Abstract
Field emission scanning electron microscopy (FE-SEM) may be used to visualize the surface morphology of samples that are permeable to electron beams, including biological samples. Probiotics attenuate host physiological functions and are characterized by their three-dimensional surface structures. In this study, we determined the effect of critical point drying (CPD) on FE-SEM observations of the surface of Lacticaseibacillus paracasei strain Shirota (LcS). We also assessed ionic liquid (IL), a non-volatile liquid salt that retains moisture, and NanoSuit®, which forms a protective polymer membrane around the sample, through FE-SEM observation of these probiotics. The results indicate that dehydration during CPD leads to reticular structures on the probiotic surface, potentially affecting the characteristics observed by FE-SEM. In addition, we examined IL and NanoSuit®, which do not involve dehydration. The initial examination involving optimal dilution using silica particles revealed that 5–10% IL and 5–20% NanoSuit® solutions maintained particle size consistency. We examined LcS specimens under these conditions and observed smooth surfaces, not reticulate structures. These results indicate that CPD affects LcS surface morphology, whereas the IL and NanoSuit® methods preserved it. This suggests their applicability for probiotic preparation before FE-SEM observations.
1. Introduction
Field emission scanning electron microscopy (FE-SEM) excels at imaging the surface morphology of highly electron-transparent biological specimens owing to its low acceleration voltage and high-resolution capabilities [1]. It is particularly effective for observing bacteria with intricate morphologies [2,3,4,5].
The gut microbiota is involved in various physiological functions [6,7,8], and probiotics have attracted attention as a tool to influence the gut microbiota. Probiotics are live microorganisms that benefit the host when consumed in sufficient quantities [9]. The three-dimensional, molecular-level structure of probiotic surfaces, such as those of lactic acid bacteria, can affect host immune function [10]. For example, the Lacticaseibacillus paracasei (formerly known as Lactobacillus casei) strain Shirota (LcS), which is a representative lactic acid bacterium, contains strain-specific polysaccharides on its surface that affect various host immune functions [11,12]. Thus, preserving the three-dimensional structures of probiotics for FE-SEM observation is important for studying these beneficial probiotics.
One limitation when observing probiotics using FE-SEM is the moisture content. The high-vacuum environment maintained by FE-SEM can cause outgassing because of the volatilization of moisture or organic solvents. This may result in the deposition of hydrocarbons and other substances onto the microscope body. Therefore, when analyzing samples containing moisture or volatile components, it is essential to ensure that they are thoroughly dried prior to analysis.
Critical point drying (CPD), freeze-drying (FD), and the hexamethyldisilazane (HMDS) method are used to dehydrate moisture-containing samples under conditions that prevent surface tension from affecting the analysis. These methods are well-established for preparing biological samples for SEM [13,14,15,16,17]. To our knowledge, there have been no reports describing how dehydration affects the surface of bacterial cells in probiotics; however, it is possible that similar changes in surface morphology, as observed in Escherichia coli and Candida albicans from dehydration [18,19], may also occur in probiotics. Conversely, Tween 20, NanoSuit®, and ionic liquids (ILs) are considered effective sample preparation techniques for SEM, as they maintain a hydrated state in the specimens and prevent dehydration [20,21,22,23,24,25,26,27]. For Tween 20 and NanoSuit®, thin polymer films form on the specimen surface upon electron beam irradiation. They retain moisture and shield the specimen from stress and damage caused by the vacuum environment and electron beam exposure [23]; however, deformation of the specimen during SEM has been reported using the Tween 20 method [23]. In contrast, the IL method uses ionic liquids, extremely low vapor pressures, and high hydrophilicity, which enables SEM observation of specimens in a hydrated state, even under vacuum conditions. The NanoSuit® and IL methods have been applied to various biological samples, including biofilms [24,25,26,27], paraffin sections [19], plankton, plant cells, and cultured cells [21,24]; however, these methods have not been thoroughly evaluated for the surface structures of probiotics.
