and ongoing metabolic and inflammatory dysregulation, and eventually presented with disseminated intravascular coagulation (DIC) and seizures, culminating in death on Day 36 of hospitalization.
4. Discussion
Several studies have demonstrated that infectious diseases represent a significant cause of mortality in individuals with DM [
15,
16,
17,
18,
19]. In the United Kingdom, they are estimated to be the third most common cause of death in this population, following cardiovascular diseases and cancer [
15]. This highlights the clinical impact of the immunological alterations associated with this condition. Although DM does not cause direct immunosuppression, as seen with certain diseases or immunosuppressive therapies, it does lead to significant immune system dysfunctions that increase susceptibility to infections [
16,
20].
Particularly in patients with poor glycemic control, chronic hyperglycemia has been shown to impair both innate and adaptive immunity [
21]. The described alterations include impaired phagocytic function of macrophages, monocytes, and neutrophils; reduced antimicrobial activity; and increased oxidative stress and systemic inflammation; as well as disruptions in cell signaling and cytokine production [
20,
22]. These immunological dysfunctions weaken the host response against various pathogens, including fungi, although the exact mechanisms are not yet fully understood and further studies are needed.
In this context, DM has emerged as a significant risk factor for the development of invasive mycoses, such as candidemia and mucormycosis [
23,
24]. It has also been increasingly associated with infections caused by species of the
Cryptococcus genus [
25]. However, further systematic studies are still required to confirm and more precisely characterize this association.
Traditionally, infections caused by
Cryptococcus spp. have been associated with patients with HIV/AIDS; however, recent reports highlight a growing proportion of cases in HIV-negative individuals [
10], particularly elderly patients with chronic comorbidities such as renal failure, liver disease, hematologic malignancies undergoing chemotherapy, lupus, or Cushing’s syndrome [
25,
26,
27]. This shift in the epidemiological profile highlights the need to consider diabetic patients as a vulnerable population, even in the absence of classical immunosuppression [
27].
In this context, we conducted a review of case reports published over the past 25 years with the aim of exploring a possible relationship between pulmonary cryptococcosis and DM. In total, 40 clinical cases documented in the literature were analyzed, offering detailed insights into predisposing factors, clinical characteristics, therapeutic management, and outcomes, as summarized in
Supplementary Table S1.
In this series, a slight male predominance was observed (57.5%), with a mean age of 53.6 years (range: 13 to 84 years). This pattern is consistent with previous studies suggesting increased susceptibility to invasive fungal infections among older adults, likely related to immunosenescence and the higher prevalence of comorbidities in this age group.
The most frequently identified risk factors were immunosuppressive conditions (62.5%), including HIV infection, neoplasms, autoimmune diseases treated with immunosuppressants, transplants, and hematologic disorders. A particularly relevant finding was the presence of DM in seven patients (17.5%), most of whom were over 60 years of age. Although these cases did not involve pharmacological immunosuppression or primary immunological diseases, diabetes as a chronic condition can impair both innate and adaptive immune responses, thereby increasing susceptibility to opportunistic infections. Notably, one patient with multiple comorbidities had a fatal outcome, underscoring the potential clinical impact of this metabolic disease on the progression of invasive mycoses.
From an etiological standpoint, C. neoformans was identified as the causative agent in 75% of cases. Infection with C. gattii was confirmed in two patients, both of whom were immunocompetent and presented with severe clinical manifestations. This finding is consistent with studies suggesting greater virulence of C. gattii in individuals without immunosuppression. In the remaining 20% of cases, the diagnosis was limited to the genus level (Cryptococcus spp.), highlighting the need to strengthen species-level mycological identification methods, both to guide appropriate treatment and to improve understanding of epidemiological patterns.