In this study, we determined the effect of the CPD method, a processing technique that includes a dehydration process, on FE-SEM observation using the probiotic strain LcS, and evaluated the IL and NS methods.
2. Materials and Methods
2.1. Evaluation of the CPD Method on the Bacterial Surface of LcS
To determine how the CPD method affects the microstructure of the LcS surfaces, LcS suspensions were sampled following each CPD step, including washing with 0.1 mol L−1 phosphate buffer (PB; pH 7.2), fixation with 2.5% glutaraldehyde, fixation with 1% osmium tetroxide, ethanol dehydration, and isoamyl acetate replacement. After each step, the supernatant was removed following centrifugation, and the suspension was prepared for the next treatment. Bacterial suspensions soaked in PB following ethanol dehydration were also sampled to confirm the dehydration effects. Each suspension (2 µL) was dropped onto a highly polished carbon stub and allowed to dry at ambient temperature (22–25 °C) for approximately 10 min to prepare the specimen for FE-SEM observation. CPD specimens were prepared by dropping 2 µL of bacterial suspension, using isoamyl acetate as a dispersant, onto a carbon stub, followed by CPD using a critical point dryer (EM CPD300, Leica Microsystems, Wetzlar, Germany) with liquefied carbon dioxide gas.
2.2. Experimental Condition Optimization for IL and NanoSuit® Methods
The high viscosity of the IL and NanoSuit® fluids raised concerns regarding trapping bacterial surface microstructures at nanometer depths, thus altering their morphology. Therefore, diluted solutions of IL and NanoSuit® were prepared, and the untreated silica particle size and morphology with silica particles from samples prepared for FE-SEM using IL or NanoSuit® were compared. The silica particle suspensions contained particles with an average diameter of 30 nm (Micromod, 43-00-301, Rostock, Germany). The IL solution was HILEM IL-1000 (Hitachi High-Tech, Tokyo, Japan), whereas the NanoSuit® solution was Type III–NanoSuit® solution for cells (NanoSuit Corporation, Shizuoka, Japan). HILEM IL-1000 is a phosphonium-based ionic liquid with a high viscosity (~300 cP) and low volatility; thus, it is suitable for SEM imaging of biological samples. NanoSuit® Type III is a proprietary polymer solution that forms a thin protective membrane following electron beam irradiation. It has moderate viscosity and low electrical conductivity. The IL and NanoSuit® solutions were diluted with purified water to 5%, 10%, 20%, or 40% concentrations. Equal-volume mixtures of silica particle suspensions with the diluted IL or NanoSuit® solution were prepared at each concentration. The final IL and NanoSuit® solution concentrations were 2.5%, 5%, 10%, and 20%. Each mixture (2 µL) was dropped onto a highly polished carbon stub and allowed to dry at ambient temperature (22–25 °C) for approximately 10 min to facilitate evaporation of the dispersant and absorption into the stub for subsequent FE-SEM observations.
2.3. LcS Preparation for FE-SEM Using the IL and NanoSuit® Methods
Cultured LcS cells were washed with PB, fixed in 2.5% glutaraldehyde, rewashed in PB, and resuspended in purified water. Equal-volume mixtures of the LcS suspension with IL (10% or 20% dilution) or NanoSuit® (10%, 20%, or 40% dilution) solutions were prepared using purified water. The final IL concentrations were 5% and 10%, whereas NanoSuit® solution concentrations were 5%, 10%, and 20%. Each mixture (2 µL) was dropped onto a highly polished carbon stub and allowed to dry at ambient temperature (22–25 °C) for approximately 10 min to facilitate evaporation of the dispersant and absorption into the stub for subsequent FE-SEM observations.
2.4. LcS Culture
LcS cells were cultured in De Man–Rogosa–Sharpe liquid medium (Becton Dickinson Biosciences, Franklin Lakes, NJ, USA) under aerobic conditions at 37 °C for 24 h prior to the experiment, as previously reported [28].
2.5. FE-SEM Observations
LcS cells were observed under a field emission scanning electron microscope (FE-SEM; SU8220, Hitachi High-Tech, Tokyo, Japan) at an acceleration voltage of 1.0 kV, an emission current of 1–10 µA, a working distance of 2.0–5.2 mm, and a magnification of 20,000×. The silica particles were observed under FE-SEM at an acceleration voltage of 1.0 kV, an emission current of 5 µA, a working distance of 2.0 mm, and a magnification of 150,000×. For the silica particle specimens, the maximum and minimum diameters of 20 particles per sample were measured using the length measurement function of the FE-SEM.
2.6. Statistical Analysis
After averaging the two particle diameter measurements for the silica particle specimens, Bartlett’s test (5% significance level) was used to determine the variance homogeneity. If the variances were equal, Dunnett’s test was used to compare each treatment group with the control; otherwise, Steel’s test, which is a non-parametric alternative, was used. These tests were selected based on the data distribution and variance characteristics. All statistical analyses were performed using EZR software (ver. 1.54) [29].
Before the experiment, a power analysis was carried out based on preliminary data comparing the IL 2.5% group with the control group, which yielded a large effect size (Cohen’s d = 1.11). The analysis indicated that a sample size of 14 particles per group would be sufficient to achieve 80% power at a 5% significance level. Therefore, the use of 20 particles per group was considered appropriate for validating morphological preservation.
3. Results
3.1. Effect of the CPD Method on the Bacterial Surface of LcS
The bacterial surface appeared smooth after PB washing (Figure 1A), glutaraldehyde fixation (Figure 1B), and osmium tetroxide fixation (Figure 1C); however, the focus was disrupted by the effects of outgassing caused by water evaporation. In contrast, irregular reticulate structures were observed on bacterial surfaces following ethanol dehydration (Figure 1D), isoamyl acetate substitution (Figure 1E), and CPD (Figure 1F), which indicates that dehydration contributed to their formation. After ethanol dehydration, the LcS specimens were re-immersed in PB; however, no reticulate structures were observed, and the bacterial surface appeared smooth. Even at this stage, the focus was disrupted by the outgassing effect caused by water evaporation (Figure 1G).
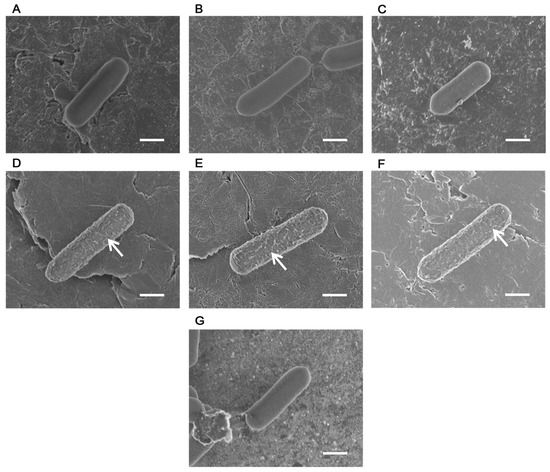
Figure 1.
Field emission scanning electron microscopy images of Lacticaseibacillus paracasei strain Shirota (LcS) at various treatment stages using the critical point drying (CPD) method. (A–C) Before dehydration, the bacterial surface of LcS appeared smooth. (D–F) After dehydration, a reticulate structure formed on the bacterial surface (arrows). (G) Following dehydration, the bacterial surface of LcS suspended in phosphate buffer (PB) was smooth. (A) After PB washing; (B) after glutaraldehyde fixation; (C) after osmium tetroxide fixation; (D) after ethanol dehydration; (E) after isoamyl acetate substitution; (F) after CPD; (G) after dehydration and immersion in PB. Scale bars: 0.5 µm.