Regarding antifungal treatment, fluconazole was the most used agent, administered in 85% of cases, generally at a dose of 400 mg/day (range: 200–800 mg/day), adjusted according to clinical severity and the patient’s immune status. Liposomal amphotericin B was used in 13 cases (32.5%), sometimes in combination with flucytosine (8 cases, 20%), primarily in immunocompromised patients or those with disseminated disease. Voriconazole was employed in five patients (12.5%), and one case required isavuconazole, reflecting the diversity of antifungal regimens applied on an individualized basis. One case was resolved surgically without the need for antifungal therapy, suggesting that in selected scenarios with localized disease, surgical management may be a viable alternative. These studies highlight the importance of standardizing treatments for Cryptococcus spp.
In terms of clinical outcomes, most patients (92.5%) experienced complete recovery or significant improvement with the instituted treatment. In two cases, pulmonary lesions persisted without progression or clinical relapse during follow-up. The only fatal case involved a patient immunocompromised due to HIV and active tuberculosis, reflecting the severity of this infection when severe and poorly controlled immunosuppressive conditions coexist.
In this context, we report a fatal case of pulmonary cryptococcosis caused by
Cryptococcus neoformans in a patient with DM, accompanied by a review of the literature published over the past 25 years. To the best of our knowledge, only the study by Nsenga et al., 2021, has explored the association between disseminated or systemic cryptococcosis and DM [
27]. However, cases of pulmonary cryptococcosis (PC) and their specific risk factors have not been thoroughly examined, highlighting the importance of case reports from different geographic regions. Such reports contribute to building a valuable chain of clinical and epidemiological knowledge; in this regard, our review helps fill an existing gap in understanding this relationship.
A previous review analyzing the association between disseminated or systemic cryptococcosis and DM reported a frequency of PC in diabetic patients of 14.9% (
n = 7) [
27]. However, our findings suggest that this proportion could potentially double, indicating a stronger link between DM and pulmonary cryptococcosis. This observation underscores the possibility that many cases of PC in diabetic patients remain underdiagnosed due to the broad spectrum of pulmonary manifestations that can mimic other respiratory conditions. These findings emphasize the need for additional clinical studies to determine the true magnitude of this association and its relationship with other opportunistic mycoses, in order to establish standardized, evidence-based diagnostic and management protocols.
Regarding diagnostic methodologies, our results show that more than 60% of cases were identified through non-culture-based techniques. This may be related to the inherent limitations of culturing respiratory samples [
28,
29], which often exhibit lower sensitivity than immunological or molecular assays [
29]. Previous studies have demonstrated that non-culture-based methods provide higher diagnostic sensitivity and enable earlier initiation of antifungal therapy [
30,
31].
Nevertheless, culture of biological specimens remains the reference “gold standard” for diagnosing systemic fungal infections due to its low cost and its ability to assess antifungal susceptibility of the causative agent. However, the limited sensitivity and prolonged incubation and identification times of culture methods can delay appropriate treatment initiation and, consequently, increase the risk of mortality [
32]. Although one limitation of our study is its focus on pulmonary infection without an in-depth evaluation of possible hematogenous dissemination, our findings underscore the importance of incorporating non-culture-based techniques—such as cryptococcal antigen detection—as complementary diagnostic tools, particularly in low- and middle-income settings where diagnostic capacities for fungal diseases remain limited. In this context and considering the inherent limitations of culture-based methods, the absence of growth in bronchoalveolar lavage samples—despite the observation of yeasts in the India ink stain and a positive result for cryptococcal antigen detection—prevented the performance of antifungal susceptibility testing. This limitation precluded establishing a correlation between the patient’s lack of clinical response to fluconazole treatment and the possible presence of resistant phenotypes. Such a constraint is particularly relevant given that the molecular type VNII of
C. neoformans identified in this case has generally been described as more susceptible to fluconazole than the VNI type. Taken together, these findings underscore the importance of maintaining microbiological cultures as an essential tool in the diagnostic and therapeutic management of cryptococcosis, even when their sensitivity and yield may be limited.