3.2. Dilution Concentrations of IL and NanoSuit® Solutions
FE-SEM observations of silica particles at 2.5%, 5%, 10%, and 20% dilutions of IL and NanoSuit® solutions are presented in Figure 2. No discernible differences were observed in silica particle surface morphology between the IL or NanoSuit® methods and the control. Table 1 lists the silica particle size measurements following treatment with various IL and NanoSuit® solutions. Notably, 2.5% and 20% IL and 2.5% NanoSuit® solutions exhibited significant (p < 0.05) size differences compared with the control, and were larger in all cases. Thus, the diameter of the silica particles treated with 5% and 10% IL and 5%, 10%, and 20% NanoSuit® methods did not differ from that of the untreated particle. Therefore, FE-SEM sample preparation of LcS was carried out using these concentrations.
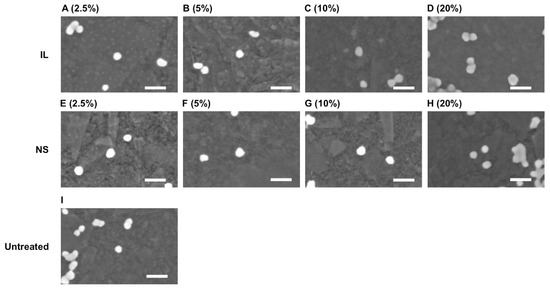
Figure 2.
Field emission scanning electron microscopy images of the silica particles. Surface morphology of the silica particles, which were prepared by the control, ionic liquid (IL), and NanoSuit® (NS) methods, exhibited no clear differences. (A–D) IL method; (E–H) NS method; (I) untreated. Liquid concentrations are shown in parentheses. Scale bars: 60 nm.

Table 1.
Silica particle size measurements obtained using the NanoSuit® (NS) or the ionic liquid (IL) method.
3.3. FE-SEM Observations of LcS Prepared by the IL and NanoSuit® Methods
FE-SEM images of the LcS specimens were captured following preparation using IL at 5% and 10% and NanoSuit® at 5%, 10%, and 20%. For all concentrations and both methods, the surface of LcS was smooth, with no reticulate structures observed (Figure 3). Moreover, no blurring of the focus caused by the outgassing effect of water evaporation occurred, which had been observed when moisture is present (i.e., Figure 1A–C,G).
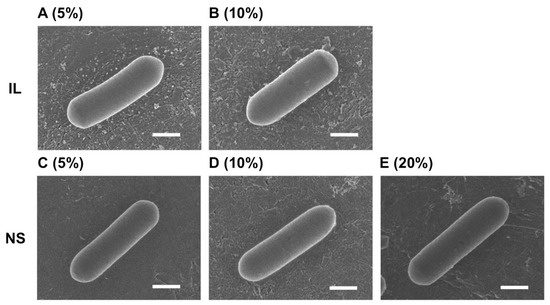
Figure 3.
Field emission scanning electron microscopy images of Lacticaseibacillus paracasei strain Shirota (LcS) prepared by the ionic liquid (IL) or NanoSuit® (NS) method. (A–E) For both methods, the surface of LcS was smooth, with no reticulate structures evident. (A) 5% IL; (B) 10% IL; (C) 5% NS solution; (D) 10% NS solution; (E) 20% NS solution. Scale bars: 0.5 µm.
4. Discussion
In this study, we examined the effect of CPD on field emission scanning electron microscopy FE-SEM images of LcS and evaluated the applicability of IL and NanoSuit® methods. To determine the effect of CPD, each procedural step from the LcS culture to CPD was analyzed. The results indicated that neither glutaraldehyde nor osmium tetroxide fixation altered the surface microstructure of LcS, which indicates that fixation does not affect the surface microstructure. Moreover, a reticulate structure was observed on the bacterial surface during the dehydration step. Furthermore, FE-SEM observations of LcS cells washed in phosphate buffer (PB) following cultivation or resuspended in PB after dehydration revealed the effect of outgassing. This prompted us to evaluate the applicability of the IL and NanoSuit® methods, which enable observation without dehydration. At optimal concentrations, no outgassing was observed, and the LcS bacterial surface was smooth and consistent with the SEM images before fixation.
Regarding the formation of a reticulate structure on the LcS bacterial surface as a result of dehydration, in C. albicans, it was demonstrated that dehydration eliminates free water between the surface glycans, thus altering the surface morphology [19]. A comparable process may have occurred in LcS, leading to modifications in the surface microstructure. LcS synthesizes a polysaccharide (designated LCPS-1) that is bound to a highly linear cell wall, with a molecular weight exceeding 100 kDa [28]. Previously, we established an LCPS-1-deficient mutant and examined its cell surface structure following CPD treatment using FE-SEM. At that time, the reticulate structure observed in the current study was not detected in the LCPS-1-deficient mutant [28]. Based on these studies and our current observations, dehydration treatment may remove free or bound water associated with LCPS-1 on the LcS cell surface, resulting in the formation of a reticulate structure through the contraction of the linear polysaccharide chains. Furthermore, with respect to the preservation of the LcS surface structure, the chemical properties of the bacterial surface layer may affect the state of preservation. LCPS-1 exhibits a high water-retention capacity and contributes to resistance against acids and bile acids [28]. Although such water-retention capacity induces structural changes during dehydration, in non-dehydration methods, such as the IL method and NanoSuit® method, the preservation of the LCPS-1 structure likely enables observation of the bacterial surface microstructure without alteration.
IL and NanoSuit® solutions are highly viscous and may obscure or distort the nanoscale structures on the sample surface. Therefore, silica particles with known particle sizes were used as a reference to evaluate the dilution conditions that preserved the particle morphology. No difference in particle size was observed when treated with 5% and 10% IL, or 5%, 10%, and 20% NanoSuit® solutions compared with that of the untreated particles. This suggests that morphological changes resulting from residual IL or NanoSuit® solution do not occur at these concentrations. Based on these findings and the fact that glutaraldehyde or osmium tetroxide fixation does not affect the surface morphology of LcS, LcS after glutaraldehyde fixation was observed at concentrations of IL or NanoSuit® solution that did not cause morphological changes in LcS. The LcS bacterial surface appeared smooth, similar to the SEM images before fixation, whereas the outgassing effect observed with untreated or re-immersed LcS was not observed. Taken together, these results indicate that LcS may be observed in near-complete conditions when these techniques are applied at optimal concentrations. This is the first study to demonstrate the use of these nondehydration preparation methods in SEM imaging of probiotics. The LcS selected for this study exhibits well-characterized water-retention properties, which are common to many other probiotic strains, suggesting that this imaging technique holds considerable promise for other probiotics.
The are several limitations to this study, for example, the lack of an evaluation of the biological effects of IL and NanoSuit®, and the absence of a quantitative assessment of surface structure preservation. As this study did not evaluate the toxicity of IL and NanoSuit® on bacterial cells or their effects on EPS solubility or membrane permeability, these treatments may potentially affect cell surface structure or physiological function [30,31].
In particular, chemical interactions with the preservation solution are possible in unfixed samples [32], necessitating future verification. The evaluation of the surface structure by FE-SEM imaging was limited to qualitative comparisons; however, quantitative morphometric and statistical analyses were not performed. This makes the objective assessment of the preservation status difficult. Nevertheless, verification using silica particles showed no significant change in particle size at appropriate concentrations of IL and NanoSuit®, suggesting that these solutions do not affect the surface structure. Future studies should focus on conducting detailed evaluations of the biological effects (toxicity, EPS solubility, and membrane permeability) of IL and NanoSuit® using unfixed samples. Furthermore, quantitative assessments of the preservation state of the surface structures using morphometric techniques (e.g., AFM or image analysis) will be necessary. These studies could lead to the establishment of high-precision visualization techniques for probiotic surface structures. Although advanced techniques, such as the TRUST method [33] and droplet spotter systems [34], have expanded the scope of biological SEM applications, we focused specifically on morphological preservation of probiotic surfaces. Nevertheless, these emerging approaches may offer valuable insights for future studies into hydrated specimen imaging and molecular-level structural analyses.