Finally, regarding treatment, the therapeutic regimens of choice include flucytosine and liposomal amphotericin B. However, in Honduras, flucytosine is not available and access to liposomal amphotericin B is limited [
33]. In the present case, amphotericin B deoxycholate was administered in combination with vancomycin, both drugs known for their nephrotoxic potential, which likely contributed to the patient’s renal function deteriorating [
34,
35]. Therefore, it is imperative to promote the implementation of liposomal amphotericin B and the inclusion of flucytosine in national treatment guidelines in order to improve clinical outcomes and reduce mortality associated with pulmonary cryptococcosis.
Implications in the Honduran Context
In Honduras, the epidemiological situation of DM is particularly alarming. It is estimated that more than 1.5 million people are affected, with a considerable number of cases remaining undiagnosed, making this condition a growing challenge for the national healthcare system [
36]. An evident example of the impact of DM on public health was the COVID-19-associated mucormycosis outbreak that occurred in 2021, during which 17 cases were reported over a period of just four months, with 70% of those cases associated with DM [
37]. This event underscores the role of DM as a key predisposing factor in the emergence of invasive fungal infections.
On the other hand, a regional study on the implementation of rapid diagnostic tests for histoplasmosis and cryptococcosis in patients with HIV/AIDS—conducted in the two main hospitals in the country, located in San Pedro Sula and Tegucigalpa—revealed that Honduras had the highest positivity rate for
Cryptococcus in the Central American region using the CrAg LFA test, with a rate of 16% [
38]. This finding suggests a high environmental burden of this fungus and reinforces the need to expand such evaluations to populations with other forms of immunosuppression beyond HIV/AIDS.
Specifically, regarding pulmonary cryptococcosis, data from the WHO Global Tuberculosis Report (2022) estimated that the incidence of tuberculosis (TB) in Honduras was 33 cases per 100,000 inhabitants in 2021—one of the highest rates in the region. Given that pulmonary TB can present with clinical and radiological features similar to other diseases, it is likely that numerous cases of pulmonary cryptococcosis are being underdiagnosed or misidentified as TB. This situation is further exacerbated by the high prevalence of diabetes, limitations in diagnostic infrastructure, and the potential for undetected coinfections—suggesting that many invasive fungal infections may go undiagnosed or be diagnosed late.
Our analysis underscores the need to strengthen fungal infection screening in individuals with diabetes, particularly those with poor glycemic control. Moreover, expanding access to sensitive and rapid diagnostic tools—beyond conventional culture methods—is essential to reduce the time to treatment initiation and improve clinical outcomes, especially in resource-limited settings such as Honduras.
Until 2021, a total of 106 publications reported the major molecular type of 5686 isolates of
C. neoformans and
C. gattii from Latin America, identified predominantly by RFLP (57.1%) [
39]. In the present study, we used ITS gene sequencing, a method considered more informative than RFLP-PCR. The internal transcribed spacer (ITS) region of ribosomal DNA comprises the partial 18S rRNA, the complete 5.8S rRNA, and the partial 28S rRNA genes, including the two variable non-coding regions known as ITS1 and ITS2. This highly polymorphic region serves as a key genetic marker for the identification, classification, and phylogenetic analysis of fungal species and their diversity [
40,
41].
According to molecular data from 5686 clinical, environmental, and veterinary
Cryptococcus isolates from member countries of the Latin American Cryptococcal Study Group,
C. neoformans molecular type VNI is the most frequent cause of cryptococcosis (76%) among HIV-infected individuals [
39]. In contrast, our phylogenetic analysis showed that the Honduran isolate clustered within molecular type VNII. This finding suggests that, unlike the predominance of VNI reported across Latin America, VNII may also be circulating in the Honduran population and could represent a relevant genotype associated with infection in this region.
In summary, to the best of our knowledge, this study represents the first molecular characterization of Cryptococcus neoformans associated with infection in Honduras.
Finally, it should be noted that flucytosine is not available in the country, and access to the different formulations of amphotericin B is limited [
33]. Considering the high rate of cryptococcosis reported in the country [
38,
42], it is imperative that policymakers actively work to ensure the timely availability of essential antifungal treatments in Honduras.