5. Conclusions
In this study, we demonstrated that CPD, a method widely used for FE-SEM sample preparation, induces morphological changes on the surface of LcS resulting from dehydration and leads to the formation of a reticulate structure. In contrast, the IL and NanoSuit® methods maintained surface water retention and prevented structural collapse, rendering LcS observable by FE-SEM in a nearly intact state. These results emphasize the importance of selecting appropriate preparation techniques when analyzing the surface structure of probiotic bacteria. Moreover, the water-retention capacity of surface polysaccharides, such as LCPS-1, plays an important role in maintaining the structural integrity of bacterial surfaces during dehydration. Consequently, the IL and NanoSuit® methods represent promising alternatives, particularly for the morphological evaluation of microorganisms containing hydrophilic extracellular components.
Author Contributions
Conceptualization, M.A.; methodology, M.A.; validation, M.A., C.H. and H.N.; formal analysis, M.A., C.H. and H.N.; investigation, M.A., C.H. and H.N.; resources, M.A.; data curation, C.H. and H.N.; writing—original draft preparation, M.A.; writing—review and editing, M.A., C.H., H.N., M.T. and K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to express their sincere gratitude to Gaku Wagai, Tomonori Aida, Yuki Ohta-Takada, Jun Otsuka, and Tomo Suzuki for their useful discussions and advice.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pretorius, E. Influence of acceleration voltage on scanning electron microscopy of human blood platelets. Microsc. Res. Tech. 2010, 73, 225–228. [Google Scholar] [CrossRef]
- Khaleel, D.S.; Mutter, T.Y.; Huang, X. Potential mechanism of gallic acid-coated iron oxide nanoparticles against associated genes of Klebsiella pneumoniae capsule, antibacterial and antibiofilm. Microsc. Res. Tech. 2024, 87, 2774–2784. [Google Scholar] [CrossRef]
- Famuyide, I.M.; Fasina, F.O.; Eloff, J.N.; McGaw, L.J. The ultrastructural damage caused by Eugenia zeyheri and Syzygium legatii acetone leaf extracts on pathogenic Escherichia coli. BMC Vet. Res. 2020, 16, 326. [Google Scholar] [CrossRef]
- Kim, M.-A.; Rosa, V.; Min, K.-S. Characterization of Enterococcus faecalis in different culture conditions. Sci. Rep. 2020, 10, 21867. [Google Scholar] [CrossRef]
- Raulio, M.; Järn, M.; Ahola, J.; Peltonen, J.; Rosenholm, J.B.; Tervakangas, S.; Kolehmainen, J.; Ruokolainen, T.; Narko, P.; Salkinoja-Salonen, M. Microbe repelling coated stainless steel analysed by field emission scanning electron microscopy and physicochemical methods. J. Ind. Microbiol. Biotechnol. 2008, 35, 751–760. [Google Scholar] [CrossRef]
- Sommer, F.; Bäckhed, F. The gut microbiota—Masters of host development and physiology. Nat. Rev. Microbiol. 2013, 11, 227–238. [Google Scholar] [CrossRef]
- Schroeder, B.O.; Bäckhed, F. Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med. 2016, 22, 1079–1089. [Google Scholar] [CrossRef]
- Togao, M.; Kurakawa, T.; Tajima, S.; Wagai, G.; Ohta-Takada, Y.; Otsuka, J.; Kurita, A.; Kawakami, K. Human gut microbiota influences drug-metabolizing enzyme hepatic Cyp3a: A human flora-associated mice study. J. Toxicol. Sci. 2023, 48, 333–343. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Chapot-Chartier, M.-P.; Kulakauskas, S. Cell wall structure and function in lactic acid bacteria. Microb. Cell Fact. 2014, 13, S9. [Google Scholar] [CrossRef]
- Yasuda, E.; Serata, M.; Sako, T. Suppressive effect on activation of macrophages by Lactobacillus casei strain Shirota genes determining the synthesis of cell wall-associated polysaccharides. Appl. Environ. Microbiol. 2008, 74, 4746–4755. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Hara, T.; Nagaoka, M.; Mike, A.; Mitsuyama, K.; Sako, T.; Yamamoto, M.; Kado, S.; Takada, T. A component of polysaccharide peptidoglycan complex on Lactobacillus induced an improvement of murine model of inflammatory bowel disease and colitis-associated cancer. Immunology 2009, 128 Pt 2, e170–e180. [Google Scholar] [CrossRef] [PubMed]
- Couvigny, B.; Lapaque, N.; Rigottier-Gois, L.; Guillot, A.; Chat, S.; Meylheuc, T.; Kulakauskas, S.; Rohde, M.; Mistou, M.; Renault, P. Three glycosylated serine-rich repeat proteins play a pivotal role in adhesion and colonization of the pioneer commensal bacterium, Streptococcus salivarius. Environ. Microbiol. 2017, 19, 3579–3594. [Google Scholar] [CrossRef] [PubMed]
- Oxaran, V.; Ledue-Clier, F.; Dieye, Y.; Herry, J.-M.; Péchoux, C.; Meylheuc, T.; Briandet, R.; Juillard, V.; Piard, J.-C. pilus biogenesis in Lactococcus lactis: Molecular characterization and role in aggregation and biofilm formation. PLoS ONE 2012, 7, e50989. [Google Scholar] [CrossRef]
- Hazrin-Chong, N.H.; Manefield, M. An Alternative SEM Drying Method Using Hexamethyldisilazane (HMDS) for Microbial Cell Attachment Studies on Sub-bituminous Coal. J. Microbiol. Methods 2012, 90, 96–99, Corrigendum in J. Microbiol. Methods 2015, 110, 102. https://doi.org/10.1016/j.mimet.2015.01.006. [Google Scholar]
- Logan, M.A.; O’Meara, D.L.; Monkowski, J.R.; Cowles, H. Particulate Contamination on Wafer Surfaces Resulting from Hexamethyldisilazane/Water Interactions. In Particles on Surfaces 1; Mittal, K.L., Ed.; Springer: Boston, MA, USA, 1988; pp. 57–68. [Google Scholar] [CrossRef]
- Malyshev, D.; Lee, C.C.; Andersson, M. Evaluating Bacterial Spore Preparation Methods for Scanning Electron Microscopy. Microsc. Microanal. 2024, 30, 564–573. [Google Scholar] [CrossRef]
- Amako, K.; Umeda, A. Bacterial surfaces as revealed by the high resolution scanning electron microscope. J. Gen. Microbiol. 1977, 98, 297–299. [Google Scholar] [CrossRef]
- Kusamichi, M.; Monodane, T.; Tokunaga, M.; Koike, H. Influence of surrounding media on preservation of cell wall ultrastructure of Candida albicans revealed by low temperature scanning electron microscopy. J. Electron. Microsc. 1990, 39, 477–486. [Google Scholar] [CrossRef]
- Kawasaki, H.; Itoh, T.; Takaku, Y.; Suzuki, H.; Kosugi, I.; Meguro, S.; Iwashita, T.; Hariyama, T. The Nanosuit method: A novel histological approach for examining paraffin sections in a nondestructive manner by correlative light and electron microscopy. Lab. Investig. 2020, 100, 161–173. [Google Scholar] [CrossRef]
- Joubert, L.-M.; McDonald, K. SEM visualization of biological samples using Hitachi ionic liquid HILEM® IL 1000: A comparative study. Microsc. Microanal. 2016, 22, 1170–1171. [Google Scholar] [CrossRef]
- Takaku, Y.; Suzuki, H.; Ohta, I.; Ishii, D.; Muranaka, Y.; Shimomura, M.; Hariyama, T. A Thin polymer membrane, nano-suit, enhancing survival across the continuum between air and high vacuum. Proc. Natl. Acad. Sci. USA 2013, 110, 7631–7635. [Google Scholar] [CrossRef]
- Takaku, Y.; Suzuki, H.; Kawasaki, H.; Ohta, I.; Ishii, D.; Hirakawa, S.; Tsutsui, T.; Matsumoto, H.; Takehara, S.; Nakane, C.; et al. A modified ‘NanoSuit®’ preserves wet samples in high vacuum: Direct observations on cells and tissues in field-emission scanning electron microscopy. R. Soc. Open Sci. 2017, 4, 160887. [Google Scholar] [CrossRef]
- Inoue, M.; Suganami, M.; Hahimoto, Y.; Iyasu, T.; Saito, H.; Moriguchi, K.; Tanaka, T. Application of ionic liquid coating method to observation of non-conductive samples by a mobile scanning electron microscope for elementary science education. J. Surf. Anal. 2011, 18, 105–109. [Google Scholar] [CrossRef][Green Version]
- Asahi, Y.; Miura, J.; Tsuda, T.; Kuwabata, S.; Tsunashima, K.; Noiri, Y.; Sakata, T.; Ebisu, S.; Hayashi, M. Simple observation of Streptococcus mutans biofilm by scanning electron microscopy using ionic liquids. AMB Express 2015, 5, 6. [Google Scholar] [CrossRef]
- Takahashi, C.; Ogawa, N.; Kawashima, Y.; Yamamoto, H. Observation of antibacterial effect of biodegradable polymeric nanoparticles on Staphylococcus epidermidis biofilm using FE-SEM with an ionic liquid. Microscopy 2015, 64, 169–180. [Google Scholar] [CrossRef]
- Takahashi, C.; Kalita, G.; Ogawa, N.; Moriguchi, K.; Tanemura, M.; Kawashima, Y.; Yamamoto, H. Electron microscopy of Staphylococcus epidermidis fibril and biofilm formation using image-enhancing ionic liquid. Anal. Bioanal. Chem. 2015, 407, 1607–1613. [Google Scholar] [CrossRef]
- Kato, K.; Serata, M.; Nakamura, M.; Ando, M.; Suzuki, T.; Okumura, T. Cell wall polysaccharide enhances Lacticaseibacillus paracasei strain Shirota growth in milk and contributes to acid and bile tolerance. Int. J. Food. Microbiol. 2024, 422, 110811. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone. Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Gonçalves, A.R.P.; Paredes, X.; Cristino, A.F.; Santos, F.J.V.; Queirós, C.S.G.P. Ionic liquids—A review of their toxicity to living organisms. Int. J. Mol. Sci. 2021, 22, 5612. [Google Scholar] [CrossRef]
- Gomez-Herrero, E.; Tobajas, M.; Polo, A.; Rodriguez, J.J.; Mohedano, A.F. Toxicity and inhibition assessment of ionic liquids by activated sludge. Ecotoxicol. Environ. Saf. 2020, 187, 109836. [Google Scholar] [CrossRef]
- Kim, K.W. Biological applications of the NanoSuit for electron imaging and X-microanalysis of insulating specimens. Appl. Microsc. 2022, 52, 4. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Kang, L.; Tsang, V.T.; Zhang, Y.; Wong, I.H.; Wong, T.T. translational rapid ultraviolet-excited sectioning tomography for whole-organ multicolor imaging with real-time molecular staining. eLife 2022, 11, e81015. [Google Scholar] [CrossRef] [PubMed]
- Yogi, O.; Kawakami, T.; Mizuno, A. On-demand mixing droplet spotter for preparing picoliter droplets on surfaces. Anal. Chem. 2004, 76, 2991–2996. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).